Revealing a Third Dissolved-Phase Xenon-129 Resonance in Blood Caused by Hemoglobin Glycation
Abstract
:1. Introduction
2. Results
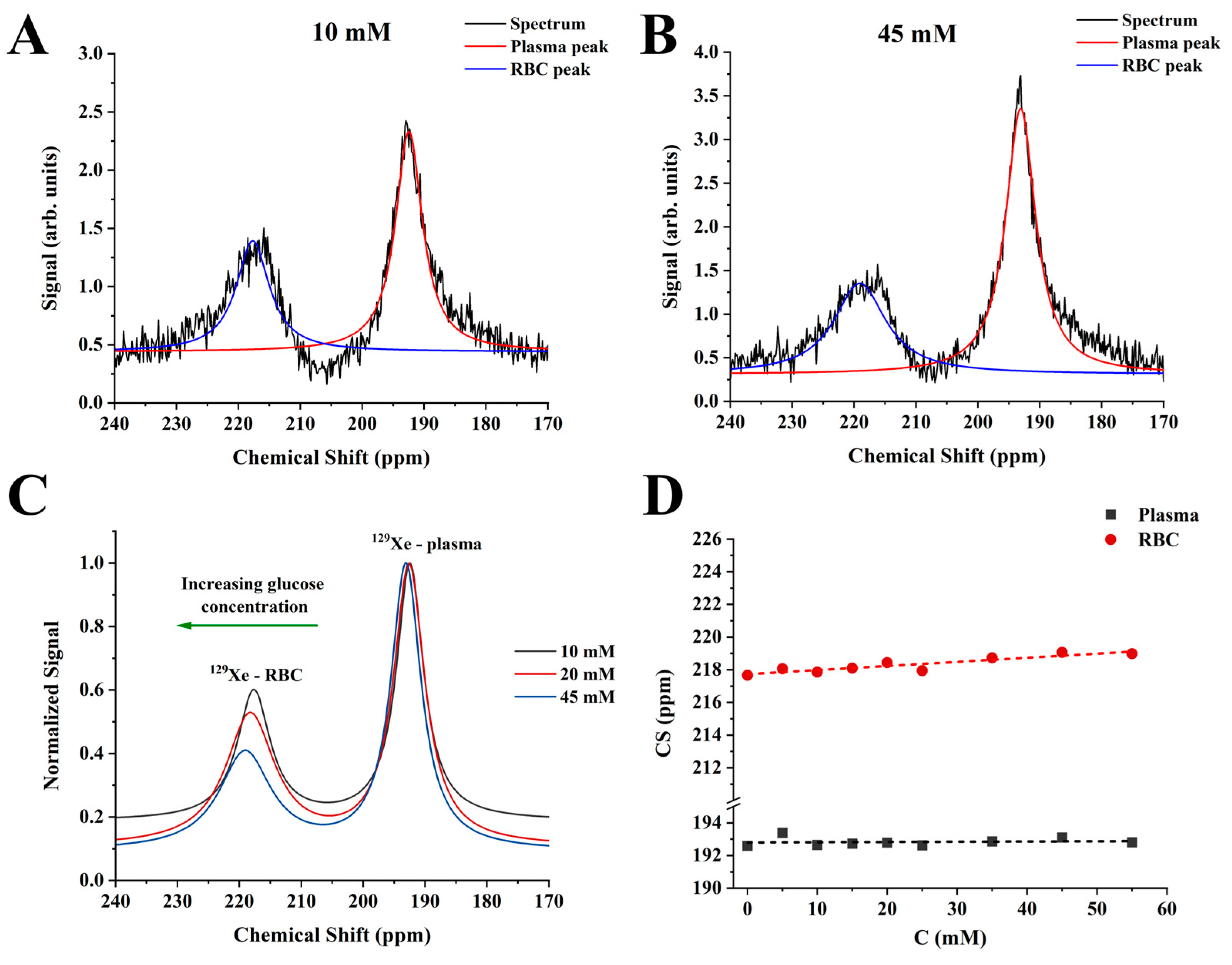
2.1. CS Analysis Using a Conventional Three-Peak Model (3PM)
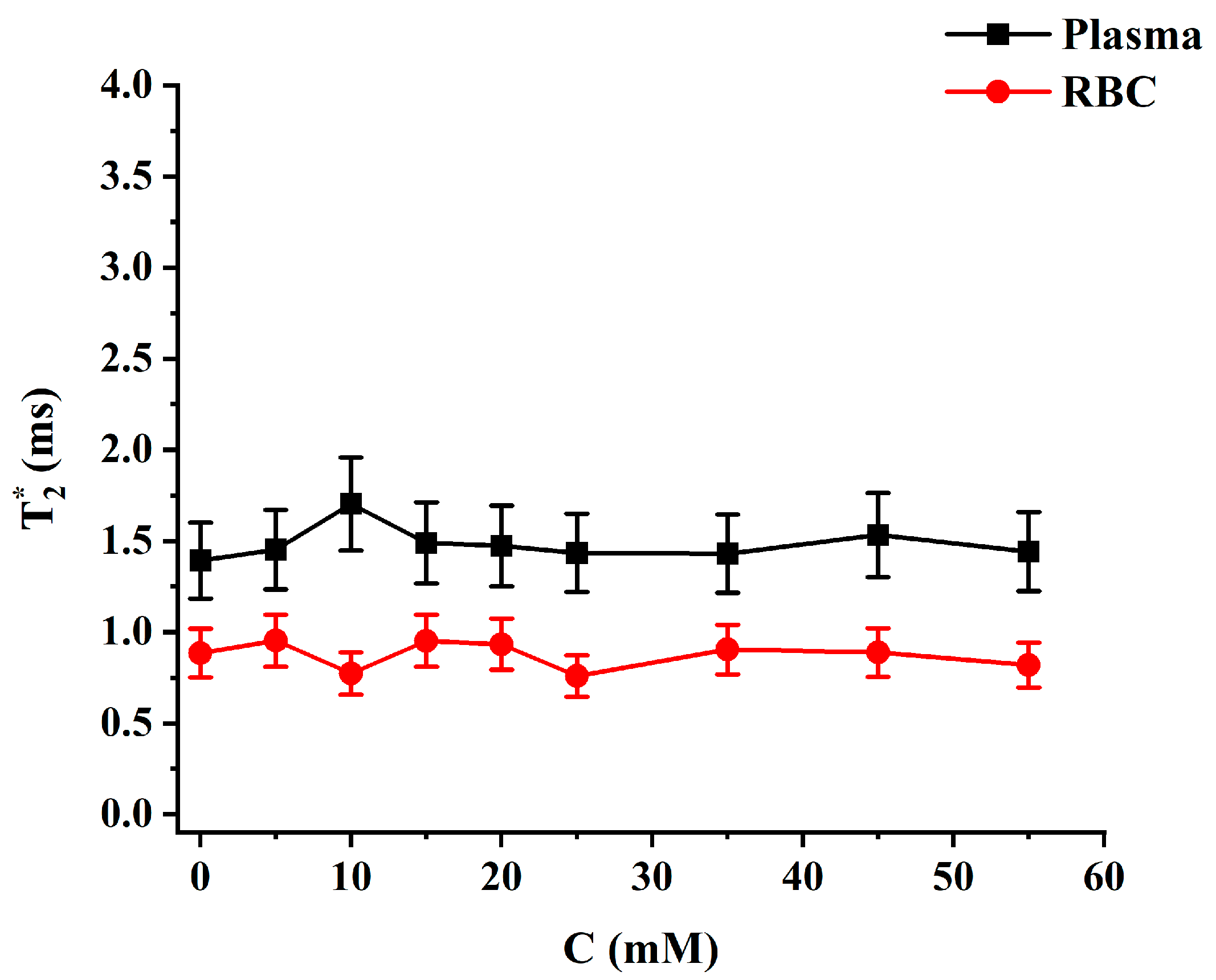
2.2. Relaxation Measurements
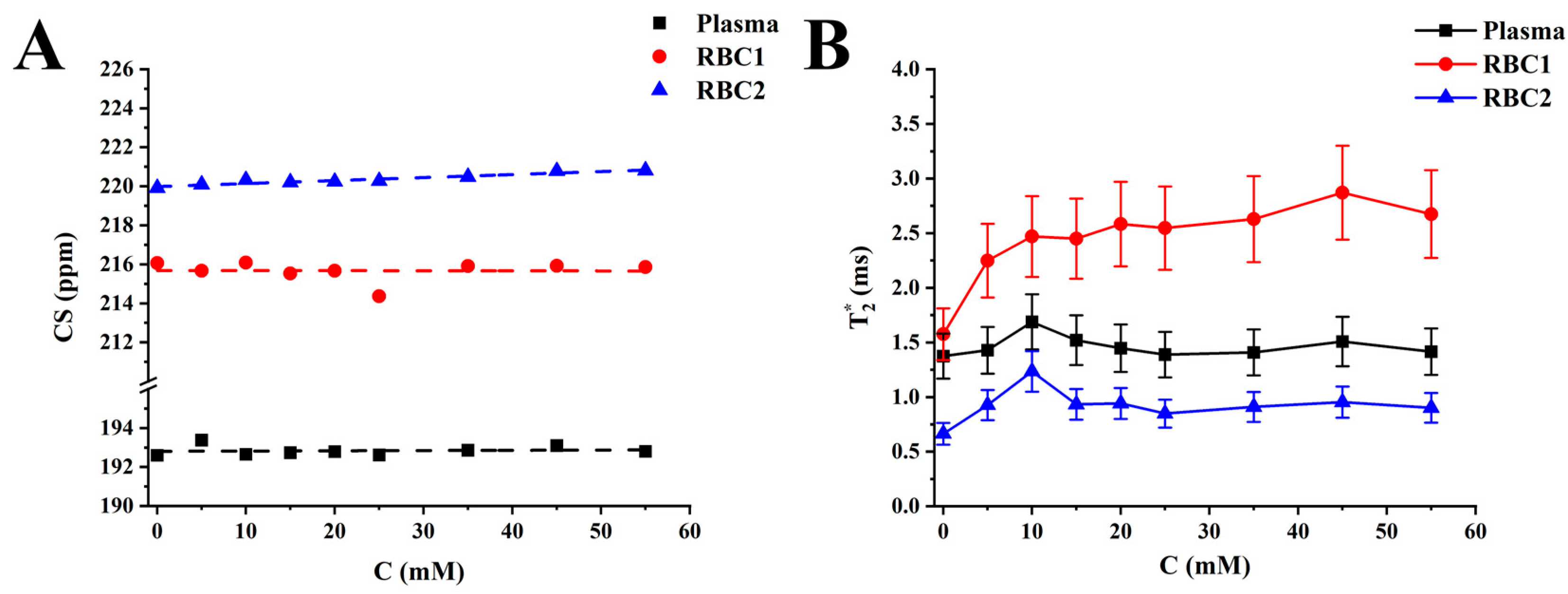
2.3. Four-Peak Spectroscopic Model (4PM) Analysis
3. Discussion
4. Materials and Methods
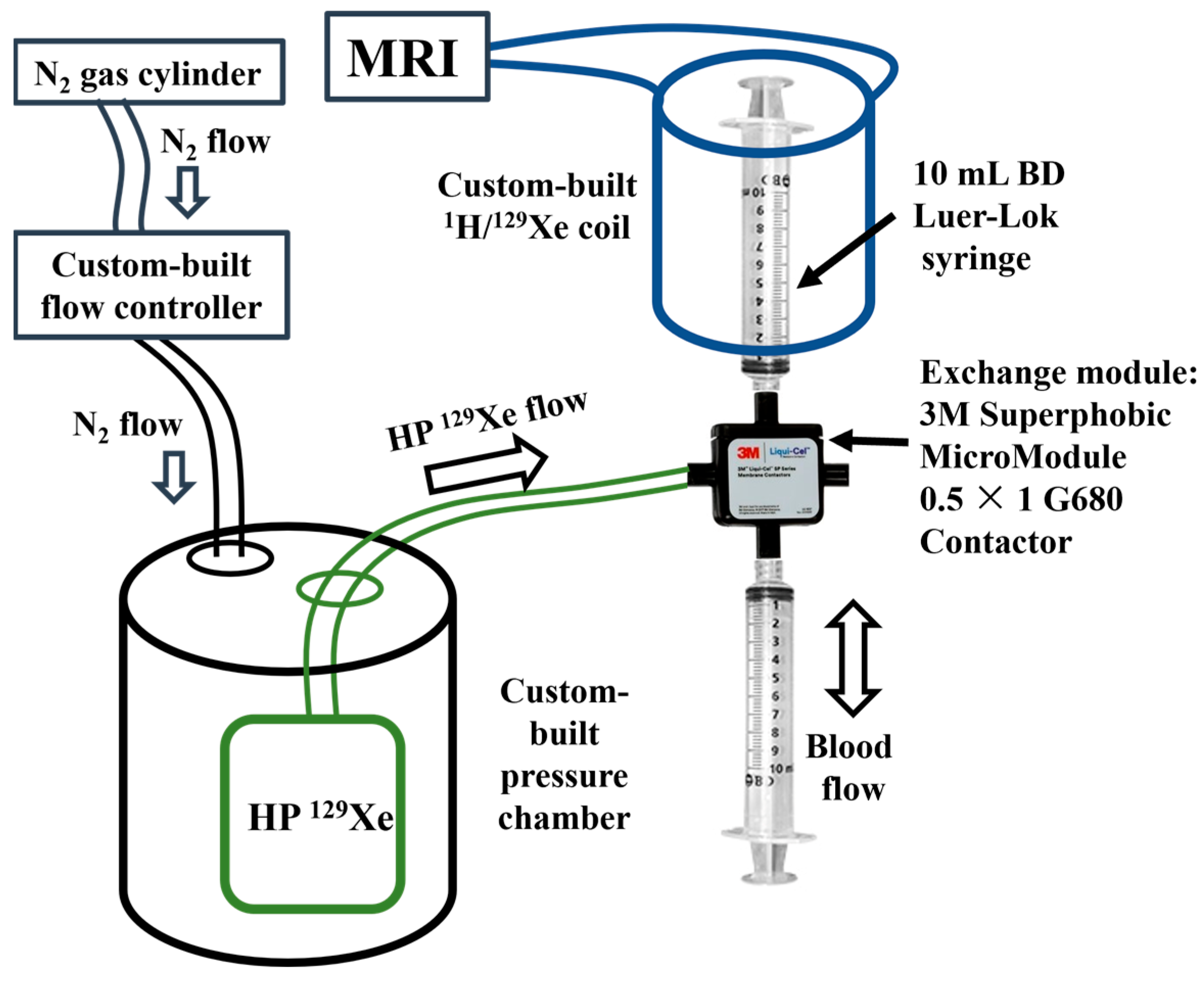
4.1. Sample Preparation
4.2. Magnetic Resonance Spectroscopy (MRS)
4.3. Data Reconstruction and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albert, M.S.; Cates, G.D.; Driehuys, B.; Happer, W.; Saam, B.; Springer, C.S.; Wishnia, A. Biological Magnetic Resonance Imaging Using Laser-Polarized 129Xe. Nature 1994, 370, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Kruger, S.J.; Nagle, S.K.; Couch, M.J.; Ohno, Y.; Albert, M.; Fain, S.B. Functional Imaging of the Lungs with Gas Agents. J. Magn. Reson. Imaging 2016, 43, 295–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patz, S.; Muradyan, I.; Hrovat, M.I.; Dabaghyan, M.; Washko, G.R.; Hatabu, H.; Butler, J.P. Diffusion of Hyperpolarized 129Xe in the Lung: A Simplified Model of 129Xe Septal Uptake and Experimental Results. New J. Phys. 2011, 13, 015009. [Google Scholar] [CrossRef]
- Ruppert, K.; Mata, J.F.; Brookeman, J.R.; Hagspiel, K.D.; Mugler, J.P. Exploring Lung Function with Hyperpolarized 129Xe Nuclear Magnetic Resonance. Magn. Reson. Med. 2004, 51, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Santyr, G.; Fox, M.; Thind, K.; Hegarty, E.; Ouriadov, A.; Jensen, M.; Scholl, T.J.; Van Dyk, J.; Wong, E. Anatomical, Functional and Metabolic Imaging of Radiation-Induced Lung Injury Using Hyperpolarized MRI. NMR Biomed. 2014, 27, 1515–1524. [Google Scholar] [CrossRef] [Green Version]
- Svenningsen, S.; Kirby, M.; Starr, D.; Leary, D.; Wheatley, A.; Maksym, G.N.; McCormack, D.G.; Parraga, G. Hyperpolarized 3He and 129Xe MRI: Differences in Asthma before Bronchodilation. J. Magn. Reson. Imaging 2013, 38, 1521–1530. [Google Scholar] [CrossRef]
- Matheson, A.M.; Mcintosh, M.J.; Kooner, H.K.; Lee, J.; Desaigoudar, V.; Bier, E.; Driehuys, B.; Svenningsen, S.; Santyr, G.E.; Kirby, M.; et al. Persistent 129Xe MRI Pulmonary and CT Vascular Abnormalities in Symptomatic Individuals with Post-Acute COVID-19 Syndrome. Radiology 2022, 305, 466–476. [Google Scholar] [CrossRef]
- Rankine, L.J.; Wang, Z.; Wang, J.M.; He, M.; McAdams, H.P.; Mammarappallil, J.; Rackley, C.R.; Driehuys, B.; Tighe, R.M. 129xenon Gas Exchange Magnetic Resonance Imaging as a Potential Prognostic Marker for Progression of Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2020, 17, 121–125. [Google Scholar] [CrossRef]
- Grist, J.T.; Chen, M.; Collier, G.J.; Raman, B.; Abueid, G.; McIntyre, A.; Matthews, V.; Fraser, E.; Ho, L.P.; Wild, J.M.; et al. Hyperpolarized 129Xe MRI Abnormalities in Dyspneic Patients 3 Months after COVID-19 Pneumonia. Radiology 2021, 301, E353–E360. [Google Scholar] [CrossRef]
- Wang, Z.; Robertson, S.H.; Wang, J.; He, M.; Virgincar, R.S.; Schrank, G.M.; Bier, E.A.; Rajagopal, S.; Huang, Y.C.; O’Riordan, T.G.; et al. Quantitative Analysis of Hyperpolarized 129Xe Gas Transfer MRI. Med. Phys. 2017, 44, 2415–2428. [Google Scholar] [CrossRef] [Green Version]
- Willmering, M.M.; Cleveland, Z.I.; Walkup, L.L.; Woods, J.C. Removal of Off-Resonance Xenon Gas Artifacts in Pulmonary Gas-Transfer MRI. Magn. Reson. Med. 2021, 86, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Niedbalski, P.J.; Hall, C.S.; Castro, M.; Eddy, R.L.; Rayment, J.H.; Svenningsen, S.; Parraga, G.; Zanette, B.; Santyr, G.E.; Thomen, R.P.; et al. Protocols for Multi-Site Trials Using Hyperpolarized 129Xe MRI for Imaging of Ventilation, Alveolar-Airspace Size, and Gas Exchange: A Position Paper from the 129Xe MRI Clinical Trials Consortium. Magn. Reson. Med. 2021, 86, 2966–2986. [Google Scholar] [CrossRef] [PubMed]
- Shepelytskyi, Y.; Grynko, V.; Rao, M.R.; Li, T.; Agostino, M.; Wild, J.M.; Albert, M.S. Hyperpolarized 129Xe Imaging of the Brain: Achievements and Future Challenges. Magn. Reson. Med. 2022, 88, 83–105. [Google Scholar] [CrossRef]
- Grynko, V.; Shepelytskyi, Y.; Li, T.; Hassan, A.; Granberg, K.; Albert, M.S. Hyperpolarized 129Xe Multi-Slice Imaging of the Human Brain Using a 3D Gradient Echo Pulse Sequence. Magn. Reson. Med. 2021, 86, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Shepelytskyi, Y.; Grynko, V.; Li, T.; Hassan, A.; Granberg, K.; Albert, M.S. The Effects of an Initial Depolarization Pulse on Dissolved Phase Hyperpolarized 129Xe Brain MRI. Magn. Reson. Med. 2021, 86, 3147–3155. [Google Scholar] [CrossRef] [PubMed]
- Shepelytskyi, Y.; Hane, F.T.; Grynko, V.; Li, T.; Hassan, A.; Albert, M.S. Hyperpolarized 129Xe Time-of-Flight MR Imaging of Perfusion and Brain Function. Diagnostics 2020, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.R.; Norquay, G.; Stewart, N.J.; Hoggard, N.; Griffiths, P.D.; Wild, J.M. Assessment of Brain Perfusion Using Hyperpolarized 129Xe MRI in a Subject with Established Stroke. J. Magn. Reson. Imaging 2019, 50, 1002–1004. [Google Scholar] [CrossRef]
- Rao, M.R.; Stewart, N.J.; Griffiths, P.D.; Norquay, G.; Wild, J.M. Imaging Human Brain Perfusion with Inhaled Hyperpolarized 129Xe MR Imaging. Radiology 2018, 286, 659–665. [Google Scholar] [CrossRef] [Green Version]
- Chacon-Caldera, J.; Maunder, A.; Rao, M.; Norquay, G.; Rodgers, O.I.; Clemence, M.; Puddu, C.; Schad, L.R.; Wild, J.M. Dissolved Hyperpolarized Xenon-129 MRI in Human Kidneys. Magn. Reson. Med. 2020, 83, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Loza, L.A.; Kadlecek, S.J.; Pourfathi, M.; Hamedani, H.; Duncan, I.F.; Ruppert, K.; Rizi, R.R. Quantification of Ventilation and Gas Uptake in Free-Breathing Mice With Hyperpolarized 129Xe MRI. IEEE Trans. Med. Imaging 2019, 38, 2081–2091. [Google Scholar] [CrossRef]
- Albert, M.S.; Balamore, D.; Kacher, D.F.; Venkatesh, A.K.; Jolesz, F.A. Hyperpolarized 129XE T1 in Oxygenated and Deoxygenated Blood. NMR Biomed. 2000, 13, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; Kacher, D.F.; Balamore, D.; Venkatesh, A.K.; Jolesz, F.A. T1 of 129Xe in Blood and the Role of Oxygenation. J. Magn. Reson. 1999, 140, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Wolber, J.; Cherubini, A.; Dzik-Jurasz, A.S.K.K.; Leach, M.O.; Bifone, A. Spin-Lattice Relaxation of Laser-Polarized Xenon in Human Blood. Proc. Natl. Acad. Sci. USA 1999, 96, 3664–3669. [Google Scholar] [CrossRef] [PubMed]
- Norquay, G.; Leung, G.; Stewart, N.J.; Wolber, J.; Wild, J.M. 129Xe Chemical Shift in Human Blood and Pulmonary Blood Oxygenation Measurement in Humans Using Hyperpolarized 129Xe NMR. Magn. Reson. Med. 2017, 77, 1399–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norquay, G.; Leung, G.; Stewart, N.J.; Tozer, G.M.; Wolber, J.; Wild, J.M. Relaxation and Exchange Dynamics of Hyperpolarized 129Xe in Human Blood. Magn. Reson. Med. 2015, 74, 303–311. [Google Scholar] [CrossRef]
- Wolber, J.; Cherubini, A.; Leach, M.O.; Bifone, A. On the Oxygenation-Dependent 129Xe T 1 in Blood. NMR Biomed. 2000, 13, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Kar, M.; Roy, A.; Chakraborti, A.S. Effect of Nonenzymatic Glycation on Functional and Structural Properties of Hemoglobin. Biophys. Chem. 2005, 113, 289–298. [Google Scholar] [CrossRef]
- Giulivi, C.; Hochstein, P.; Davies, K.J.A. Hydrogen Peroxide Production by Red Blood Cells. Free Radic. Biol. Med. 1994, 16, 123–129. [Google Scholar] [CrossRef]
- De Rosa, M.C.; Sanna, M.T.; Messana, I.; Castagnola, M.; Galtieri, A.; Tellone, E.; Scatena, R.; Botta, B.; Botta, M.; Giardina, B. Glycated Human Hemoglobin HbA1c: Functional Characteristics and Molecular Modeling Studies. Biophys. Chem. 1998, 72, 323–335. [Google Scholar] [CrossRef]
- Sacks, D.B. Correlation between Hemoglobin A1c (HbA1c) and Average Blood Glucose: Can HbA1c Be Reported as Estimated Blood Glucose Concentration? J. Diabetes Sci. Technol. 2007, 1, 801–803. [Google Scholar] [CrossRef] [Green Version]
- Coletta, M.; Amiconi, G.; Bellellil, A.; Carsky, J.; Castagnola, M.; Condb, S.; Brunori, M. Alteration of T-State Binding Properties of Naturally Glycated. J. Mol. Biol. 1988, 203, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Freeman, V.S. Glucose and Hemoglobin A1c. Lab. Med. 2014, 45, e21–e24. [Google Scholar] [CrossRef] [Green Version]
- Penttilä, I.; Penttilä, K.; Holm, P.; Laitinen, H.; Ranta, P.; Törrönen, J.; Rauramaa, R. Methods, Units and Quality Requirements for the Analysis of Haemoglobin A 1c in Diabetes Mellitus. World J. Methodol. 2016, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Inouye, M.; Mio, T.; Sumino, K. Glycated Hemoglobin and Lipid Peroxidation in Erythrocytes of Diabetic Patients. Metabolism 1999, 48, 205–209. [Google Scholar] [CrossRef]
- Viskupicova, J.; Blaskovic, D.; Galiniak, S.; Soszyński, M.; Bartosz, G.; Horakova, L.; Sadowska-Bartosz, I. Effect of High Glucose Concentrations on Human Erythrocytes in Vitro. Redox Biol. 2015, 5, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Kar, M.; Chakraborti, A.S. Effect of Glucosylation on Iron-Mediated Free Radical Reactions of Haemoglobin. Curr. Sci. 2001, 80, 770–773. [Google Scholar]
- Buys, A.V.; Van Rooy, M.J.; Soma, P.; Van Papendorp, D.; Lipinski, B.; Pretorius, E. Changes in Red Blood Cell Membrane Structure in Type 2 Diabetes: A Scanning Electron and Atomic Force Microscopy Study. Cardiovasc. Diabetol. 2013, 12, 25. [Google Scholar] [CrossRef] [Green Version]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red Blood Cell Distribution Width: A Simple Parameter with Multiple Clinical Applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef]
- Schoenborn, B.P. Binding of Xenon to Horse Haemoglobin. Nature 1965, 208, 760–762. [Google Scholar] [CrossRef]
- Peterson, K.P.; Pavlovich, J.G.; Goldstein, D.; Little, R.; England, J.; Peterson, C.M. What Is Hemoglobin A1c? An Analysis of Glycated Hemoglobins by Electrospray Ionization Mass Spectrometry Endocrinology and Metabolism. Clin. Chem. 1998, 44, 1951–1958. [Google Scholar] [CrossRef] [Green Version]
- Watala, C.; Gwozdzinski, K.; Malek’, M. Direct Evidence for the Alterations in Protein Structure and Conformation upon In Vitro Nonenzymatic Glycosylation. J. Biochem. 1992, 24, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Ruan, P.; Yong, J.; Shen, H.; Liao, Z.; Dong, X. The Impact of the HbA1c Level of Type 2 Diabetics on the Structure of Haemoglobin. Sci. Rep. 2016, 6, 33352. [Google Scholar] [CrossRef] [PubMed]
- Savino, C.; Miele, A.E.; Draghi, F.; Johnson, K.A.; Sciara, G.; Brunori, M.; Vallone, B. Pattern of Cavities in Globins: The Case of Human Hemoglobin. Biopolym. Pept. Sci. Sect. 2009, 91, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.P.; Jiang, Z.Y.; Huwr, J.V. Protein Glycation and Oxidative Stress in Diabetes Mellitus and Ageing. Free Radic. Biol. Med. 1991, 10, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Role of Free Radicals and Catalytic Metal Ions in Human Disease: An Overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef]
- Koga, M.; Inada, S.; Shimizu, S.; Hatazaki, M.; Umayahara, Y.; Nishihara, E. Aldimine Formation Reaction, the First Step of the Maillard Early-Phase Reaction, Might Be Enhanced in Variant Hemoglobin, Hb Himeji. Ann. Clin. Lab. Sci. 2015, 45, 643–649. [Google Scholar] [PubMed]
- Kerber, R.C. The A1c Blood Test: An Illustration of Principles from General and Organic Chemistry. J. Chem. Educ. 2007, 84, 1541–1545. [Google Scholar] [CrossRef]
- Lowrey, C.H.; Lyness, S.J.; Soeldner, J.S. The Effect of Hemoglobin Ligands on the Kinetics of Human Hemoglobin A(1c) Formation. J. Biol. Chem. 1985, 260, 11611–11618. [Google Scholar] [CrossRef]
- Umbreit, J. Methemoglobin—It’s Not Just Blue: A Concise Review. Am. J. Hematol. 2007, 82, 134–144. [Google Scholar] [CrossRef]
- da Silva, M.V.B.; Alet, A.I.; Castellini, H.V.; Riquelme, B.D. Methods: A New Protocol for in Vitro Red Blood Cell Glycation. Comp. Biochem. Physiol. Part A 2022, 264, 111109. [Google Scholar] [CrossRef]
- Shepelytskyi, Y.; Li, T.; Grynko, V.; Newman, C.; Hane, F.T.; Albert, M.S. Evaluation of Fluorine-19 Magnetic Resonance Imaging of the Lungs Using Octafluorocyclobutane in a Rat Model. Magn. Reson. Med. 2021, 85, 987–994. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikowska, L.; Grynko, V.; Shepelytskyi, Y.; Ruset, I.C.; Deschamps, J.; Aalto, H.; Targosz-Korecka, M.; Balamore, D.; Harańczyk, H.; Albert, M.S. Revealing a Third Dissolved-Phase Xenon-129 Resonance in Blood Caused by Hemoglobin Glycation. Int. J. Mol. Sci. 2023, 24, 11311. https://doi.org/10.3390/ijms241411311
Mikowska L, Grynko V, Shepelytskyi Y, Ruset IC, Deschamps J, Aalto H, Targosz-Korecka M, Balamore D, Harańczyk H, Albert MS. Revealing a Third Dissolved-Phase Xenon-129 Resonance in Blood Caused by Hemoglobin Glycation. International Journal of Molecular Sciences. 2023; 24(14):11311. https://doi.org/10.3390/ijms241411311
Chicago/Turabian StyleMikowska, Lutosława, Vira Grynko, Yurii Shepelytskyi, Iullian C. Ruset, Joseph Deschamps, Hannah Aalto, Marta Targosz-Korecka, Dilip Balamore, Hubert Harańczyk, and Mitchell S. Albert. 2023. "Revealing a Third Dissolved-Phase Xenon-129 Resonance in Blood Caused by Hemoglobin Glycation" International Journal of Molecular Sciences 24, no. 14: 11311. https://doi.org/10.3390/ijms241411311





