Glycine: The Smallest Anti-Inflammatory Micronutrient
Abstract
:1. Introduction
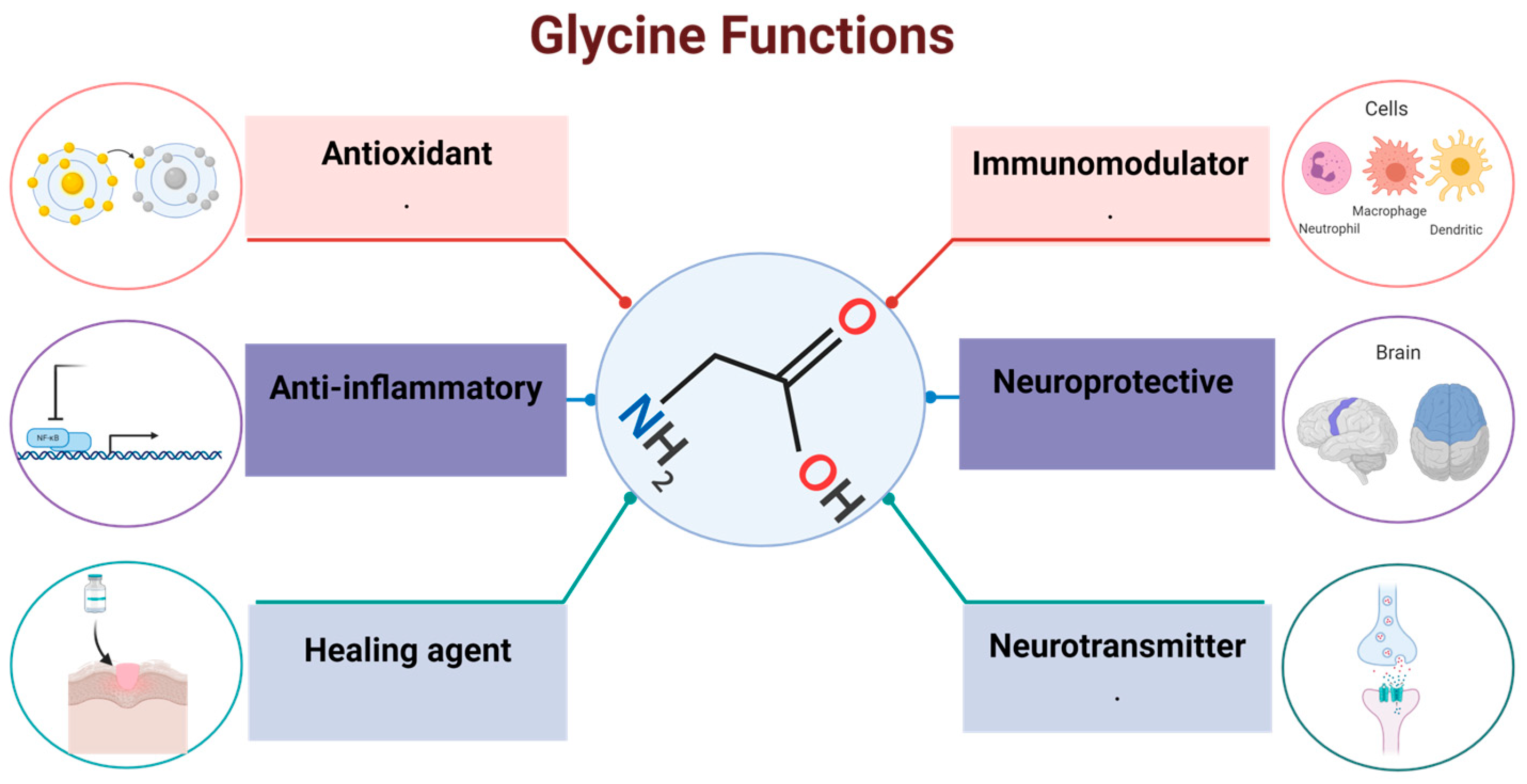
2. Glycine Targets
3. Receptors
3.1. GlyRs
3.2. NMDA Receptor
3.3. GPRC6 Receptor
4. Glycine Transporters
4.1. GlyT1
4.2. GlyT2
5. Effects of Glycine in the Organism
5.1. Neurotransmission
5.2. Antioxidant
5.3. Dietary Supplementation
5.4. Immunomodulation
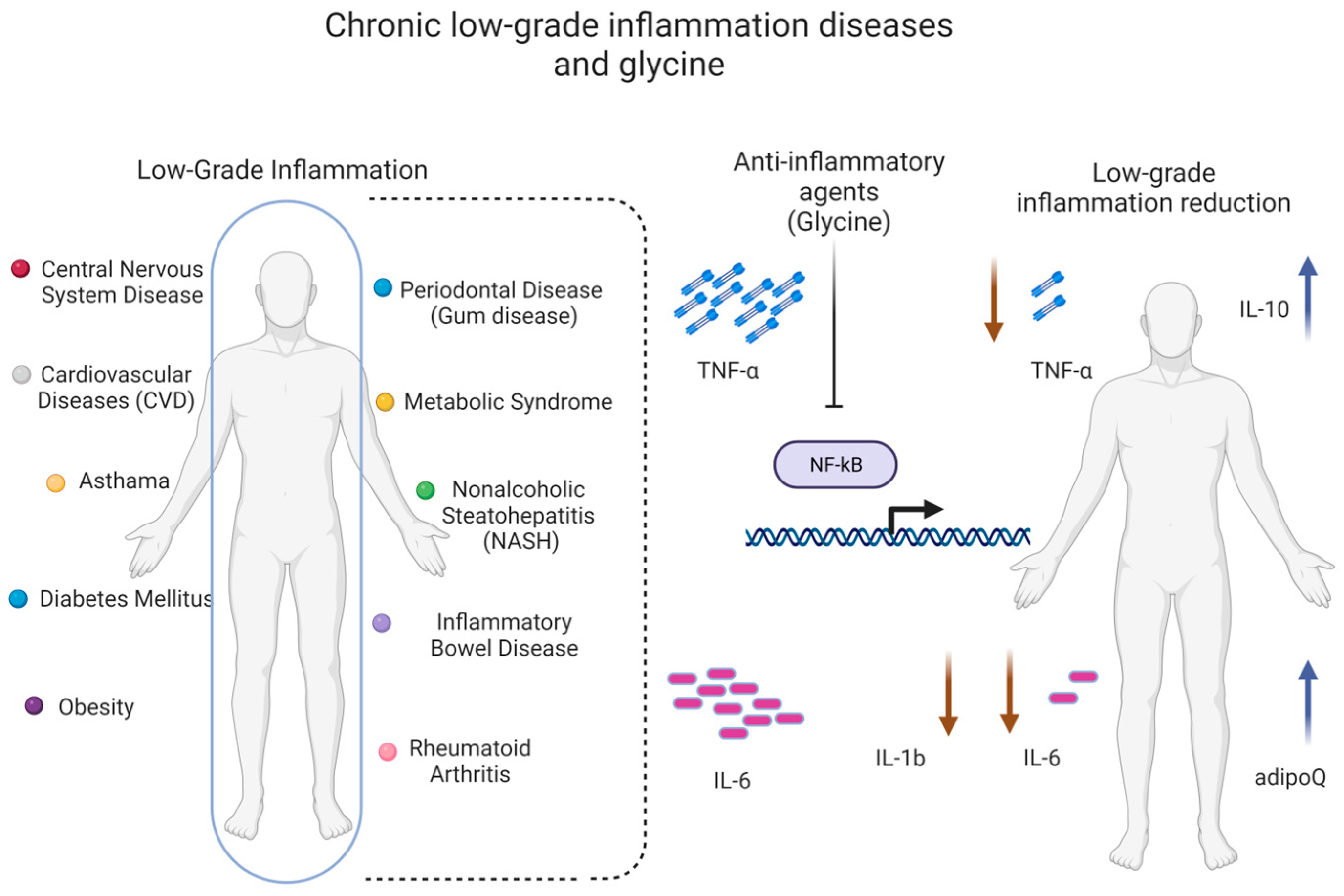
5.5. Low Glycine Plasma Levels Are Associated with Low-Grade Inflammation
6. The Role of Glycine in Inflammation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tibbetts, A.S.; Appling, D.R. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 2010, 30, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.A.; Badaloo, A.V.; Forrester, T.; Hibbert, J.M.; Persaud, C. Urinary excretion of 5-oxoproline (pyroglutamic aciduria) as an index of glycine insufficiency in normal man. Br. J. Nutr. 1987, 58, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, A.; Bassot, A.; Bulteau, A.L.; Pirola, L.; Morio, B. Glycine metabolism and its alterations in obesity and metabolic diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Robert, J.J.; Bier, D.M.; Zhao, X.H.; Matthews, D.E.; Young, V.R. Glucose and insulin effects on de novo amino acid synthesis in young men: Studies with stable isotope labeled alanine, glycine, leucine, and lysine. Metabolism 1982, 31, 1210–1218. [Google Scholar] [CrossRef]
- Lamers, Y.; Williamson, J.; Gilbert, L.R.; Stacpoole, P.W.; Gregory, J.F., III. Glycine turnover and decarboxylation rate quantified in healthy men and women using primed, constant infusions of [1, 2-13C2] glycine and [2H3] leucine. J. Nutr. 2007, 137, 2647–2652. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.M.; Yang, R.D.; Matthews, D.E.; Wen, Z.M.; Burke, J.F.; Bier, D.M.; Young, V.R. Quantitative aspects of glycine and alanine nitrogen metabolism in postabsorptive young men: Effects of level of nitrogen and dispensable amino acid intake. J. Nutr. 1985, 115, 399–410. [Google Scholar] [CrossRef]
- Meléndez-Hevia, E.; de Paz-Lugo, P.; Cornish-Bowden, A.; Cárdenas, M.L. A weak link in metabolism: The metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J. Biosci. 2009, 34, 853–872. [Google Scholar] [CrossRef]
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wu, Z.; Lin, G.; Hu, S.; Wang, B.; Dai, Z.; Wu, G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J. Nutr. 2014, 144, 1540–1548. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jia, H.; Jin, Y.; Liu, N.; Chen, J.; Yang, Y.; Dai, Z.; Wang, C.; Wu, G.; Wu, Z. Glycine attenuates LPS-induced apoptosis and inflammatory cell infiltration in mouse liver. J. Nutr. 2020, 150, 1116–1125. [Google Scholar] [CrossRef]
- Eulenburg, V.S. Glycine transporters: Essential regulators of synaptic transmission. Eur. Neuropsychopharmacol. 2011, 21, S230. [Google Scholar] [CrossRef]
- Lynch, J.W. Native glycine receptor subtypes and their physiological roles. Neuropharmacology 2009, 56, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Foster, E.; Wildner, H.; Tudeau, L.; Haueter, S.; Ralvenius, W.T.; Jegen, M.; Johannssen, H.; Hösli, L.; Haenraets, K.; Ghanem, A.; et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 2015, 85, 1289–1304. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Piña, R.; Nuño-Licona, A. Effects of glycine on motor performance in rats after traumatic spinal cord injury. Proc. West. Pharmacol. Soc. 2007, 50, 131–133. [Google Scholar]
- Luka, Z.; Cerone, R.; Phillips, J.A.; Mudd, H.S.; Wagner, C. Mutations in human glycine N-methyltransferase give insights into its role in methionine metabolism. Hum. Genet. 2002, 110, 68–74. [Google Scholar] [CrossRef]
- Martínez-Chantar, M.L.; Vázquez-Chantada, M.; Ariz, U.; Martínez, N.; Varela, M.; Luka, Z.; Capdevila, A.; Rodríguez, J.; Aransay, A.M.; Matthiesen, R.; et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology 2008, 47, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- Walrand, S.; Chiotelli, E.; Noirt, F.; Mwewa, S.; Lassel, T. Consumption of a functional fermented milk containing collagen hydrolysate improves the concentration of collagen-specific amino acids in plasma. J. Agric. Food Chem. 2008, 56, 7790–7795. [Google Scholar] [CrossRef]
- Picanço, E.D.A.; Lopes-Paulo, F.; Marques, R.G.; Diestel, C.F.; Caetano, C.E.R.; de Souza, M.V.M.; Moscoso, G.M.; Pazos, H.M.F. L-arginine and glycine supplementation in the repair of the irradiated colonic wall of rats. Int. J. Color. Dis. 2011, 26, 561–568. [Google Scholar] [CrossRef]
- Gameiro, A.; Reimann, F.; Habib, A.M.; O’malley, D.; Williams, L.; Simpson, A.K.; Gribble, F.M. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J. Physiol. 2005, 569, 761–772. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, M.; Medina-Santillan, R.; Martinez-Abundis, E.; von Drateln, C.R. Effect of glycine on insulin secretion and action in healthy first-degree relatives of type 2 diabetes mellitus patients. Horm. Metab. Res. 2001, 33, 358–360. [Google Scholar] [CrossRef]
- Gannon, M.C.; Nuttall, J.A.; Nuttall, F.Q. The metabolic response to ingested glycine. Am. J. Clin. Nutr. 2002, 76, 1302–1307. [Google Scholar] [CrossRef] [Green Version]
- Yan-Do, R.; Duong, E.; Fox, J.E.M.; Dai, X.; Suzuki, K.; Khan, S.; Bautista, A.; Ferdaoussi, M.; Lyon, J.; Wu, X.; et al. A glycine-insulin autocrine feedback loop enhances insulin secretion from human β-cells and is impaired in type 2 diabetes. Diabetes 2016, 65, 2311–2321. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Wang, Y.; Sun, G. High dietary choline and betaine intake is associated with low insulin resistance in the Newfoundland population. Nutrition 2017, 33, 28–34. [Google Scholar] [CrossRef]
- Bröer, S. The SLC6 orphans are forming a family of amino acid transporters. Neurochem. Int. 2006, 48, 559–567. [Google Scholar] [CrossRef]
- Vanslambrouck, J.M.; Bröer, A.; Thavyogarajah, T.; Holst, J.; Bailey, C.G.; Bröer, S.; Rasko, J.E. Renal imino acid and glycine transport system ontogeny and involvement in developmental iminoglycinuria. Biochem. J. 2010, 428, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Christopoulos, A.; Changeux, J.-P.; Catterall, W.A.; Fabbro, D.; Burris, T.P.; Cidlowski, J.A.; Olsen, R.W.; Peters, J.A.; Neubig, R.R.; Pin, J.-P.; et al. International Union of Basic and Clinical Pharmacology. XC. Multisite Pharmacology: Recommendations for the Nomenclature of Receptor Allosterism and Allosteric Ligands. Pharmacol. Rev. 2014, 66, 918–947. [Google Scholar] [CrossRef] [Green Version]
- Thwaites, D.T.; Anderson, C.M. The SLC36 family of proton-coupled amino acid transporters and their potential role in drug transport. Br. J. Pharmacol. 2011, 164, 1802–1816. [Google Scholar] [CrossRef] [Green Version]
- Olsen, R.W.; Sieghart, W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acidA receptors: Classification on the basis of subunit composition, pharmacology, and function. Pharmacol. Rev. 2008, 60, 243–260. [Google Scholar] [CrossRef] [Green Version]
- Velázquez-Flores, M.A.; Salceda, R. Modulación de los receptores ionotrópicos de tipo cys-loop por proteincinasas A y C. Rev. Neurol. 2011, 52, 81. [Google Scholar] [CrossRef]
- Avila, A.; Nguyen, L.; Rigo, J.M. Glycine receptors and brain development. Front. Cell. Neurosci. 2013, 7, 184. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Taran, E.; Webb, T.I.; Lynch, J.W. Stoichiometry and subunit arrangement of α1β glycine receptors as determined by atomic force microscopy. Biochemistry 2012, 51, 5229–5231. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.W.; Callister, R.J. Glycine receptors: A new therapeutic target in pain pathways. Curr. Opin. Investig. Drugs 2006, 7, 48–53. [Google Scholar]
- Grudzinska, J.; Schemm, R.; Haeger, S.; Nicke, A.; Schmalzing, G.; Betz, H.; Laube, B. The β subunit determines the ligand binding properties of synaptic glycine receptors. Neuron 2005, 45, 727–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betz, H.; Laube, B. Glycine receptors: Recent insights into their structural organization and functional diversity. J. Neurochem. 2006, 97, 1600–1610. [Google Scholar] [CrossRef]
- Durisic, N.; Godin, A.G.; Wever, C.M.; Heyes, C.D.; Lakadamyali, M.; Dent, J.A. Stoichiometry of the human glycine receptor revealed by direct subunit counting. J. Neurosci. 2012, 32, 12915–12920. [Google Scholar] [CrossRef] [Green Version]
- Matzenbach, B.; Maulet, Y.; Sefton, L.; Courtier, B.; Avner, P.; Guenet, J.L.; Betz, H. Structural analysis of mouse glycine receptor alpha subunit genes. Identification and chromosomal localization of a novel variant. J. Biol. Chem. 1994, 269, 2607–2612. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.J.; Schmieden, V.; Von Holst, A.; Laube, B.; Rohrer, H.; Betz, H. Glycine receptors containing the α4 subunit in the embryonic sympathetic nervous system, spinal cord and male genital ridge. Eur. J. Neurosci. 2000, 12, 994–1001. [Google Scholar] [CrossRef]
- Kuhse, J.; Kuryatov, A.; Maulet, Y.; Malosio, M.; Schmieden, V.; Betz, H. Alternative splicing generates two isoforms of the α2 subunit of the inhibitory glycine receptor. FEBS Lett. 1991, 283, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wong-Riley, M.T. Postnatal development of glycine receptor subunits α1, α2, α3, and β immunoreactivity in multiple brain stem respiratory-related nuclear groups of the rat. Brain Res. 2013, 1538, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malosio, M.L.; Marquèze-Pouey, B.; Kuhse, J.; Betz, H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991, 10, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Greferath, U.; Brandstätter, J.H.; Wässle, H.; Kirsch, J.; Kuhse, J.; Grünert, U. Differential expression of glycine receptor subunits in the retina of the rat: A study using immunohistochemistry and in situ hybridization. Vis. Neurosci. 1994, 11, 721–729. [Google Scholar] [CrossRef]
- Harvey, R.J.; Depner, U.B.; Wassle, H.; Ahmadi, S.; Heindl, C.; Reinold, H.; Smart, T.G.; Harvey, K.; Schutz, B.; Abo-Salem, O.M.; et al. GlyR α3: An essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 2004, 304, 884–887. [Google Scholar] [CrossRef] [Green Version]
- Manzke, T.; Niebert, M.; Koch, U.R.; Caley, A.; Vogelgesang, S.; Hülsmann, S.; Ponimaskin, E.; Müller, U.; Smart, T.G.; Harvey, R.J.; et al. Serotonin receptor 1A–modulated phosphorylation of glycine receptor α3 controls breathing in mice. J. Clin. Investig. 2010, 120, 4118–4128. [Google Scholar] [CrossRef] [Green Version]
- Grenningloh, G.; Pribilla, I.; Prior, P.; Multhaup, G.; Beyreuther, K.; Taleb, O.; Betz, H. Cloning and expression of the 58 kd β subunit of the inhibitory glycine receptor. Neuron 1990, 4, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Sato, K.; Sato, M.; Inoue, T.; Kozuka, T.; Tohyama, M. Regional distribution of the cells expressing glycine receptor beta subunit mRNA in the rat brain. Brain Res. 1991, 560, 23–37. [Google Scholar] [CrossRef]
- Meyer, G.; Kirsch, J.; Betz, H.; Langosch, D. Identification of a gephyrin binding motif on the glycine receptor β subunit. Neuron 1995, 15, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Ottersen, O.P.; Storm-Mathisen, J.; Laake, J.H. Cellular and subcellular localization of glycine studied by quantitative electron microscopic immunocytochemistry. In Glycine Neurotransmission; Ottersen, O.P., Storm-Mathisen, J., Eds.; Wiley: Chichester, UK, 1990; pp. 303–328. [Google Scholar]
- Gundersen, Y.; Vaagenes, P.; Dreiem, A.; Fonnum, F. Glysin. Tidsskr. Nor. Legeforening 2004, 124, 773–775. [Google Scholar]
- Manousopoulou, A.; Koutmani, Y.; Karaliota, S.; Woelk, C.H.; Manolakos, E.S.; Karalis, K.; Garbis, S.D. Hypothalamus proteomics from mouse models with obesity and anorexia reveals therapeutic targets of appetite regulation. Nutr. Diabetes 2016, 6, e204. [Google Scholar] [CrossRef] [Green Version]
- Danysz, W.; Parsons, C.G. Glycine and N-methyl-D-aspartate receptors: Physiological significance and possible therapeutic applications. Pharmacol. Rev. 1998, 50, 597–664. [Google Scholar] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, U.; Sjödin, J.; Angery Möller, K.; Johansson, S.; Wiksröm, L.; Nasström, J. Glutamate-induced currents reveal three functionally distinct NMDA receptor populations in rat dorsal horn-effects of peripheral nerve lesion and inflammation. Neuroscience 2002, 112, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, M.; Martineau, M.; Sacchi, S.; Yatsenko, N.; Radzishevsky, I.; Conrod, S.; Ouares, K.A.; Wolosker, H.; Pollegioni, L.; Billard, J.-M.; et al. Identity of the NMDA receptor coagonist is synapse specific and developmentally regulated in the hippocampus. Proc. Natl. Acad. Sci. USA 2015, 112, E204–E213. [Google Scholar] [CrossRef]
- Johnson, J.W.; Ascher, P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 1987, 325, 529–531. [Google Scholar] [CrossRef]
- Song, Z.H.; Young, W.S., III; Brownstein, M.J.; Bonner, T.I. Molecular cloning of a novel candidate G protein-coupled receptor from rat brain. FEBS Lett. 1994, 351, 375–379. [Google Scholar] [CrossRef] [Green Version]
- Eggerickx, D.; Denef, J.F.; Labbé, O.; Hayashi, Y.; Refetoff, S.; Vassart, G.; Parmentier, M.; Libert, F. Molecular cloning of an orphan G-protein-coupled receptor that constitutively activates adenylate cyclase. Biochem. J. 1995, 309, 837–843. [Google Scholar] [CrossRef] [Green Version]
- Isawi, I.H.; Morales, P.; Sotudeh, N.; Hurst, D.P.; Lynch, D.L.; Reggio, P.H. GPR6 structural insights: Homology model construction and docking studies. Molecules 2020, 25, 725. [Google Scholar] [CrossRef] [Green Version]
- Fredriksson, R.; Lagerström, M.C.; Lundin, L.G.; Schiöth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef] [Green Version]
- Wellendorph, P.; Hansen, K.B.; Balsgaard, A.; Greenwood, J.R.; Egebjerg, J.; Bräuner-Osborne, H. Deorphanization of GPRC6A: A promiscuous L-alpha-amino acid receptor with preference for basic amino acids. Mol. Pharmacol. 2005, 67, 589–597. [Google Scholar] [CrossRef]
- Uhlenbrock, K.; Gassenhuber, H.; Kostenis, E. Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell. Signal. 2002, 14, 941–953. [Google Scholar] [CrossRef]
- Ignatov, A.; Lintzel, J.; Kreienkamp, H.J.; Schaller, H.C. Sphingosine-1-phosphate is a high-affinity ligand for the G protein-coupled receptor GPR6 from mouse and induces intracellular Ca2+ release by activating the sphingosine-kinase pathway. Biochem. Biophys. Res. Commun. 2003, 311, 329–336. [Google Scholar] [CrossRef]
- Alexander, S.P.; Battey, J.; Benson, H.E.; Benya, R.V.; Bonner, T.I.; Davenport, A.P.; Eguchi, S.; Harmar, A.; Holliday, N.; Jensen, R.T.; et al. Class A orphans (version 2019.5) in the IUPHAR/BPS guide to pharmacology database. IUPHAR/BPS Guide Pharmacol. CITE 2019. [Google Scholar] [CrossRef]
- Martin, A.L.; Steurer, M.A.; Aronstam, R.S. Constitutive activity among orphan class-A G protein coupled receptors. PLoS ONE 2015, 10, e0138463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, P.; Isawi, I.; Reggio, P.H. Towards a better understanding of the cannabinoid-related orphan receptors GPR3, GPR6, and GPR12. Drug Metab. Rev. 2018, 50, 74–93. [Google Scholar] [CrossRef]
- Wellendorph, P.; Bräuner-Osborne, H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene 2004, 335, 37–46. [Google Scholar] [CrossRef]
- Gutiérrez-Rojas, R.A.; Aguayo-Cerón, K.A.; Vargas-De-León, C.; Cabrera-Becerra, S.E.; Almanza-Pérez, J.C.; Huang, F.; Villafaña, S.; Romero-Nava, R. Glycine Effect on the Expression Profile of Orphan Receptors GPR21, GPR26, GPR39, GPR82 and GPR6 in a Model of Inflammation in 3T3-L1 Cells. Life 2022, 12, 1687. [Google Scholar] [CrossRef] [PubMed]
- Laun, A.S.; Shrader, S.H.; Brown, K.J.; Song, Z.H. GPR3, GPR6, and GPR12 as novel molecular targets: Their biological functions and interaction with cannabidiol. Acta Pharmacol. Sin. 2019, 40, 300–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boll, M.; Daniel, H.; Gasnier, B. The SLC36 family: Proton-coupled transporters for the absorption of selected amino acids from extracellular and intracellular proteolysis. Pflügers Arch. 2004, 447, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Supplisson, S.; Roux, M.J. Why glycine transporters have different stoichiometries. FEBS Lett. 2002, 529, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Eulenburg, V.; Armsen, W.; Betz, H.; Gomeza, J. Glycine transporters: Essential regulators of neurotransmission. Trends Biochem. Sci. 2005, 30, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Gomeza, J.; Armsen, W.; Betz, H.; Eulenburg, V. Lessons from the Knocked-out Glycine Transporters; Springer: Berlin/Heidelberg, Germany, 2006; pp. 457–483. [Google Scholar]
- Roux, M.J.; Supplisson, S. Neuronal and glial glycine transporters have different stoichiometries. Neuron 2000, 25, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Corcuera, B.; Martínez-Maza, R.; Núñez, E.; Roux, M.; Supplisson, S.; Aragón, C. Differential properties of two stably expressed brain-specific glycine transporters. J. Neurochem. 1998, 71, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Erdem, F.A.; Ilic, M.; Koppensteiner, P.; Gołacki, J.; Lubec, G.; Freissmuth, M.; Sandtner, W. A comparison of the transport kinetics of glycine transporter 1 and glycine transporter 2. J. Gen. Physiol. 2019, 151, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, R.; Meyer, T.M.; Coyle, J.T.; Greene, R.W. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc. Natl. Acad. Sci. USA 1998, 95, 15730–15734. [Google Scholar] [CrossRef]
- Supplisson, S.; Bergman, C. Control of NMDA receptor activation by a glycine transporter co-expressed in Xenopus oocytes. J. Neurosci. 1997, 17, 4580–4590. [Google Scholar] [CrossRef] [Green Version]
- Howard, A.; Hirst, B.H. The glycine transporter GLYT1 in human intestine: Expression and function. Biol. Pharm. Bull. 2011, 34, 784–788. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.-R.; Corcuera, B.L.; Mandiyan, S.; Nelson, H.; Nelson, N. Cloning and expression of a spinal cord-and brain-specific glycine transporter with novel structural features. J. Biol. Chem. 1993, 268, 22802–22808. [Google Scholar] [CrossRef]
- Carta, E.; Chung, S.-K.; James, V.M.; Robinson, A.; Gill, J.L.; Remy, N.; Vanbellinghen, J.-F.; Drew, C.J.G.; Cagdas, S.; Cameron, D.; et al. Mutations in the GlyT2 gene (SLC6A5) are a second major cause of startle disease. J. Biol. Chem. 2012, 287, 28975–28985. [Google Scholar] [CrossRef] [Green Version]
- Benveniste, M.; Mayer, M.L. Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. Biophys. J. 1991, 59, 560–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clements, J.D.; Westbrook, G.L. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron 1991, 7, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Mizusawa, N.; Kimura, Y.; Ishii, A.; Yamanari, T.; Nakazawa, S.; Teramoto, H.; Ono, T.A. Impact of replacement of D1 C-terminal alanine with glycine on structure and function of photosynthetic oxygen-evolving complex. J. Biol. Chem. 2004, 279, 29622–29627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collingridge, G.L.; Volianskis, A.; Bannister, N.; France, G.; Hanna, L.; Mercier, M.; Tidball, P.; Fang, G.; Irvine, M.W.; Costa, B.M.; et al. The NMDA receptor as a target for cognitive enhancement. Neuropharmacology 2013, 64, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Zafra, F.; Ibáñez, I.; Bartolomé-Martín, D.; Piniella, D.; Arribas-Blázquez, M.; Giménez, C. Glycine transporters and its coupling with NMDA receptors. Glial Amino Acid Transp. 2017, 16, 55–83. [Google Scholar]
- Fujita, H.; Sato, K.; Wen, T.C.; Peng, Y.; Sakanaka, M. Differential expressions of glycine transporter 1 and three glutamate transporter mRNA in the hippocampus of gerbils with transient forebrain ischemia. J. Cereb. Blood Flow Metab. 1999, 19, 604–615. [Google Scholar] [CrossRef] [Green Version]
- Zafra, F.; Aragón, C.; Giménez, C. Molecular biology of glycinergic neurotransmission. Mol. Neurobiol. 1997, 14, 117–142. [Google Scholar] [CrossRef]
- Al-Khrasani, M.; Mohammadzadeh, A.; Balogh, M.; Király, K.; Barsi, S.; Hajnal, B.; Köles, L.; Zádori, Z.S.; Harsing, L.G., Jr. Glycine transporter inhibitors: A new avenue for managing neuropathic pain. Brain Res. Bull. 2019, 152, 143–158. [Google Scholar] [CrossRef]
- Gomeza, J.; Hülsmann, S.; Ohno, K.; Eulenburg, V.; Szöke, K.; Richter, D.; Betz, H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron 2003, 40, 785–796. [Google Scholar] [CrossRef] [Green Version]
- Tsai, G.; Ralph-Williams, R.J.; Martina, M.; Bergeron, R.; Berger-Sweeney, J.; Dunham, K.S.; Jiang, Z.; Caine, S.B.; Coyle, J.T. Gene knockout of glycine transporter 1: Characterization of the behavioral phenotype. Proc. Natl. Acad. Sci. USA 2004, 101, 8485–8490. [Google Scholar] [CrossRef]
- Oeckl, P.; Hengerer, B.; Ferger, B. G-protein coupled receptor 6 deficiency alters striatal dopamine and cAMP concentrations and reduces dyskinesia in a mouse model of Parkinson’s disease. Exp. Neurol. 2014, 257, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oeckl, P.; Ferger, B. Increased susceptibility of G-protein coupled receptor 6 deficient mice to MPTP neurotoxicity. Neuroscience 2016, 337, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liao, X.Y.; Pan, M.X.; Tang, J.C.; Chen, S.F.; Zhang, Y.; Lu, P.X.; Lu, L.J.; Zou, Y.Y.; Qin, X.P.; et al. Glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of M1 microglial polarization via the NF-κB p65/Hif-1α signaling pathway. J. Immunol. 2019, 202, 1704–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Hu, B.; Wang, F.; Du, L.; Huang, B.; Li, L.; Qi, J.; Wang, X. Glycine bidirectionally regulates ischemic tolerance via different mechanisms including NR2A-dependent CREB phosphorylation. J. Neurochem. 2015, 133, 397–408. [Google Scholar] [CrossRef]
- Gusev, E.I.; Skvortsova, V.I.; Dambinova, S.; Raevskiy, K.S.; Alekseev, A.A.; Bashkatova, V.G.; Kovalenko, A.V.; Kudrin, V.S.; Yakovleva, E.V. Neuroprotective effects of glycine for therapy of acute ischaemic stroke. Cerebrovasc. Dis. 2000, 10, 49–60. [Google Scholar] [CrossRef]
- Ikejima, K.E.N.I.C.H.I.; Iimuro, Y.U.J.I.; Forman, D.T.; Thurman, R.G. A diet containing glycine improves survival in endotoxin shock in the rat. Am. J. Physiol.-Gastrointest. Liver Physiol. 1996, 271, G97–G103. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Viswanathan, P.; Nalini, N. Glycine modulates hepatic lipid accumulation in alcohol-induced liver injury. Pol. J. Pharmacol. 2003, 55, 603–611. [Google Scholar]
- Zeb, A.; Rahman, S.U. Protective effects of dietary glycine and glutamic acid toward the toxic effects of oxidized mustard oil in rabbits. Food Funct. 2017, 8, 429–436. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, M.; Wang, T.J.; Clish, C.; Engström, G.; Nilsson, P.; Gerszten, R.E.; Melander, O. Dimethylglycine deficiency and the development of diabetes mellitus. Diabetes 2015, 64, 3010–3016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lv, S.J.; Yan, H.; Wang, L.; Liang, G.P.; Wan, Q.X.; Peng, X. Effects of glycine supplementation on myocardial damage and cardiac function after severe burn. Burns 2013, 39, 729–735. [Google Scholar] [CrossRef]
- Ascher, E.; Hanson, J.N.; Cheng, W.; Hingorani, A.; Scheinman, M. Glycine preserves function and decreases necrosis in skeletal muscle undergoing ischemia and reperfusion injury. Surgery 2001, 129, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Grotz, M.R.W.; Pape, H.C.; van Griensven, M.; Stalp, M.; Rohde, F.; Bock, D.; Krettek, C. Glycine reduces the inflammatory response and organ damage in a two-hit sepsis model in rats. Shock 2001, 16, 116–121. [Google Scholar] [CrossRef]
- Yang, S.; Koo, D.J.; Chaudry, I.H.; Wang, P. Glycine attenuates hepatocellular depression during early sepsis and reduces sepsis-induced mortality. Crit. Care Med. 2001, 29, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Enomoto, N.; Connor, H.D.; Moss, N.; Mason, R.P.; Thurman, R.G. Glycine improves survival after hemorrhagic shock in the rat. Shock 1999, 12, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Ikejima, K.; Zhong, Z.; Waalkes, M.P.; Thurman, R.G. Glycine blocks the increase in intracellular free Ca2+ due to vasoactive mediators in hepatic parenchymal cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 283, G1249–G1256. [Google Scholar] [CrossRef] [Green Version]
- Alvarado-Vásquez, N.; Zamudio, P.; Cerón, E.; Vanda, B.; Zenteno, E.; Carvajal-Sandoval, G. Effect of glycine in streptozotocin-induced diabetic rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 134, 521–527. [Google Scholar] [CrossRef]
- Hafidi, M.E.; Pérez, I.; Zamora, J.; Soto, V.; Carvajal-Sandoval, G.; Banos, G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R1387–R1393. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, S.; Sulochana, K.N. Decrease in glycation of lens proteins by lysine and glycine by scavenging of glucose and possible mitigation of cataractogenesis. Exp. Eye Res. 1993, 57, 623–628. [Google Scholar] [CrossRef]
- Sandoval, G.C.; Santillan, R.M.; Juarez, E.; Martlnez, G.R.; Juärez, M.E.C. Effect of glycine on hemoglobin glycation in diabetic patients. Proc. West. Pharmacol. Soc. 1999, 42, 31–32. [Google Scholar]
- Bahmani, F.; Bathaie, S.Z.; Aldavood, S.J.; Ghahghaei, A. Glycine therapy inhibits the progression of cataract in streptozotocin-induced diabetic rats. Mol. Vis. 2012, 18, 439. [Google Scholar]
- Zhong, Z.; Wheeler, M.D.; Li, X.; Froh, M.; Schemmer, P.; Yin, M.; Bunzendaul, H.; Bradford, B.; Lemasters, J.J. L-Glycine: A novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 229–240. [Google Scholar] [CrossRef]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxidative Med. Cell. Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, A.; Tahir, I.; Javed, S.; Waring, S.M.; Ford, D.; Hirst, B.H. Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. J. Physiol. 2010, 588, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- van Bergenhenegouwen, J.; Braber, S.; Loonstra, R.; Buurman, N.; Rutten, L.; Knipping, K.; Savelkoul, P.J.; Harthoorn, L.F.; Jahnsen, F.L.; Garssen, J.; et al. Oral exposure to the free amino acid glycine inhibits the acute allergic response in a model of cow’s milk allergy in mice. Nutr. Res. 2018, 58, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Ham, D.J.; Murphy, K.T.; Chee, A.; Lynch, G.S.; Koopman, R. Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clin. Nutr. 2014, 33, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, G.; Timm, A.-K.; Bergmann, F.; Günther, A.; Aghdassi, A.; Demir, I.; Mayerle, J.; Kern, M.; Lerch, M.; Büchler, M.; et al. Prophylactic glycine administration attenuates pancreatic damage and inflammation in experimental acute pancreatitis. Pancreatology 2011, 11, 57–67. [Google Scholar] [CrossRef]
- Hartog, A.; Leenders, I.; van der Kraan, P.M.; Garssen, J. Anti-inflammatory effects of orally ingested lactoferrin and glycine in different zymosan-induced inflammation models: Evidence for synergistic activity. Int. Immunopharmacol. 2007, 7, 1784–1792. [Google Scholar] [CrossRef]
- Wheeler, M.D.; Thurman, R.G. Production of superoxide and TNF-α from alveolar macrophages is blunted by glycine. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 277, L952–L959. [Google Scholar] [CrossRef]
- Froh, M.; Thurman, R.G.; Wheeler, M.D. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 283, G856–G863. [Google Scholar] [CrossRef] [Green Version]
- Stoffels, B.; Türler, A.; Schmidt, J.; Nazir, A.; Tsukamoto, T.; Moore, B.A.; Schnurr, C.; Kalff, J.C.; Bauer, A.J. Anti-inflammatory role of glycine in reducing rodent postoperative inflammatory ileus. Neurogastroenterol. Motil. 2011, 23, 76-e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, J.M.; Bienholz, A.; Venkatachalam, M.A. The role of glycine in regulated cell death. Cell. Mol. Life Sci. 2016, 73, 2285–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashina, S.; Ikejima, K.; Enomoto, N.; Takei, Y.; Sato, N. Glycine as a Therapeutic Immuno-Nutrient for Alcoholic Liver Disease. Alcohol. Clin. Exp. Res. 2005, 29, 162S–165S. [Google Scholar] [CrossRef] [PubMed]
- AlShakweer, W.; Alwelaie, Y.; Mankung, A.M.; Graeber, M.B. Bone marrow-derived microglia in pilocytic astrocytoma. Front. Biosci. 2011, 3, 371–379. [Google Scholar]
- Xia, C.Y.; Zhang, S.; Gao, Y.; Wang, Z.Z.; Chen, N.H. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int. Immunopharmacol. 2015, 25, 377–382. [Google Scholar] [CrossRef]
- Zhu, T.; Miller, A.G.; Deliyanti, D.; Berka, D.R.; Agrotis, A.; Campbell, D.J.; Wilkinson-Berka, J.L. Prorenin stimulates a pro-angiogenic and proinflammatory response in retinal endothelial cells and an M1 phenotype in retinal microglia. Clin. Exp. Pharmacol. Physiol. 2015, 42, 537–548. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Y.; Wang, Y.; Wang, Y.; He, L.; Jiang, Z.; Huang, Z.; Liao, H.; Li, J.; Saavedra, J.M.; et al. Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKb-dependent AMPK activation. Brain Behav. Immun. 2015, 50, 298–313. [Google Scholar] [CrossRef]
- Rose, M.L.; Cattley, R.C.; Dunn, C.; Wong, V.; Li, X.; Thurman, R.G. Dietary glycine prevents the development of liver tumors caused by the peroxisome proliferator WY-14,643. Carcinogenesis 1999, 20, 2075–2081. [Google Scholar] [CrossRef] [Green Version]
- Spittler, A.; Reissner, C.M.; Oehler, R.; Gornikiewicz, A.; Gruenberger, T.; Manhart, N.; Brodowicz, T.; Mittlboeck, M.; Boltz-Nitulescu, G.; Roth, E. Immunomodulatory effects of glycine on LPS-treated monocytes: Reduced TNF-alpha production and accelerated IL-10 expression. FASEB J. 1999, 13, 563–571. [Google Scholar] [CrossRef]
- Li, X.; Bradford, B.U.; Wheeler, M.D.; Stimpson, S.A.; Pink, H.M.; Brodie, T.A.; Schwab, J.H.; Thurman, R.G. Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: Role for glycine-gated chloride channel. Infect. Immun. 2001, 69, 5883–5891. [Google Scholar] [CrossRef] [Green Version]
- Okekunle, A.P.; Li, Y.; Liu, L.; Du, S.; Wu, X.; Chen, Y.; Li, Y.; Qi, J.; Sun, C.; Feng, R. Abnormal circulating amino acid profiles in multiple metabolic disorders. Diabetes Res. Clin. Pract. 2017, 132, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Gosmanov, N.R.; Gosmanov, A.R.; Gerich, J.E. Glucagon Physiology. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Yan-Do, R.; MacDonald, P.E. Impaired “glycine”-mia in type 2 diabetes and potential mechanisms contributing to glucose homeostasis. Endocrinology 2017, 158, 1064–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, P.J.; Lapworth, A.L.; An, J.; Wang, L.; McGarrah, R.W.; Stevens, R.D.; Ilkayeva, O.; George, T.; Muehlbauer, M.J.; Bain, J.R.; et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 2016, 5, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Hsu, J.W.; Jahoor, F.; Sekhar, R.V. Eect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J. Clin. Endocrinol. Metab. 2014, 99, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Beato, M. The time course of transmitter at glycinergic synapses onto motoneurons. J. Neurosci. 2008, 28, 7412–7425. [Google Scholar] [CrossRef] [Green Version]
- Xiong, W.; Cui, T.; Cheng, K.; Yang, F.; Chen, S.-R.; Willenbring, D.; Guan, Y.; Pan, H.-L.; Ren, K.; Xu, Y.; et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha3 glycine receptors. J. Exp. Med. 2012, 209, 1121–1134. [Google Scholar] [CrossRef] [Green Version]
- Bregman, H.; Simard, J.R.; Andrews, K.L.; Ayube, S.; Chen, H.; Gunaydin, H.; Guzman-Perez, A.; Hu, J.; Huang, L.; Huang, X.; et al. discovery and hit-to-lead optimization of tricyclic sulfonamides as potent and efficacious potentiators of glycine receptors. J. Med. Chem. 2017, 60, 1105–1125. [Google Scholar] [CrossRef]
- Wheeler, M.; Stachlewitz, R.F.; Yamashina, S.; Ikehima, K.; Morrow, A.L.; Thurman, R.G. Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J. 2000, 14, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Kawada, N.; Mizoguchi, Y.; Kobayashi, K.; Monna, T.; Morisawa, S.; Ueda, N.; Omoto, Y.; Takahashi, Y.; Yamamoto, S. Possible induction of fatty acid cyclo-oxygenase in lipopolysaccharide-stimulated rat Kupffer cells. Gastroenterology 1992, 103, 1026–1033. [Google Scholar] [CrossRef]
- Garcia-Macedo, R.; Sanchez-Muñoz, F.; Almanza-Perez, J.C.; Duran-Reyes, G.; Alarcon-Aguilar, F.; Cruz, M. Glycine increases mRNA adiponectin and diminishes pro-inflammatory adipokines expression in 3T3-L1 cells. Eur. J. Pharmacol. 2008, 587, 317–321. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Jiang, D.; Yang, Y.; Wu, G.; Wu, Z. Protective effects of functional amino acids on apoptosis, inflammatory response, and pulmonary fibrosis in lipopolysaccharide-challenged mice. J. Agric. Food Chem. 2019, 67, 4915–4922. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Metabolomics and metabolic diseases: Where do we stand? Cell Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takashina, C.; Tsujino, I.; Watanabe, T.; Sakaue, S.; Ikeda, D.; Yamada, A.; Sato, T.; Ohira, H.; Otsuka, Y.; Oyama-Manabe, N.; et al. Associations among the plasma amino acid profile, obesity, and glucose metabolism in Japanese adults with normal glucose tolerance. Nutr. Metab. 2016, 13, 5. [Google Scholar] [CrossRef]
- Tariq, M.; Al Moutaery, A.R. Studies on the antisecretory, gastric anti-ulcer and cytoprotective properties of glycine. Res. Commun. Mol. Pathol. Pharmacol. 1997, 97, 185–198. [Google Scholar] [PubMed]
- Rose, M.L.; Madren, J.; Bunzendahl, H.; Thurman, R.G. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis 1999, 20, 793–798. [Google Scholar] [CrossRef] [Green Version]
- El Kamouni, S.; El Kebbaj, R.; Andreoletti, P.; El Ktaibi, A.; Rharrassi, I.; Essamadi, A.; El Kebbaj, M.S.; Mandard, S.; Latruffe, N.; Vamecq, J.; et al. Protective Effect of Argan and Olive Oils against LPS-Induced Oxidative Stress and Inflammation in Mice Livers. Int. J. Mol. Sci. 2017, 18, 2181. [Google Scholar] [CrossRef] [Green Version]
- Mauriz, J.L.; Matilla, B.; Culebras, J.M.; González, P.; González-Gallego, J. Dietary glycine decreases liver injury after haemorrhagic shock in rats. Br. J. Surg. 2000, 87, 945–946. [Google Scholar] [CrossRef]
- Stachlewitz, R.F.; Li, X.; Smith, S.; Bunzendahl, H.; Graves, L.M.; Thurman, R.G. Glycine Inhibits Growth of T Lymphocytes by an IL-2-Independent Mechanism. J. Immunol. 2000, 164, 176–182. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Okada, S.; Wakiguchi, H.; Kudo, K.; Kittaka, S.; Hara, M.; Furukawa, S. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin. Exp. Immunol. 2011, 167, 269–274. [Google Scholar] [CrossRef]
- Matilla, B.; Mauriz, J.L.; Culebras, J.M.; Gonzalez-Gallego, J.; Gonzalez, P. La glicina: Un nutriente antioxidante protector celular. Nutr. Hosp. 2002, 17, 2–9. [Google Scholar]
- Chen, J.; Ma, X.; Yang, Y.; Dai, Z.; Wu, Z.; Wu, G. Glycine enhances expression of adiponectin and IL-10 in 3T3-L1 adipocytes without affecting adipogenesis and lipolysis. Amino Acids 2018, 50, 629–640. [Google Scholar] [CrossRef]
- Alarcon-Aguilar, F.; Almanza-Perez, J.; Blancas, G.; Angeles, S.; Garcia-Macedo, R.; Roman, R.; Cruz, M. Glycine regulates the production of pro-inflammatory cytokines in lean and monosodium glutamate-obese mice. Eur. J. Pharmacol. 2008, 599, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Blancas-Flores, G.; Alarcón-Aguilar, F.J.; García-Macedo, R.; Almanza-Pérez, J.C.; Flores-Sáenz, J.L.; Román-Ramos, R.; Ventura-Gallegos, J.L.; Kumate, J.; Zentella-Dehesa, A.; Cruz, M. Glycine suppresses TNF-alpha-induced activation of NF-κB in differentiated 3T3-L1 adipocytes. Eur. J. Pharmacol. 2012, 689, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, L.K.; Wallace, J.M.; Livingstone, M.B. Obesity and inflammation: The effects of weight loss. Nutr. Res. Rev. 2008, 21, 117–133. [Google Scholar] [CrossRef]
- Romero-Nava, R.; Alarcón-Aguilar, F.J.; Giacoman-Martínez, A.; Blancas-Flores, G.; Aguayo-Cerón, K.A.; Ballinas-Verdugo, M.A.; Sánchez-Muñoz, F.; Huang, F.; Villafaña-Rauda, S.; Almanza-Pérez, J.C. Glycine is a competitive antagonist of the TNF receptor mediating the expression of inflammatory cytokines in 3T3-L1 adipocytes. Inflamm. Res. 2021, 70, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.; Li, J.; Chao, W.R.; Dewhirst, M.W.; Haroon, Z.A. Dietary glycine inhibits angiogenesis during wound healing and tumor growth. Cancer Biol. Ther. 2003, 2, 173–178. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguayo-Cerón, K.A.; Sánchez-Muñoz, F.; Gutierrez-Rojas, R.A.; Acevedo-Villavicencio, L.N.; Flores-Zarate, A.V.; Huang, F.; Giacoman-Martinez, A.; Villafaña, S.; Romero-Nava, R. Glycine: The Smallest Anti-Inflammatory Micronutrient. Int. J. Mol. Sci. 2023, 24, 11236. https://doi.org/10.3390/ijms241411236
Aguayo-Cerón KA, Sánchez-Muñoz F, Gutierrez-Rojas RA, Acevedo-Villavicencio LN, Flores-Zarate AV, Huang F, Giacoman-Martinez A, Villafaña S, Romero-Nava R. Glycine: The Smallest Anti-Inflammatory Micronutrient. International Journal of Molecular Sciences. 2023; 24(14):11236. https://doi.org/10.3390/ijms241411236
Chicago/Turabian StyleAguayo-Cerón, Karla Aidee, Fausto Sánchez-Muñoz, Rocío Alejandra Gutierrez-Rojas, Lourdes Nallely Acevedo-Villavicencio, Aurora Vanessa Flores-Zarate, Fengyang Huang, Abraham Giacoman-Martinez, Santiago Villafaña, and Rodrigo Romero-Nava. 2023. "Glycine: The Smallest Anti-Inflammatory Micronutrient" International Journal of Molecular Sciences 24, no. 14: 11236. https://doi.org/10.3390/ijms241411236
APA StyleAguayo-Cerón, K. A., Sánchez-Muñoz, F., Gutierrez-Rojas, R. A., Acevedo-Villavicencio, L. N., Flores-Zarate, A. V., Huang, F., Giacoman-Martinez, A., Villafaña, S., & Romero-Nava, R. (2023). Glycine: The Smallest Anti-Inflammatory Micronutrient. International Journal of Molecular Sciences, 24(14), 11236. https://doi.org/10.3390/ijms241411236





