New Insights on the Role of Marinobufagenin from Bench to Bedside in Cardiovascular and Kidney Diseases
Abstract
:1. Introduction
2. Biochemical Structure and Production
Mechanism of Action
3. Extraction Techniques
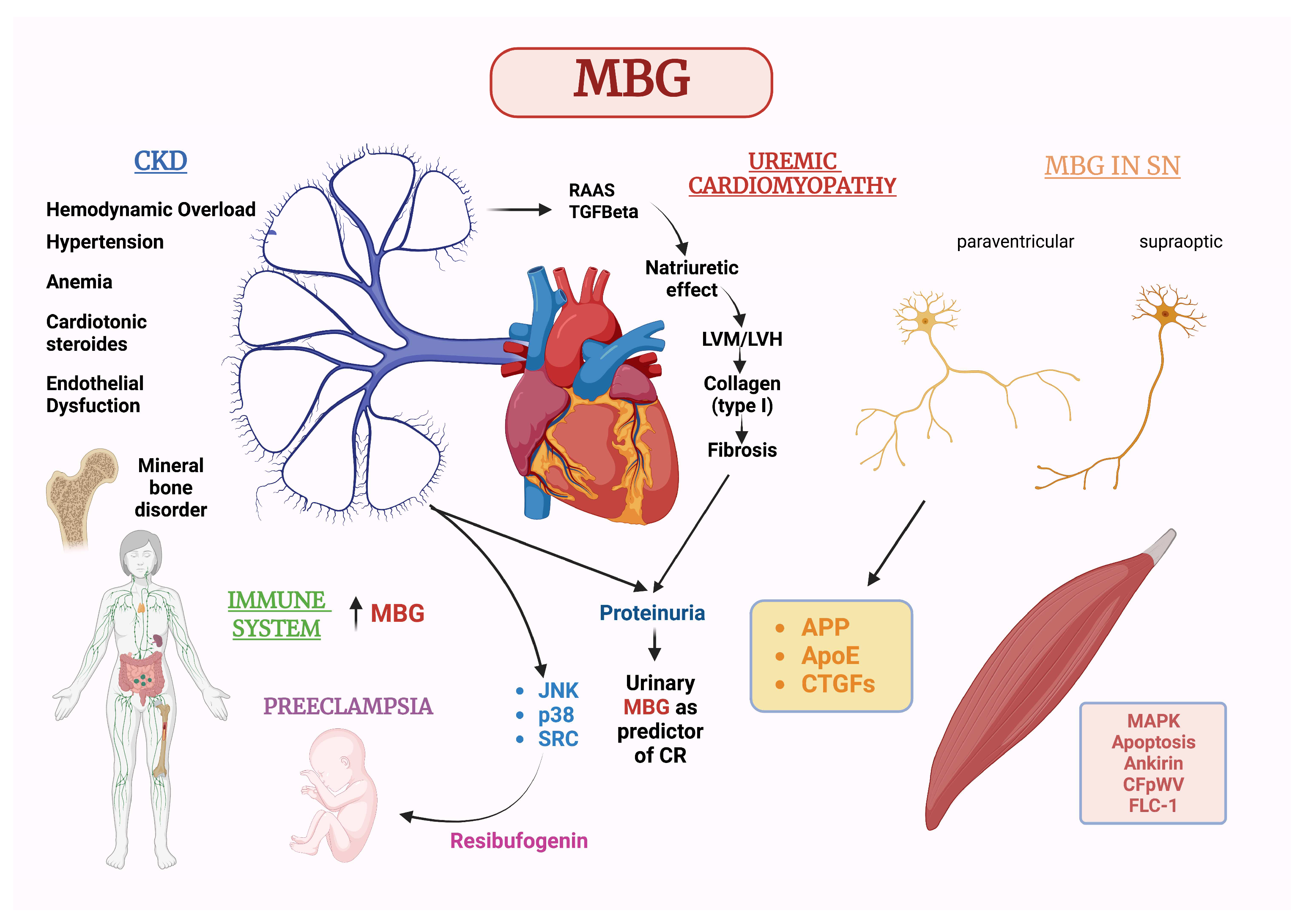
4. Marinobufagenin and Chronic Kidney Disease
5. Marinobufagenin and CV Diseases
6. MBG in Relation to Sex and Gender Medicine with a Special Focus on Pre-Eclampsia
7. MBG Action on the Nervous System
8. MBG Action on Cells of the Immune System
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schoner, W. Endogenous cardiac glycosides, a new class of steroid hormones. Eur. J. Biochem. 2002, 269, 2440–24488. [Google Scholar] [CrossRef] [PubMed]
- Schoner, W.; Scheiner-Bobis, G. Endogenous cardiac glycosides: Hormones using the sodium pump as signal transducer. Semin. Nephrol. 2005, 25, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and exogenous cardiac glycosides: Their roles in hypertension, salt metabolism, and cell growth. Am. J. Physiol. Cell Physiol. 2007, 293, C509–C536. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Lakatta, E.; Bagrov, A.Y. Endogenous Na,K pump ligands are differentially regulated during acute NaCl loading of Dahl rats. Circulation 2000, 102, 3009–3014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagrov, A.Y.; Fedorova, O.V.; Dmitriev, R.I.; French, A.W.; Anderson, D.E. Plasma marinobufagenin-like and ouabain-like immunoreactivity during saline volume expansion in anesthetized dogs. Cardiovasc. Res. 1996, 31, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.J.; Elkareh, J.; Shidyak, A.; Shapiro, A.P.; Smaili, S.; Mutgi, K.; Gupta, S.; Tian, J.; Morgan, E.; Khouri, S.; et al. Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. Am. J. Physiol. Renal Physiol. 2008, 294, F450–F454. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, S.; Valentine, B.; Han, C.; Fedorova, O.V.; Bagrov, A.Y.; Liu, J.; Periyasamy, S.M.; Kennedy, D.; Malhotra, D.; Xie, Z.; et al. Effect of green tea extract on cardiac hypertrophy following 5/6 nephrectomy in the rat. Kidney Int. 2003, 63, 1785–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, D.J.; Shrestha, K.; Sheehey, B.; Li, X.S.; Guggilam, A.; Wu, Y.; Finucan, M.; Gabi, A.; Medert, C.M.; Westfall, K.M.; et al. Elevated Plasma Marinobufagenin, An Endogenous Cardiotonic Steroid, Is Associated with Right Ventricular Dysfunction and Nitrative Stress in Heart Failure. Circ. Heart Fail. 2015, 8, 1068–1076. [Google Scholar] [CrossRef] [Green Version]
- Elkareh, J.; Kennedy, D.J.; Yashaswi, B.; Vetteth, S.; Shidyak, A.; Kim, E.G.R.; Smaili, S.; Periyasamy, S.M.; Hariri, I.M.; Fedorova, L.; et al. Marinobufagenin Stimulates Fibroblast Collagen Production and Causes Fibrosis in Experimental Uremic Cardiomyopathy. Hypertension 2007, 49, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Kometiani, P.; Liu, J.; Li, J.; Shapiro, J.I.; Askari, A. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J. Biol. Chem. 1999, 274, 19323–19328. [Google Scholar] [CrossRef] [Green Version]
- Pamnani, M.B.; Chen, S.; Yuan, C.M.; Haddy, F.J. Chronic blood pressure effects of bufalin, a sodium-potassium ATPase inhibitor, in rats. Hypertension 1994, 23, I106–I109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, H.; Ianosi-Irimie, M.; Danchuk, S.; Rabon; Nogawa, T.; Kamano, Y.; Pettit, G.R.; Wiese, T.; Puschett, J.B. Resibufogenin Corrects Hypertension in a Rat Model of Human Preeclampsia. Exp. Biol. Med. 2006, 231, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.V.; Ianosi-Irimie, M.R.; Pridjian, C.A.; Whitbred, J.M.; Durst, J.M.; Bagrov, A.Y.; Fedorova, O.V.; Pridjian, G.; Puschett, J.B. Involvement of marinobufagenin in a rat model of human preeclampsia. Am. J. Nephrol. 2005, 25, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Agunanne, E.; Horvat, D.; Harrison, R.; Uddin, M.; Jones, R.; Kuehl, T.; Ghanem, D.; Berghman, L.; Lai, X.; Li, J.; et al. Marinobufagenin Levels in Preeclamptic Patients: A Preliminary Report. Am. J. Perinatol. 2011, 28, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Horvat, D.; Glaser, S.S.; Mitchell, B.M.; Puschett, J.B. Examination of the Cellular Mechanisms by Which Marinobufagenin Inhibits Cytotrophoblast Function. J. Biol. Chem. 2008, 283, 17946–17953. [Google Scholar] [CrossRef] [Green Version]
- LaMarca, H.; Morris, C.; Pettit, G.; Nagowa, T.; Puschett, J. Marinobufagenin Impairs First Trimester Cytotrophoblast Differentiation. Placenta 2006, 27, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Grigorova, Y.N.; Juhasz, O.; Long, J.M.; Zernetkina, V.I.; Hall, M.L.; Wei, W.; Morrell, C.H.; Petrashevskaya, N.; Morrow, A.; LaNasa, K.H.; et al. Effect of Cardiotonic Steroid Marinobufagenin on Vascular Remodeling and Cognitive Impairment in Young Dahl-S Rats. Int. J. Mol. Sci. 2022, 23, 4563. [Google Scholar] [CrossRef]
- Carvalho, D.C.M.; Cavalcante-Silva, L.H.A.; Lima, D.A.; Galvão, J.G.F.M.; Alves, A.K.D.A.; Feijó, P.R.O.; Quintas, L.E.M.; Rodrigues-Mascarenhas, S. Marinobufagenin Inhibits Neutrophil Migration and Proinflammatory Cytokines. J. Immunol. Res. 2019, 2019, 1094520. [Google Scholar] [CrossRef] [Green Version]
- Keppel, M.H.; Piecha, G.; März, W.; Cadamuro, J.; Auer, S.; Felder, T.K.; Mrazek, C.; Oberkofler, H.; Trummer, C.; Grübler, M.R.; et al. The endogenous cardiotonic steroid Marinobufagenin and decline in estimated glomerular filtration rate at follow-up in patients with arterial hypertension. PLoS ONE 2019, 14, e0212973. [Google Scholar] [CrossRef]
- Bolignano, D.; Greco, M.; Presta, P.; Crugliano, G.; Sabatino, J.; Carullo, N.; Arena, R.; Leo, I.; Comi, A.; Andreucci, M.; et al. Altered circulating marinobufagenin levels and recurrent intradialytic hypotensive episodes in chronic hemodialysis patients: A pilot, prospective study. Rev. Cardiovasc. Med. 2021, 22, 1577–1587. [Google Scholar] [CrossRef]
- Piecha, G.; Kujawa-Szewieczek, A.; Kuczera, P.; Skiba, K.; Sikora-Grabka, E.; Więcek, A. Plasma marinobufagenin immunoreactivity in patients with chronic kidney disease: A case control study. Am. J. Physiol. Renal Physiol. 2018, 315, F637–F643. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; De Rosa, S.; Greco, M.; Presta, P.; Patella, G.; Crugliano, G.; Sabatino, J.; Strangio, A.; Romano, L.R.; Comi, A.; et al. Marinobufagenin, left ventricular geometry and cardiac dysfunction in end-stage kidney disease patients. Int. Urol. Nephrol. 2022, 54, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.L.; Racine, M.L.; Geolfos, C.J.; Gates, P.E.; Chonchol, M.; McQueen, M.B.; Seals, D.R. Seals Faculty Opinions recommendation of Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J. Am. Coll. Cardiol. 2013, 61, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauss, M.; Smith, W.; Fedorova, O.V.; Schutte, A.E. The Na+K+-ATPase Inhibitor Marinobufagenin and Early Cardiovascular Risk in Humans: A Review of Recent Evidence. Curr. Hypertens. Rep. 2019, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Strauss, M.; Smith, W.; Kruger, R.; Wei, W.; Fedorova, O.V.; Schutte, A.E. Marinobufagenin and left ventricular mass in young adults: The African-PREDICT study. Eur. J. Prev. Cardiol. 2018, 25, 1587–1595. [Google Scholar] [CrossRef]
- Lopatin, D.A.; Ailamazian, E.K.; Dmitrieva, R.I.; Shpen, V.M.; Fedorova, O.V.; Doris, P.A.; Bagrov, A.Y. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J. Hypertens. 1999, 17, 1179–1187. [Google Scholar] [CrossRef]
- Nikitina, E.R.; Mikhailov, A.V.; Nikandrova, E.S.; Frolova, E.V.; Fadeev, A.V.; Shman, V.V.; Shilova, V.Y.; Tapilskaya, N.I.; Shapiro, J.I.; Fedorova, O.V.; et al. In preeclampsia endogenous cardiotonic steroids induce vascular fibrosis and impair relaxation of umbilical arteries. J. Hypertens. 2011, 29, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Lenaerts, C.; Wells, M.; Hambye, S.; Blankert, B. Marinobufagenin extraction from Rhinella marina toad glands: Alternative approaches for a systematized strategy. J. Sep. Sci. 2019, 42, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Puschett, J.B.; Agunanne, E.; Uddin, M.N. Emerging role of the bufadienolides in cardiovascular and kidney diseases. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2010, 56, 359–370. [Google Scholar] [CrossRef]
- Krenn, L.; Kopp, B. Bufadienolides from animal and plant sources. Phytochemistry 1998, 48, 1–29. [Google Scholar] [CrossRef]
- Steyn, P.S.; van Heerden, F.R. Bufadienolides of plant and animal origin. Nat. Prod. Rep. 1998, 15, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Hilton, P.J.; White, R.W.; Lord, G.A.; Garner, G.V.; Gordon, D.B.; Hilton, M.J.; Forni, L.G.; McKinnon, W.; Ismail, F.M.; Keenan, M.; et al. An inhibitor of the sodium pump obtained from human placenta. Lancet 1996, 348, 303–305. [Google Scholar] [CrossRef]
- Dmitrieva, R.I.; Bagrov, A.Y.; Lalli, E.; Sassone-Corsi, P.; Stocco, D.M.; Doris, P.A. Mammalian bufadienolide is synthesized from cholesterol in the adrenal cortex by a pathway that Is independent of cholesterol side-chain cleavage. Hypertension 2000, 36, 442–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedorova, O.V.; Zernetkina, V.I.; Shilova, V.Y.; Grigorova, Y.N.; Juhasz, O.; Wei, W.; Marshall, C.A.; Lakatta, E.G.; Bagrov, A.Y. Synthesis of an Endogenous Steroidal Na Pump Inhibitor Marinobufagenin, Implicated in Human Cardiovascular Diseases, Is Initiated by CYP27A1 via Bile Acid Pathway. Circ. Cardiovasc. Genet. 2015, 8, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Lichtstein, D.; Gati, I.; Haver, E.; Katz, U. Digitalis-like compounds in the toad Bufo viridis: Tissue and plasma levels and significance in osmotic stress. Life Sci. 1992, 51, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; D’Arrigo, G.; Pisano, A.; Coppolino, G. Pentoxifylline for Anemia in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0134104. [Google Scholar]
- Jing, J.; Ren, W.C.; Li, C.; Bose, U.; Parekh, H.S.; Wei, M.Q. Rapid identification of primary constituents in parotoid gland secretions of the Australian cane toad using HPLC/MS-Q-TOF. Biomed. Chromatogr. 2013, 27, 685–687. [Google Scholar] [CrossRef]
- Rash, L.D.; Morales, R.A.V.; Vink, S.; Alewood, P.F. De novo sequencing of peptides from the parotid secretion of the cane toad, Bufo marinus (Rhinella marina). Toxicon 2011, 57, 208–216. [Google Scholar] [CrossRef]
- Tian, H.-Y.; Luo, S.-L.; Liu, J.-S.; Wang, L.; Wang, Y.; Zhang, D.-M.; Zhang, X.-Q.; Jiang, R.-W.; Ye, W.-C. C23 Steroids from the Venom of Bufo bufo gargarizans. J. Nat. Prod. 2013, 76, 1842–1847. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Quispe, C.; Theoduloz, C.; de Sousa, P.T.; Parizotto, C. Antiproliferative activity and new argininyl bufadienolide esters from the “cururú” toad Rhinella (Bufo) schneideri. J. Ethnopharmacol. 2014, 155, 1076–1085. [Google Scholar] [CrossRef]
- Jared, C.; Antoniazzi, M.M.; Jordão, A.E.; Silva, J.R.M.; Greven, H.; Rodrigues, M.T. Parotoid macroglands in toad (Rhinella jimi): Their structure and functioning in passive defence. Toxicon 2009, 54, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Toledo, R.C.; Jared, C. Cutaneous granular glands and amphibian venoms. Comp. Biochem. Physiol. Part A Physiol. 1995, 111, 1–29. [Google Scholar] [CrossRef]
- Mailho-Fontana, P.L.; Antoniazzi, M.M.; Toledo, L.F.; Verdade, V.K.; Sciani, J.M.; Barbaro, K.C.; Pimenta, D.C.; Rodrigues, M.T.; Jared, C. Passive and active defense in toads: The parotoid macroglands in Rhinella marina and Rhaebo guttatus. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2014, 321, 65–77. [Google Scholar] [CrossRef]
- Chen, K.; Chen, A.L. Notes on the poisonous secretions of twelve species of toads. J. Pharmacol. Exp. Ther. 1933, 47, 281–293. [Google Scholar]
- Low, B.S. Evidence from parotoid-gland secretions. Evol. Genus Bufo 1972, 55, 244–264. [Google Scholar]
- Fedorova, O.V.; Talan, M.I.; Agalakova, N.I.; Lakatta, E.G.; Bagrov, A.Y. Endogenous ligand of alpha(1) sodium pump, marinobufagenin, is a novel mediator of sodium chloride—Dependent hypertension. Circulation 2002, 105, 1122–1127. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.N.; Allen, S.R.; Jones, R.O.; Zawieja, D.C.; Kuehl, T.J. Pathogenesis of pre-eclampsia: Marinobufagenin and angiogenic imbalance as biomarkers of the syndrome. Translational research: J. Lab. Clin. Med. 2012, 160, 99–113. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and Exogenous Cardiac Glycosides and their Mechanisms of Action. Am. J. Cardiovasc. Drugs Drugs Devices Interv. 2007, 7, 173–189. [Google Scholar] [CrossRef]
- Paczula, A.; Wiecek, A.; Piecha, G. Cardiotonic Steroids—A Possible Link Between High-Salt Diet and Organ Damage. Int. J. Mol. Sci. 2019, 20, 590. [Google Scholar] [CrossRef] [Green Version]
- Bagrov, A.Y.; Shapiro, J.I.; Fedorova, O.V. Endogenous Cardiotonic Steroids: Physiology, Pharmacology, and Novel Therapeutic Targets. Pharmacol. Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef]
- Liang, M.; Tian, J.; Liu, L.; Pierre, S.; Liu, J.; Shapiro, J.; Xie, Z.-J. Identification of a Pool of Non-pumping Na/K-ATPase. J. Biol. Chem. 2007, 282, 10585–10593. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Haas, M.; Liang, M.; Cai, T.; Tian, J.; Li, S.; Xie, Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J. Biol. Chem. 2004, 27, 17250–17259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolignano, D.; Coppolino, G.; Criseo, M.; Campo, S.; Romeo, A.; Buemi, M. Aquaretic agents: What’s beyond the treatment of hyponatremia? Curr. Pharm. Des. 2007, 13, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Bagrov, A.; Dmitrieva, R.; Fedorova, O.V.; Kazakov, G.P.; Roukoyatkina, N.I.; Shpen, V.M. Endogenous marinobufagenin-like immunoreactive substance*A possible endogenous Na,K-ATPase inhibitor with vasoconstrictor activity. Am. J. Hypertens. 1996, 9, 982–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagrov, A.Y.; Fedorova, O.V. Effects of two putative endogenous digitalis-like factors, marinobufagenin and ouabain, on the Na+,K+-pump in human mesenteric arteries. J. Hypertens. 1998, 16, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Adrogué, H.J.; Madias, N.E. Sodium and Potassium in the Pathogenesis of Hypertension. N. Engl. J. Med. 2007, 356, 1966–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppolino, G.; Leonardi, G.; Andreucci, M.; Bolignano, D. Oxidative Stress and Kidney Function: A Brief Update. Curr. Pharm. Des. 2018, 24, 4794–4799. [Google Scholar] [CrossRef] [PubMed]
- Elkareh, J.; Periyasamy, S.M.; Shidyak, A.; Vetteth, S.; Schroeder, J.; Raju, V.; Hariri, I.M.; El-Okdi, N.; Gupta, S.; Fedorova, L.; et al. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: Implications for uremic cardiomyopathy. Am. J. Physiol. Physiol. 2009, 296, F1219–F1226. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Emelianov, I.V.; Bagrov, K.A.; Grigorova, Y.N.; Wei, W.; Juhasz, O.; Frolova, E.V.; Marshall, C.A.; Lakatta, E.G.; Konradi, A.O.; et al. Marinobufagenin-induced vascular fibrosis is a likely target for mineralocorticoid antagonists. J. Hypertens. 2015, 33, 1602–1610. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Kesiry, R.; Periyasamy, S.M.; Malhotra, D.; Xie, Z.; Shapiro, J.I. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 2004, 66, 227–241. [Google Scholar] [CrossRef] [Green Version]
- Fedorova, L.V.; Raju, V.; El-Okdi, N.; Shidyak, A.; Kennedy, D.J.; Vetteth, S.; Giovannucci, D.R.; Bagrov, A.Y.; Fedorova, O.V.; Shapiro, J.I.; et al. The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: Implication of epithelial-to-mesenchymal transition. Am. J. Physiol. Renal Physiol. 2009, 296, F922–F934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedorova, O.V.; Agalakova, N.I.; Talan, M.I.; Lakatta, E.G.; Bagrov, A.Y. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signalling in NaCl-loaded Dahl-S rats. J. Hypertens. 2005, 23, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.T.; Drummond, C.A.; Yan, Y.; Liu, J.; Tian, J.; Malhotra, D.; Shapiro, J.I. Passive Immunization Against Marinobufagenin Attenuates Renal Fibrosis and Improves Renal Function in Experimental Renal Disease. Am. J. Hypertens. 2013, 27, 603–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Shidyak, A.; Periyasamy, S.M.; Haller, S.; Taleb, M.; El-Okdi, N.; Elkareh, J.; Gupta, S.; Gohara, S.; Fedorova, O.V.; et al. Spironolactone Attenuates Experimental Uremic Cardiomyopathy by Antagonizing Marinobufagenin. Hypertension 2009, 54, 1313–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, C.A.; Fan, X.; Haller, S.T.; Kennedy, D.J.; Liu, J.; Tian, J. Na/K-ATPase signaling mediates miR-29b-3p regulation and cardiac fibrosis formation in mice with chronic kidney disease. PLoS ONE 2018, 13, e0197688. [Google Scholar] [CrossRef] [Green Version]
- Drummond, C.A.; Hill, M.C.; Shi, H.; Fan, X.; Xie, J.X.; Haller, S.T.; Kennedy, D.J.; Liu, J.; Garrett, M.R.; Xie, Z.; et al. Na/K-ATPase signaling regulates collagen synthesis through microRNA-29b-3p in cardiac fibroblasts. Physiol. Genom. 2016, 48, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Bolignano, D.; Greco, M.; Presta, P.; Duni, A.; Vita, C.; Pappas, E.; Mirabelli, M.; Lakkas, L.; Naka, K.K.; Brunetti, A.; et al. A small circulating miRNAs signature predicts mortality and adverse cardiovascular outcomes in chronic hemodialysis patients. Clin. Kidney J. 2023, 16, 868–878. [Google Scholar] [CrossRef]
- Lenaerts, C.; Demeyer, M.; Gerbaux, P.; Blankert, B. Analytical aspects of marinobufagenin. Clin. Chim. Acta Int. J. Clin. Chem. 2013, 421, 193–201. [Google Scholar] [CrossRef]
- Komiyama, Y.; Dong, X.H.; Nishimura, N.; Masaki, H.; Yoshika, M.; Masuda, M.; Takahashi, H. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin. Biochem. 2005, 38, 36–45. [Google Scholar] [CrossRef]
- Kerkhoff, J.; Noronha, J.D.C.; Bonfilio, R.; Sinhorin, A.P.; Rodrigues, D.D.J.; Chaves, M.H.; Vieira, G.M. Quantification of bufadienolides in the poisons of Rhinella marina and Rhaebo guttatus by HPLC-UV. Toxicon 2016, 119, 311–318. [Google Scholar] [CrossRef]
- Jiang, P.; Dou, S.; Liu, L.; Zhang, W.; Chen, Z.; Xu, R.; Ding, J.; Liu, R. Identification of Multiple Constituents in the TCM-Formula Shexiang Baoxin Pill by LC Coupled with DAD-ESI-MS-MS. Chromatographia 2009, 70, 133–142. [Google Scholar] [CrossRef]
- Miyashiro, Y.; Nishio, T.; Shimada, K. Characterization of In Vivo Metabolites of Toad Venom Using Liquid Chromatography-Mass Spectrometry. J. Chromatogr. Sci. 2008, 46, 534–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha-Filho, G.A.; Resck, I.S.; Cavalcanti, B.C.; Pessoa, C.; Moraes, M.O.; Ferreira, J.R.; Rodrigues, F.A.; dos Santos, M.L. Cytotoxic profile of natural and some modified bufadienolides from toad Rhinella schneideri parotoid gland secretion. Toxicon 2010, 56, 339–348. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Roukoyatkina, N.I.; Pinaev, A.G.; Dmitrieva, R.I.; Fedorova, O.V. Effects of two endogenous Na+,K(+)-ATPase inhibitors, marinobufagenin and ouabain, on isolated rat aorta. Eur. J. Pharmacol. 1995, 274, 151–158. [Google Scholar] [CrossRef]
- Shimada, K.; Kurata, Y.; Oe, T. Utility of Cyclodextrin in Mobile Phase for High-Performance Liquid Chromatographic Separation of Bufadienolides. J. Liq. Chromatogr. 1990, 13, 493–504. [Google Scholar] [CrossRef]
- Cunha Filho, G.A.; Schwartz, C.A.; Resck, I.S.; Murta, M.M.; Lemos, S.S.; Castro, M.S.; Kyaw, C.; Pires, O.R., Jr.; Leite, J.R.; Bloch, C.; et al. Antimicrobial activity of the bufadienolides marinobufagin and telocinobufagin isolated as major components from skin secretion of the toad Bufo rubescens. Toxicon 2005, 45, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, Z.; Wang, C.; Shen, A.; Liu, Y.; Zhang, X.; Zhao, W.; Liang, X. Purification of bufadienolides from the skin of Bufo bufo gargarizans Cantor with positively charged C18 column. J. Pharm. Biomed. Anal. 2014, 92, 105–113. [Google Scholar] [CrossRef]
- Banfi, F.F.; Guedes, K.D.S.; Andrighetti, C.R.; Aguiar, A.C.; Debiasi, B.W.; Noronha, J.D.C.; Rodrigues, D.D.J.; Júnior, G.M.V.; Sanchez, B.A.M. Antiplasmodial and Cytotoxic Activities of Toad Venoms from Southern Amazon, Brazil. Korean J. Parasitol. 2016, 54, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Shimada, K.; Nambara, T. Isolation and characterization of cardiotonic steroid conjugates from the skin of Bufo marinus (L.) Schneider. Chem. Pharm. Bull. 1979, 27, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Strauss, M.; Smith, W.; Wei, W.; Bagrov, A.Y.; Fedorova, O.V.; Schutte, A.E. Large artery stiffness is associated with marinobufagenin in young adults: The African-PREDICT study. J. Hypertens. 2018, 36, 2333–2339. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Lakatta, E.G.; Bagrov, A.Y.; Melander, O. Plasma level of the endogenous sodium pump ligand marinobufagenin is related to the salt-sensitivity in men. J. Hypertens. 2015, 33, 534–541, discussion 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.E.; Fedorova, O.V.; Morrell, C.H.; Longo, D.L.; Kashkin, V.A.; Metzler, J.D.; Bagrov, A.Y.; Lakatta, E.G.; Piecha, G.; Kujawa-Szewieczek, A.; et al. Endogenous sodium pump inhibitors and age-associated increases in salt sensitivity of blood pressure in normotensives. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R1248–R1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauss, M.; Smith, W.; Wei, W.; Fedorova, O.V.; Schutte, A.E. Marinobufagenin is related to elevated central and 24-h systolic blood pressures in young black women: The African-PREDICT Study. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2018, 41, 183–192. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [Green Version]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.-y. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Coppolino, G.; Faga, T.; Garofalo, C.; Serra, R.; Andreucci, M. Epidemiology of cardiovascular risk in chronic kidney disease patients: The real silent killer. Rev. Cardiovasc. Med. 2019, 20, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provenzano, M.; Coppolino, G.; De Nicola, L.; Serra, R.; Garofalo, C.; Andreucci, M.; Bolignano, D. Unraveling Cardiovascular Risk in Renal Patients: A New Take on Old Tale. Front. Cell Dev. Biol. 2019, 7, 314. [Google Scholar] [CrossRef] [Green Version]
- Bolignano, D.; Pisano, A.; Coppolino, G. The Dark Side of Blocking RAS in Diabetic Patients with Incipient or Manifested Nephropathy. Exp. Clin. Endocrinol. Diabetes 2015, 124, 350–360. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Doris, P.A.; Bagrov, A.Y. Endogenous Marinobufagenin-Like Factor in Acute Plasma Volume Expansion. Clin. Exp. Hypertens. 1998, 20, 581–591. [Google Scholar] [CrossRef]
- Hauck, C.; Frishman, W.H. Systemic hypertension: The roles of salt, vascular Na+/K+ ATPase and the endogenous glycosides, ouabain and marinobufagenin. Cardiol. Rev. 2012, 20, 130–138. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Vetteth, S.; Periyasamy, S.M.; Kanj, M.; Fedorova, L.; Khouri, S.; Kahaleh, M.B.; Xie, Z.; Malhotra, D.; Kolodkin, N.I.; et al. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension 2006, 47, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shapiro, J.I. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat. Rev. Nephrol. 2019, 15, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Suassuna, P.G.D.A.; Sanders-Pinheiro, H.; de Paula, R.B. Uremic Cardiomyopathy: A New Piece in the Chronic Kidney Disease-Mineral and Bone Disorder Puzzle. Front. Med. 2018, 5, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, R.J.; Parfrey, P.S.; Foley, R.N. Left ventricular hypertrophy in the renal patient. J. Am. Soc. Nephrol. JASN 2001, 12, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Rigatto, C.; Parfrey, P.; Foley, R.; Negrijn, C.; Tribula, C.; Jeffery, J. Congestive heart failure in renal transplant recipients: Risk factors, outcomes, and relationship with ischemic heart disease. J. Am. Soc. Nephrol. 2002, 13, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Parfrey, P.S. Is renal insufficiency an atherogenic state? Reflections on prevalence, incidence, and risk. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2001, 37, 154–156. [Google Scholar] [CrossRef]
- D’Agostino, M.; Mauro, D.; Zicarelli, M.; Carullo, N.; Greco, M.; Andreucci, M.; Coppolino, G.; Bolignano, D. miRNAs in Uremic Cardiomyopathy: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 5425. [Google Scholar] [CrossRef]
- Zeisberg, M.; Kalluri, R. Fibroblasts emerge via epithelial-mesenchymal transition in chronic kidney fibrosis. Front Biosci. 2008, 13, 6991–6998. [Google Scholar] [CrossRef] [Green Version]
- Kometiani, P.; Li, J.; Gnudi, L.; Kahn, B.B.; Askari, A.; Xie, Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J. Biol. Chem. 1998, 273, 15249–15256. [Google Scholar] [CrossRef] [Green Version]
- Bolignano, D.; Greco, M.; Presta, P.; Caglioti, A.; Carullo, N.; Zicarelli, M.; Foti, D.P.; Dragone, F.; Andreucci, M.; Coppolino, G. Marinobufagenin, Left Ventricular Hypertrophy and Residual Renal Function in Kidney Transplant Recipients. J. Clin. Med. 2023, 12, 3072. [Google Scholar] [CrossRef]
- Coppolino, G.; Bolignano, D.; Presta, P.; Ferrari, F.F.; Lionetti, G.; Borselli, M.; Randazzo, G.; Andreucci, M.; Bonelli, A.; Errante, A.; et al. Acquisition of optical coherence tomography angiography metrics during hemodialysis procedures: A pilot study. Front. Med. 2022, 9, 1057165. [Google Scholar] [CrossRef]
- Coppolino, G.; Lucisano, G.; Bolignano, D.; Buemi, M. Acute cardiovascular complications of hemodialysis. Minerva Urol. Nefrol. 2010, 62, 67–80. [Google Scholar] [PubMed]
- Uddin, M.N.; Horvat, D.; Childs, E.W.; Puschett, J.B. Marinobufagenin causes endothelial cell monolayer hyperpermeability by altering apoptotic signaling. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R1726–R1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedorova, O.V.; Kolodkin, N.I.; Agalakova, N.I.; Lakatta, E.G.; Bagrov, A.Y. Marinobufagenin, an endogenous alpha-1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension 2001, 37, 462–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedorova, O.V.; Kolodkin, N.I.; Agalakova, N.I.; Namikas, A.R.; Bzhelyansky, A.; St-Louis, J.; Lakatta, E.G.; Bagrov, A.Y. Antibody to marinobufagenin lowers blood pressure in pregnant rats on a high NaCl intake. J. Hypertens. 2005, 23, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Bagrov, A.Y.; Fedorova, O.V.; Dmitrieva, R.I.; Howald, W.N.; Hunter, A.P.; Kuznetsova, E.A.; Shpen, V.M. Characterization of a urinary bufodienolide Na+,K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension 1998, 31, 1097–1103. [Google Scholar] [CrossRef] [Green Version]
- Tomaschitz, A.; Piecha, G.; Ritz, E.; Meinitzer, A.; Haas, J.; Pieske, B.; Wiecek, A.; Rus-Machan, J.; Toplak, H.; März, W.; et al. Marinobufagenin in essential hypertension and primary aldosteronism: A cardiotonic steroid with clinical and diagnostic implications. Clin. Exp. Hypertens. 2014, 37, 108–115. [Google Scholar] [CrossRef]
- Tian, J.; Haller, S.; Periyasamy, S.; Brewster, P.; Zhang, H.; Adlakha, S.; Fedorova, O.V.; Xie, Z.-J.; Bagrov, A.Y.; Shapiro, J.I.; et al. Renal Ischemia Regulates Marinobufagenin Release in Humans. Hypertension 2010, 56, 914–919. [Google Scholar] [CrossRef] [Green Version]
- Kolmakova, E.V.; Haller, S.T.; Kennedy, D.J.; Isachkina, A.N.; Budny, G.V.; Frolova, E.V.; Piecha, G.; Nikitina, E.R.; Malhotra, D.; Fedorova, O.V.; et al. Endogenous cardiotonic steroids in chronic renal failure. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2011, 26, 2912–2919. [Google Scholar] [CrossRef] [Green Version]
- Meneton, P.; Jeunemaitre, X.; de Wardener, H.E.; Macgregor, G.A. Links Between Dietary Salt Intake, Renal Salt Handling, Blood Pressure, and Cardiovascular Diseases. Physiol. Rev. 2005, 85, 679–715. [Google Scholar] [CrossRef] [Green Version]
- Pamnani, M.B.; Chen, S.; Bryant, H.J.; Schooley, J.F.; Eliades, D.C.; Yuan, C.M.; Haddy, F.J. Effects of three sodium-potassium adenosine triphosphatase inhibitors. Hypertension 1991, 18, 316–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvat, D.; Severson, J.; Uddin, M.N.; Mitchell, B.; Puschett, J.B. Resibufogenin prevents the manifestations of preeclampsia in an animal model of the syndrome. Hypertens. Pregnancy 2010, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Simbirtsev, A.S.; Kolodkin, N.I.; Kotov, A.Y.; Agalakova, N.I.; Kashkin, V.A.; Tapilskaya, N.I.; Bzhelyansky, A.; Reznik, V.A.; Frolova, E.V.; et al. Monoclonal antibody to an endogenous bufadienolide, marinobufagenin, reverses preeclampsia-induced Na/K-ATPase inhibition and lowers blood pressure in NaCl-sensitive hypertension. J. Hypertens. 2008, 26, 2414–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonski, K.L.; Fedorova, O.V.; Racine, M.L.; Geolfos, C.J.; Gates, P.E.; Chonchol, M.; Fleenor, B.S.; Lakatta, E.G.; Bagrov, A.Y.; Seals, D.R. Dietary Sodium Restriction and Association with Urinary Marinobufagenin, Blood Pressure, and Aortic Stiffness. Clin. J. Am. Soc. Nephrol. 2013, 8, 1952–1959. [Google Scholar] [CrossRef] [Green Version]
- Titze, J. Sodium balance is not just a renal affair. Curr. Opin. Nephrol. Hypertens. 2014, 23, 101–105. [Google Scholar] [CrossRef]
- Oberleithner, H.; Kusche-Vihrog, K.; Schillers, H. Endothelial cells as vascular salt sensors. Kidney Int. 2010, 77, 490–494. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Xie, Z.J. The sodium pump and cardiotonic steroids-induced signal transduction protein kinases and calcium-signaling microdomain in regulation of transporter trafficking. Biochim. Biophys. Acta 2010, 1802, 1237–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, M.; Askari, A.; Xie, Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J. Biol. Chem. 2000, 275, 27832–27837. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Li, H.; Xie, Z. Ouabain-induced Hypertrophy in Cultured Cardiac Myocytes is Accompanied by Changes in Expression of Several Late Response Genes. J. Mol. Cell. Cardiol. 1997, 29, 429–437. [Google Scholar] [CrossRef]
- Liu, J.; Tian, J.; Haas, M.; Shapiro, J.I.; Askari, A.; Xie, Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J. Biol. Chem. 2000, 275, 27838–27844. [Google Scholar] [CrossRef]
- Liu, L.; Mohammadi, K.; Aynafshar, B.; Wang, H.; Li, D.; Liu, J.; Ivanov, A.V.; Xie, Z.; Askari, A. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am. J. Physiol. Cell Physiol. 2003, 284, C1550–C1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liang, M.; Liu, L.; Malhotra, D.; Xie, Z.; Shapiro, J.I. Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int. 2005, 67, 1844–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [Green Version]
- Todd, A.S.; MacGinley, R.J.; Schollum, J.B.; Johnson, R.J.; Williams, S.M.; Sutherland, W.H.; Mann, J.I.; Walker, R.J. Dietary salt loading impairs arterial vascular reactivity. Am. J. Clin. Nutr. 2010, 91, 557–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.J.; Marciniak, M.; Visagie, E.; Markandu, N.D.; Anand, V.; Dalton, R.N.; MacGregor, G.A. Effect of Modest Salt Reduction on Blood Pressure, Urinary Albumin, and Pulse Wave Velocity in White, Black, and Asian Mild Hypertensives. Hypertension 2009, 54, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, G.F. Arterial Stiffness and Hypertension. Hypertension 2014, 64, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in the Framingham Heart Study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef]
- Rodriguez, C.J.; Bibbins-Domingo, K.; Jin, Z.; Daviglus, M.L.; Goff, D.C., Jr.; Jacobs, D.R., Jr. Association of sodium and potassium intake with left ventricular mass: Coronary artery risk development in young adults. Hypertension 2011, 58, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.C.M.; Burrell, L.M.; Black, M.J.; Wu, L.L.; Dilley, R.J.; Cooper, M.E.; Johnston, C.I. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation 1998, 98, 2621–2628. [Google Scholar] [CrossRef] [Green Version]
- Grigorova, Y.N.; Wei, W.; Petrashevskaya, N.; Zernetkina, V.; Juhasz, O.; Fenner, R.; Gilbert, C.; Lakatta, E.G.; Shapiro, J.I.; Bagrov, A.Y.; et al. Dietary Sodium Restriction Reduces Arterial Stiffness, Vascular TGF-β-Dependent Fibrosis and Marinobufagenin in Young Normotensive Rats. Int. J. Mol. Sci. 2018, 19, 3168. [Google Scholar] [CrossRef] [Green Version]
- Grigorova, Y.N.; Juhasz, O.; Zernetkina, V.; Fishbein, K.W.; Lakatta, E.; Fedorova, O.V.; Bagrov, A.Y. Aortic Fibrosis, Induced by High Salt Intake in the Absence of Hypertensive Response, Is Reduced by a Monoclonal Antibody to Marinobufagenin. Am. J. Hypertens. 2015, 29, 641–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wei, W.; Shilova, V.; Petrashevskaya, N.N.; Zernetkina, V.I.; Grigorova, Y.N.; Marshall, C.A.; Fenner, R.C.; Lehrmann, E.; Wood, W.H., 3rd; et al. Monoclonal Antibody to Marinobufagenin Downregulates TGFbeta Profibrotic Signaling in Left Ventricle and Kidney and Reduces Tissue Remodeling in Salt-Sensitive Hypertension. J. Am. Heart Assoc. 2019, 8, e012138. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, M.H.; Miller, J.Z.; Luft, F.C.; Grim, C.E.; Fineberg, N.S. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 1986, 8, II127–II134. [Google Scholar] [CrossRef] [Green Version]
- Pisano, A.; D’arrigo, G.; Coppolino, G.; Bolignano, D. Biotic Supplements for Renal Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1224. [Google Scholar] [CrossRef] [Green Version]
- Regitz-Zagrosek, V.; Seeland, U. Sex and Gender Differences in Clinical Medicine. In Handbook of Experimental Pharmacolog; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–22. [Google Scholar] [CrossRef]
- Grego, S.; Pasotti, E.; Moccetti, T.; Maggioni, A.P. “Sex and gender medicine”: Il principio della medicina di genere. G. Ital. Cardiol. 2020, 21, 602–606. [Google Scholar]
- Tokatli, M.R.; Sisti, L.G.; Marziali, E.; Nachira, L.; Rossi, M.F.; Amantea, C.; Moscato, U.; Malorni, W. Hormones and Sex-Specific Medicine in Human Physiopathology. Biomolecules 2022, 12, 413. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Buemi, M.; Bolignano, D.; Barilla, A.; Nostro, L.; Crasci, E.; Campo, S.; Coppolino, G.; D’Anna, R. Preeclampsia and cardiovascular risk: General characteristics, counseling and follow-up. J. Nephrol. 2008, 21, 663–672. [Google Scholar]
- Bolignano, D.; Coppolino, G.; Aloisi, C.; Romeo, A.; Nicocia, G.; Buemi, M. Effect of a Single Intravenous Immunoglobulin Infusion on Neutrophil Gelatinase-Associated Lipocalin Levels in Proteinuric Patients with Normal Renal Function. J. Investig. Med. 2008, 56, 997–1003. [Google Scholar] [CrossRef]
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef]
- Duley, L. The Global Impact of Pre-eclampsia and Eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef] [PubMed]
- MacKay, A.P.; Berg, C.J.; Liu, X.; Duran, C.; Hoyert, D.L. Changes in pregnancy mortality ascertainment: United States, 1999–2005. Obstet. Gynecol. 2011, 118, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Main, E.K. Maternal mortality: New strategies for measurement and prevention. Curr. Opin. Obstet. Gynecol. 2010, 22, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Elam-Evans, L.D.; Berg, C.J.; Herndon, J.; Flowers, L.; Seed, K.A.; Syverson, C.J. Pregnancy-related mortality surveillance—United States, 1991–1999. In Morbidity and Mortality Weekly Report Surveillance Summaries; Center for Disease Control and Prevention: Washington, DC, USA, 2003; Volume 52, pp. 1–8. [Google Scholar]
- Sturiale, A.; Coppolino, G.; Loddo, S.; Criseo, M.; Campo, S.; Crascì, E.; Bolignano, D.; Nostro, L.; Teti, D.; Buemi, M. Effects of Haemodialysis on Circulating Endothelial Progenitor Cell Count. Blood Purif. 2007, 25, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Agunanne, E.; Horvat, D.; Uddin, M.N.; Puschett, J. The Treatment of Preeclampsia in a Rat Model Employing Digibind®. Am. J. Perinatol. 2009, 27, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Puschett, J.B. Marinobufagenin Predicts and Resibufogenin Prevents Preeclampsia: A Review of the Evidence. Am. J. Perinatol. 2012, 29, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Puschett, J.; Agunanne, E.; Uddin, M. Marinobufagenin, resibufogenin and preeclampsia. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 1246–1253. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.J.M.; Bakar, N.S.; Aziz, M.A.; Basah, N.K.; Singh, H.J. Leptin-induced increase in blood pressure and markers of endothelial activation during pregnancy in Sprague Dawley rats is prevented by resibufogenin, a marinobufagenin antagonist. Reprod. Biol. 2020, 20, 184–190. [Google Scholar] [CrossRef]
- Uddin, M.N.; Agunanne, E.E.; Horvat, D.; Puschett, J.B. Resibufogenin Administration Prevents Oxidative Stress in a Rat Model of Human Preeclampsia. Hypertens. Pregnancy 2010, 31, 70–78. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Ishkaraeva, V.V.; Grigorova, Y.N.; Reznik, V.A.; Kolodkin, N.I.; Zazerskaya, I.E.; Zernetkina, V.; Agalakova, N.I.; Tapilskaya, N.I.; Adair, C.D.; et al. Antibody to Marinobufagenin Reverses Placenta-Induced Fibrosis of Umbilical Arteries in Preeclampsia. Int. J. Mol. Sci. 2018, 19, 2377. [Google Scholar] [CrossRef] [Green Version]
- Agalakova, N.I.; Grigorova, Y.N.; Ershov, I.A.; Reznik, V.A.; Mikhailova, E.V.; Nadei, O.V.; Samuilovskaya, L.; Romanova, L.A.; Adair, C.D.; Romanova, I.V.; et al. Canrenone Restores Vasorelaxation Impaired by Marinobufagenin in Human Preeclampsia. Int. J. Mol. Sci. 2022, 23, 3336. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Scuteri, A.; Agalakova, N.; Parsons, D.J.; Bagrov, A. Racial differences in resting end-tidal CO2 and circulating sodium pump inhibitor. Am. J. Hypertens. 2001, 14, 761–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantaria, N.; Pantsulaia, I.; Andronikashvili, I.; Simonia, G. Assosiation of Endogenous Cardiotonic Steroids with Salt-sensitivity of Blood Pressure in Georgian Population. Georgian Med. News 2016, 258, 33–37. [Google Scholar]
- Orlov, S.N.; Mongin, A.A. Salt-sensing mechanisms in blood pressure regulation and hypertension. Am. J. Physiol. Circ. Physiol. 2007, 293, H2039–H2053. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef] [PubMed]
| Organs and Systems | Pathological Conditions | Effects of MBG | Pre-Clinical Studies | |
|---|---|---|---|---|
| Kidney and CV system | Volume-expanding conditions: essential hypertension, heart failure, PE, CKD complications: LVH, UC, myocardial fibrosis, diastolic dysfunction | Sodium and fluid retention, organ fibrosis and remodeling, vascular and microcirculation alterations, activation of oxidative stress pathways | Model | Results |
| Fedorova, O.V., et al. [4]; male Fisher 344XNB rats and anesthetized dogs | IV saline infusion to anesthetized rats induced a significant increase in MLF plasma levels and pituitary OLC. No changes in pituitary MLF levels. Two hours of plasma volume expansion in anesthetized dogs increased urinary release of MLF. No change in OLC immunoreactivity. Evidence for the presence of a bufadienolide EDLF in mammals. Volume expansion stimulates EDLF response with the stimulation of brain OLC and plasma bufadienolide. | |||
| Bagrov, A.Y., et al. [5]; anesthetized dogs | Decreased urinary release of MLF after volume expansion, but no changes in OLC, suggesting a bufadienolide nature of mammalian EDLF. | |||
| Kennedy, D.J., et al. [6]; male CD1 mice | Plasma MBG increased after PNx. PNx caused cardiac hypertrophy and fibrosis, inducing UC. | |||
| Priyadarshi, S., et al. [7]; male Sprague Dawley rats, isolated cardiac myocytes | In rats subjected to remnant kidney surgery, the administration of green tea extract at the induced attenuation of LVH, hypertension and preserved cardiac Na-K-ATP-ase activity. In isolated cardiac myocytes, both MBG and ouabain increased ROS production, whereas the addition of green tea prevented the increase in ROS production. | |||
| Kennedy, D.J., et al. [8]; male Sprague Dawley rats | Rats with PNx had a significant increase in MBG plasma levels and urinary excretion rates and developed UC. | |||
| Elkareh, J., et al. [9]; male Sprague Dawley rats, isolated cardiac fibroblasts | PNx increased MBG levels. Heart tissue samples from rats subjected to MBG-infusion and PNx showed an important increase in collagen-1 and α smooth muscle actin, whereas immunization against MBG attenuated these effects. Cardiotonic steroids, such as MBG, play a substantial role in the pathogenesis of cardiac fibrosis. | |||
| Xie, Z., et al. [10]; neonatal ventricular myocytes cultures | Ouabain increases ROS production in cardiac myocytes, while preincubation with NAC and vitamin E reduced these effects. In cultured myocytes, the effects of ouabain on growth and growth-related genes can be dissociated from its effect on the resting intracellular Ca2+, the latter being responsible for the positive inotropic effect of this drug. It remains to be clarified whether the redox state of the myocyte or the intact heart may alter the effects of cardiac glycosides on cardiac hypertrophy without affecting the positive inotropic effect of these drugs. | |||
| Pamnani, M.B., et al. [11]; male Wistar rats | Bufalin infusion increased mean the arterial BP, HR and renal excretion of Na+ and water, while ouabain infusion in an equimolar dose produced a significantly smaller increase in these effects and had no effect on HR. Bufalin has a greater effect on CV contractility and renal excretion of Na+ and water in rats than ouabain. | |||
| Feto-placental unit | Sex and gender medicine: pregnancy diseases, PE | Endothelial dysfunction, apoptosis, release of angiogenic factors, umbilical arteries fibrosis | Vu, H.V., et al. [12]; pregnant female rats | MBG levels increased in pregnant female rats treated with deoxycorticosterone acetate and 0.9% saline compared with normal pregnant rats. The administration of MBG in normal pregnant female rats caused a significant increase in blood pressure and vasoconstrictive activity of uterine vessels, while no changes were observed with the infusion of ouabain or digoxin at the same concentration. There is a relationship between MBG and a PE-like syndrome in rats. |
| Vu, H.V., et al. [13]; pregnant female rats | RBG administration reversed MBG effects on BP. Antagonism of MBG could be a future therapeutic strategy for PE. | |||
| Agunanne, E., et al. [14]; pregnant female Sprague Dawley rats | RBG administration in early pregnancy prevented PE syndrome in a rat model. RBG also prevented IUGR and had no teratogenic effects. Treatment of PE can focus on drugs that do not compromise the fetus. | |||
| Uddin, M.N., et al. [15]; human extra-villous CTB cell line SGHPL-4 derived from first trimester chronic villous tissue | MBG induces a negative effect on CTB cell function, including apoptosis. MBG has a role in abnormal placentation and altered vascular function typical of PE. Targeting the MBG signaling pathway may be a future therapeutic strategy in PE treatment. | |||
| La Marca, H.L., et al. [16]; human extra-villous CTB cell line SGHPL-4 derived from first trimester chorionic villous tissues | MBG has an anti-proliferative effect on CTB before CTB differentiation into an invasive pathway. MBG inhibits CTB cell migration and growth factor-induced invasion processes. MBG expression in the early phase of pregnancy has a role in abnormal placentation and altered vascular function. | |||
| Nervous system | Synaptic dysfunction, genesis of neurofibrillary tangles, neuronal death | Genesis of new isoforms of sodium Nax channels | Grigorova, Y.N., et al. [17]; young Dahl salt sensitive rats | HS diet prohypertensive and profibrotic effects can be at least partially attributed to MBG increase. MBG and HS diet had similar effects on CV system. MBG and HS diet upregulated the expression of fibrosis and Alzheimer’s disease genes in LV of the rat model. Hippocampal neuronal density was not affected by MBG or HS diet. Brain plasticity in young rats probably helped the animals to sustain the MBG-induced central arterial stiffness, which is one of the underlying mechanisms of cognitive impairment. |
| Immune system | Inhibition of neutrophil migration, inhibition of pro-inflammatory cytokines | Anti-inflammatory activity in a dose-dependent manner | Carvalho, D.C.M., et al. [18]; Swiss mice peritoneal fluid | MBG inhibited polymorphonuclear leukocyte migration to the peritoneal cavity. MBG reduced the expression of different pro-inflammatory cytokines. MBG had no cytotoxicity effects on macrophages in peritoneum. |
| Organs and Systems | Pathological Processes | Effects of MBG | Clinical Studies | |
|---|---|---|---|---|
| Population | Results | |||
| Kidney and CV system | Volume-expanding conditions: essential hypertension, heart failure, PE CKD complications: LVH, UC, myocardial fibrosis, diastolic dysfunction | Sodium and fluid retention, organ fibrosis and remodeling, vascular and microcirculation alterations, activation of oxidative stress pathways | Keppel, M.H., [19] et al.; in patients with arterial hypertension; plasma MBG levels were measured in 40 patients, of whom 11 patients had primary aldosteronism (PA) and 29 patients had essential hypertension after exclusion of PA. | MBG concentrations increased, but not significantly, and showed a direct correlation trend with albuminuria and proteinuria. |
| Bolignano, D., [20] et al.; cohort of 29 patients on HD vs. healthy controls. | MBG levels in HD patients were significantly higher than in healthy controls and significantly reduced in HD patients experiencing IDH during follow-up. Inverse correlations were found between the absolute number of IDH episodes per person and, respectively pre-dialysis MBG, 2 h MBG and HD-end MBG. MBG levels remained basically unchanged in HD patients with no documented IDH episodes during follow-up. | |||
| Piecha, G., et al. [21]; 68 HD patients vs. 68 age-, gender- and blood pressure-matched subjects without CKD. | Mean plasma MBG immunoreactivity was significantly higher in HD patients compared with subjects with normal kidney function. In HD patients, plasma MBG was higher in men than in women, while this difference was not observed in subjects with normal kidney function. | |||
| Bolignano, D., et al. [22]; 46 HD patients vs. healthy controls. | MBG levels were significantly higher in HD patients than in healthy controls. A statistically significant trend in MBG levels was found across different patterns of LV geometry, with the highest values in eccentric LVH. MBG levels were higher in presence of diastolic dysfunction. | |||
| Jablonski, K.L., et al. [23]; middle-aged/older adults with moderately elevated systolic BP, but otherwise free of CV disease, diabetes, kidney disease and other clinical disorders. | Urinary MBG excretion decreased after 5 weeks of low sodium diet compared with 5 weeks of high sodium, while plasma MBG levels were not different between sodium conditions. Urinary MBG excretion was related to urinary sodium excretion and blood pressure measurements | |||
| Strauss, M., et al. [24]; young, apparently healthy Black and White adults (60). | A persistent positive association between carotid-femoral pulse wave velocity and MBG excretion was found in women but not in men. High endogenous MBG levels may contribute to large artery stiffness in women through pressure-independent mechanisms | |||
| Strauss, M., et al. [25]; young, apparently healthy Black and White adults (63) | LV mass, end diastolic volume and stroke volume were positively related to MBG excretion. The relationship between LV mass and MBG excretion was evident in women but not in men. Women may be more sensitive to MBG effects on early structural cardiac changes | |||
| Feto-placental unit | Sex and gender medicine: pregnancy diseases, PE | Endothelial dysfunction, apoptosis, release of angiogenic factors, umbilical arteries fibrosis | Lopatin, D.A., et al. [26]; 6 non-pregnant women, 6 normotensive age-matched pregnant controls and 11 patients with PE. | MBG levels significantly increased in PE pregnant women. MBG induced a contractile response of isolated rings of human mesenteric arteries in a concentration-dependent manner. MBG has a pathogenic role in PE. |
| Agunanne, E., et al. [14]; 17 pre-eclamptic women and 46 normotensive pregnant women in various gestational periods. | Serum and urinary levels of MBG were significantly greater in pre-eclamptic women than normotensive pregnant women. MBG can be used for prediction and diagnosis of PE. | |||
| Nikitina, E.R., et al. [27]; 16 pre-eclamptic pregnant women and 14 gestational age-matched normal pregnant women. | Serum and urinary levels of MBG increased in pre-eclamptic women compared with normal pregnant women. MBG, through a Fli-1-dependent mechanism stimulates collagen synthesis in umbilical arteries, leading to the impairment of vasorelaxation. MBG may represent a potential target for PE therapy. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carullo, N.; Fabiano, G.; D'Agostino, M.; Zicarelli, M.T.; Musolino, M.; Presta, P.; Michael, A.; Andreucci, M.; Bolignano, D.; Coppolino, G. New Insights on the Role of Marinobufagenin from Bench to Bedside in Cardiovascular and Kidney Diseases. Int. J. Mol. Sci. 2023, 24, 11186. https://doi.org/10.3390/ijms241311186
Carullo N, Fabiano G, D'Agostino M, Zicarelli MT, Musolino M, Presta P, Michael A, Andreucci M, Bolignano D, Coppolino G. New Insights on the Role of Marinobufagenin from Bench to Bedside in Cardiovascular and Kidney Diseases. International Journal of Molecular Sciences. 2023; 24(13):11186. https://doi.org/10.3390/ijms241311186
Chicago/Turabian StyleCarullo, Nazareno, Giuseppe Fabiano, Mario D'Agostino, Maria Teresa Zicarelli, Michela Musolino, Pierangela Presta, Ashour Michael, Michele Andreucci, Davide Bolignano, and Giuseppe Coppolino. 2023. "New Insights on the Role of Marinobufagenin from Bench to Bedside in Cardiovascular and Kidney Diseases" International Journal of Molecular Sciences 24, no. 13: 11186. https://doi.org/10.3390/ijms241311186
APA StyleCarullo, N., Fabiano, G., D'Agostino, M., Zicarelli, M. T., Musolino, M., Presta, P., Michael, A., Andreucci, M., Bolignano, D., & Coppolino, G. (2023). New Insights on the Role of Marinobufagenin from Bench to Bedside in Cardiovascular and Kidney Diseases. International Journal of Molecular Sciences, 24(13), 11186. https://doi.org/10.3390/ijms241311186










