Toward Two-Dimensional Tessellation through Halogen Bonding between Molecules and On-Surface-Synthesized Covalent Multimers
Abstract
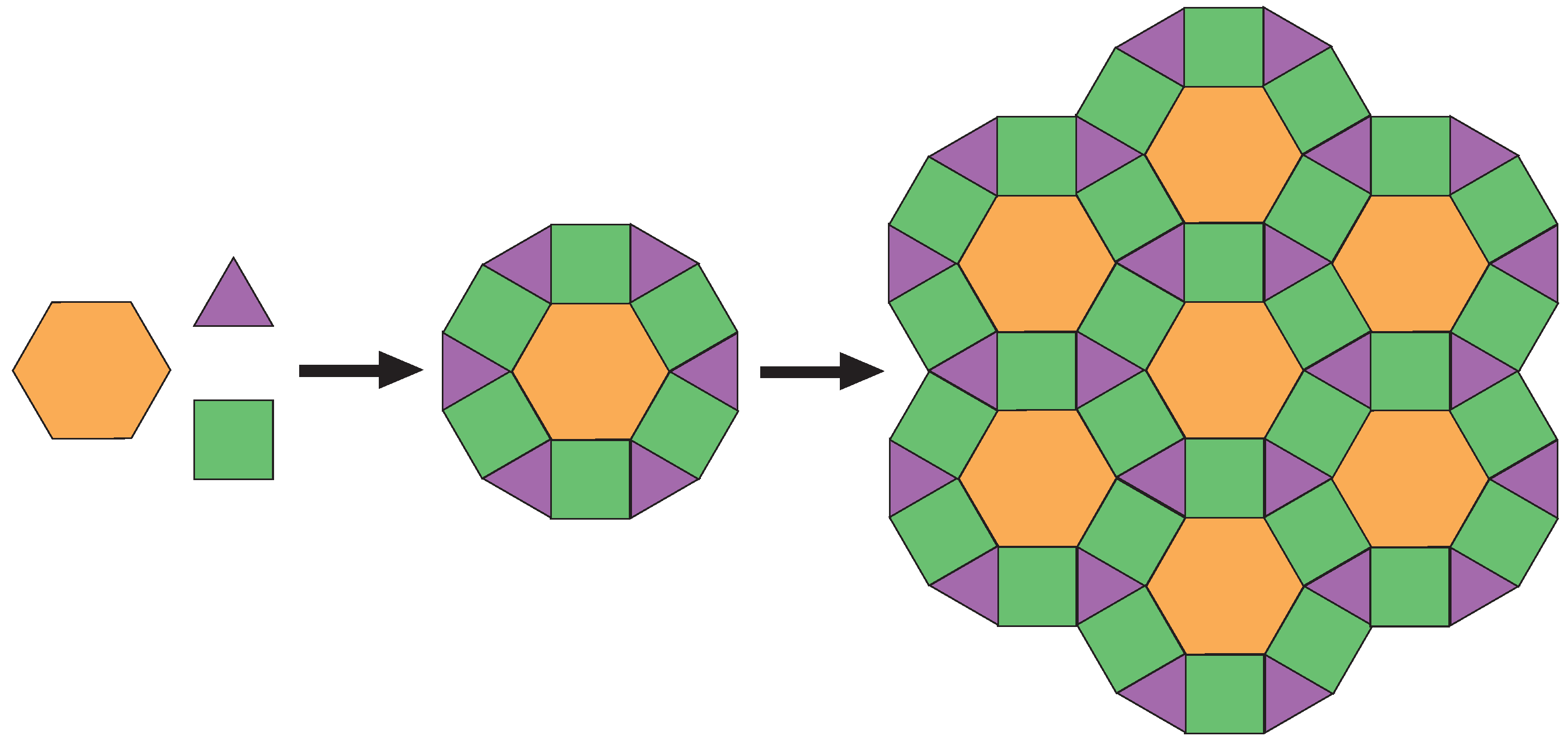
:1. Introduction
2. Results
2.1. Halogen-Bonded Nanoarchitecture of Intact Molecules at Room-Temperature Deposition
2.2. Formation of a 2D Halogen-Bonded Superstructure Composed of Monomers and Covalent Multimers after Deposition Onto a 145 Surface
2.3. Disappearance of 2D Halogen-Bonded Arrangements after Deposition Onto a 165 Surface
2.4. Formation of Covalent Hexagons after Deposition Onto a 175 Surface
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| STM Scanning tunneling microscopy |
References
- Liang, H.; He, Y.; Ye, Y.; Xu, X.; Cheng, F.; Sun, W.; Shao, X.; Wang, Y.; Li, J.; Wu, K. Two-Dimensional Molecular Porous Networks Constructed by Surface Assembling. Coord. Chem. Rev. 2009, 253, 2959–2979. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C. Hierarchical Construction of Self-Assembled Low-Dimensional Molecular Architectures Observed by Using Scanning Tunneling Microscopy. Chem. Soc. Rev. 2009, 38, 2576. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.V. Fresh Perspectives for Surface Coordination Chemistry. Surf. Sci. 2009, 603, 1533–1541. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Kepcija, N.; Kleinschrodt, M.; Diller, K.; Fischer, S.; Papageorgiou, A.C.; Allegretti, F.; Björk, J.; Klyatskaya, S.; Klappenberger, F.; et al. Homo-Coupling of Terminal Alkynes on a Noble Metal Surface. Nat. Commun. 2012, 3, 1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Z.; Gan, F.; Liu, H.; Shen, C.; Qiu, H.; Yang, B.; Yu, P. Helical Conformation Tunability via Hydrogen Bonding in Supramolecular Frameworks. CCS Chem. 2021, 4, 1405–1413. [Google Scholar] [CrossRef]
- Xu, L.; Miao, X.; Cui, L.; Liu, P.; Miao, K.; Chen, X.; Deng, W. Chiral Transition of the Supramolecular Assembly by Concentration Modulation at the Liquid/Solid Interface. J. Phys. Chem. C 2015, 119, 17920–17929. [Google Scholar] [CrossRef]
- Silly, F. Two-Dimensional 1,3,5-Tris(4-carboxyphenyl)benzene Self-Assembly at the 1-Phenyloctane/Graphite Interface Revisited. J. Phys. Chem. C 2012, 116, 10029–10032. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zou, H.; Liu, Y.; Miao, X.; Deng, W.; Yuan, Q. F⋯Br and F⋯S Heterohalogen-Bond-Directed 2D Self-Assemblies of a Benzothiadiazole Derivative. Langmuir 2023, 39, 8314–8322. [Google Scholar] [CrossRef]
- Yi, Z.Y.; Yang, X.Q.; Duan, J.J.; Zhou, X.; Chen, T.; Wang, D.; Wan, L.J. Evolution of Br⋯Br Contacts in Enantioselective Molecular Recognition during Chiral 2D Crystallization. Nat. Commun. 2022, 13, 5850. [Google Scholar] [CrossRef]
- Zeng, X.; Ding, W.; Zou, H.; Ke, M.; Miao, X.; Cheng, X.; Deng, W. Solvent Coadsorption Effect on I⋯O Halogen-Bonded 2D Self-Assembled Nanostructures. J. Phys. Chem. C 2022, 126, 5777–5783. [Google Scholar] [CrossRef]
- Sharber, S.A.; Mullin, W.J.; Thomas, S.W.I. Bridging the Void: Halogen Bonding and Aromatic Interactions to Program Luminescence and Electronic Properties of π-Conjugated Materials in the Solid State. Chem. Mater. 2021, 33, 6640–6661. [Google Scholar] [CrossRef]
- Pang, P.; Li, B.; Zeng, X.; Miao, X.; Wang, Y.; Deng, W. Solvent Effect on Halogen-Bonded Self-Assembled Nanostructures of a 2,9-Dibromo-Phenanthridine Derivative at the Liquid–Solid Interface. J. Phys. Chem. C 2021, 125, 1378–1383. [Google Scholar] [CrossRef]
- Davidson, J.A.; Sacchi, M.; Gorrec, F.; Clarke, S.M.; Jenkins, S.J. Halogen Bonding in Bicomponent Monolayers: Self-Assembly of a Homologous Series of Iodinated Perfluoroalkanes with Bipyridine. Langmuir 2021, 37, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Hua, C.; Zhou, M. On-Surface Synthesis toward Two-Dimensional Polymers. J. Phys. Chem. Lett. 2022, 13, 8062–8077. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, T.; Yan, H.J.; Wang, D.; Fan, Q.H.; Wan, L.J.; Ghosh, K.; Yang, H.B.; Stang, P.J. Engineering of Linear Molecular Nanostructures by a Hydrogen-Bond-Mediated Modular and Flexible Host-Guest Assembly. ACS Nano 2010, 4, 5685–5692. [Google Scholar] [CrossRef]
- Madueno, R.; Räisänen, M.T.; Silien, C.; Buck, M. Functionalizing hydrogen-bonded surface networks with self-assembled monolayers. Nature 2008, 454, 618–621. [Google Scholar] [CrossRef]
- Canas-Ventura, M.E.; Aït-Mansour, K.; Ruffieux, P.; Rieger, R.; Müllen, K.; Brune, H.; Fasel, R. Complex Interplay and Hierarchy of Interactions in Two-Dimensional Supramolecular Assemblies. ACS Nano 2011, 5, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Silien, C.; Räisänen, M.T.; Buck, M. A Supramolecular Network as Sacrificial Mask for the Generation of a Nanopatterned Binary Self-Assembled Monolayer. Small 2010, 6, 391–394. [Google Scholar] [CrossRef]
- Sun, X.; Jonkman, H.T.; Silly, F. Tailoring Two-Dimensional PTCDA-melamine Self-Assembled Architectures at Room Temperature by Tuning Molecular Ratio. Nanotechnology 2010, 21, 165602. [Google Scholar] [CrossRef]
- Bouju, X.; Mattioli, C.; Franc, G.; Pujol, A.; Gourdon, A. Bicomponent Supramolecular Architectures at the Vacuum–Solid Interface. Chem. Rev. 2017, 117, 1407–1444. [Google Scholar] [CrossRef] [Green Version]
- Wäckerlin, C.; Li, J.; Mairena, A.; Martin, K.; Avarvari, N.; Ernst, K.H. Surface-Assisted Diastereoselective Ullmann Coupling of Bishelicenes. Chem. Commun. 2016, 52, 12694–12697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grill, L.; Dyer, M.; Lafferentz, L.; Persson, M.; Peters, M.V.; Hecht, S. Nano-Architectures by Covalent Assembly of Molecular Building Blocks. Nat. Nanotech. 2007, 2, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Clair, S.; de Oteyza, D.G. Controlling a Chemical Coupling Reaction on a Surface: Tools and Strategies for On-Surface Synthesis. Chem. Rev. 2019, 119, 4717–4776. [Google Scholar] [CrossRef] [PubMed]
- Mairena, A.; Wäckerlin, C.; Wienke, M.; Grenader, K.; Terfort, A.; Ernst, K.H. Diastereoselective Ullmann Coupling to Bishelicenes by Surface Topochemistry. J. Am. Chem. Soc. 2018, 140, 15186–15189. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.F.; Yang, B.; Jiang, N. Direct Observation of the Geometric Isomer Selectivity of a Reaction Controlled via Adsorbed Bromine. Nanoscale 2020, 12, 2726–2731. [Google Scholar] [CrossRef]
- Dai, J.; Zhao, W.; Xing, L.; Shang, J.; Ju, H.; Zhou, X.; Liu, J.; Chen, Q.; Wang, Y.; Zhu, J.; et al. Dechlorinated Ullmann Coupling Reaction of Aryl Chlorides on Ag(111): A Combined STM and XPS Study. ChemPhysChem 2019, 20, 2367–2375. [Google Scholar] [CrossRef]
- Stepanenko, V.; Kandanelli, R.; Uemura, S.; Würthner, F.; Fernández, G. Concentration-Dependent Rhombitrihexagonal Tiling Patterns at the Liquid/Solid Interface. Chem. Sci. 2015, 6, 5853–5858. [Google Scholar] [CrossRef] [Green Version]
- Tao, Z.; Wang, T.; Wu, D.; Feng, L.; Huang, J.; Wu, X.; Zhu, J. Construction of Molecular Regular Tessellations on a Cu(111) Surface. Chem. Commun. 2018, 54, 7010–7013. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Paszkiewicz, M.; Du, P.; Zhang, L.; Lin, T.; Chen, Z.; Klyatskaya, S.; Ruben, M.; Seitsonen, A.P.; Barth, J.V.; et al. Complex Supramolecular Interfacial Tessellation through Convergent Multi-Step Reaction of a Dissymmetric Simple Organic Precursor. Nature Chem. 2018, 10, 296–304. [Google Scholar] [CrossRef]
- Iritani, K.; Ikeda, M.; Yang, A.; Tahara, K.; Hirose, K.; Moore, J.S.; Tobe, Y. Hexagonal Molecular Tiling by Hexagonal Macrocycles at the Liquid/Solid Interface: Structural Effects on Packing Geometry. Langmuir 2017, 33, 12453–12462. [Google Scholar] [CrossRef]
- Feng, L.; Wang, T.; Tao, Z.; Huang, J.; Li, G.; Xu, Q.; Tait, S.L.; Zhu, J. Supramolecular Tessellations at Surfaces by Vertex Design. ACS Nano 2019, 13, 10603–10611. [Google Scholar] [CrossRef] [PubMed]
- Écija, D.; Urgel, J.I.; Papageorgiou, A.C.; Joshi, S.; Auwärter, W.; Seitsonen, A.P.; Klyatskaya, S.; Ruben, M.; Fischer, S.; Vijayaraghavan, S.; et al. Five-vertex Archimedean Surface Tessellation by Lanthanide-Directed Molecular Self-Assembly. Proc. Natl. Acad. Sci. USA 2013, 110, 6678–6681. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wang, Y.; Chen, M.; Dai, J.; Zhou, X.; Kuttner, J.; Hilt, G.; Shao, X.; Gottfried, J.M.; Wu, K. Assembling Molecular Sierpiński Triangle Fractals. Nat. Chem. 2015, 7, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wu, X.J.; Hu, Z.; Lu, X.; Ding, Z.; Shao, Y.; Xu, H.; Ji, W.; Wu, J.; Loh, K.P. Two-Dimensional Tessellation by Molecular Tiles constructed from Halogen–Halogen and Halogen–Metal Networks. Nat. Commun. 2018, 9, 4871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silly, F. Selecting Two-Dimensional Halogen-Halogen Bonded Self-Assembled 1,3,5-Tris(4-iodophenyl)benzene Porous Nanoarchitectures at the Solid-Liquid Interface. J. Phys. Chem. C 2013, 117, 20244–20249. [Google Scholar] [CrossRef]
- Bui, T.T.T.; Dahaoui, S.; Lecomte, C.; Desiraju, G.R.; Espinosa, E. The Nature of Halogen⋯Halogen Interactions: A Model Derived from Experimental Charge-Density Analysis. Angew. Chem. Int. Ed. 2009, 48, 3838–3841. [Google Scholar] [CrossRef]
- Silly, F. A Robust Method For Processing Scanning Probe Microscopy Images and Determining Nanoobject Position and Dimensions. J. Microsc.-Oxf. 2009, 236, 211–218. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peyrot, D.; Silly, F. Toward Two-Dimensional Tessellation through Halogen Bonding between Molecules and On-Surface-Synthesized Covalent Multimers. Int. J. Mol. Sci. 2023, 24, 11291. https://doi.org/10.3390/ijms241411291
Peyrot D, Silly F. Toward Two-Dimensional Tessellation through Halogen Bonding between Molecules and On-Surface-Synthesized Covalent Multimers. International Journal of Molecular Sciences. 2023; 24(14):11291. https://doi.org/10.3390/ijms241411291
Chicago/Turabian StylePeyrot, David, and Fabien Silly. 2023. "Toward Two-Dimensional Tessellation through Halogen Bonding between Molecules and On-Surface-Synthesized Covalent Multimers" International Journal of Molecular Sciences 24, no. 14: 11291. https://doi.org/10.3390/ijms241411291




