Impact of the Protein Environment on Two-Photon Absorption Cross-Sections of the GFP Chromophore Anion Resolved at the XMCQDPT2 Level of Theory
Abstract
1. Introduction
2. Results and Discussion
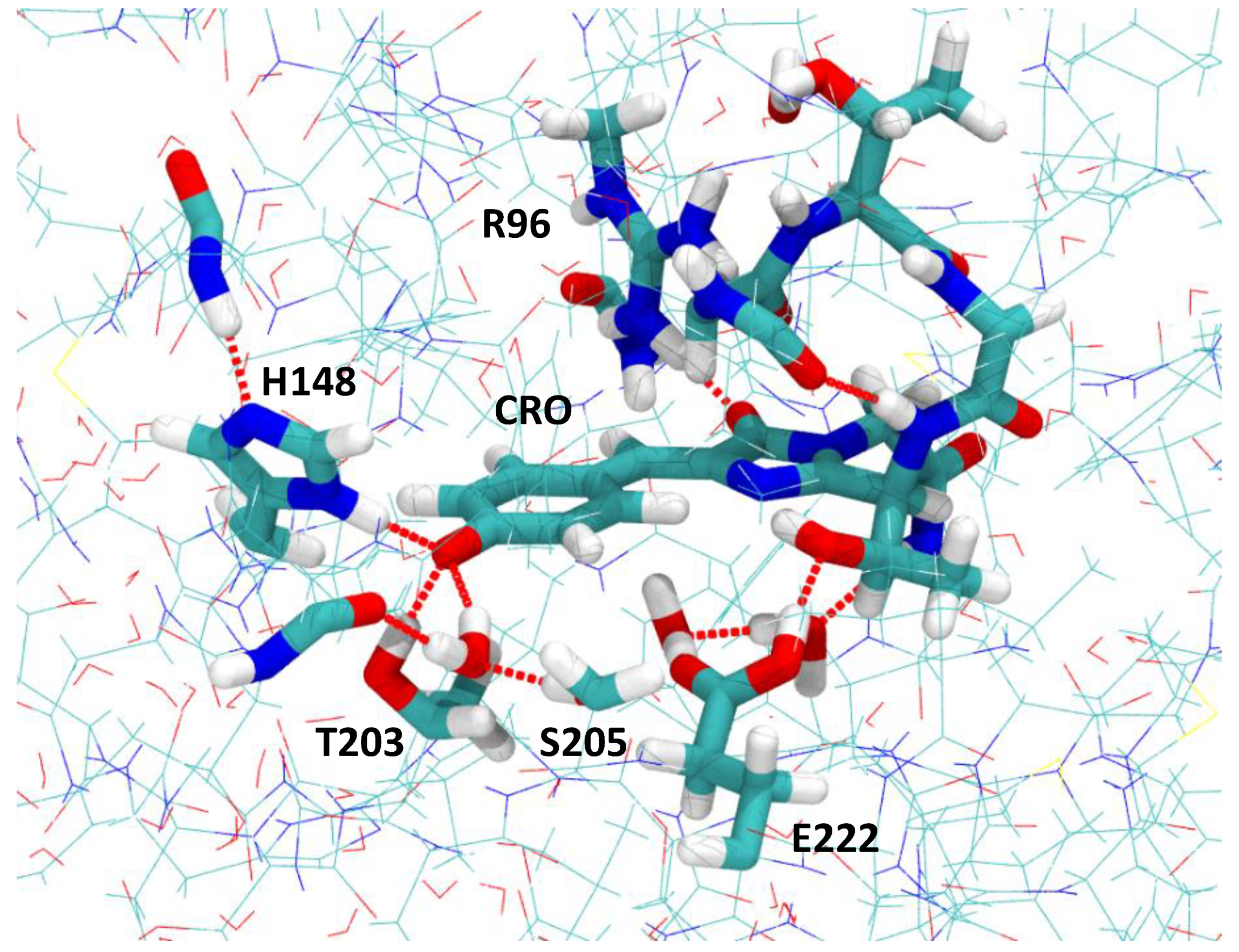
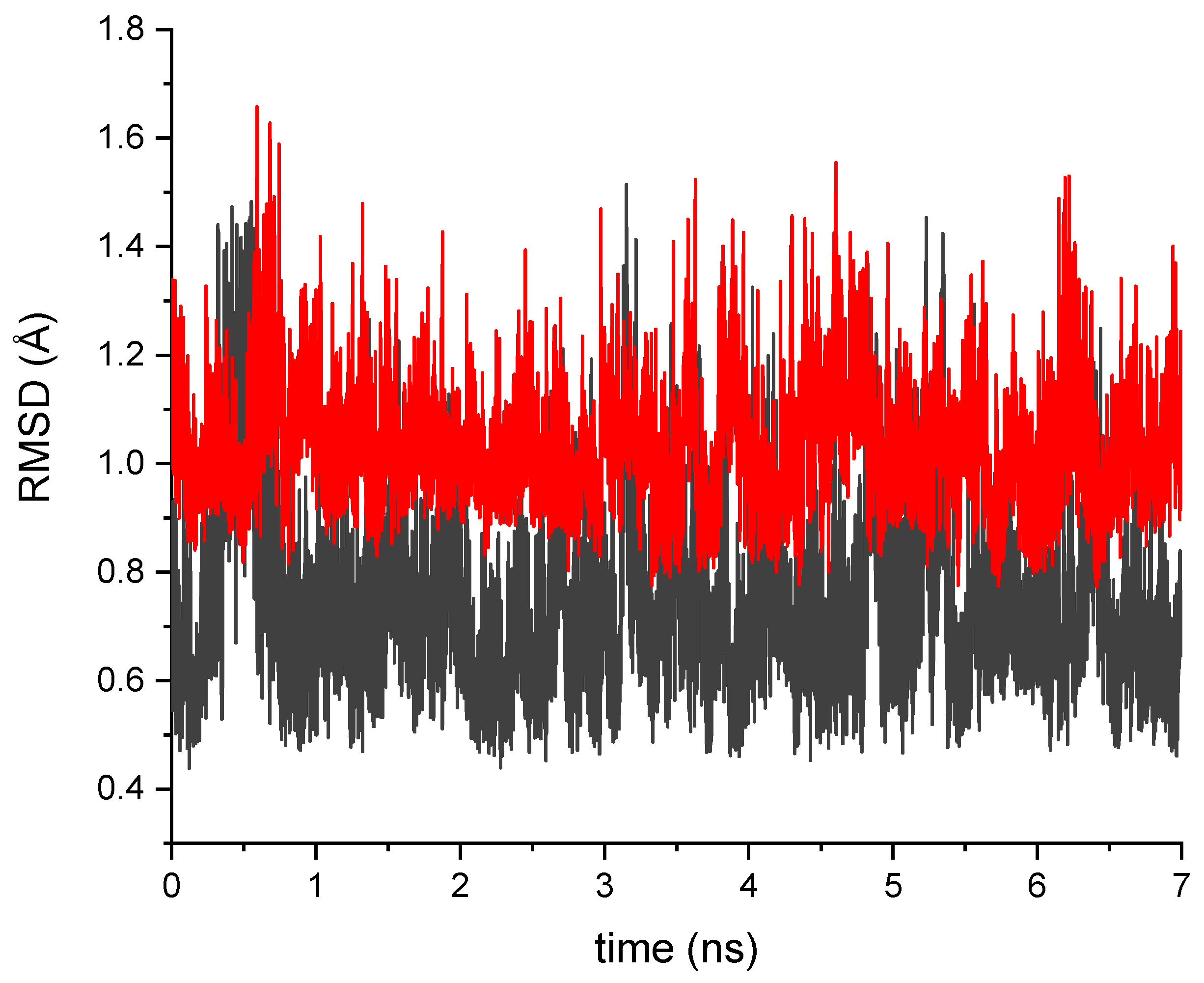
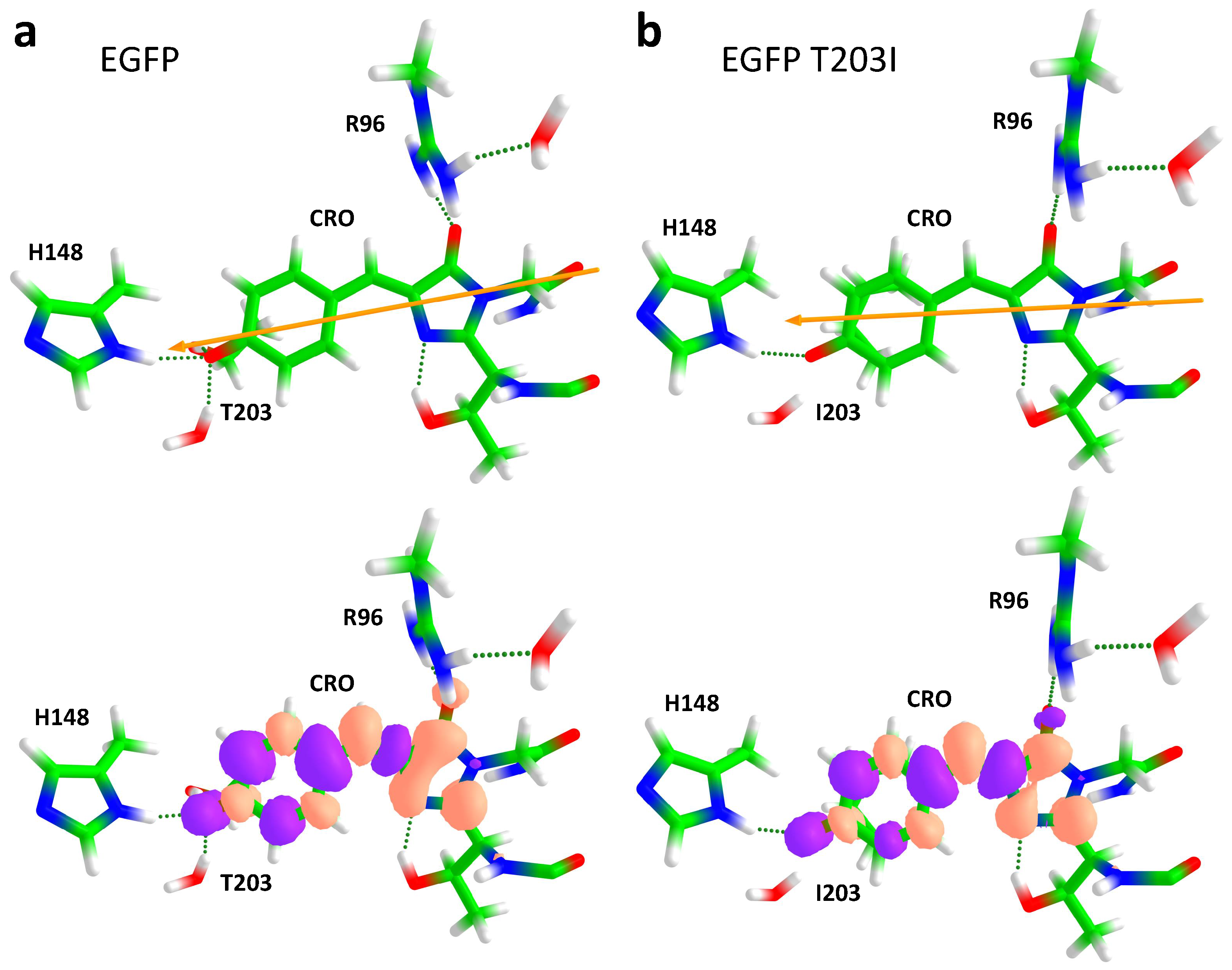
2.1. Impact of the T203I Mutation and Conformational Sampling
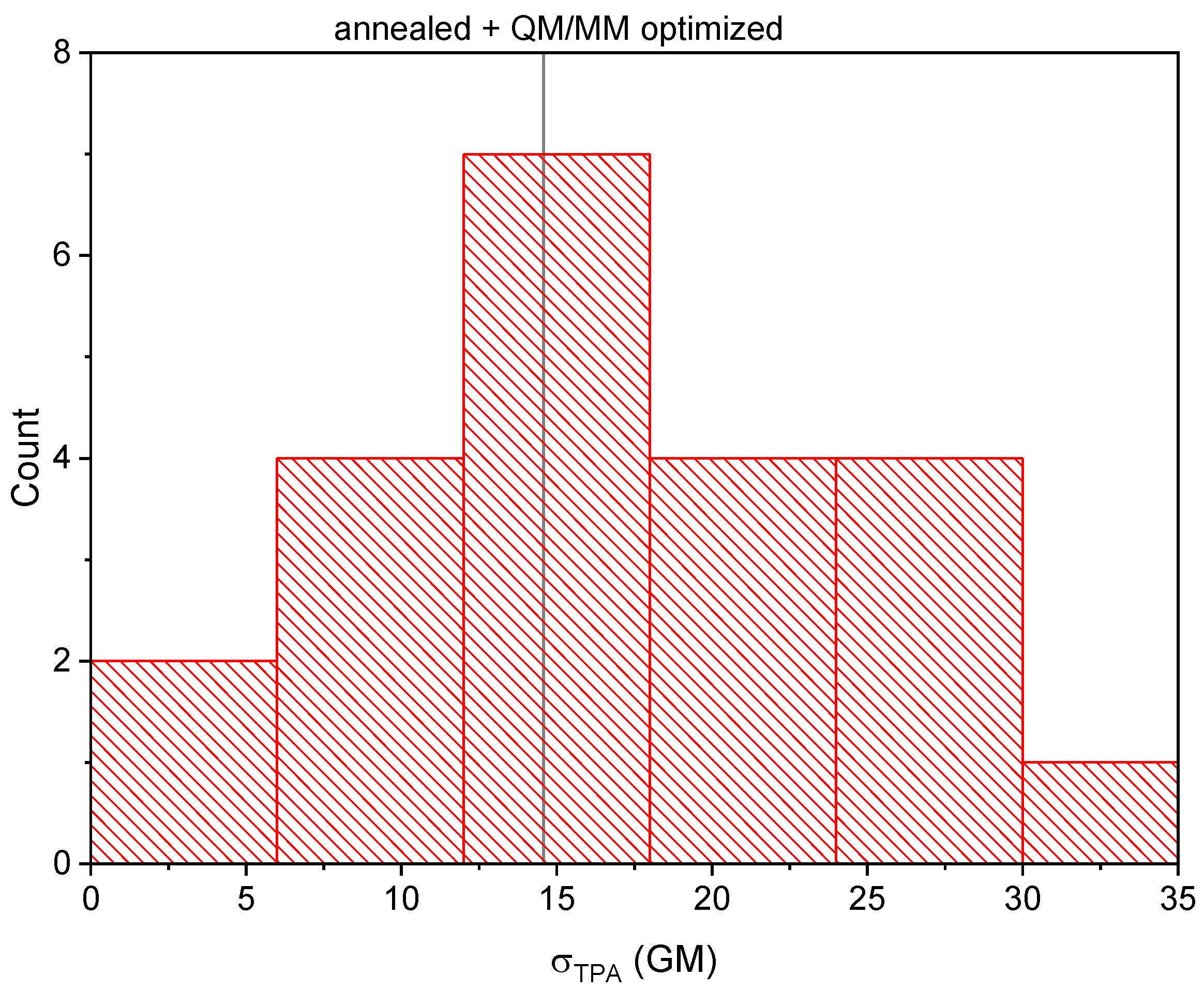
2.2. Analysis of the Calculated TPA Cross-Sections
3. Materials and Methods
3.1. Molecular Dynamics Simulations
3.2. Ground State QM/MM Optimization
3.3. Excited State QM/MM Calculations
3.4. Gas-Phase Calculations
3.5. Calculation of the TPA Cross-Section
Two-Level Model and N-Level Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-Photon Laser Scanning Fluorescence Microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef]
- Xu, C.; Zipfel, W.; Shear, J.B.; Williams, R.M.; Webb, W.W. Multiphoton fluorescence excitation: New spectral windows for biological nonlinear microscopy. Proc. Natl. Acad. Sci. USA 1996, 93, 10763–10768. [Google Scholar] [CrossRef]
- Zipfel, W.; Williams, R.; Webb, W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.S.; Tran, T.M.; Campbell, R.E.; Lambert, G.G.; Salih, A.; Shaner, N.C.; Hughes, T.E.; Drobizhev, M. Blue-Shifted Green Fluorescent Protein Homologues Are Brighter than Enhanced Green Fluorescent Protein under Two-Photon Excitation. J. Phys. Chem. Lett. 2017, 8, 2548–2554. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Cheng, L.; Kain, S.R. Optimized Codon Usage and Chromophore Mutations Provide Enhanced Sensitivity with the Green Fluorescent Protein. Nucleic Acids Res. 1996, 24, 4592–4593. [Google Scholar] [CrossRef] [PubMed]
- Osamu, S.; Johnson, F.H.; Yo, S. Extraction, Purification and Properties of Aequorin, a Bioluminescent Protein from the Luminous Hydromedusan, Aequorea. J. Cell. Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef]
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef]
- Heim, R.; Prasher, D.C.; Tsien, R.Y. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl. Acad. Sci. USA 1994, 91, 12501–12504. [Google Scholar] [CrossRef]
- Arpino, J.A.J.; Rizkallah, P.J.; Jones, D.D. Crystal Structure of Enhanced Green Fluorescent Protein to 1.35 Å Resolution Reveals Alternative Conformations for Glu222. PLoS ONE 2012, 7, e47132. [Google Scholar] [CrossRef]
- Heim, R.; Cubitt, A.B.; Tsien, R.Y. Improved green fluorescence. Nature 1995, 373, 663–664. [Google Scholar] [CrossRef]
- Bochenkova, A.V.; Andersen, L.H. Ultrafast dual photoresponse of isolated biological chromophores: Link to the photoinduced mode-specific non-adiabatic dynamics in proteins. Faraday Discuss. 2013, 163, 297–319. [Google Scholar] [CrossRef]
- Chudakov, D.M.; Matz, M.V.; Lukyanov, S.; Lukyanov, K.A. Fluorescent Proteins and Their Applications in Imaging Living Cells and Tissues. Physiol. Rev. 2010, 90, 1103–1163. [Google Scholar] [CrossRef] [PubMed]
- Cubitt, A.B.; Heim, R.; Adams, S.R.; Boyd, A.E.; Gross, L.A.; Tsien, R.Y. Understanding, improving and using green fluorescent proteins. Trends Biochem. Sci. 1995, 20, 448–455. [Google Scholar] [CrossRef]
- Drobizhev, M.; Makarov, N.; Tillo, S.; Hughes, T.; Rebane, A. Two-photon absorption properties of fluorescent proteins. Nat. Methods 2011, 8, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, C.; Barnett, L.; Drobizhev, M.; Wicks, G.; Mikhaylov, A.; Hughes, T.; Rebane, A. Two-photon directed evolution of green fluorescent proteins. Sci. Rep. 2015, 5, 11968. [Google Scholar] [CrossRef] [PubMed]
- Drobizhev, M.; Makarov, N.S.; Tillo, S.E.; Hughes, T.E.; Rebane, A. Describing Two-Photon Absorptivity of Fluorescent Proteins with a New Vibronic Coupling Mechanism. J. Phys. Chem. B. 2012, 116, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Drobizhev, M.; Callis, P.; Nifosì, R.; Wicks, G.; Stoltzfus, C.; Barnett, L.; Hughes, T.; Sullivan, P.; Rebane, A. Long- and Short-Range Electrostatic Fields in GFP Mutants: Implications for Spectral Tuning. Sci. Rep. 2015, 5, 13223. [Google Scholar] [CrossRef]
- Olsen, J.; Jørgensen, P. Linear and nonlinear response function for an exact state and for an MCSCF state. J. Chem. Phys. 1985, 82, 3235–3264. [Google Scholar] [CrossRef]
- Nanda, K.D.; Krylov, A.I. Two-photon absorption cross sections within equation-of-motion coupled-cluster formalism using resolution-of-the-identity and Cholesky decomposition representations: Theory, implementation, and benchmarks. J. Chem. Phys. 2015, 142, 064118. [Google Scholar] [CrossRef]
- Dalgaard, E. Quadratic response functions within the time-dependent Hartree-Fock approximation. Phys. Rev. A 1982, 26, 42–52. [Google Scholar] [CrossRef]
- Parkinson, W.A.; Sengeløv, P.W.; Oddershede, J. Two-photon transition moments as determined from the quadratic response function. Int. J. Quantum Chem. 1990, 38, 487–499. [Google Scholar] [CrossRef]
- Hettema, H.; Jensen, H.J.A.; Jørgensen, P.; Olsen, J. Quadratic response functions for a multiconfigurational self-consistent field wave function. J. Chem. Phys. 1992, 97, 1174–1190. [Google Scholar] [CrossRef]
- Koch, H.; Jørgensen, P. Coupled cluster response functions. J. Chem. Phys. 1990, 93, 3333–3344. [Google Scholar] [CrossRef]
- Hättig, C.; Christiansen, O.; Koch, H.; Jørgensen, P. Frequency-dependent first hyperpolarizabilities using coupled cluster quadratic response theory. Chem. Phys. Lett. 1997, 269, 428–434. [Google Scholar] [CrossRef]
- Hättig, C.; Christiansen, O.; Jørgensen, P. Multiphoton transition moments and absorption cross sections in coupled cluster response theory employing variational transition moment functionals. J. Chem. Phys. 1998, 108, 8331–8354. [Google Scholar] [CrossRef]
- Hättig, C.; Christiansen, O.; Jørgensen, P. Coupled cluster response calculations of two-photon transition probability rate constants for helium, neon and argon. J. Chem. Phys. 1998, 108, 8355–8359. [Google Scholar] [CrossRef]
- Iwata, J.I.; Yabana, K.; Bertsch, G.F. Real-space computation of dynamic hyperpolarizabilities. J. Chem. Phys. 2001, 115, 8773–8783. [Google Scholar] [CrossRef]
- Hait Heinze, H.; Della Sala, F.; Görling, A. Efficient methods to calculate dynamic hyperpolarizability tensors by time-dependent density-functional theory. J. Chem. Phys. 2002, 116, 9624–9640. [Google Scholar] [CrossRef]
- Sałek, P.; Vahtras, O.; Helgaker, T.; Ågren, H. Density-functional theory of linear and nonlinear time-dependent molecular properties. J. Chem. Phys. 2002, 117, 9630–9645. [Google Scholar] [CrossRef]
- Wang, F.; Yam, C.Y.; Chen, G. Time-dependent density-functional theory/localized density matrix method for dynamic hyperpolarizability. J. Chem. Phys. 2007, 126, 244102. [Google Scholar] [CrossRef]
- Zahariev, F.; Gordon, M.S. Nonlinear response time-dependent density functional theory combined with the effective fragment potential method. J. Chem. Phys. 2014, 140, 18A523. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, S.; Rehn, D.R.; Wormit, M.; Starcke, J.H.; Rusakova, I.L.; Trofimov, A.B.; Dreuw, A. Calculations of nonlinear response properties using the intermediate state representation and the algebraic-diagrammatic construction polarization propagator approach: Two-photon absorption spectra. J. Chem. Phys. 2012, 136, 064107. [Google Scholar] [CrossRef] [PubMed]
- Nanda, K.D.; Gulania, S.; Krylov, A.I. Theory, implementation, and disappointing results for two-photon absorption cross sections within the doubly electron-attached equation-of-motion coupled-cluster framework. J. Chem. Phys. 2023, 158, 054102. [Google Scholar] [CrossRef] [PubMed]
- Beerepoot, M.T.P.; Friese, D.H.; List, N.H.; Kongsted, J.; Ruud, K. Benchmarking two-photon absorption cross sections: Performance of CC2 and CAM-B3LYP. Phys. Chem. Chem. Phys. 2015, 17, 19306–19314. [Google Scholar] [CrossRef] [PubMed]
- Steindal, A.H.; Olsen, J.M.H.; Ruud, K.; Frediani, L.; Kongsted, J. A combined quantum mechanics/molecular mechanics study of the one- and two-photon absorption in the green fluorescent protein. Phys. Chem. Chem. Phys. 2012, 14, 5440–5451. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, Y.; Liang, W. Assessment of mode-mixing and Herzberg-Teller effects on two-photon absorption and resonance hyper-Raman spectra from a time-dependent approach. J. Chem. Phys. 2014, 140, 094107. [Google Scholar] [CrossRef] [PubMed]
- de Wergifosse, M.; Beaujean, P.; Grimme, S. Ultrafast Evaluation of Two-Photon Absorption with Simplified Time-Dependent Density Functional Theory. J. Phys. Chem. A 2022, 126, 7534–7547. [Google Scholar] [CrossRef]
- Gholami, S.; Pedraza-González, L.; Yang, X.; Granovsky, A.; Ioffe, I.; Olivucci, M. Multi-State Multi-Configuration Quantum Chemical Computation of the Two-Photon Absorption Spectra of Bovine Rhodopsin. J. Phys. Chem. Lett. 2019, 2019, 6293–6300. [Google Scholar] [CrossRef]
- Dick, B.; Hohlneicher, G. Importance of initial and final states as intermediate states in two-photon spectroscopy of polar molecules. J. Chem. Phys. 1982, 76, 5755–5760. [Google Scholar] [CrossRef]
- Birge, R.R.; Zhang, C. Two-photon double resonance spectroscopy of bacteriorhodopsin. Assignment of the electronic and dipolar properties of the low-lying 1Ag*--like and 1Bu*+-like π, π* states. J. Chem. Phys. 1990, 92, 7178–7195. [Google Scholar] [CrossRef]
- Drobizhev, M.; Tillo, S.; Makarov, N.S.; Hughes, T.E.; Rebane, A. Color Hues in Red Fluorescent Proteins Are Due to Internal Quadratic Stark Effect. J. Phys. Chem. B 2009, 113, 12860–12864. [Google Scholar] [CrossRef]
- Grabarek, D.; Andruniów, T. Illuminating the origins of two-photon absorption properties in fluorescent protein chromophores. Int. J. Quantum Chem. 2019, 120, e26086. [Google Scholar] [CrossRef]
- Drobizhev, M.; Molina, R.S.; Callis, P.R.; Scott, J.N.; Lambert, G.G.; Salih, A.; Shaner, N.C.; Hughes, T.E. Local Electric Field Controls Fluorescence Quantum Yield of Red and Far-Red Fluorescent Proteins. Front. Mol. Biosci. 2021, 8, 633217. [Google Scholar] [CrossRef] [PubMed]
- Delysse, S.; Raimond, P.; Nunzi, J.M. Two-photon absorption in non-centrosymmetric dyes. Chem. Phys. 1997, 219, 341–351. [Google Scholar] [CrossRef]
- Rebane, A.; Makarov, N.S.; Drobizhev, M.; Spangler, B.; Tarter, E.S.; Reeves, B.D.; Spangler, C.W.; Meng, F.; Suo, Z. Quantitative Prediction of Two-Photon Absorption Cross Section Based on Linear Spectroscopic Properties. J. Phys. Chem. C 2008, 112, 7997–8004. [Google Scholar] [CrossRef]
- Rebane, A.; Drobizhev, M.; Makarov, N.S.; Beuerman, E.; Haley, J.E.; Krein, D.M.; Burke, A.R.; Flikkema, J.L.; Cooper, T.M. Relation between Two-Photon Absorption and Dipolar Properties in a Series of Fluorenyl-Based Chromophores with Electron Donating or Electron Withdrawing Substituents. J. Phys. Chem. A 2011, 115, 4255–4262. [Google Scholar] [CrossRef]
- Cronstrand, P.; Luo, Y.; Ågren, H. Generalized few-state models for two-photon absorption of conjugated molecules. Chem. Phys. Lett. 2002, 352, 262–269. [Google Scholar] [CrossRef]
- Cronstrand, P.; Luo, Y.; Ågren, H. Effects of dipole alignment and channel interference on two-photon absorption cross sections of two-dimensional charge-transfer systems. J. Chem. Phys. 2002, 117, 11102–11106. [Google Scholar] [CrossRef]
- Alam, M.M.; Chattopadhyaya, M.; Chakrabarti, S. Solvent induced channel interference in the two-photon absorption process—A theoretical study with a generalized few-state-model in three dimensions. Phys. Chem. Chem. Phys. 2012, 14, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Beerepoot, M.; Alam, M.; Bednarska, J.; Bartkowiak, W.; Ruud, K.; Zalesny, R. Benchmarking the Performance of Exchange-Correlation Functionals for Predicting Two-Photon Absorption Strengths. J. Chem. Theory Comput. 2018, 14, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Ośmiałowski, B.; Petrusevich, E.F.; Antoniak, M.A.; Grela, I.; Bin Jassar, M.A.; Nyk, M.; Luis, J.M.; Jędrzejewska, B.; Zaleśny, R.; Jacquemin, D. Controlling Two-Photon Action Cross Section by Changing a Single Heteroatom Position in Fluorescent Dyes. J. Phys. Chem. Lett. 2020, 11, 5920–5925. [Google Scholar] [CrossRef] [PubMed]
- Ośmiałowski, B.; Petrusevich, E.F.; Nawrot, K.C.; Paszkiewicz, B.K.; Nyk, M.; Zielak, J.; Jedrzejewska, B.; Luis, J.M.; Jacquemin, D.; Zaleśny, R. Tailoring the nonlinear absorption of fluorescent dyes by substitution at a boron center. J. Mater. Chem. C 2021, 9, 6225–6233. [Google Scholar] [CrossRef]
- Petrusevich, E.F.; Ośmiałowski, B.; Zaleśny, R.; Alam, M.M. Two-Photon Absorption Activity of BOPHY Derivatives: Insights from Theory. J. Phys. Chem. A 2021, 125, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Chołuj, M.; Behera, R.; Petrusevich, E.F.; Bartkowiak, W.; Alam, M.M.; Zaleśny, R. Much of a Muchness: On the Origins of Two- and Three-Photon Absorption Activity of Dipolar Y-Shaped Chromophores. J. Phys. Chem. A 2022, 126, 752–759. [Google Scholar] [CrossRef]
- Chołuj, M.; Alam, M.; Beerepoot, M.; Sitkiewicz, S.; Matito, E.; Ruud, K.; Zaleśny, R. Choosing Bad versus Worse: Predictions of Two-Photon-Absorption Strengths Based on Popular Density Functional Approximations. J. Chem. Theory Comput. 2022, 18, 1046–1060. [Google Scholar] [CrossRef]
- Takaba, K.; Tai, Y.; Eki, H.; Dao, H.A.; Hanazono, Y.; Hasegawa, K.; Miki, K.; Takeda, K. Subatomic resolution X-ray structures of green fluorescent protein. IUCrJ 2019, 6, 387–400. [Google Scholar] [CrossRef]
- Ehrig, T.; O’Kane, D.J.; Prendergast, F.G. Green-fluorescent protein mutants with altered fluorescence excitation spectra. FEBS Lett. 1995, 367, 163–166. [Google Scholar] [CrossRef]
- Kummer, A.D.; Wiehler, J.; Rehaber, H.; Kompa, C.; Steipe, B.; Michel-Beyerle, M.E. Effects of Threonine 203 Replacements on Excited-State Dynamics and Fluorescence Properties of the Green Fluorescent Protein (GFP). J. Phys. Chem. B 2000, 104, 4791–4798. [Google Scholar] [CrossRef]
- Ormö, M.; Cubitt, A.B.; Kallio, K.; Gross, L.A.; Tsien, R.Y.; Remington, S.J. Crystal Structure of the Aequorea victoria Green Fluorescent Protein. Science 1996, 273, 1392–1395. [Google Scholar] [CrossRef]
- Phillips, J.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Regmi, C.K. Structural Flexibility and Oxygen Diffusion Pathways in Monomeric Fluorescent Proteins. Ph.D. Thesis, FIU Electronic Theses and Dissertations, Florida International University, Miami, FL, USA, 2014. Available online: https://digitalcommons.fiu.edu/etd/1122 (accessed on 3 July 2023).
- Granovsky, A.A. Firefly Version 8. Available online: http://classic.chem.msu.su/gran/firefly/index.html (accessed on 3 July 2023).
- Schmidt, M.; Baldridge, K.; Boatz, J.; Elbert, S.; Gordon, M.; Jenson, J.; Koseki, S.; Matsunaga, N.; Nguyen, K.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comp. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Rackers, J.A.; Wang, Z.; Lu, C.; Laury, M.L.; Lagardère, L.; Schnieders, M.J.; Piquemal, J.P.; Ren, P.; Ponder, J.W. Tinker 8: Software Tools for Molecular Design. J. Chem. Theory Comput. 2018, 14, 5273–5289. [Google Scholar] [CrossRef] [PubMed]
- Day, P.N.; Jensen, J.H.; Gordon, M.S.; Webb, S.P.; Stevens, W.J.; Morris, K.; David, G.; Harold, B.; Drora, C. An effective fragment method for modeling solvent effects in quantum mechanical calculations. J. Chem. Phys. 1996, 105, 1968–1986. [Google Scholar] [CrossRef]
- Gordon, M.S.; Freitag, M.A.; Bandyopadhyay, P.; Jensen, J.H.; Kairys, V.; Stevens, W.J. The Effective Fragment Potential Method: A QM-Based MM Approach to Modeling Environmental Effects in Chemistry. J. Phys. Chem. A 2001, 105, 293–307. [Google Scholar] [CrossRef]
- Granovsky, A. Extended multi-configuration quasi-degenerate perturbation theory: The new approach to multi-state multi-reference perturbation theory. J. Chem. Phys. 2011, 134, 214113. [Google Scholar] [CrossRef]
- Macak, P.; Luo, Y.; Ågren, H. Simulations of vibronic profiles in two-photon absorption. Chem. Phys. Lett. 2000, 330, 447–456. [Google Scholar] [CrossRef]
- Monson, P.R.; McClain, W.M. Polarization Dependence of the Two-Photon Absorption of Tumbling Molecules with Application to Liquid 1-Chloronaphthalene and Benzene. J. Chem. Phys. 1970, 53, 29–37. [Google Scholar] [CrossRef]

| HBDI | EGFP T203I | EGFP | |
|---|---|---|---|
| , D | 10.5 | 10.9 | 10.5 |
| , D | 2.9 | 2.5 | 4.4 |
| VEE, eV | 2.62 | 2.43 | 2.52 |
| N | |||
| 2 | 19 (7448) | 15 (6953) | 45 (19,262) |
| 3 | 19 (7439) | 15 (6991) | 45 (19,333) |
| 4 | 18 (7340) | 15 (7011) | 45 (19,474) |
| 5 | 18 (7270) | 15 (6762) | 45 (19,213) |
| 6 | 18 (7312) | 15 (6817) | 45 (19,209) |
| 7 | 19 (7396) | 15 (6759) | 44 (19,184) |
| Experiment [15] | — | 19 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslopovsky, V.R.; Scherbinin, A.V.; Kleshchina, N.N.; Bochenkova, A.V. Impact of the Protein Environment on Two-Photon Absorption Cross-Sections of the GFP Chromophore Anion Resolved at the XMCQDPT2 Level of Theory. Int. J. Mol. Sci. 2023, 24, 11266. https://doi.org/10.3390/ijms241411266
Aslopovsky VR, Scherbinin AV, Kleshchina NN, Bochenkova AV. Impact of the Protein Environment on Two-Photon Absorption Cross-Sections of the GFP Chromophore Anion Resolved at the XMCQDPT2 Level of Theory. International Journal of Molecular Sciences. 2023; 24(14):11266. https://doi.org/10.3390/ijms241411266
Chicago/Turabian StyleAslopovsky, Vladislav R., Andrei V. Scherbinin, Nadezhda N. Kleshchina, and Anastasia V. Bochenkova. 2023. "Impact of the Protein Environment on Two-Photon Absorption Cross-Sections of the GFP Chromophore Anion Resolved at the XMCQDPT2 Level of Theory" International Journal of Molecular Sciences 24, no. 14: 11266. https://doi.org/10.3390/ijms241411266
APA StyleAslopovsky, V. R., Scherbinin, A. V., Kleshchina, N. N., & Bochenkova, A. V. (2023). Impact of the Protein Environment on Two-Photon Absorption Cross-Sections of the GFP Chromophore Anion Resolved at the XMCQDPT2 Level of Theory. International Journal of Molecular Sciences, 24(14), 11266. https://doi.org/10.3390/ijms241411266







