Preparation and Characterization of a Photo-Crosslinked Methacryloyl-Collagen Composite Film to Promote Corneal Nerve Regeneration via Surface Grafting of Taurine Molecules
Abstract
:1. Introduction
2. Results and Discussion
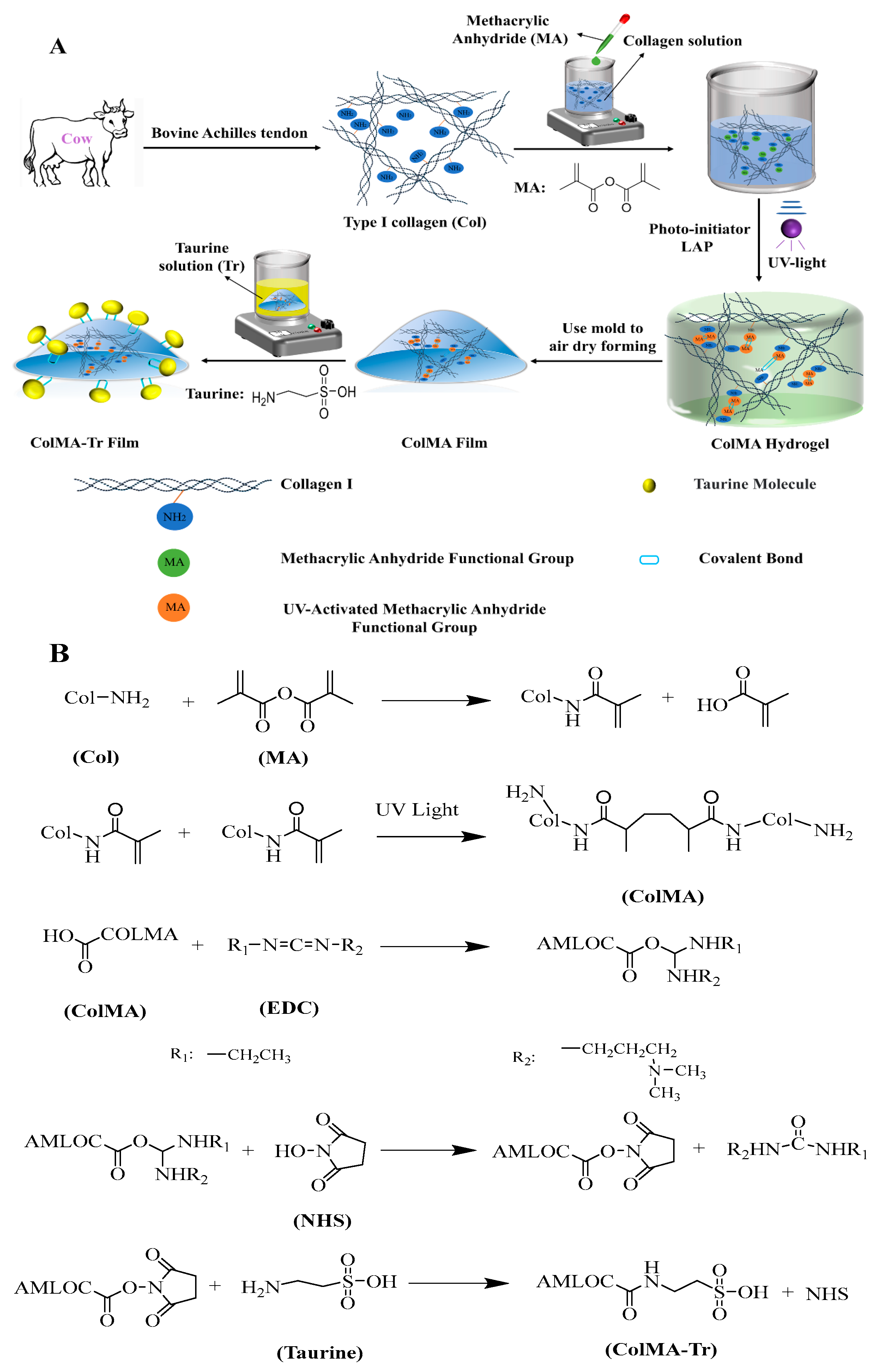
2.1. Infrared Spectroscopy Analysis
2.2. Evaluation of Contact Angle and Water Absorption
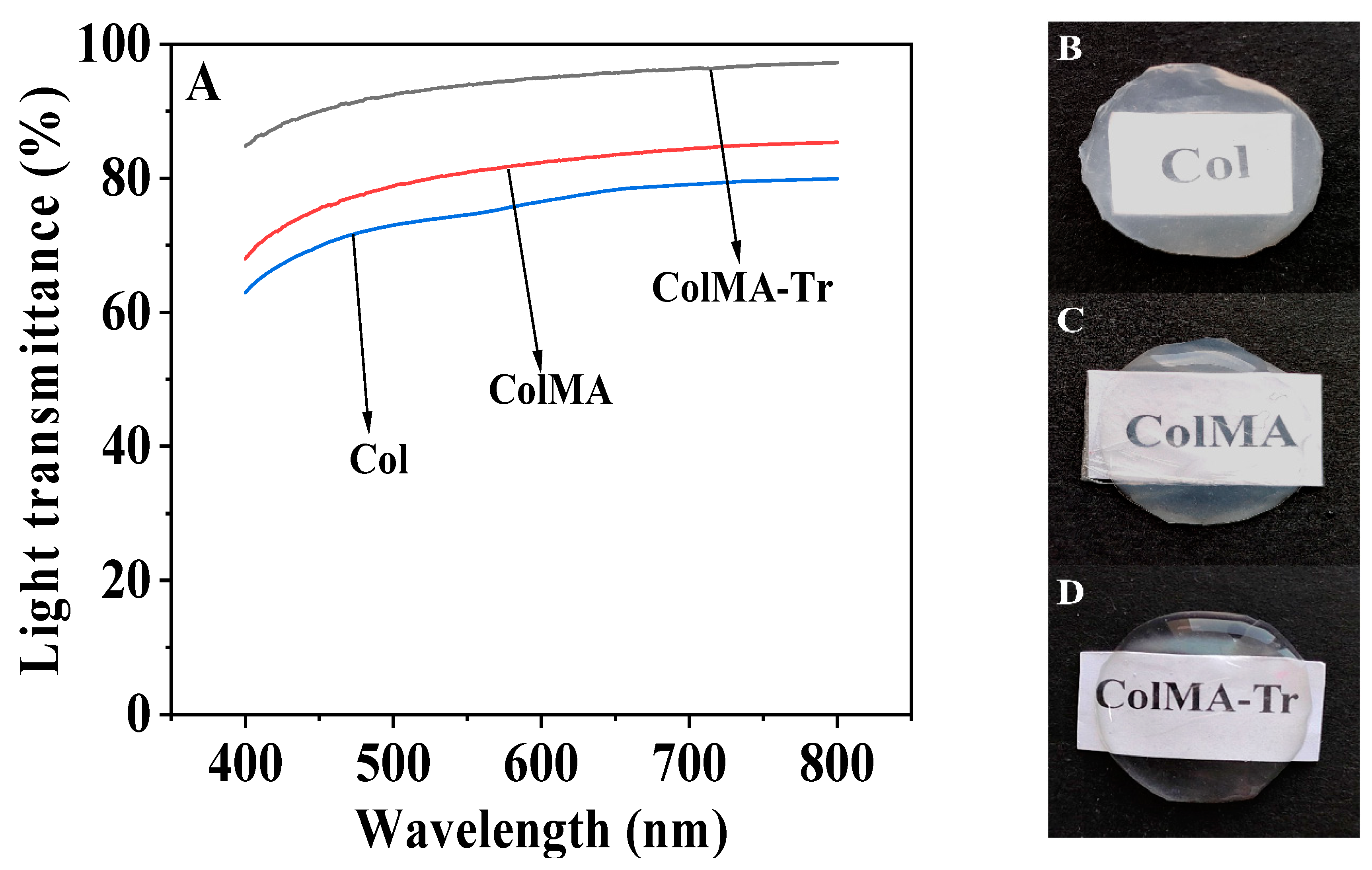
2.3. Optical Performance Evaluation
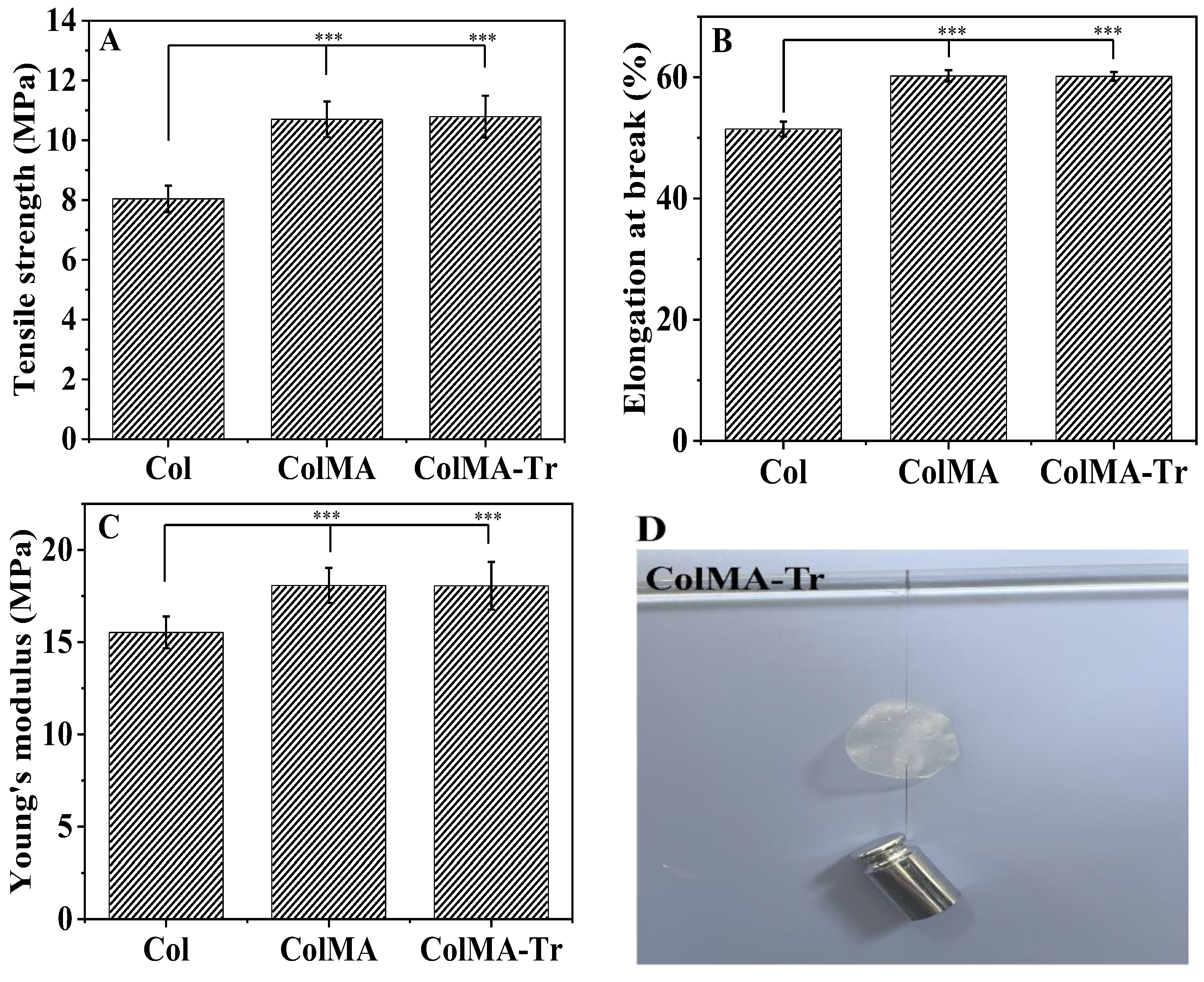
2.4. Mechanical Property Analysis
2.5. Analysis of Drug Loading and Sustained Release of ColMA-Tr Film
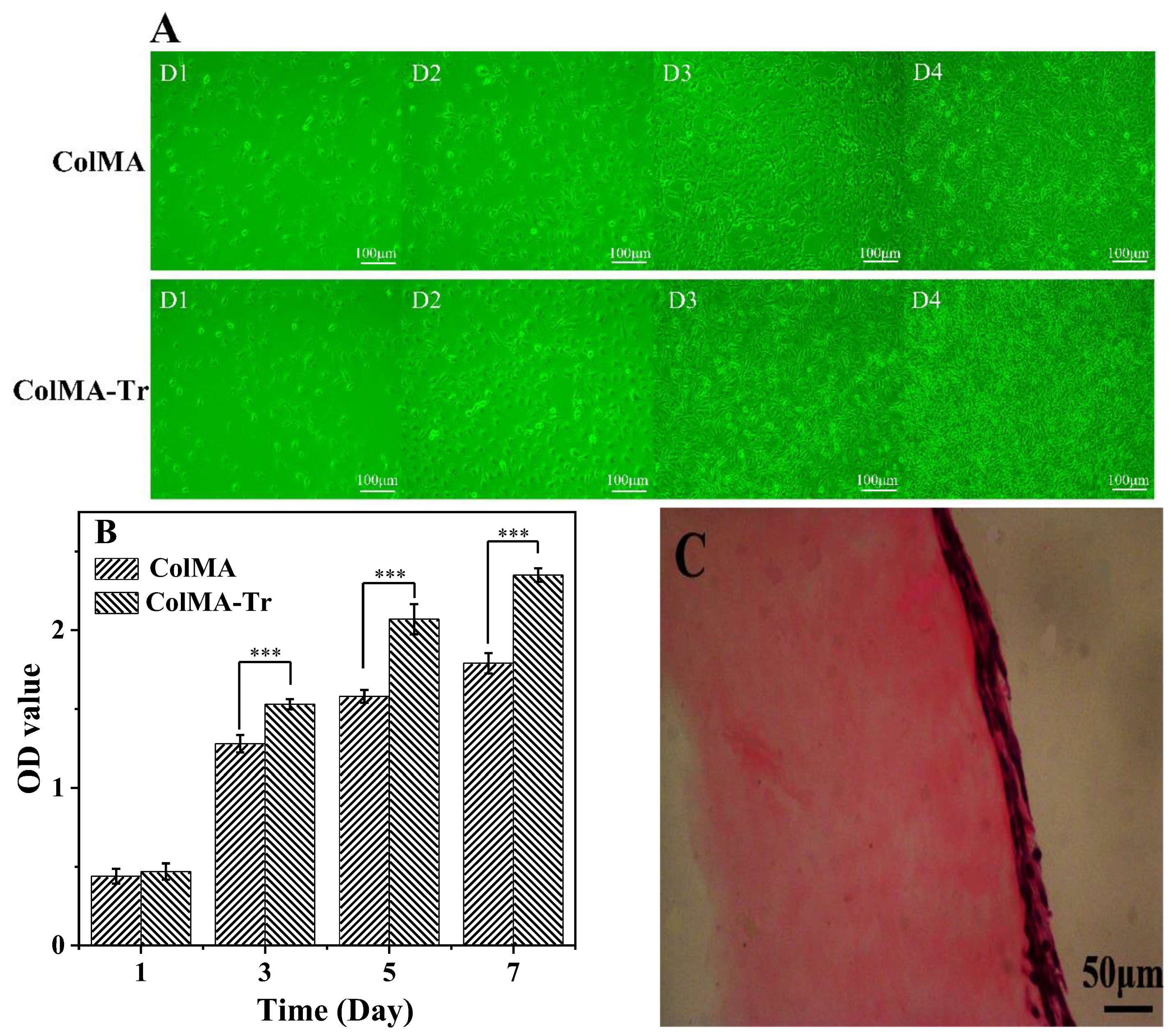
2.6. Growth of PC12 Nerve Cells on ColMA and ColMA-Tr Film
2.7. Adhesion and Proliferation of HCECs on ColMA and ColMA-Tr Film
3. Materials and Methods
3.1. Materials
3.2. Preparation Method of ColMA-Tr Film
3.3. Fourier Transform Infrared Spectroscopy
3.4. Contact Angle and Swelling Property Tests
3.5. Light Transmittance
3.6. Mechanical Properties Measurement
3.7. Assessment of Drug Loading and In Vitro Drug Release
3.8. Adhesion and Proliferation of PC12 Cells on ColMA and ColMA-Tr Films
3.9. HCECs Growth on the Surface of ColMA and ColMA-Tr Film
3.10. Histology
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palchesko, R.N.; Carrasquilla, S.D.; Feinberg, A.W. Natural Biomaterials for Corneal Tissue Engineering, Repair, and Regeneration. Adv. Healthc. Mater. 2018, 7, 1701434. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Saeed, H.N. Disparities in Access to Corneal Tissue in the Developing World. Semin. Ophthalmol. 2023, 38, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.; Pandit, A.; Sánchez-Abella, L.; Haiek, A.; Loinaz, I.; Dupin, D.; Gonzalez, M.; Larra, E.; Bidaguren, A.; Lagali, N.; et al. Artificial Cornea: Past, Current, and Future Directions. Front. Med. 2021, 8, 770780. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wu, X.; Pang, K.; Yang, Y. Histological Evaluation and Biomechanical Characterisation of an Acellular Porcine Cornea Scaffold. Br. J. Ophthalmol. 2011, 95, 410–414. [Google Scholar] [CrossRef]
- Kumar, P.; Pandit, A.; Zeugolis, D.I. Progress in Corneal Stromal Repair: From Tissue Grafts and Biomaterials to Modular Supramolecular Tissue-Like Assemblies. Adv. Mater. 2016, 28, 5381–5399. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in Vitro Characterization of Cross-Linked Collagen–Gelatin Hydrogel Using EDC/NHS for Corneal Tissue Engineering Applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef]
- Dong, Q.; Wu, D.; Li, M.; Dong, W. Polysaccharides, as Biological Macromolecule-Based Scaffolding Biomaterials in Cornea Tissue Engineering: A Review. Tissue Cell 2022, 76, 101782. [Google Scholar] [CrossRef]
- Kong, B.; Sun, L.; Liu, R.; Chen, Y.; Shang, Y.; Tan, H.; Zhao, Y.; Sun, L. Recombinant Human Collagen Hydrogels with Hierarchically Ordered Microstructures for Corneal Stroma Regeneration. Chem. Eng. J. 2022, 428, 131012. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Peng, Y.; Eichenbaum, J.V.; Ren, L.; Liu, Y. Effects of Different Radiation Sources on the Performance of Collagen-Based Corneal Repair Materials and Macrophage Polarization. ACS Omega 2022, 7, 22559–22566. [Google Scholar] [CrossRef]
- Jung, O.; Radenkovic, M.; Stojanović, S.; Lindner, C.; Batinic, M.; Görke, O.; Pissarek, J.; Pröhl, A.; Najman, S.; Barbeck, M. In Vitro and In Vivo Biocompatibility Analysis of a New Transparent Collagen-Based Wound Membrane for Tissue Regeneration in Different Clinical Indications. In Vivo 2020, 34, 2287–2295. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, L.; Long, K.; Wang, L.; Wang, Y. Preparation and Characterization of a Novel Tobramycin-Containing Antibacterial Collagen Film for Corneal Tissue Engineering. Acta Biomater. 2014, 10, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, H.; Ren, L.; Xue, G.; Wang, Y. Improving the Moisturizing Properties of Collagen Film by Surface Grafting of Chondroitin Sulfate for Corneal Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2016, 27, 758–772. [Google Scholar] [CrossRef]
- Liu, C.Y.; Arteaga, A.C.; Fung, S.E.; Cortina, M.S.; Leyngold, I.M.; Aakalu, V.K. Corneal Neurotization for Neurotrophic Keratopathy: Review of Surgical Techniques and Outcomes. Ocul. Surf. 2021, 20, 163–172. [Google Scholar] [CrossRef]
- Cruzat, A.; Qazi, Y.; Hamrah, P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul. Surf. 2017, 15, 15–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Militante, J.D.; Lombardini, J.B. Taurine: Evidence of Physiological Function in the Retina. Nutr. Neurosci. 2002, 5, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Taurine Alleviates Chronic Social Defeat Stress-Induced Depression by Protecting Cortical Neurons from Dendritic Spine Loss|SpringerLink. Available online: https://link.springer.com/article/10.1007/s10571-022-01218-3 (accessed on 10 April 2023).
- Ramírez-Guerrero, S.; Guardo-Maya, S.; Medina-Rincón, G.J.; Orrego-González, E.E.; Cabezas-Pérez, R.; González-Reyes, R.E. Taurine and Astrocytes: A Homeostatic and Neuroprotective Relationship. Front. Mol. Neurosci. 2022, 15, 937789. [Google Scholar] [CrossRef]
- Li, X.; Nie, S.; Kong, J.; Li, N.; Ju, C.; Pan, W. A Controlled-Release Ocular Delivery System for Ibuprofen Based on Nanostructured Lipid Carriers. Int. J. Pharm. 2008, 363, 177–182. [Google Scholar] [CrossRef]
- Ran, W.; Ma, H.; Li, M. In Vitro and In Vivo Studies of Polyvinyl Pyrrolidone–Coated Sparfloxacin-Loaded Ring Contact Lens to Treat Conjunctivitis. J. Pharm. Sci. 2020, 109, 1951–1957. [Google Scholar] [CrossRef]
- Yao, Q.Y.; Wang, L.; Du, Z.; Li, K.; Lin, Y. Characterization and Biological Evaluation of Structurally Modified Taurine Using Benzaldehydes. Asian J. Chem. 2013, 25, 7843–7846. [Google Scholar] [CrossRef]
- Luo, Y.; Li, G.; Chen, L.; Hong, F.F. Preparation and Evaluation of Bacterial Nanocellulose/Hyaluronic Acid Composite Artificial Cornea for Application of Corneal Transplantation. Biomacromolecules 2023, 24, 201–212. [Google Scholar] [CrossRef]
- Li, H.C.; Sun, X.M.; Huang, Y.R.; Peng, Y.H.; Liu, J.; Ren, L. Synthetic Crosslinker Based on Amino–Yne Click to Enhance the Suture Tension of Collagen-Based Corneal Repair Materials. ACS Appl. Polym. Mater. 2022, 4, 4495–4507. [Google Scholar] [CrossRef]
- Dong, L.; Liu, Q.; Gao, Y.; Jia, H.; Dai, W.; Guo, L.; Fan, H.; Fan, Y.; Zhang, X. The Effect of Collagen Hydrogels on Chondrocyte Behaviors through Restricting the Contraction of Cell/Hydrogel Constructs. Regen. Biomater. 2021, 8, rbab030. [Google Scholar] [CrossRef] [PubMed]
- Long, K.; Liu, Y.; Li, W.; Wang, L.; Liu, S.; Wang, Y.; Wang, Z.; Ren, L. Improving the Mechanical Properties of Collagen-Based Membranes Using Silk Fibroin for Corneal Tissue Engineering: Improving the Mechanical Properties of Collagen-Based Membranes. J. Biomed. Mater. Res. A 2015, 103, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Jia, Y.G.; Song, W.; Qi, D.; Jin, J.; Liu, J.; Ren, L. Mechanical and Optical Properties of Reinforced Collagen Membranes for Corneal Regeneration through Polyrotaxane Cross-Linking. ACS Appl. Bio Mater. 2019, 2, 3861–3869. [Google Scholar] [CrossRef]
- Qin, L.; Gao, H.; Xiong, S.; Jia, Y.; Ren, L. Preparation of Collagen/Cellulose Nanocrystals Composite Films and Their Potential Applications in Corneal Repair. J. Mater. Sci. Mater. Med. 2020, 31, 55. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Yoshii, F. Hydrogels and Their Medical Applications. Nucl. Instrum. Methods Phys. Res. Sect. B 1999, 151, 56–64. [Google Scholar] [CrossRef]
- Farasatkia, A.; Kharaziha, M.; Ashrafizadeh, F.; Salehi, S. Transparent Silk/Gelatin Methacrylate (GelMA) Fibrillar Film for Corneal Regeneration. Mater. Sci. Eng. C 2021, 120, 111744. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Y.; Wang, B.; Chen, Y.; Huang, C. Facile Preparation and Characterization of Photopolymerized Adhesive Hydrogels Based on Methacrylated Catechol-Chitosan. J. Mater. Sci. 2022, 57, 20974–20986. [Google Scholar] [CrossRef]
- Elomaa, L.; Teixeira, S.; Hakala, R.; Korhonen, H.; Grijpma, D.W.; Seppälä, J.V. Preparation of Poly(ε-Caprolactone)-Based Tissue Engineering Scaffolds by Stereolithography. Acta Biomater. 2011, 7, 3850–3856. [Google Scholar] [CrossRef]
- Kang, W.; Bi, B.; Zhuo, R.; Jiang, X. Photocrosslinked Methacrylated Carboxymethyl Chitin Hydrogels with Tunable Degradation and Mechanical Behavior. Carbohydr. Polym. 2017, 160, 18–25. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Liu, J.; Huang, Y.; Peng, Y.; Xu, Y.; Ren, L. Photo-Crosslinked GelMA/Collagen Membrane Loaded with Lysozyme as an Antibacterial Corneal Implant. Int. J. Biol. Macromol. 2021, 191, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar] [CrossRef]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherland, 2011; p. 130. [Google Scholar] [CrossRef]
- Pircher, M.; Götzinger, E.; Leitgeb, R.; Fercher, A.F.; Hitzenberger, C.K. Measurement and Imaging of Water Concentration in Human Cornea with Differential Absorption Optical Coherence Tomography. Opt. Express 2003, 11, 2190. [Google Scholar] [CrossRef]
- Dragan, E.S. Advances in Interpenetrating Polymer Network Hydrogels and Their Applications. Pure Appl. Chem. 2014, 86, 1707–1721. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Kong, Y.; Liu, H.; Guo, J.; Yang, H.; Deng, L. Modification of Collagen Film via Surface Grafting of Taurine Molecular to Promote Corneal Nerve Repair and Epithelization Process. J. Funct. Biomater. 2022, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Li, Y.T.; Cho, C.H.; Yu, T.C. Nanoscale Modification of Porous Gelatin Scaffolds with Chondroitin Sulfate for Corneal Stromal Tissue Engineering. Int. J. Nanomed. 2012, 7, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Kong, B.; Mi, S. Electrospun Scaffolds for Corneal Tissue Engineering: A Review. Materials 2016, 9, 614. [Google Scholar] [CrossRef] [Green Version]
- Knoke, S.; Bunjes, H. Transfer of Lipophilic Drugs from Nanoemulsions into Lipid-Containing Alginate Microspheres. Pharmaceutics 2021, 13, 173. [Google Scholar] [CrossRef]
- Chen, T.; Jiang, H.; Zhu, Y.; Chen, X.; Zhang, D.; Li, X.; Shen, F.; Xia, H.; Min, Y.; Xie, K. Highly Ordered 3D Tissue Engineering Scaffolds as a Versatile Culture Platform for Nerve Cells Growth. Macromol. Biosci. 2021, 21, 2100047. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.W.; Du, L.Q.; Wu, X.Y. Acellular Porcine Corneal Matrix as a Carrier Scaffold for Cultivating Human Corneal Epithelial Cells and Fibroblasts In Vitro. Int. J. Ophthalmol. 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Tian, F.; Li, S. Study of Spectrophotometric Characteristics of the Charge Transfer Complex of Taurine Drug with 7,7,8,8-Tetracyanoquinodimethane. Quimica Nova 2020, 43, 1205–1209. [Google Scholar] [CrossRef]
- Brăzdaru, L.; Staicu, T.; Albu Kaya, M.G.; Chelaru, C.; Ghica, C.; Cîrcu, V.; Leca, M.; Ghica, M.V.; Micutz, M. 3D Porous Collagen Matrices—A Reservoir for In Vitro Simultaneous Release of Tannic Acid and Chlorhexidine. Pharmaceutics 2022, 15, 76. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, C.; Kong, Y.; Liu, H.; Chen, C.; Gao, W.; Xi, X.; Yang, H.; Deng, L. Preparation and Characterization of a Photo-Crosslinked Methacryloyl-Collagen Composite Film to Promote Corneal Nerve Regeneration via Surface Grafting of Taurine Molecules. Int. J. Mol. Sci. 2023, 24, 11248. https://doi.org/10.3390/ijms241411248
Liu Y, Zhang C, Kong Y, Liu H, Chen C, Gao W, Xi X, Yang H, Deng L. Preparation and Characterization of a Photo-Crosslinked Methacryloyl-Collagen Composite Film to Promote Corneal Nerve Regeneration via Surface Grafting of Taurine Molecules. International Journal of Molecular Sciences. 2023; 24(14):11248. https://doi.org/10.3390/ijms241411248
Chicago/Turabian StyleLiu, Yang, Chuanlei Zhang, Yanhui Kong, Huiyu Liu, Cheng Chen, Wenyu Gao, Xiaowei Xi, Hui Yang, and Linhong Deng. 2023. "Preparation and Characterization of a Photo-Crosslinked Methacryloyl-Collagen Composite Film to Promote Corneal Nerve Regeneration via Surface Grafting of Taurine Molecules" International Journal of Molecular Sciences 24, no. 14: 11248. https://doi.org/10.3390/ijms241411248





