Numerous Trigger-like Interactions of Kinases/Protein Phosphatases in Human Skeletal Muscles Can Underlie Transient Processes in Activation of Signaling Pathways during Exercise
Abstract
1. Introduction
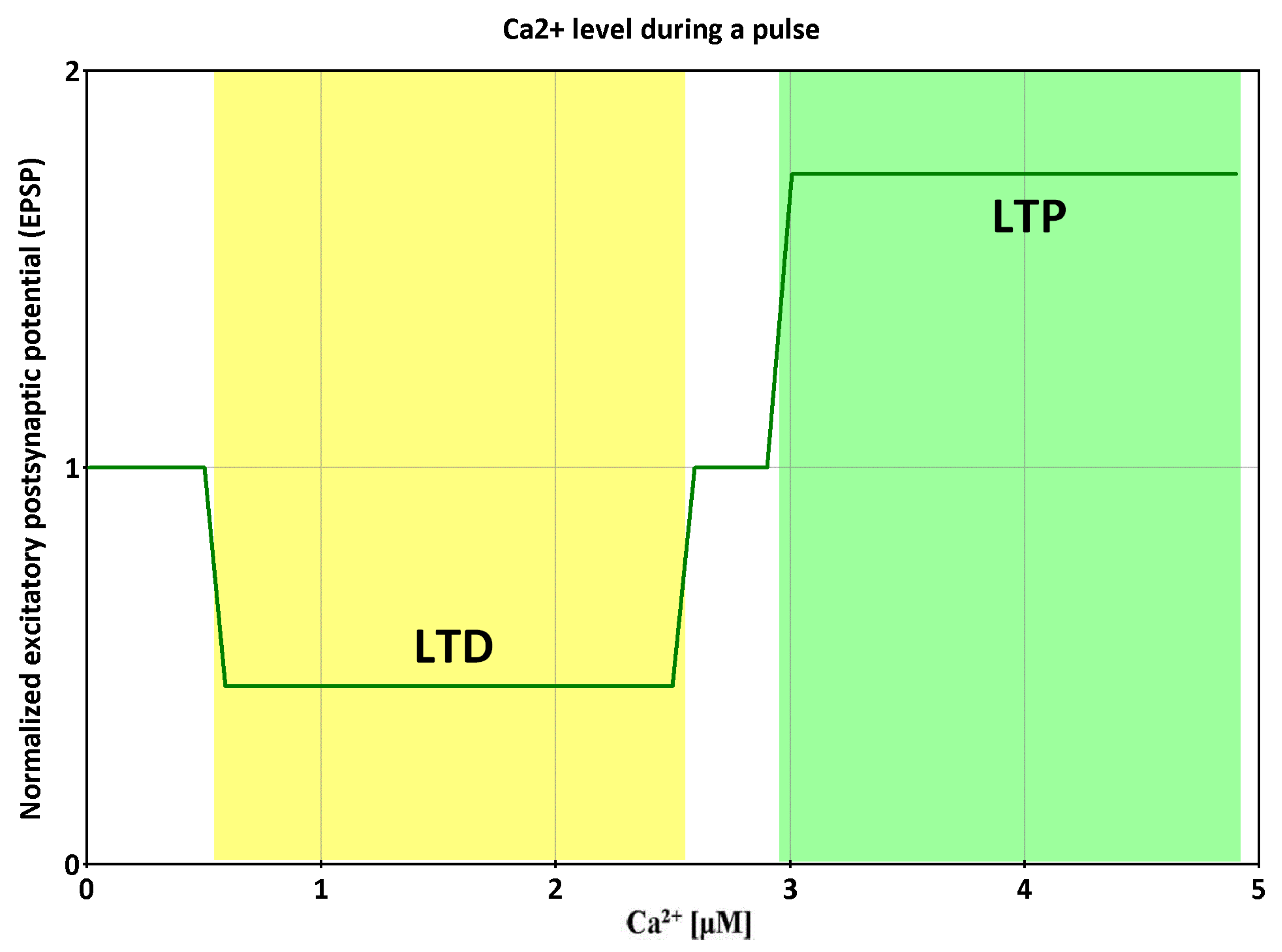
2. AMPK and Ca2+—Dependent Signaling Transients
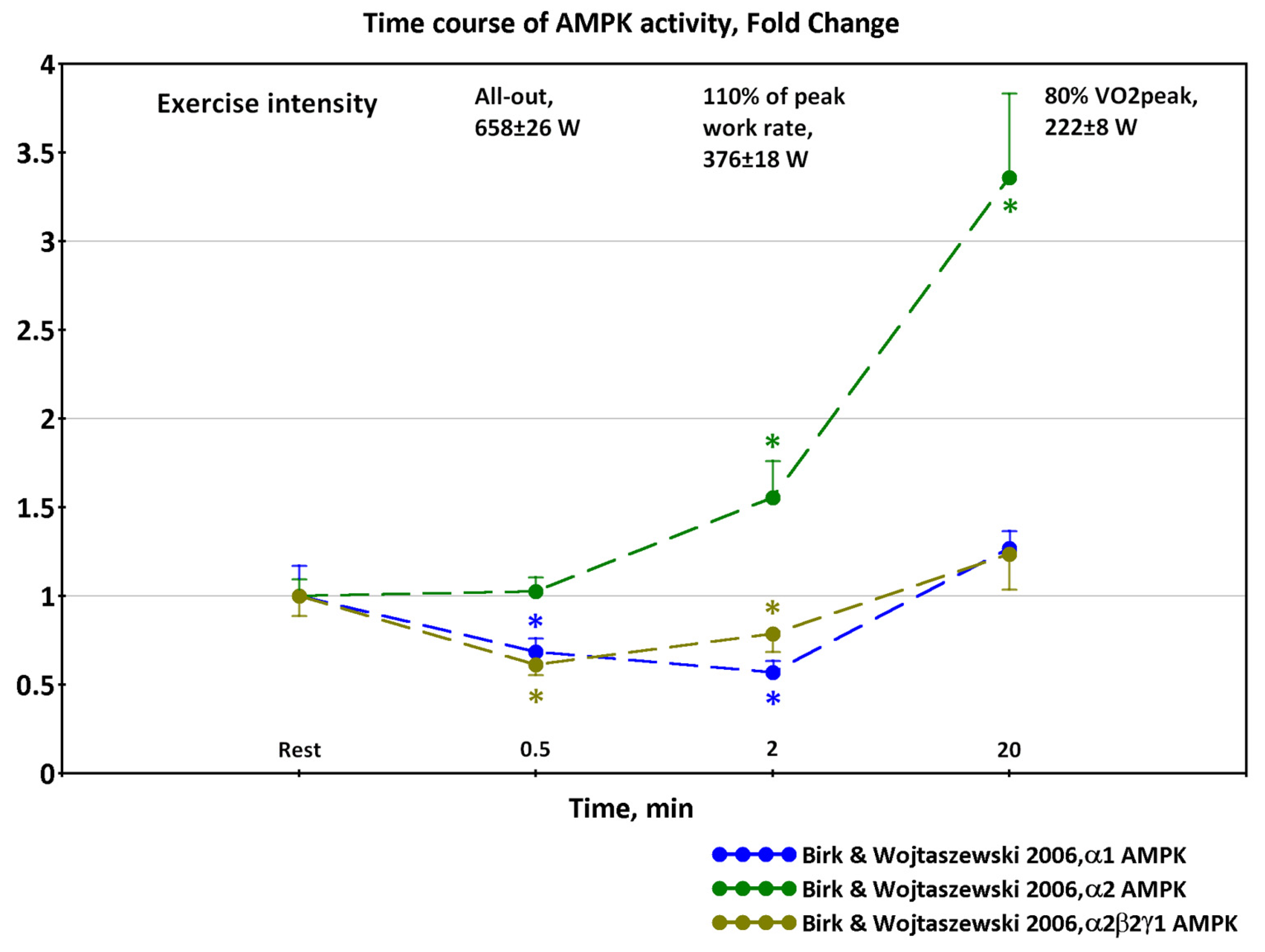
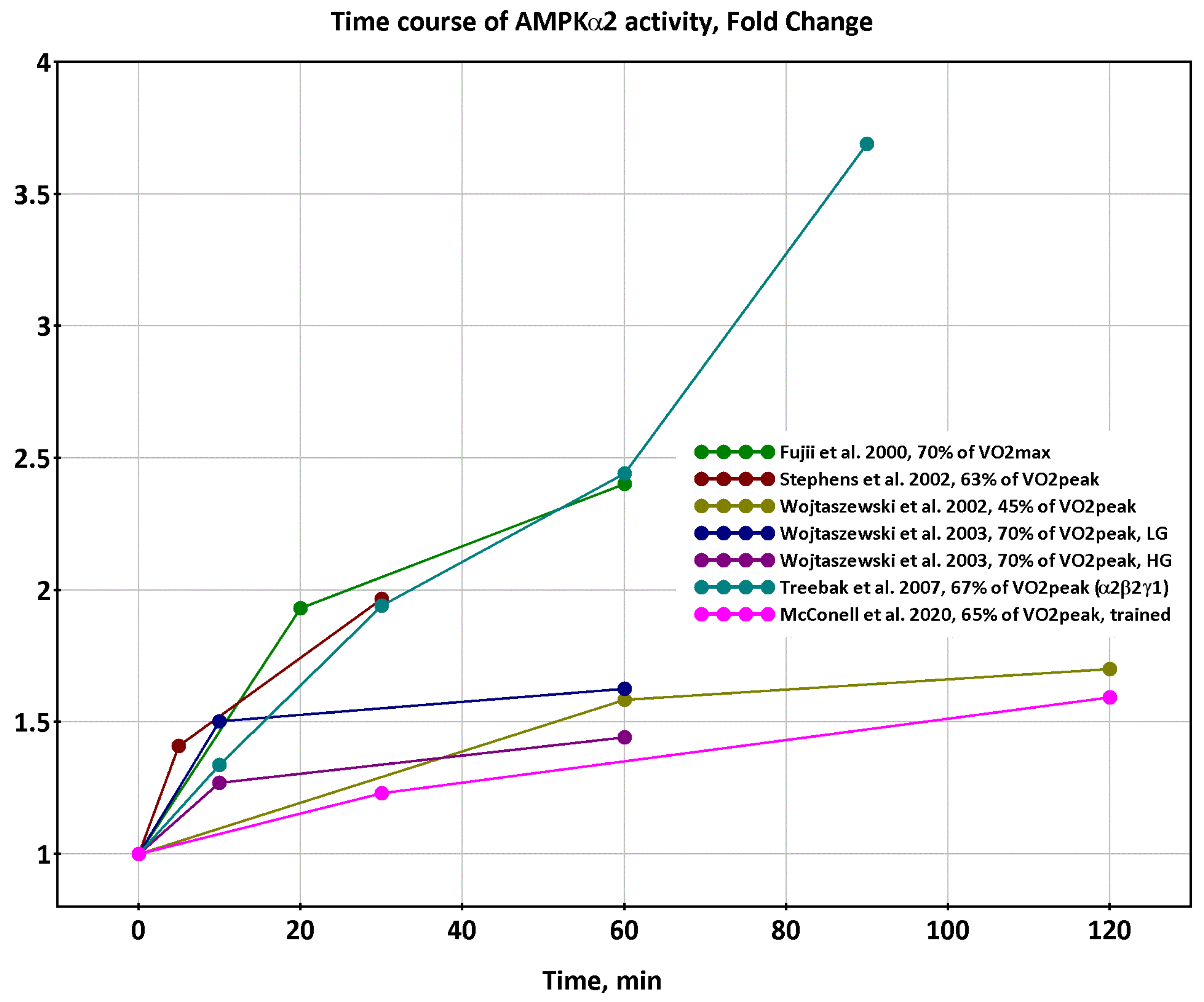
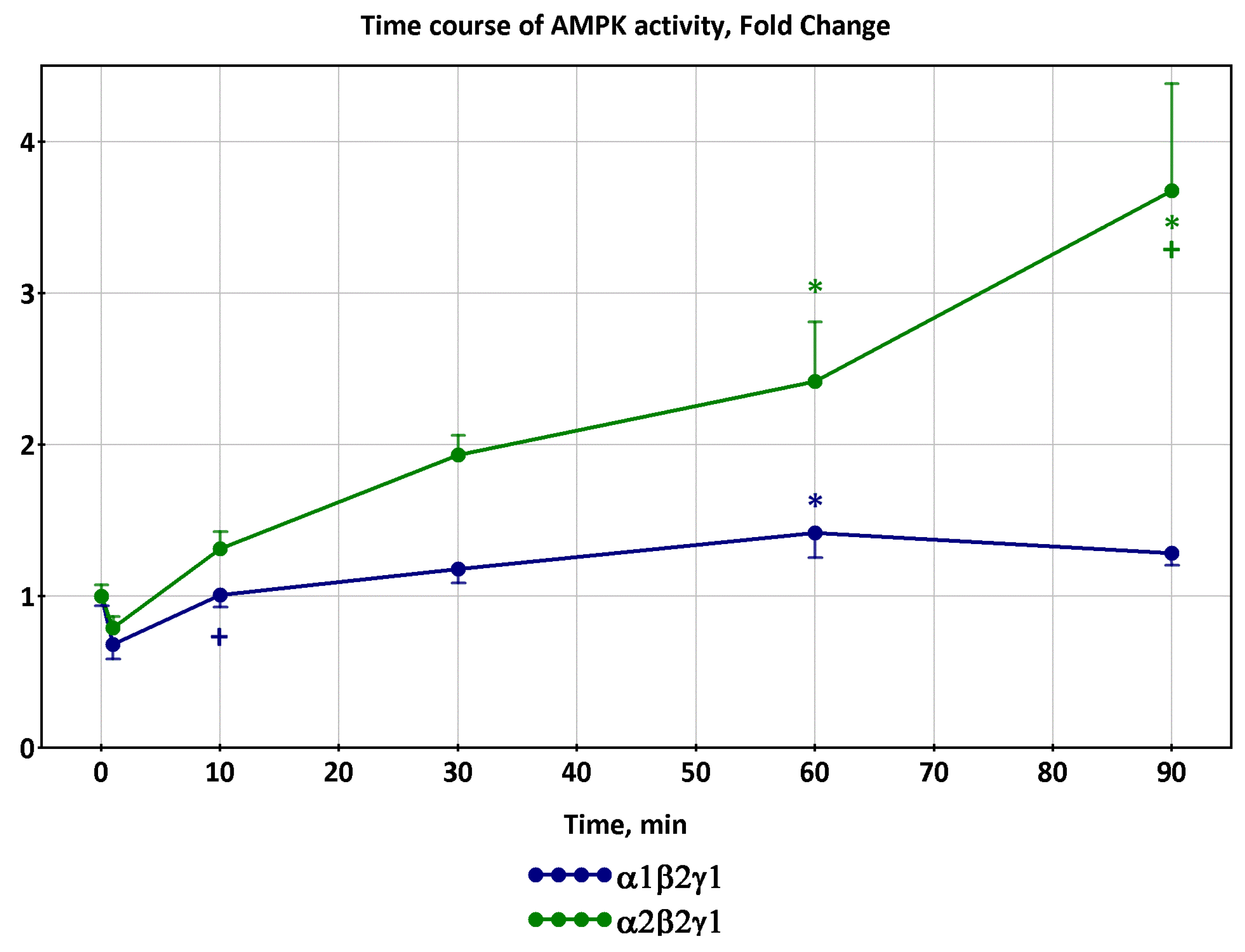
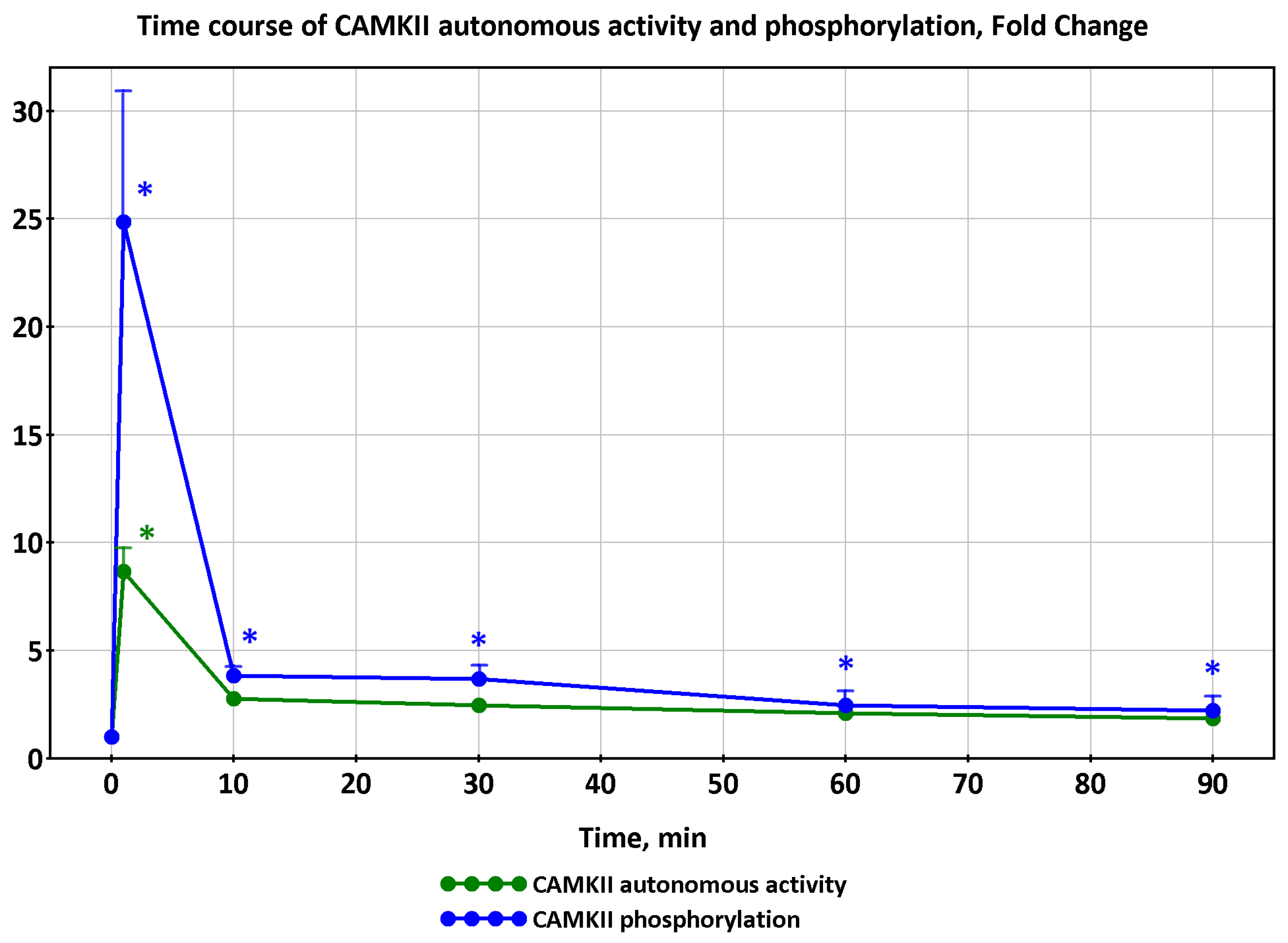
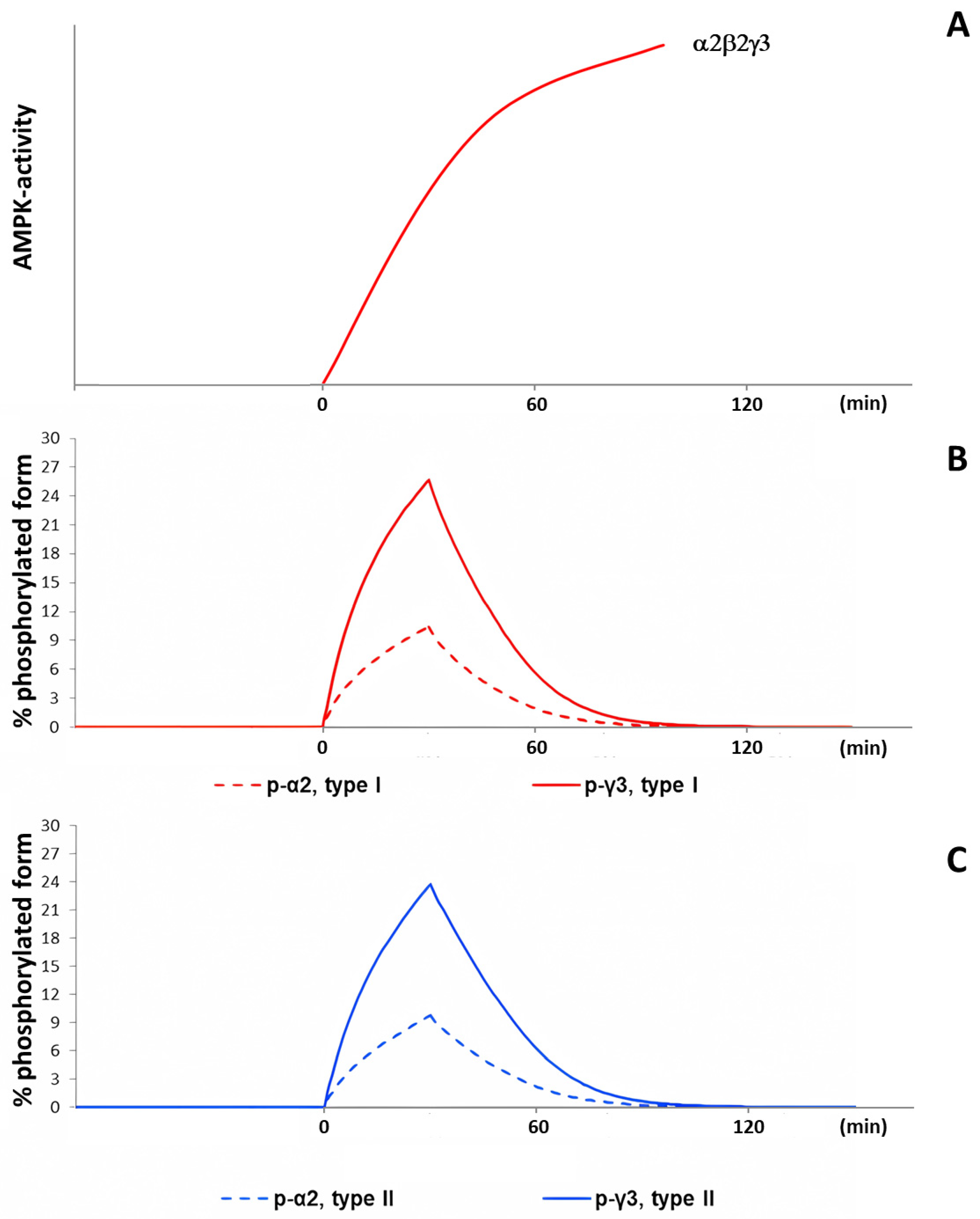
2.1. Dynamics of AMPK and CaMKII Activity during Exercise
2.2. Possible Effects of Repeated Exercise
2.3. Simulations Based on the Current Model and Their Limitations
3. Trigger-like Kinase/Protein Phosphatase Interaction Networks in Central Nervous System (CNS)
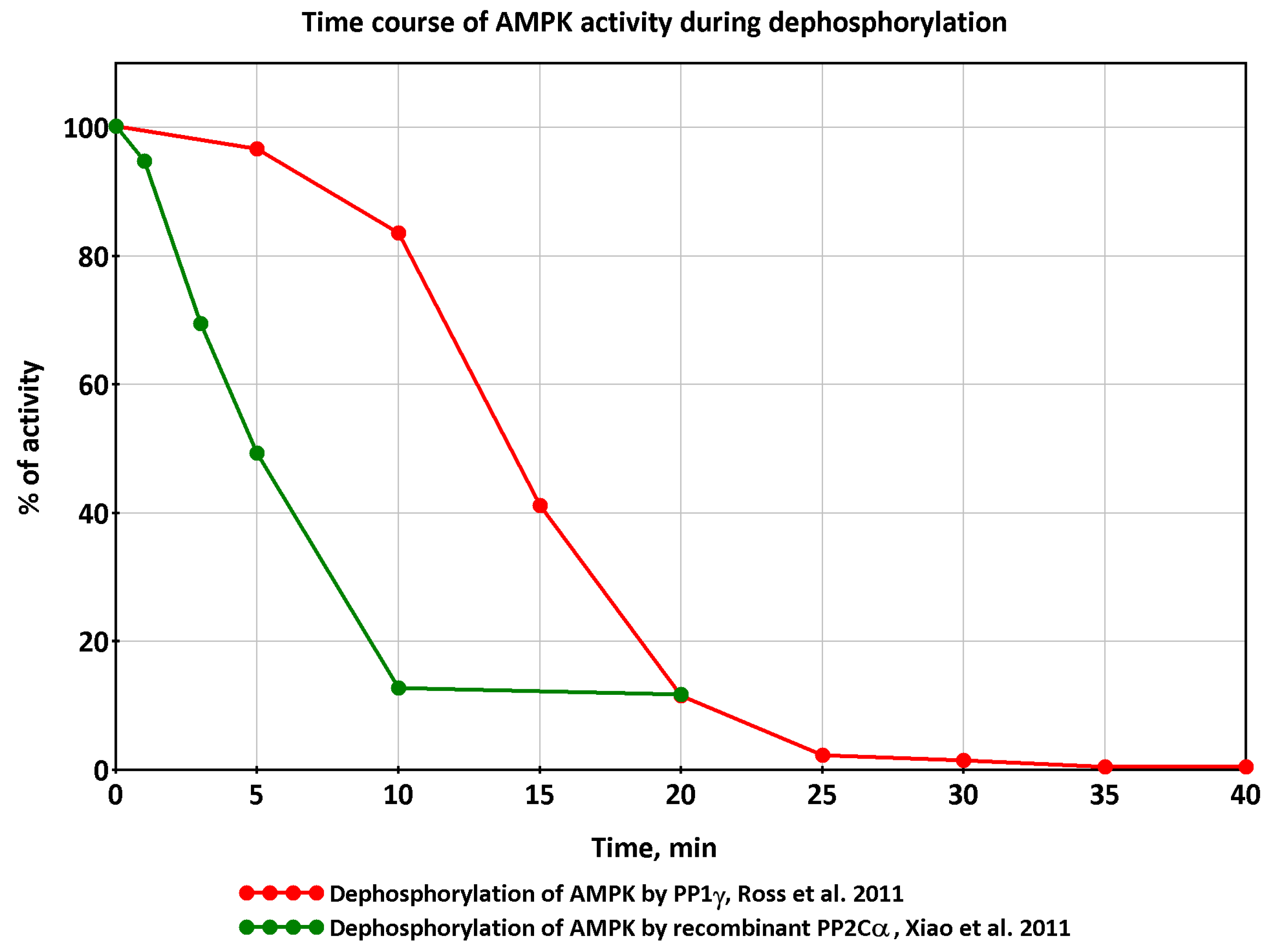
3.1. Regulation of Protein Phosphatases in the CNS
- Regulators of the expression of protein phosphatases and regulatory proteins; modulators of the assembly of protein phosphatase complexes (long-term processes, hours, days);
- Regulators of post-translational modifications associated with long-term processes; pathways related to hormones and nutrients (minutes, hours);
- Regulators of activity and post-translational modifications that have a higher speed of action, comparable to the speed of processes during the onset and cessation of the exercise (seconds, minutes).
3.2. Are Similar Regulation Loops Possible in Skeletal Muscles?
4. Additional Regulatory Factors in Skeletal Muscles
4.1. Reactive Oxygen and Nitrogen Species
4.2. Muscle Glycogen
4.3. Insulin
4.4. Adrenaline
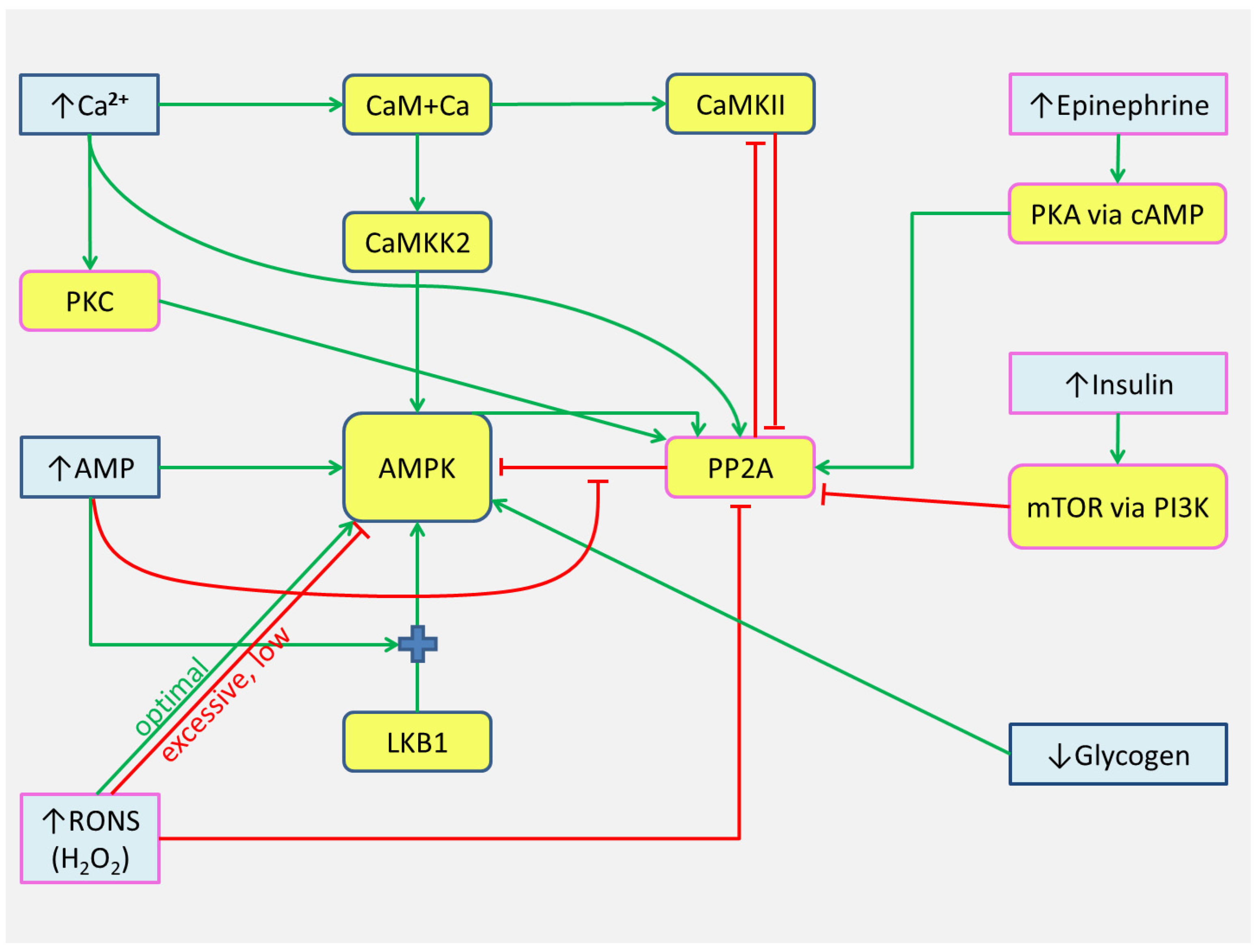
5. Hypothesis
5.1. Generalized Scheme
- Observed decreases in activity of α1β2γ1 and α2β2γ1 isoforms of AMPK and delayed increases in activity of α2β2γ3 isoforms at exercise intensity 70% of VO2max and above [12,16] are determined by coupled metabolic and signaling transient processes at the onset of exercise in a time window of about 1–10 min;
- These transients may in part be due to putative trigger-like kinase/protein phosphatase interactions similar to the nature of interactions in the CNS;
- Numerous dynamically changing factors: [Ca2+], metabolite concentration, RONS, and intramuscular glycogen hormones, can shift switching thresholds and change the state of «triggers», thereby affecting the kinase activity.
5.2. View at the Level of the Individual Muscle Fibre and Fibre Type
6. Issues
7. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Dash, R.K.; Kim, J.; Saidel, G.M.; Cabrera, M.E.; White, A.T.; Schenk, S.; Solomon, T.P.J.; Haus, J.M.; Kirwan, J.P. Role of NADH/NAD+ transport activity and glycogen store on skeletal muscle energy metabolism during exercise: In silico studies. Am. J. Physiol.-Cell Physiol. 2009, 296, C25–C46. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, I.; Akberdin, I.; Vertyshev, A.; Popov, D.; Kolpakov, F. A Modular Visual Model of Energy Metabolism in Human Skeletal Muscle. Math. Biol. Bioinform. 2019, 14, 373–392. [Google Scholar] [CrossRef]
- Akberdin, I.R.; Kiselev, I.N.; Pintus, S.S.; Sharipov, R.N.; Vertyshev, A.Y.; Vinogradova, O.L.; Popov, D.V.; Kolpakov, F.A. A Modular Mathematical Model of Exercise-Induced Changes in Metabolism, Signaling, and Gene Expression in Human Skeletal Muscle. Int. J. Mol. Sci. 2021, 22, 10353. [Google Scholar] [CrossRef]
- Soderlund, K.; Greenhaff, P.L.; Hultman, E. Energy metabolism in type I and type II human muscle fibres during short term electrical stimulation at different frequencies. Acta Physiol. Scand. 1992, 144, 15–22. [Google Scholar] [CrossRef]
- Ivarsson, N.; Mattsson, C.M.; Cheng, A.J.; Bruton, J.D.; Ekblom, B.; Lanner, J.T.; Westerblad, H. SR Ca2+ leak in skeletal muscle fibers acts as an intracellular signal to increase fatigue resistance. J. Gen. Physiol. 2019, 151, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Racioppi, L.; Means, A.R. Calcium/Calmodulin-dependent Protein Kinase Kinase 2: Roles in Signaling and Pathophysiology. J. Biol. Chem. 2012, 287, 31658–31665. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Gehlert, S.; Bloch, W.; Suhr, F. Ca2+-Dependent Regulations and Signaling in Skeletal Muscle: From Electro-Mechanical Coupling to Adaptation. Int. J. Mol. Sci. 2015, 16, 1066–1095. [Google Scholar] [CrossRef]
- Camera, D.M.; Smiles, W.J.; Hawley, J.A. Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free. Radic. Biol. Med. 2016, 98, 131–143. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.-N.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef]
- McGee, S.L.; Hargreaves, M. Exercise adaptations: Molecular mechanisms and potential targets for therapeutic benefit. Nat. Rev. Endocrinol. 2020, 16, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Treebak, J.T.; Birk, J.B.; Rose, A.J.; Kiens, B.; Richter, E.A.; Wojtaszewski, J.F.P. AS160 phosphorylation is associated with activation of α2β2γ1- but not α2β2γ3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am. J. Physiol. Metab. 2007, 292, E715–E722. [Google Scholar] [CrossRef] [PubMed]
- Birk, J.B.; Wojtaszewski, J.F.P. Kinase Activity Determination of Specific AMPK Complexes/Heterotrimers in the Skeletal Muscle. Methods Mol. Biol. 2018, 1732, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Wojtaszewski, J.F.P.; Nielsen, P.; Hansen, B.F.; Richter, E.A.; Kiens, B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J. Physiol. 2000, 528 Pt 1, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Wojtaszewski, J.F.; Mourtzakis, M.; Hillig, T.; Saltin, B.; Pilegaard, H. Dissociation of AMPK activity and ACCβ phosphorylation in human muscle during prolonged exercise. Biochem. Biophys. Res. Commun. 2002, 298, 309–316. [Google Scholar] [CrossRef]
- Birk, J.B.; Wojtaszewski, J.F.P. Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J. Physiol. 2006, 577 Pt 3, 1021–1032. [Google Scholar] [CrossRef]
- Chen, Z.-P.; McConell, G.K.; Michell, B.J.; Snow, R.J.; Canny, B.J.; Kemp, B.E. AMPK signaling in contracting human skeletal muscle: Acetyl-CoA carboxylase and NO synthase phosphorylation. Am. J. Physiol. Metab. 2000, 279, E1202–E1206. [Google Scholar] [CrossRef]
- Nielsen, J.N.; Wojtaszewski, J.F.P.; Haller, R.G.; Hardie, D.G.; Kemp, B.E.; Richter, E.A.; Vissing, J. Role of 5′AMP-activated protein kinase in glycogen synthase activity and glucose utilization: Insights from patients with McArdle’s disease. J. Physiol. 2002, 541 Pt 3, 979–989. [Google Scholar] [CrossRef]
- Nielsen, J.N.; Mustard, K.J.W.; Graham, D.A.; Yu, H.; MacDonald, C.S.; Pilegaard, H.; Goodyear, L.J.; Hardie, D.G.; Richter, E.A.; Wojtaszewski, J.F.P.; et al. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J. Appl. Physiol. 2003, 94, 631–641. [Google Scholar] [CrossRef]
- Fujii, N.; Hayashi, T.; Hirshman, M.F.; Smith, J.T.; Habinowski, S.A.; Kaijser, L.; Mu, J.; Ljungqvist, O.; Birnbaum, M.J.; Witters, L.A.; et al. Exercise Induces Isoform-Specific Increase in 5′AMP-Activated Protein Kinase Activity in Human Skeletal Muscle. Biochem. Biophys. Res. Commun. 2000, 273, 1150–1155. [Google Scholar] [CrossRef]
- Roepstorff, C.; Thiele, M.; Hillig, T.; Pilegaard, H.; Richter, E.A.; Wojtaszewski, J.F.P.; Kiens, B. Higher skeletal muscle α2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J. Physiol. 2006, 574 Pt 1, 125–138. [Google Scholar] [CrossRef]
- Kristensen, D.E.; Albers, P.H.; Prats, C.; Baba, O.; Birk, J.B.; Wojtaszewski, J.F.P. Human muscle fibre type-specific regulation of AMPK and downstream targets by exercise. J. Physiol. 2015, 593, 2053–2069. [Google Scholar] [CrossRef]
- McConell, G.K.; Wadley, G.D.; Le Plastrier, K.; Linden, K.C. Skeletal muscle AMPK is not activated during 2 h of moderate intensity exercise at ∼65% VO2 peak in endurance trained men. J. Physiol. 2020, 598, 3859–3870. [Google Scholar] [CrossRef]
- Jensen, T.E.; Wojtaszewski, J.F.P.; Richter, E.A. AMP-activated protein kinase in contraction regulation of skeletal muscle metabolism: Necessary and/or sufficient? Acta Physiol. 2009, 196, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Stephens, T.J.; Chen, Z.-P.; Canny, B.J.; Michell, B.J.; Kemp, B.E.; McConell, G.K. Progressive increase in human skeletal muscle AMPKα2 activity and ACC phosphorylation during exercise. Am. J. Physiol. Metab. 2002, 282, E688–E694. [Google Scholar] [CrossRef]
- Wojtaszewski, J.F.P.; MacDonald, C.; Nielsen, J.N.; Hellsten, Y.; Hardie, D.G.; Kemp, B.E.; Kiens, B.; Richter, E.A.; Miller, B.F.; Konopka, A.R.; et al. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am. J. Physiol. Metab. 2003, 284, E813–E822. [Google Scholar] [CrossRef]
- Rose, A.J.; Kiens, B.; Richter, E.A. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J. Physiol. 2006, 574 Pt 3, 889–903. [Google Scholar] [CrossRef]
- Bangsbo, J.; Krustrup, P.; González-Alonso, J.; Saltin, B. ATP production and efficiency of human skeletal muscle during intense exercise: Effect of previous exercise. Am. J. Physiol. Metab. 2001, 280, E956–E964. [Google Scholar] [CrossRef] [PubMed]
- Burnley, M.; Doust, J.H.; Ball, D.; Jones, A.M. Effects of prior heavy exercise on VO(2) kinetics during heavy exercise are related to changes in muscle activity. J. Appl. Physiol. 2002, 93, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Gurd, B.J.; Scheuermann, B.W.; Paterson, D.H.; Kowalchuk, J.M.; Niemeijer, V.M.; Spee, R.F.; Schoots, T.; Wijn, P.F.F.; Kemps, H.M.C.; Williams, A.M.; et al. Prior heavy-intensity exercise speeds VO2 kinetics during moderate-intensity exercise in young adults. J. Appl. Physiol. 2005, 98, 1371–1378. [Google Scholar] [CrossRef]
- Niemeyer, M.; Leithäuser, R.; Beneke, R. Effect of intensive prior exercise on muscle fiber activation, oxygen uptake kinetics, and oxygen uptake plateau occurrence. Eur. J. Appl. Physiol. 2020, 120, 2019–2028. [Google Scholar] [CrossRef]
- Parolin, M.L.; Chesley, A.; Matsos, M.P.; Spriet, L.L.; Jones, N.L.; Heigenhauser, G.J.F. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am. J. Physiol. Metab. 1999, 277, E890–E900. [Google Scholar] [CrossRef] [PubMed]
- Tupling, A.R.; Green, H.J.; Roy, B.D.; Grant, S.; Ouyang, J. Paradoxical effects of prior activity on human sarcoplasmic reticulum Ca2+-ATPase response to exercise. J. Appl. Physiol. 2003, 95, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Gurd, B.J.; Peters, S.J.; Heigenhauser, G.J.F.; LeBlanc, P.J.; Doherty, T.J.; Paterson, D.H.; Kowalchuk, J.M. Prior heavy exercise elevates pyruvate dehydrogenase activity and speeds O2 uptake kinetics during subsequent moderate-intensity exercise in healthy young adults. J. Physiol. 2006, 577 Pt 3, 985–996. [Google Scholar] [CrossRef]
- Rico-Sanz, J. Progressive decrease of intramyocellular accumulation of H+ and Pi in human skeletal muscle during repeated isotonic exercise. Am. J. Physiol.-Cell Physiol. 2003, 284, C1490–C1496. [Google Scholar] [CrossRef]
- Jones, A.M.; Fulford, J.; Wilkerson, D.P. Influence of prior exercise on muscle [phosphorylcreatine] and deoxygenation kinetics during high-intensity exercise in men. Exp. Physiol. 2008, 93, 468–478. [Google Scholar] [CrossRef]
- Layec, G.; Bringard, A.; Le Fur, Y.; Vilmen, C.; Micallef, J.-P.; Perrey, S.; Cozzone, P.J.; Bendahan, D. Effects of a prior high-intensity knee-extension exercise on muscle recruitment and energy cost: A combined local and global investigation in humans. Exp. Physiol. 2009, 94, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.A.; Rafferty, J.N.; Dallas, M.L.; Ogunbayo, O.; Ikematsu, N.; McClafferty, H.; Tian, L.; Widmer, H.; Rowe, I.C.M.; Wyatt, C.N.; et al. Selective Expression in Carotid Body Type I Cells of a Single Splice Variant of the Large Conductance Calcium- and Voltage-activated Potassium Channel Confers Regulation by AMP-activated Protein Kinase. J. Biol. Chem. 2011, 286, 11929–11936. [Google Scholar] [CrossRef]
- Xiao, B.; Sanders, M.J.; Underwood, E.; Heath, R.; Mayer, F.V.; Carmena, D.; Jing, C.; Walker, P.A.; Eccleston, J.F.; Haire, L.F.; et al. Structure of mammalian AMPK and its regulation by ADP. Nature 2011, 472, 230–233. [Google Scholar] [CrossRef]
- Brautigan, D.L.; Shenolikar, S. Protein Serine/Threonine Phosphatases: Keys to Unlocking Regulators and Substrates. Annu. Rev. Biochem. 2018, 87, 921–964. [Google Scholar] [CrossRef]
- Elgenaidi, I.; Spiers, J. Regulation of the phosphoprotein phosphatase 2A system and its modulation during oxidative stress: A potential therapeutic target? Pharmacol. Ther. 2019, 198, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.N.; Nairn, A.C. cAMP regulation of protein phosphatases PP1 and PP2A in brain. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Raman, D.; Pervaiz, S. Redox inhibition of protein phosphatase PP2A: Potential implications in oncogenesis and its progression. Redox Biol. 2019, 27, 101105. [Google Scholar] [CrossRef]
- Sandal, P.; Jong, C.J.; Merrill, R.A.; Song, J.; Strack, S. Protein phosphatase 2A—Structure, function and role in neurodevelopmental disorders. J. Cell Sci. 2021, 134, jcs248187. [Google Scholar] [CrossRef]
- Pi, H.J.; Lisman, J.E. Coupled Phosphatase and Kinase Switches Produce the Tristability Required for Long-Term Potentiation and Long-Term Depression. J. Neurosci. 2008, 28, 13132–13138. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Hirano, T.; Kawaguchi, S. Prediction and validation of a mechanism to control the threshold for inhibitory synaptic plasticity. Mol. Syst. Biol. 2009, 5, 280. [Google Scholar] [CrossRef]
- Kawaguchi, S.-Y.; Hirano, T. Gating of long-term depression by Ca2+/calmodulin-dependent protein kinase II through enhanced cGMP signalling in cerebellar Purkinje cells. J. Physiol. 2013, 591, 1707–1730. [Google Scholar] [CrossRef]
- Gallimore, A.R.; Kim, T.; Tanaka-Yamamoto, K.; De Schutter, E. Switching On Depression and Potentiation in the Cerebellum. Cell Rep. 2018, 22, 722–733. [Google Scholar] [CrossRef]
- Fukunaga, K.; Muller, D.; Ohmitsu, M.; Bakó, E.; DePaoli-Roach, A.A.; Miyamoto, E. Decreased Protein Phosphatase 2A Activity in Hippocampal Long-Term Potentiation. J. Neurochem. 2001, 74, 807–817. [Google Scholar] [CrossRef]
- Colbran, R.J. Protein Phosphatases and Calcium/Calmodulin-Dependent Protein Kinase II-Dependent Synaptic Plasticity: Figure 1. J. Neurosci. 2004, 24, 8404–8409. [Google Scholar] [CrossRef]
- Huang, B.; Yang, C.-S.; Wojton, J.; Huang, N.-J.; Chen, C.; Soderblom, E.J.; Zhang, L.; Kornbluth, S. Metabolic Control of Ca2+/Calmodulin-dependent Protein Kinase II (CaMKII)-mediated Caspase-2 Suppression by the B55β/Protein Phosphatase 2A (PP2A). J. Biol. Chem. 2014, 289, 35882–35890. [Google Scholar] [CrossRef]
- Kubota, Y.; Bower, J.M. Transient versus asymptotic dynamics of CaM kinase II: Possible roles of phosphatase. J. Comput. Neurosci. 2001, 11, 263–279. [Google Scholar] [CrossRef]
- Bradshaw, J.M.; Kubota, Y.; Meyer, T.; Schulman, H. An ultrasensitive Ca 2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 10512–10517. [Google Scholar] [CrossRef]
- Erondu, N.; Kennedy, M. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J. Neurosci. 1985, 5, 3270–3277. [Google Scholar] [CrossRef]
- Duda, P.; Wójcicka, O.; Wiśniewski, J.R.; Rakus, D. Global quantitative TPA-based proteomics of mouse brain structures reveals significant alterations in expression of proteins involved in neuronal plasticity during aging. Aging 2018, 10, 1682–1697. [Google Scholar] [CrossRef]
- Murgia, M.; Toniolo, L.; Nagaraj, N.; Ciciliot, S.; Vindigni, V.; Schiaffino, S.; Reggiani, C.; Mann, M. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Rep. 2017, 19, 2396–2409. [Google Scholar] [CrossRef] [PubMed]
- Kanosue, K.; Yoshida, M.; Akazawa, K.; Fujii, K. The Number of Active Motor Units and Their Firing Rates in Voluntary Contraction of Human Brachialis Muscle. Jpn. J. Physiol. 1979, 29, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Sale, D.G. 5 Influence of Exercise and Training on Motor Unit Activation. Exerc. Sport Sci. Rev. 1987, 15, 95–152. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, M.; Duchateau, J.; Hainaut, K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J. Physiol. 1998, 513 Pt 1, 295–305. [Google Scholar] [CrossRef]
- Baylor, S.M.; Hollingworth, S. Simulation of Ca2+ Movements within the Sarcomere of Fast-Twitch Mouse Fibers Stimulated by Action Potentials. J. Gen. Physiol. 2007, 130, 283–302. [Google Scholar] [CrossRef]
- Eilers, W.; Gevers, W.; van Overbeek, D.; de Haan, A.; Jaspers, R.T.; Hilbers, P.A.; van Riel, N.; Flück, M. Muscle-Type Specific Autophosphorylation of CaMKII Isoforms after Paced Contractions. BioMed Res. Int. 2014, 2014, 943806. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-Y.; Baek, A.; Hwang, J.-E.; Choi, Y.A.; Jeong, J.; Lee, M.-S.; Cho, D.H.; Lim, J.-S.; Kim, K.I.; Yang, Y. Adiponectin-Activated AMPK Stimulates Dephosphorylation of AKT through Protein Phosphatase 2A Activation. Cancer Res. 2009, 69, 4018–4026. [Google Scholar] [CrossRef] [PubMed]
- Chedere, A.; Hari, K.; Kumar, S.; Rangarajan, A.; Jolly, M.K. Multi-Stability and Consequent Phenotypic Plasticity in AMPK-Akt Double Negative Feedback Loop in Cancer Cells. J. Clin. Med. 2021, 10, 472. [Google Scholar] [CrossRef]
- Su, K.-H.; Dai, S.; Tang, Z.; Xu, M.; Dai, C. Heat Shock Factor 1 Is a Direct Antagonist of AMP-Activated Protein Kinase. Mol. Cell 2019, 76, 546–561.e8. [Google Scholar] [CrossRef] [PubMed]
- Morales-Alamo, D.; Calbet, J.A. AMPK signaling in skeletal muscle during exercise: Role of reactive oxygen and nitrogen species. Free. Radic. Biol. Med. 2016, 98, 68–77. [Google Scholar] [CrossRef]
- Erickson, J.R.; Joiner, M.-L.A.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A Dynamic Pathway for Calcium-Independent Activation of CaMKII by Methionine Oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Yin, J.; Luo, Y.; Huang, S. Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int. J. Biochem. Cell Biol. 2009, 41, 1284–1295. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Ponce-González, J.G.; Guadalupe-Grau, A.; Rodríguez-García, L.; Santana, A.; Cusso, R.; Guerrero, M.; Dorado, C.; Guerra, B.; Calbet, J.A.L. Critical role for free radicals on sprint exercise-induced CaMKII and AMPKα phosphorylation in human skeletal muscle. J. Appl. Physiol. 2013, 114, 566–577. [Google Scholar] [CrossRef]
- Yeo, W.K.; McGee, S.L.; Carey, A.L.; Paton, C.D.; Garnham, A.P.; Hargreaves, M.; Hawley, J.A. Acute signalling responses to intense endurance training commenced with low or normal muscle glycogen. Exp. Physiol. 2009, 95, 351–358. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Watt, M.J.; McGee, S.L.; Chan, S.; Hargreaves, M.; Febbraio, M.A.; Stapleton, D.; Kemp, B.E. Reduced glycogen availability is associated with increased AMPKα2 activity, nuclear AMPKα2 protein abundance, and GLUT4 mRNA expression in contracting human skeletal muscle. Appl. Physiol. Nutr. Metab. 2006, 31, 302–312. [Google Scholar] [CrossRef]
- Psilander, N.; Frank, P.; Flockhart, M.; Sahlin, K. Exercise with low glycogen increases PGC-1α gene expression in human skeletal muscle. Eur. J. Appl. Physiol. 2012, 113, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Janzen, N.R.; Whitfield, J.; Hoffman, N.J. Interactive Roles for AMPK and Glycogen from Cellular Energy Sensing to Exercise Metabolism. Int. J. Mol. Sci. 2018, 19, 3344. [Google Scholar] [CrossRef] [PubMed]
- Ørtenblad, N.; Nielsen, J.; Saltin, B.; Holmberg, H.-C. Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J. Physiol. 2011, 589 Pt 3, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Gejl, K.D.; Hvid, L.G.; Frandsen, U.; Jensen, K.; Sahlin, K.; Ørtenblad, N. Muscle Glycogen Content Modifies SR Ca2+ Release Rate in Elite Endurance Athletes. Med. Sci. Sports Exerc. 2014, 46, 496–505. [Google Scholar] [CrossRef]
- Gejl, K.D.; Andersson, E.P.; Nielsen, J.; Holmberg, H.-C.; Ørtenblad, N. Effects of Acute Exercise and Training on the Sarcoplasmic Reticulum Ca2+ Release and Uptake Rates in Highly Trained Endurance Athletes. Front. Physiol. 2020, 11, 810. [Google Scholar] [CrossRef]
- Shifman, J.M.; Choi, M.H.; Mihalas, S.; Mayo, S.L.; Kennedy, M.B. Ca2+/calmodulin-dependent protein kinase II (CaMKII) is activated by calmodulin with two bound calciums. Proc. Natl. Acad. Sci. USA 2006, 103, 13968–13973. [Google Scholar] [CrossRef]
- Li, L.; Stefan, M.I.; Le Novère, N. Calcium Input Frequency, Duration and Amplitude Differentially Modulate the Relative Activation of Calcineurin and CaMKII. PLoS ONE 2012, 7, e43810. [Google Scholar] [CrossRef]
- Bhattacharyya, M.; Karandur, D.; Kuriyan, J. Structural Insights into the Regulation of Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII). Cold Spring Harb. Perspect. Biol. 2019, 12, a035147. [Google Scholar] [CrossRef]
- Valentine, R.J.; Coughlan, K.A.; Ruderman, N.B.; Saha, A.K. Insulin inhibits AMPK activity and phosphorylates AMPK Ser485/491 through Akt in hepatocytes, myotubes and incubated rat skeletal muscle. Arch. Biochem. Biophys. 2014, 562, 62–69. [Google Scholar] [CrossRef]
- Srinivasan, M.; Begum, N. Regulation of protein phosphatase 1 and 2A activities by insulin during myogenesis in rat skeletal muscle cells in culture. J. Biol. Chem. 1994, 269, 12514–12520. [Google Scholar] [CrossRef]
- Vasudevan, N.T.; Mohan, M.L.; Gupta, M.K.; Hussain, A.K.; Prasad, S.V.N. Inhibition of Protein Phosphatase 2A Activity by PI3Kγ Regulates β-Adrenergic Receptor Function. Mol. Cell 2011, 41, 636–648. [Google Scholar] [CrossRef]
- Beck, J.R.; Truong, T.; Stains, C.I. Temporal Analysis of PP2A Phosphatase Activity During Insulin Stimulation Using a Direct Activity Probe. ACS Chem. Biol. 2016, 11, 3284–3288. [Google Scholar] [CrossRef] [PubMed]
- Wlodarchak, N.; Xing, Y. PP2A as a master regulator of the cell cycle. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Marliss, E.B.; Vranic, M. Intense Exercise Has Unique Effects on Both Insulin Release and Its Roles in Glucoregulation. Diabetes 2002, 51 (Suppl. S1), S271–S283. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Guadalupe-Grau, A.; Fuentes, T.; Ponce-González, J.G.; Morales-Alamo, D.; Olmedillas, H.; Guillén-Salgado, J.; Santana, A.; Calbet, J.A.L. SIRT1, AMP-activated protein kinase phosphorylation and downstream kinases in response to a single bout of sprint exercise: Influence of glucose ingestion. Eur. J. Appl. Physiol. 2010, 109, 731–743. [Google Scholar] [CrossRef]
- Aslam, M.; Ladilov, Y. Emerging Role of cAMP/AMPK Signaling. Cells 2022, 11, 308. [Google Scholar] [CrossRef]
- Kelly, M.; Gauthier, M.-S.; Saha, A.K.; Ruderman, N.B.; Hong, E.-G.; Ko, H.J.; Cho, Y.-R.; Kim, H.-J.; Ma, Z.; Yu, T.Y.; et al. Activation of AMP-Activated Protein Kinase by Interleukin-6 in Rat Skeletal Muscle. Diabetes 2009, 58, 1953–1960. [Google Scholar] [CrossRef]
- Pullar, C.E.; Chen, J.; Isseroff, R.R. PP2A Activation by β2-Adrenergic Receptor Agonists. J. Biol. Chem. 2003, 278, 22555–22562. [Google Scholar] [CrossRef]
- Ranieri, A.; Kemp, E.; Burgoyne, J.R.; Avkiran, M. β-Adrenergic regulation of cardiac type 2A protein phosphatase through phosphorylation of regulatory subunit B56δ at S573. J. Mol. Cell. Cardiol. 2017, 115, 20–31. [Google Scholar] [CrossRef]
- Puhl, S.-L.; Weeks, K.L.; Güran, A.; Ranieri, A.; Boknik, P.; Kirchhefer, U.; Müller, F.U.; Avkiran, M. Role of type 2A phosphatase regulatory subunit B56α in regulating cardiac responses to β-adrenergic stimulation in vivo. Cardiovasc. Res. 2018, 115, 519–529. [Google Scholar] [CrossRef]
- Galassetti, P.; Mann, S.; Tate, D.; Neill, R.A.; Wasserman, D.H.; Davis, S.N. Effect of morning exercise on counterregulatory responses to subsequent, afternoon exercise. J. Appl. Physiol. 2001, 91, 91–99. [Google Scholar] [CrossRef] [PubMed]
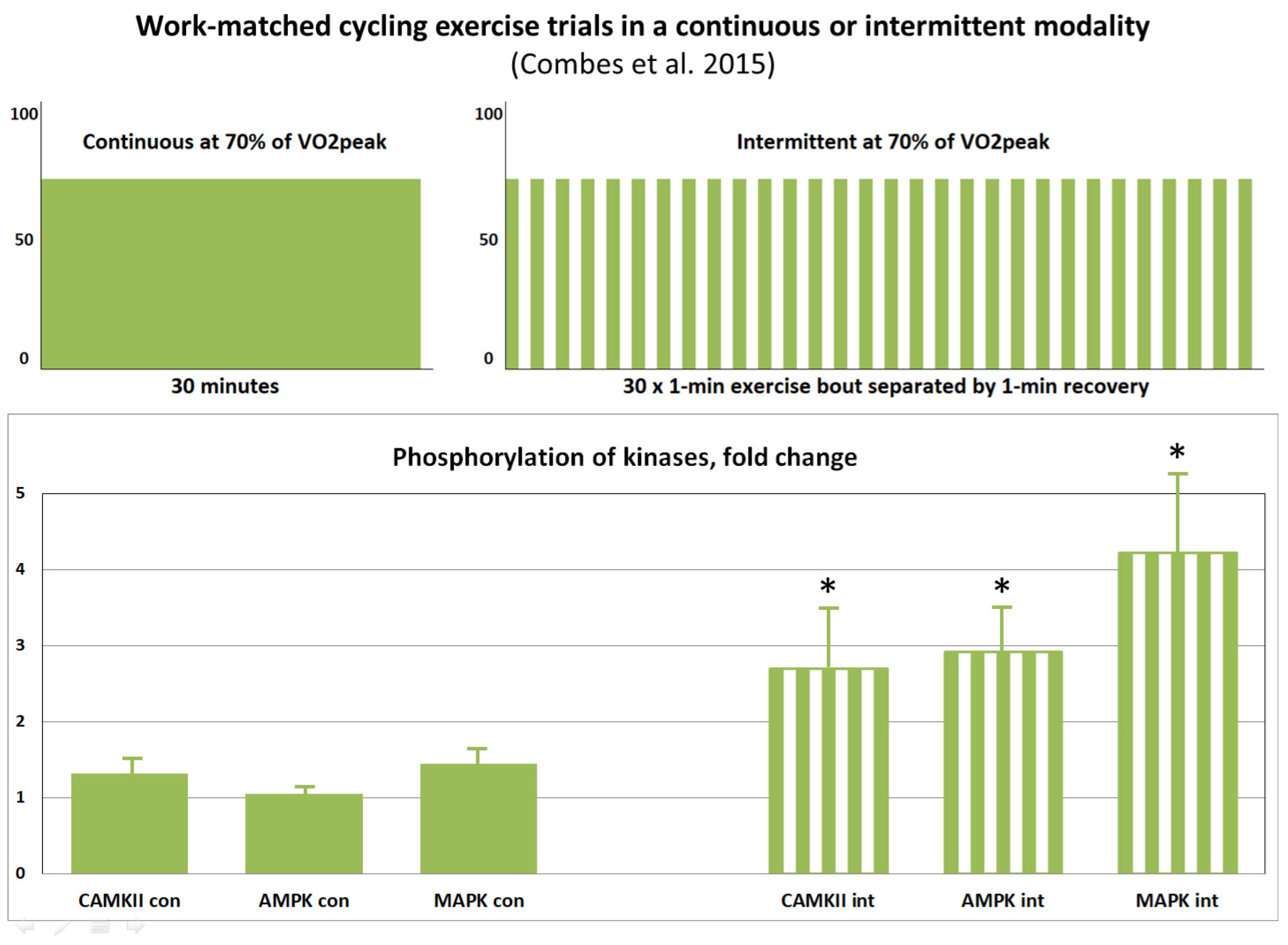
- Combes, A.; Dekerle, J.; Webborn, N.; Watt, P.; Bougault, V.; Daussin, F.N. Exercise-induced metabolic fluctuations influence AMPK, p38-MAPK and CaMKII phosphorylation in human skeletal muscle. Physiol. Rep. 2015, 3, e12462. [Google Scholar] [CrossRef] [PubMed]
- Popov, D.V.; Lysenko, E.A.; Miller, T.F.; Bachinin, A.V.; Perfilov, D.V.; Vinogradova, O.L. The effect of single aerobic exercise on the regulation of mitochondrial biogenesis in skeletal muscles of trained men: A time-course study. Hum. Physiol. 2015, 41, 296–303. [Google Scholar] [CrossRef]
- Rubinstein, S.; Kamen, G. Decreases in motor unit firing rate during sustained maximal-effort contractions in young and older adults. J. Electromyogr. Kinesiol. 2005, 15, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Kent-Braun, J.A.; Fitts, R.H.; Christie, A. Skeletal Muscle Fatigue. Compr. Physiol. 2012, 2, 997–1044. [Google Scholar] [CrossRef]
- Nielsen, J.; Cheng, A.J.; Ørtenblad, N.; Westerblad, H. Subcellular distribution of glycogen and decreased tetanic Ca2+ in fatigued single intact mouse muscle fibres. J. Physiol. 2014, 592, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.; Nogara, L.; Baraldo, M.; Reggiani, C.; Mann, M.; Schiaffino, S. Protein profile of fiber types in human skeletal muscle: A single-fiber proteomics study. Skelet. Muscle 2021, 11, 24. [Google Scholar] [CrossRef]
- Frøsig, C.; Jørgensen, S.B.; Hardie, D.G.; Richter, E.A.; Wojtaszewski, J.F.P.; Thomassen, M.; Gunnarsson, T.P.; Christensen, P.M.; Pavlovic, D.; Shattock, M.J.; et al. 5′-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am. J. Physiol. Metab. 2004, 286, E411–E417. [Google Scholar] [CrossRef] [PubMed]
- Wojtaszewski, J.F.P.; Birk, J.B.; Frøsig, C.; Holten, M.; Pilegaard, H.; Dela, F. 5′AMP activated protein kinase expression in human skeletal muscle: Effects of strength training and type 2 diabetes. J. Physiol. 2005, 564 Pt 2, 563–573. [Google Scholar] [CrossRef]
- Nemeth, P.M.; Pette, D.; Vrbová, G. Comparison of enzyme activities among single muscle fibres within defined motor units. J. Physiol. 1981, 311, 489–495. [Google Scholar] [CrossRef]
- Sieck, G.C.; Fournier, M.; Prakash, Y.S.; Blanco, C.E. Myosin phenotype and SDH enzyme variability among motor unit fibers. J. Appl. Physiol. 1996, 80, 2179–2189. [Google Scholar] [CrossRef]
- Rossiter, H.B.; Ward, S.A.; Howe, F.A.; Kowalchuk, J.M.; Griffiths, J.R.; Whipp, B.J. Dynamics of intramuscular 31P-MRS Pi peak splitting and the slow components of PCr and O2 uptake during exercise. J. Appl. Physiol. 2002, 93, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Cannon, D.T.; Bimson, W.E.; Hampson, S.A.; Bowen, T.S.; Murgatroyd, S.R.; Marwood, S.; Kemp, G.J.; Rossiter, H.B. Skeletal muscle ATP turnover by 31P magnetic resonance spectroscopy during moderate and heavy bilateral knee extension. J. Physiol. 2014, 592, 5287–5300. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H. The cross-bridge cycle and skeletal muscle fatigue. J. Appl. Physiol. 2008, 104, 551–558. [Google Scholar] [CrossRef]
- Sundberg, C.W.; Hunter, S.K.; Trappe, S.W.; Smith, C.S.; Fitts, R.H. Effects of elevated H+ and Pi on the contractile mechanics of skeletal muscle fibres from young and old men: Implications for muscle fatigue in humans. J. Physiol. 2018, 596, 3993–4015. [Google Scholar] [CrossRef]
- Woodward, M.; Debold, E.P. Acidosis and Phosphate Directly Reduce Myosin’s Force-Generating Capacity Through Distinct Molecular Mechanisms. Front. Physiol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Walsh, B.; Tiivel, T.; Tonkonogi, M.; Sahlin, K. Increased concentrations of P(i) and lactic acid reduce creatine-stimulated respiration in muscle fibers. J. Appl. Physiol. 2002, 92, 2273–2276. [Google Scholar] [CrossRef]
- Jubrias, S.A.; Crowther, G.J.; Shankland, E.G.; Gronka, R.K.; Conley, K.E. Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J. Physiol. 2003, 553 Pt 2, 589–599. [Google Scholar] [CrossRef]
- Broek, N.M.A.v.D.; De Feyter, H.M.M.L.; de Graaf, L.; Nicolay, K.; Prompers, J.J. Intersubject differences in the effect of acidosis on phosphocreatine recovery kinetics in muscle after exercise are due to differences in proton efflux rates. Am. J. Physiol.-Cell Physiol. 2007, 293, C228–C237. [Google Scholar] [CrossRef] [PubMed]
- Layec, G.; Malucelli, E.; Le Fur, Y.; Manners, D.; Yashiro, K.; Testa, C.; Cozzone, P.J.; Iotti, S.; Bendahan, D. Effects of exercise-induced intracellular acidosis on the phosphocreatine recovery kinetics: A 31P MRS study in three muscle groups in humans. NMR Biomed. 2013, 26, 1403–1411. [Google Scholar] [CrossRef]
- Spriet, L.L.; Söderlund, K.; Bergstrom, M.; Hultman, E. Skeletal muscle glycogenolysis, glycolysis, and pH during electrical stimulation in men. J. Appl. Physiol. 1987, 62, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Connett, R.J.; Sahlin, K. Control of Glycolysis and Glycogen Metabolism. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Gursahani, H.I.; Schaefer, S. Acidification reduces mitochondrial calcium uptake in rat cardiac mitochondria. Am. J. Physiol. Circ. Physiol. 2004, 287, H2659–H2665. [Google Scholar] [CrossRef] [PubMed]
- Reggiani, C.; Marcucci, L. A controversial issue: Can mitochondria modulate cytosolic calcium and contraction of skeletal muscle fibers? J. Gen. Physiol. 2022, 154, e202213167. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Rossi, A.E.; Micaroni, M.; Beznoussenko, G.V.; Polishchuk, R.S.; Dirksen, R.T.; Protasi, F. Mitochondria Are Linked to Calcium Stores in Striated Muscle by Developmentally Regulated Tethering Structures. Mol. Biol. Cell 2009, 20, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Pinter, K.; Grignani, R.T.; Watkins, H.; Redwood, C. Localisation of AMPK γ subunits in cardiac and skeletal muscles. J. Muscle Res. Cell Motil. 2013, 34, 369–378. [Google Scholar] [CrossRef]
- Dutka, T.L.; Lamb, G.D. Na+-K+ pumps in the transverse tubular system of skeletal muscle fibers preferentially use ATP from glycolysis. Am. J. Physiol.-Cell Physiol. 2007, 293, C967–C977. [Google Scholar] [CrossRef]
- Xu, Z.; Williams, B.R.G. The B56α Regulatory Subunit of Protein Phosphatase 2A Is a Target for Regulation by Double-Stranded RNA-Dependent Protein Kinase PKR. Mol. Cell. Biol. 2000, 20, 5285–5299. [Google Scholar] [CrossRef]
- Ahn, J.-H.; McAvoy, T.; Rakhilin, S.V.; Nishi, A.; Greengard, P.; Nairn, A.C. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56δ subunit. Proc. Natl. Acad. Sci. USA 2007, 104, 2979–2984. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Sung, J.Y.; McAvoy, T.; Nishi, A.; Janssens, V.; Goris, J.; Greengard, P.; Nairn, A.C. The B″/PR72 subunit mediates Ca 2+-dependent dephosphorylation of DARPP-32 by protein phosphatase 2A. Proc. Natl. Acad. Sci. USA 2007, 104, 9876–9881. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Kim, Y.; Kim, H.-S.; Greengard, P.; Nairn, A.C. Protein Kinase C-Dependent Dephosphorylation of Tyrosine Hydroxylase Requires the B56δ Heterotrimeric Form of Protein Phosphatase 2A. PLoS ONE 2011, 6, e26292. [Google Scholar] [CrossRef]
- Park, S.; Scheffler, T.; Rossie, S.; Gerrard, D. AMPK activity is regulated by calcium-mediated protein phosphatase 2A activity. Cell Calcium 2013, 53, 217–223. [Google Scholar] [CrossRef]
- Kirchhefer, U.; Heinick, A.; König, S.; Kristensen, T.; Müller, F.U.; Seidl, M.D.; Boknik, P. Protein Phosphatase 2A Is Regulated by Protein Kinase Cα (PKCα)-dependent Phosphorylation of Its Targeting Subunit B56α at Ser41. J. Biol. Chem. 2014, 289, 163–176. [Google Scholar] [CrossRef]
- Zhu, X.-N.; Chen, L.-P.; Bai, Q.; Ma, L.; Li, D.-C.; Zhang, J.-M.; Gao, C.; Lei, Z.-N.; Zhang, Z.-B.; Xing, X.-M.; et al. PP2A–AMPKα–HSF1 axis regulates the metal-inducible expression of HSPs and ROS clearance. Cell. Signal. 2014, 26, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.K.; Liu, H.-Y.; Francisco, J.; Pandya, D.; Donigan, M.; Gallo-Ebert, C.; Giordano, C.; Bata, A.; Nickels, J.T., Jr. Inhibition of AMP Kinase by the Protein Phosphatase 2A Heterotrimer, PP2APpp2r2d. J. Biol. Chem. 2015, 290, 10588–10598. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Merrill, R.A.; Wilkerson, E.M.; Herring, L.E.; Graves, L.M.; Strack, S. Reduction of protein phosphatase 2A (PP2A) complexity reveals cellular functions and dephosphorylation motifs of the PP2A/B′δ holoenzyme. J. Biol. Chem. 2020, 295, 5654–5668. [Google Scholar] [CrossRef] [PubMed]
- Kruse, T.; Gnosa, S.P.; Nasa, I.; Garvanska, D.H.; Hein, J.B.; Nguyen, H.; Samsøe-Petersen, J.; Lopez-Mendez, B.; Hertz, E.P.T.; Schwarz, J.; et al. Mechanisms of site-specific dephosphorylation and kinase opposition imposed by PP2A regulatory subunits. EMBO J. 2020, 39, e103695. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Martin, B.L.; Brautigan, D.L. Regulation of Protein Serine-Threonine Phosphatase Type-2A by Tyrosine Phosphorylation. Science 1992, 257, 1261–1264. [Google Scholar] [CrossRef]
- Guo, H.; Damuni, Z. Autophosphorylation-activated protein kinase phosphorylates and inactivates protein phosphatase 2A. Proc. Natl. Acad. Sci. USA 1993, 90, 2500–2504. [Google Scholar] [CrossRef]
- Hoermann, B.; Kokot, T.; Helm, D.; Heinzlmeir, S.; Chojnacki, J.E.; Schubert, T.; Ludwig, C.; Berteotti, A.; Kurzawa, N.; Kuster, B.; et al. Dissecting the sequence determinants for dephosphorylation by the catalytic subunits of phosphatases PP1 and PP2A. Nat. Commun. 2020, 11, 3583. [Google Scholar] [CrossRef]
- Moorhead, G.; Johnson, D.; Morrice, N.; Cohen, P. The major myosin phosphatase in skeletal muscle is a complex between the β-isoform of protein phosphatase 1 and the MYPT2 gene product. FEBS Lett. 1998, 438, 141–144. [Google Scholar] [CrossRef]
- Hartshorne, D.J.; Ito, M.; Erdödi, F. Role of Protein Phosphatase Type 1 in Contractile Functions: Myosin Phosphatase. J. Biol. Chem. 2004, 279, 37211–37214. [Google Scholar] [CrossRef]
- Kumar, G.S.; Choy, M.S.; Koveal, D.M.; Lorinsky, M.K.; Lyons, S.P.; Kettenbach, A.N.; Page, R.; Peti, W. Identification of the substrate recruitment mechanism of the muscle glycogen protein phosphatase 1 holoenzyme. Sci. Adv. 2018, 4, eaau6044. [Google Scholar] [CrossRef] [PubMed]
- Castermans, D.; Somers, I.; Kriel, J.; Louwet, W.; Wera, S.; Versele, M.; Janssens, V.; Thevelein, J.M. Glucose-induced posttranslational activation of protein phosphatases PP2A and PP1 in yeast. Cell Res. 2012, 22, 1058–1077. [Google Scholar] [CrossRef] [PubMed]
- Grallert, A.; Boke, E.; Hagting, A.; Hodgson, B.; Connolly, Y.; Griffiths, J.R.; Smith, D.L.; Pines, J.; Hagan, I.M. A PP1–PP2A phosphatase relay controls mitotic progression. Nature 2014, 517, 94–98. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vertyshev, A.Y.; Akberdin, I.R.; Kolpakov, F.A. Numerous Trigger-like Interactions of Kinases/Protein Phosphatases in Human Skeletal Muscles Can Underlie Transient Processes in Activation of Signaling Pathways during Exercise. Int. J. Mol. Sci. 2023, 24, 11223. https://doi.org/10.3390/ijms241311223
Vertyshev AY, Akberdin IR, Kolpakov FA. Numerous Trigger-like Interactions of Kinases/Protein Phosphatases in Human Skeletal Muscles Can Underlie Transient Processes in Activation of Signaling Pathways during Exercise. International Journal of Molecular Sciences. 2023; 24(13):11223. https://doi.org/10.3390/ijms241311223
Chicago/Turabian StyleVertyshev, Alexander Yu., Ilya R. Akberdin, and Fedor A. Kolpakov. 2023. "Numerous Trigger-like Interactions of Kinases/Protein Phosphatases in Human Skeletal Muscles Can Underlie Transient Processes in Activation of Signaling Pathways during Exercise" International Journal of Molecular Sciences 24, no. 13: 11223. https://doi.org/10.3390/ijms241311223
APA StyleVertyshev, A. Y., Akberdin, I. R., & Kolpakov, F. A. (2023). Numerous Trigger-like Interactions of Kinases/Protein Phosphatases in Human Skeletal Muscles Can Underlie Transient Processes in Activation of Signaling Pathways during Exercise. International Journal of Molecular Sciences, 24(13), 11223. https://doi.org/10.3390/ijms241311223





