Endothelial Senescence and Its Impact on Angiogenesis in Alzheimer’s Disease
Abstract
1. Introduction
2. The Dual Nature of Cellular Senescence
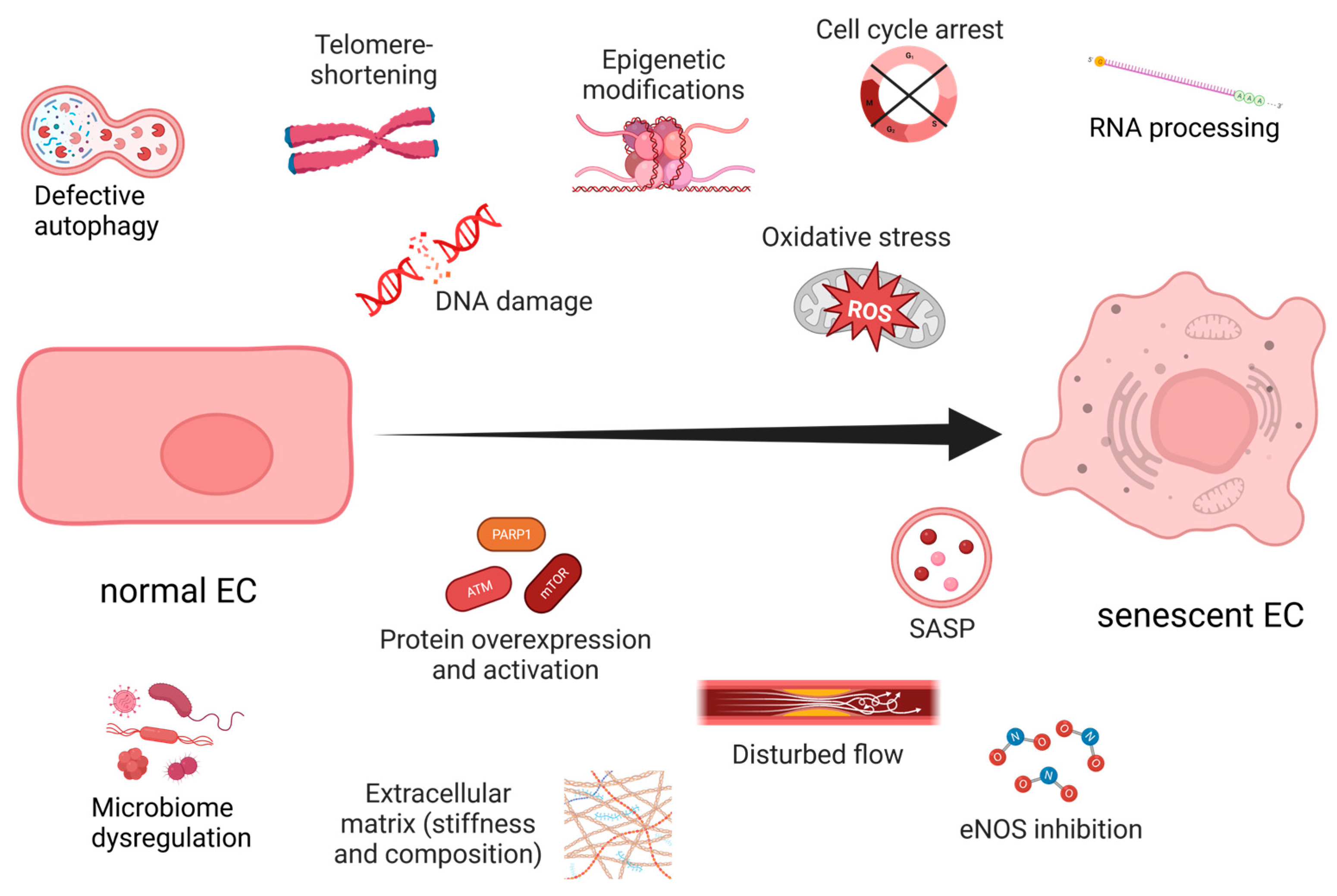
2.1. Hallmarks of Aging
2.2. The Dose Makes the Poison
3. Endothelial Senescence
3.1. ECM Disruption Accelerates Vascular Aging
3.2. Navigating the Currents: Shear Stress and Its Impact on Endothelial Cells’ Function
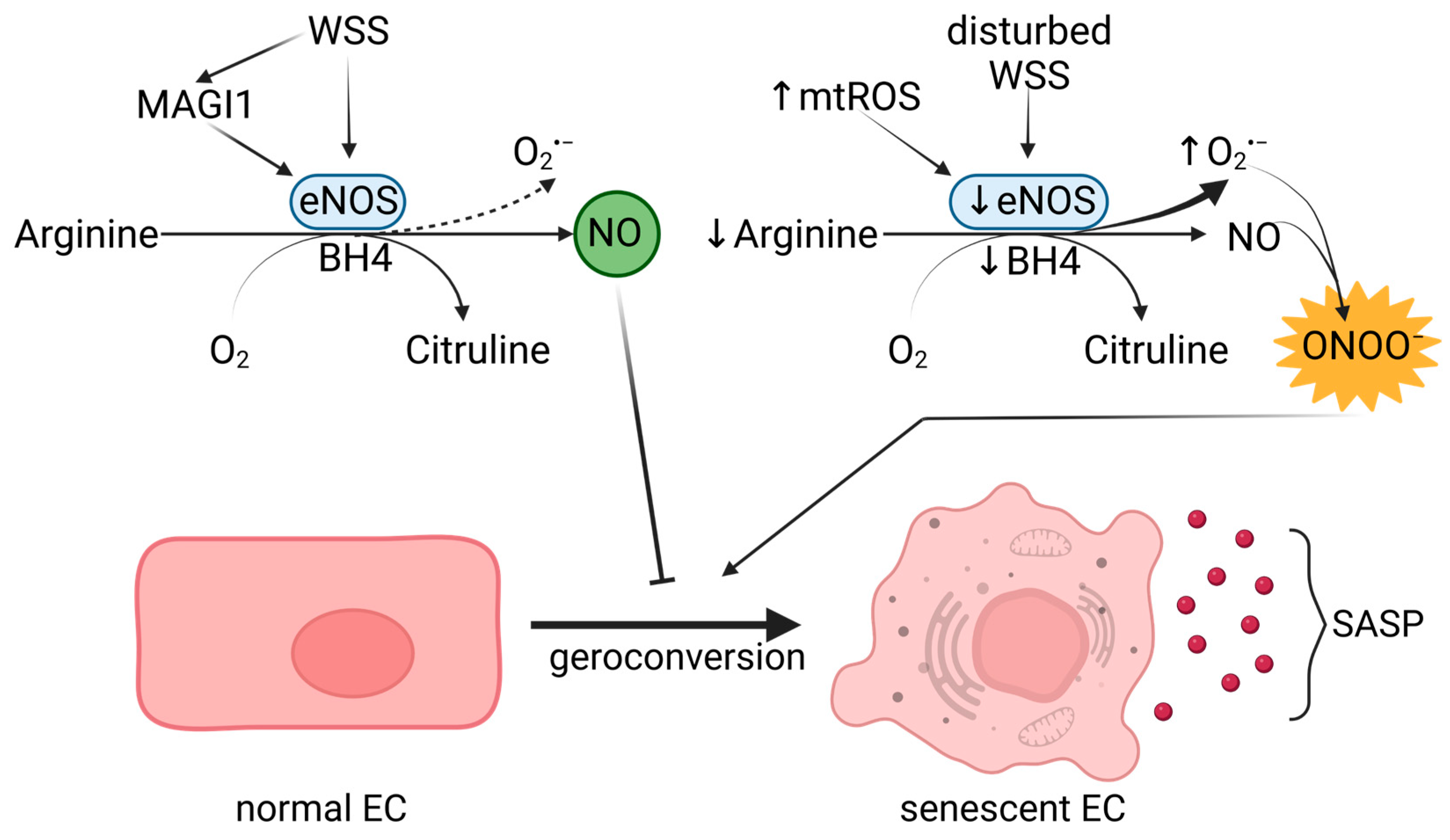
3.3. Linking NO Signaling with Endothelial Senescence
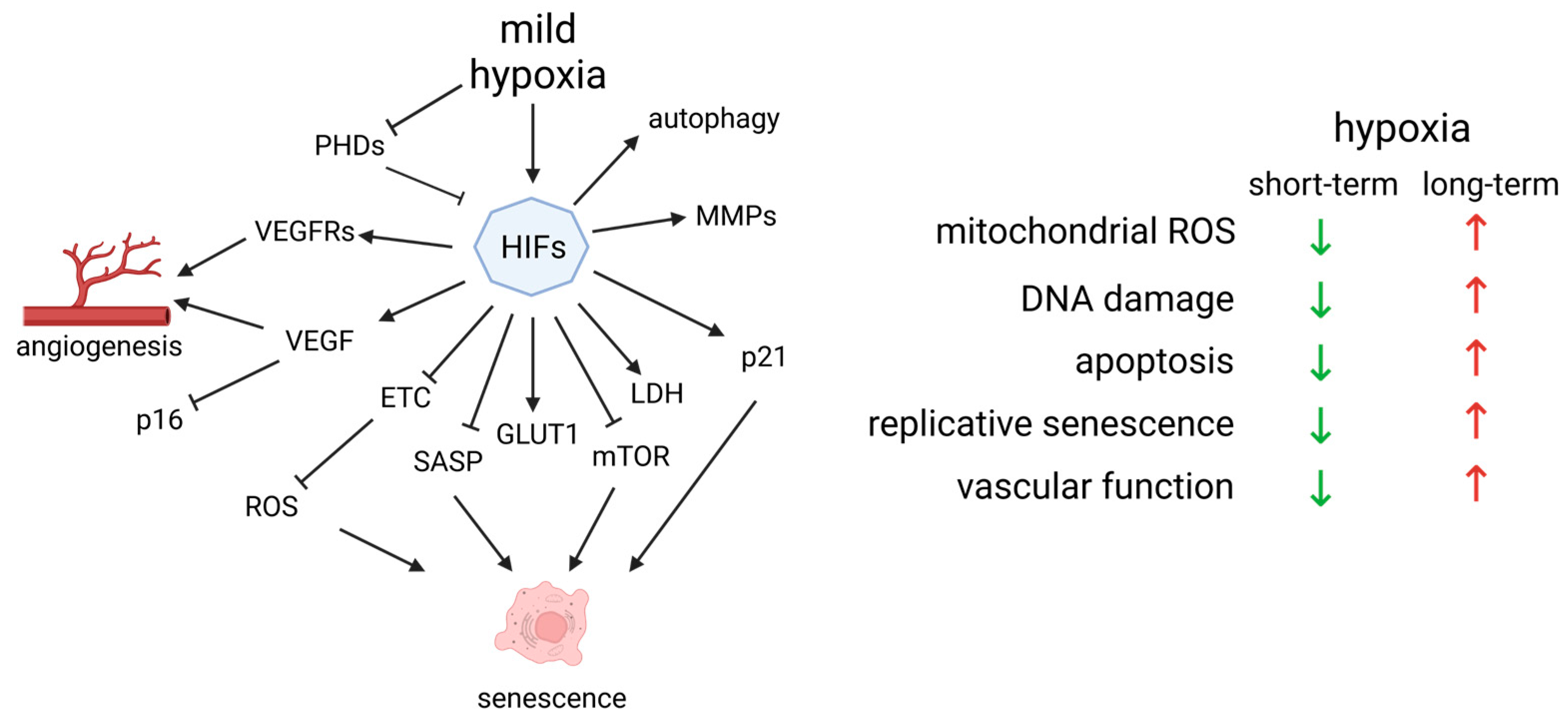
4. Unveiling the Interplay between Hypoxia and Oxidative Stress-Induced Endothelial Senescence
4.1. HIF-1α in Angiogenesis
4.2. Hypoxia Is Essential for the Day–Night Cycle
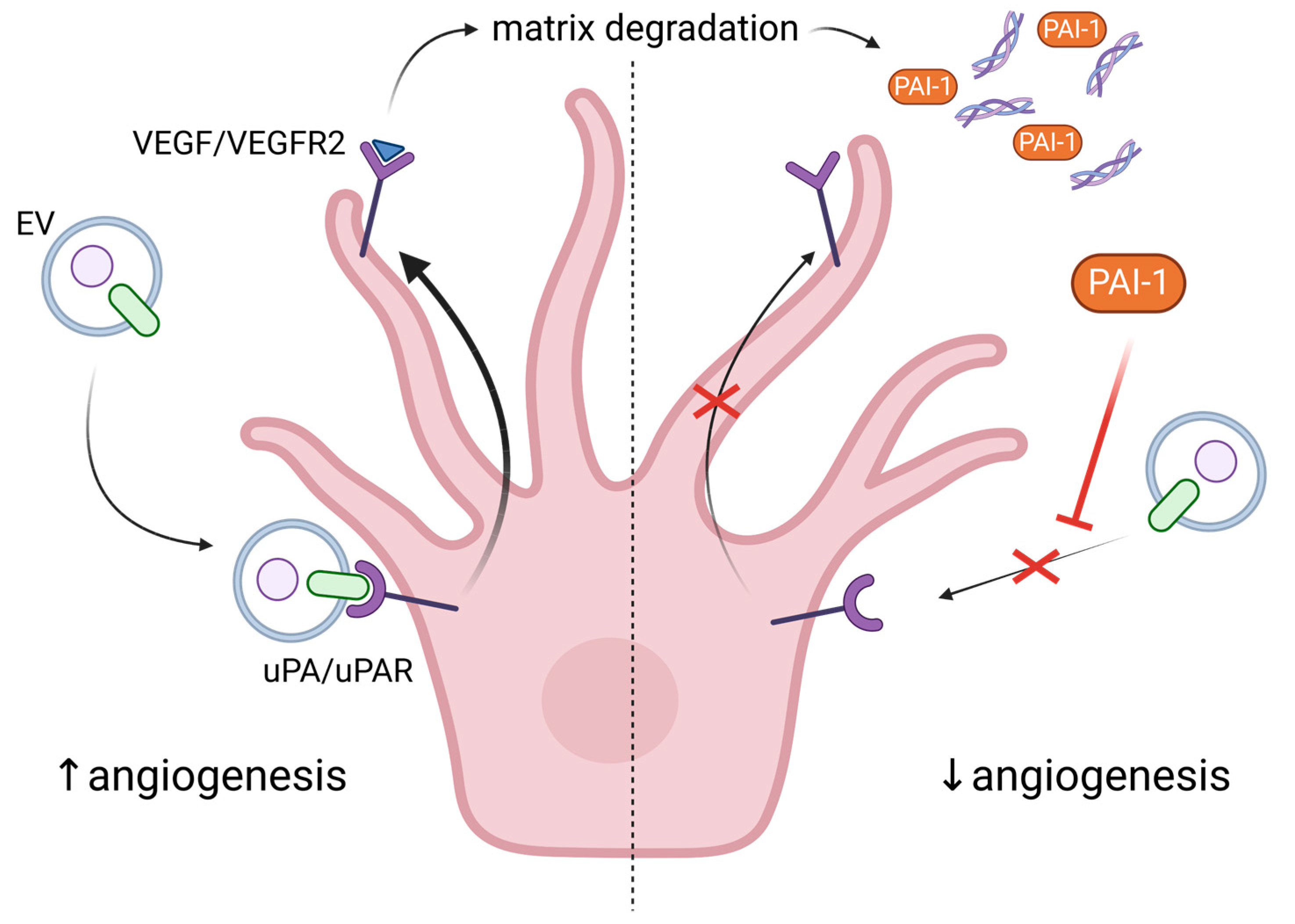
5. Exploring the Role of Extracellular Vesicles in Angiogenesis and Senescence
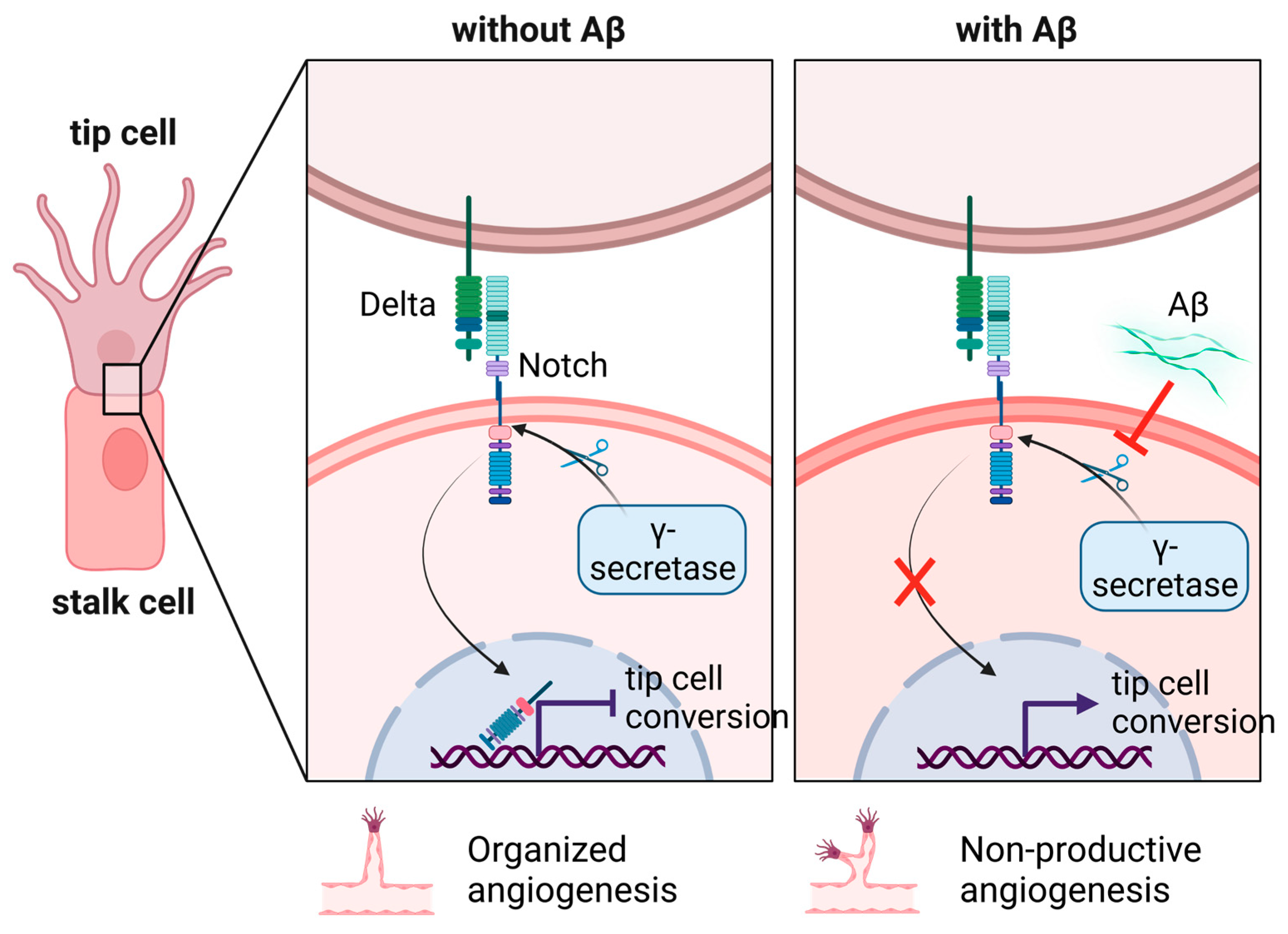
6. The Non-Productive Angiogenesis in Alzheimer’s Disease
6.1. How Does Aβ Stimulate Cerebral Angiogenesis?
6.2. EVs Can Be Used as Biomarkers for Early AD Detection
6.3. The Role of Endothelial Progenitor Cells (EPCs) as a Biomarker and Potential Therapeutic Target in AD
7. Therapeutic Approaches to Endothelial Senescence and Dysfunction
7.1. Exercise Improves CBF, Vascular Function and Cognitive Performance
7.2. Caloric Restriction Reduces OS and Vascular Aging
7.3. Role of Resveratrol in the Vascular Biology and Senescence Process
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (R)AGE | (Receptor for) Advanced Glycation End products |
| AD | Alzheimer’s disease |
| APCs | Antigen Presenting Cells |
| ApoE | Apolipoprotein E |
| APP | Amyloid Precursor Protein |
| ATM | Ataxia-telangiectasia mutated protein kinase |
| Aβ | Beta-amyloid protein |
| BAECs | Bovine Aortic Endothelial Cells |
| BBB | Blood-Brain Barrier |
| BH4 | Tetrahydrobiopterin |
| CAA | Cerebral Amyloid Angiopathy |
| CBF | Cerebral Blood Flow |
| CR | Caloric Restriction |
| CVD | Cardio Vascular Disease |
| DDR | DNA Damage Response |
| Dll4 | Delta-like 4 protein |
| ECM | Extracellular Matrix |
| ECs | Endothelial cells |
| eNOS | Endothelial nitric oxide synthase |
| EPO | Erythropoietin |
| ETC | Electron Transport Chain |
| EVs | Extracellular Vesicles |
| GLUT1 | Glucose Transporter 1 |
| GSH | Glutathione |
| H(B)MVECs | Human (Brain) Microvascular Endothelial Cells |
| HIF | Hypoxia-inducible factor |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| KO | Knock-out |
| LDH | Lactate Dehydrogenase |
| MMP | Metalloproteinase |
| mTOR | Mammalian Target of Rapamycin |
| NAD+ | Nicotinamide Adenine Dinucleotide |
| NEXT | Notch extracellular domain |
| NF-kB | Nuclear Factor–kappa Beta |
| NICD | Notch intracellular domain |
| NMN | Nicotinamide Mononucleotide |
| NO | Nitric oxide |
| NOX | NADPH oxidases |
| ONOO− (PN) | Peroxynitrite |
| OS | Oxidative Stress |
| PAI-1 | plasminogen activator inhibitor |
| PARP1 | Poly (ADP-ribose) polymerase 1 |
| PHD | Prolyl hydroxylases |
| PrP | Prion protein |
| p-Tau | Phosphorylated-Tau |
| ROS | Reactive Oxygen Species |
| SASP | Senescence-Associated Secretory Phenotype |
| SA-β-Gal | Senescence-Associated β-galactosidase |
| SCs | Senescent cells |
| sen-ECs | Senescent endothelial cells |
| SIRT | Sirtuins |
| SOD | Superoxide dismutase |
| TCA | Three Carboxylic Acid cycle |
| uPA(R) | urokinase plasminogen activator/uPA receptor |
| VEGF | Vascular Endothelial Growth Factor |
| VEGFR1(2) | Vascular Endothelial Growth Factor Receptor 1(2) |
| WSS | Wall Shear Stress |
| WT | Wild Type |
References
- Patel-Hett, S.; D’Amore, P.A. Signal transduction in vasculogenesis and developmental angiogenesis. Int. J. Dev. Biol. 2011, 55, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Adair, T.H.; Montani, J.-P. Overview of Angiogenesis. In Angiogenesis; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Weinstein, N.; Mendoza, L.; Gitler, I.; Klapp, J. A network model to explore the effect of the micro-environment on endothelial cell behavior during angiogenesis. Front. Physiol. 2017, 8, 960. [Google Scholar] [CrossRef] [PubMed]
- Brassard-Jollive, N.; Monnot, C.; Muller, L.; Germain, S. In vitro 3D Systems to Model Tumor Angiogenesis and Interactions With Stromal Cells. Front. Cell Dev. Biol. 2020, 8, 594903. [Google Scholar] [CrossRef]
- Stryker, Z.I.; Rajabi, M.; Davis, P.J.; Mousa, S.A. Evaluation of angiogenesis assays. Biomedicines 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Lähteenvuo, J.; Rosenzweig, A. Effects of aging on angiogenesis. Circ. Res. 2012, 110, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Kiss, T.; Wren, J.D.; Giles, C.B.; Griffin, C.T.; Murfee, W.L.; Pacher, P.; Csiszar, A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018, 15, 555–565. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Ambrose, C.T. Pro-Angiogenesis Therapy and Aging: A Mini-Review. Gerontology 2017, 63, 393–400. [Google Scholar] [CrossRef]
- Vinters, H.V.; Gilbert, J.J. Cerebral amyloid angiopathy: Incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke 1983, 14, 924–928. [Google Scholar] [CrossRef]
- Beckmann, N.; Schuler, A.; Mueggler, T.; Meyer, E.P.; Wiederhold, K.H.; Staufenbiel, M.; Krucker, T. Age-Dependent Cerebrovascular Abnormalities and Blood Flow Disturbances in APP23 Mice Modeling Alzheimer’s Disease. J. Neurosci. 2003, 23, 8453–8459. [Google Scholar] [CrossRef]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; Van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef]
- Singh, C.; Pfeifer, C.G.; Jefferies, W.A. Pathogenic Angiogenic Mechanisms in Alzheimer’s Disease. In Physiologic and Pathologic Angiogenesis-Signaling Mechanisms and Targeted Therapy; BoD–Books on Demand: Norderstedt, Germany, 2017. [Google Scholar] [CrossRef]
- Bradaric, B.D.; Patel, A.; Schneider, J.A.; Carvey, P.M.; Hendey, B. Evidence for Angiogenesis in Parkinson’s disease, Incidental Lewy Body disease, and Progressive Supranuclear Palsy. J. Neural Transm. 2012, 119, 59. [Google Scholar] [CrossRef]
- Ellison, S.M.; Trabalza, A.; Tisato, V.; Pazarentzos, E.; Lee, S.; Papadaki, V.; Goniotaki, D.; Morgan, S.; Mirzaei, N.; Mazarakis, N.D. Dose-dependent Neuroprotection of VEGF165 in Huntington’s DiseaseStriatum. Mol. Ther. 2013, 21, 1862. [Google Scholar] [CrossRef]
- Fontana, I.C.; Zimmer, A.R.; Rocha, A.S.; Gosmann, G.; Souza, D.O.; Lourenco, M.V.; Ferreira, S.T.; Zimmer, E.R. Amyloid-β oligomers in cellular models of Alzheimer’s disease. J. Neurochem. 2020, 155, 348–369. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Jefferies, W.A.; Price, K.A.; Biron, K.E.; Fenninger, F.; Pfeifer, C.G.; Dickstein, D.L. Adjusting the compass: New insights into the role of angiogenesis in Alzheimer’s disease. Alzheimer’s Res. Ther. 2013, 5, 64–69. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Tzoneva, R. Oxidative Stress and Aging as Risk Factors for Alzheimer’s Disease and Parkinson’s Disease: The Role of the Antioxidant Melatonin. Int. J. Mol. Sci. 2023, 24, 3022. [Google Scholar] [CrossRef]
- Tadokoro, K.; Ohta, Y.; Inufusa, H.; Loon, A.F.N.; Abe, K. Prevention of Cognitive Decline in Alzheimer’s Disease by Novel Antioxidative Supplements. Int. J. Mol. Sci. 2020, 21, 1974. [Google Scholar] [CrossRef]
- Greenberg, D.A.; Jin, K. From angiogenesis to neuropathology. Nature 2005, 438, 954–959. [Google Scholar] [CrossRef]
- Ribatti, D.; Guidolin, D. Morphogenesis of vascular and neuronal networks and the relationships between their remodeling processes. Brain Res. Bull. 2022, 186, 62–69. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194. [Google Scholar] [CrossRef] [PubMed]
- Schmauck-Medina, T.; Molière, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 485. [Google Scholar] [CrossRef]
- Han, Y.; Kim, S.Y. Endothelial senescence in vascular diseases: Current understanding and future opportunities in senotherapeutics. Exp. Mol. Med. 2023, 55, 1–12. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Gimeno, I.; Cañón-Beltrán, K.; Gutiérrez-Adán, A.; Rizos, D.; Gómez, E. Senescence and Apoptosis During in vitro Embryo Development in a Bovine Model. Front. Cell Dev. Biol. 2020, 8, 1646. [Google Scholar] [CrossRef]
- De-Carvalho, D.P.; Jacinto, A.; Saúde, L. The right time for senescence. Elife 2021, 10, e72449. [Google Scholar] [CrossRef]
- Vujin, A.; Dick, K. The information theory of aging: Hacking immortality? Health Sci. Inq. 2020, 11, 148–154. [Google Scholar] [CrossRef]
- Harley, C.B.; Vaziri, H.; Counter, C.M.; Allsopp, R.C. The telomere hypothesis of cellular aging. Exp. Gerontol. 1992, 27, 375–382. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S.; et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 2020, 588, 124–129. [Google Scholar] [CrossRef]
- Yang, J.-H.; Hayano, M.; Griffin, P.T.; Amorim, J.A.; Bonkowski, M.S.; Apostolides, J.K.; Salfati, E.L.; Blanchette, M.; Munding, E.M.; Bhakta, M.; et al. Loss of epigenetic information as a cause of mammalian aging. Cell 2023, 186, 305–326.e27. [Google Scholar] [CrossRef]
- Gill, D.; Parry, A.; Santos, F.; Okkenhaug, H.; Todd, C.D.; Hernando-Herraez, I.; Stubbs, T.M.; Milagre, I.; Reik, W. Multi-omic rejuvenation of human cells by maturation phase transient reprogramming. Elife 2022, 11, e71624. [Google Scholar] [CrossRef]
- Aleksandrov, R.; Dotchev, A.; Poser, I.; Krastev, D.; Georgiev, G.; Panova, G.; Babukov, Y.; Danovski, G.; Dyankova, T.; Hubatsch, L.; et al. Protein Dynamics in Complex DNA Lesions. Mol. Cell 2018, 69, 1046–1061.e5. [Google Scholar] [CrossRef]
- Lanna, A.; Vaz, B.; D’Ambra, C.; Valvo, S.; Vuotto, C.; Chiurchiù, V.; Devine, O.; Sanchez, M.; Borsellino, G.; Akbar, A.N.; et al. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nat. Cell Biol. 2022, 24, 1461–1474. [Google Scholar] [CrossRef]
- Gorbunova, V.; Seluanov, A.; Pereira-Smith, O.M. Expression of human telomerase (hTERT) does not prevent stress-induced senescence in normal human fibroblasts but protects the cells from stress-induced apoptosis and necrosis. J. Biol. Chem. 2002, 277, 38540–38549. [Google Scholar] [CrossRef]
- Chen, Q.M.; Prowse, K.R.; Tu, V.C.; Purdom, S.; Linskens, M.H.K. Uncoupling the Senescent Phenotype from Telomere Shortening in Hydrogen Peroxide-Treated Fibroblasts. Exp. Cell Res. 2001, 265, 294–303. [Google Scholar] [CrossRef]
- Kirsch, V.; Ramge, J.M.; Schoppa, A.; Ignatius, A.; Riegger, J. In Vitro Characterization of Doxorubicin-Mediated Stress-Induced Premature Senescence in Human Chondrocytes. Cells 2022, 11, 1106. [Google Scholar] [CrossRef]
- Hettinger, Z.R.; Kargl, C.K.; Shannahan, J.H.; Kuang, S.; Gavin, T.P. Extracellular vesicles released from stress-induced prematurely senescent myoblasts impair endothelial function and proliferation. Exp. Physiol. 2021, 106, 2083–2095. [Google Scholar] [CrossRef]
- Merdji, H.; Kassem, M.; Chomel, L.; Clere-Jehl, R.; Helms, J.; Kurihara, K.; Chaker, A.B.; Auger, C.; Schini-Kerth, V.; Toti, F.; et al. Septic shock as a trigger of arterial stress-induced premature senescence: A new pathway involved in the post sepsis long-term cardiovascular complications. Vascul. Pharmacol. 2021, 141, 106922. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Ben Ameur, R.; Bauwens, E.; Dumortier, E.; Toutfaire, M.; Toussaint, O. Stress-Induced (Premature) Senescence. In Healthy Ageing and Longevity; Springer: Cham, Switzerland, 2016; pp. 243–262. [Google Scholar]
- Xi, L.; Peng, M.; Liu, S.; Liu, Y.; Wan, X.; Hou, Y.; Qin, Y.; Yang, L.; Chen, S.; Zeng, H.; et al. Hypoxia-stimulated ATM activation regulates autophagy-associated exosome release from cancer-associated fibroblasts to promote cancer cell invasion. J. Extracell. Vesicles 2021, 10, e12146. [Google Scholar] [CrossRef] [PubMed]
- Olcina, M.M.; Grand, R.J.A.; Hammond, E.M. ATM activation in hypoxia-causes and consequences. Mol. Cell. Oncol. 2014, 1, e29903. [Google Scholar] [CrossRef] [PubMed]
- Stagni, V.; Ferri, A.; Cirotti, C.; Barilà, D. ATM Kinase-Dependent Regulation of Autophagy: A Key Player in Senescence? Front. Cell Dev. Biol. 2021, 8, 1582. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Suzuki, T.; Aizawa, K.; Miyagawa, K.; Nagai, R. Ataxia Telangiectasia Mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence. J. Biol. Chem. 2010, 285, 29662–29670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, L.; Lu, A.; Han, Y.; Colangelo, D.; Bukata, C.; Scibetta, A.; Yousefzadeh, M.J.; Li, X.; Gurkar, A.U.; et al. ATM is a key driver of NF-κB-dependent DNA-damage-induced senescence, stem cell dysfunction and aging. Aging 2020, 12, 4688. [Google Scholar] [CrossRef] [PubMed]
- Correia-Melo, C.; Marques, F.D.; Anderson, R.; Hewitt, G.; Hewitt, R.; Cole, J.; Carroll, B.M.; Miwa, S.; Birch, J.; Merz, A.; et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016, 35, 724–742. [Google Scholar] [CrossRef]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+ in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef]
- Lee, O.H.; Woo, Y.M.; Moon, S.; Lee, J.; Park, H.; Jang, H.; Park, Y.Y.; Bae, S.K.; Park, K.H.; Heo, J.H.; et al. Sirtuin 6 deficiency induces endothelial cell senescence via downregulation of forkhead box M1 expression. Aging 2020, 12, 20946. [Google Scholar] [CrossRef]
- Duan, J.L.; Ruan, B.; Song, P.; Fang, Z.Q.; Yue, Z.S.; Liu, J.J.; Dou, G.R.; Han, H.; Wang, L. Shear stress–induced cellular senescence blunts liver regeneration through Notch–sirtuin 1–P21/P16 axis. Hepatology 2022, 75, 584–599. [Google Scholar] [CrossRef]
- Brenner, C. Sirtuins are not conserved longevity genes. Life Metab. 2022, 1, 122–133. [Google Scholar] [CrossRef]
- Huang, P.; Chen, G.; Jin, W.; Mao, K.; Wan, H.; He, Y. Molecular Mechanisms of Parthanatos and Its Role in Diverse Diseases. Int. J. Mol. Sci. 2022, 23, 7292. [Google Scholar] [CrossRef]
- Ohanna, M.; Giuliano, S.; Bonet, C.; Imbert, V.; Hofman, V.; Zangari, J.; Bille, K.; Robert, C.; Bressac-de Paillerets, B.; Hofman, P.; et al. Senescent cells develop a PARP-1 and nuclear factor-{kappa}B-associated secretome (PNAS). Genes Dev. 2011, 25, 1245–1261. [Google Scholar] [CrossRef]
- de Picciotto, N.E.; Gano, L.B.; Johnson, L.C.; Martens, C.R.; Sindler, A.L.; Mills, K.F.; Imai, S.; Seals, D.R. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 2016, 15, 522–530. [Google Scholar] [CrossRef]
- Oomen, C.A.; Farkas, E.; Roman, V.; van der Beek, E.M.; Luiten, P.G.M.; Meerlo, P. Resveratrol Preserves Cerebrovascular Density and Cognitive Function in Aging Mice. Front. Aging Neurosci. 2009, 1, 4. [Google Scholar] [CrossRef]
- Maizel, J.; Xavier, S.; Chen, J.; Lin, C.H.S.; Vasko, R.; Goligorsky, M.S. Sirtuin 1 ablation in endothelial cells is associated with impaired angiogenesis and diastolic dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1691–H1704. [Google Scholar] [CrossRef]
- Sheekey, E.; Narita, M. p53 in senescence–it’s a marathon not a sprint. FEBS J. 2021, 290, 1212–1220. [Google Scholar] [CrossRef]
- Otero-Albiol, D.; Carnero, A. Cellular senescence or stemness: Hypoxia flips the coin. J. Exp. Clin. Cancer Res. 2021, 40, 243. [Google Scholar] [CrossRef]
- Gonzalez-Meljem, J.M.; Apps, J.R.; Fraser, H.C.; Martinez-Barbera, J.P. Paracrine roles of cellular senescence in promoting tumourigenesis. Br. J. Cancer 2018, 118, 1283–1288. [Google Scholar] [CrossRef]
- Ito, Y.; Hoare, M.; Narita, M. Spatial and Temporal Control of Senescence. Trends Cell Biol. 2017, 27, 820–832. [Google Scholar] [CrossRef]
- Wagner, M.; Hampel, B.; Bernhard, D.; Hala, M.; Zwerschke, W.; Jansen-Dürr, P. Replicative senescence of human endothelial cells in vitro involves G1 arrest, polyploidization and senescence-associated apoptosis. Exp. Gerontol. 2001, 36, 1327–1347. [Google Scholar] [CrossRef]
- Lafargue, A.; Degorre, C.; Corre, I.; Alves-Guerra, M.C.; Gaugler, M.H.; Vallette, F.; Pecqueur, C.; Paris, F. Ionizing radiation induces long-term senescence in endothelial cells through mitochondrial respiratory complex II dysfunction and superoxide generation. Free Radic. Biol. Med. 2017, 108, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Nakae, I.; Fujita, M.; Miwa, K.; Hasegawa, K.; Kihara, Y.; Nohara, R.; Miyamoto, S.; Ueda, K.; Tamaki, S.I.; Sasayama, S. Age-dependent impairment of coronary collateral development in humans. Heart Vessels 2000, 15, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of vascular aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Baffert, F.; Thurston, G.; Rochon-Duck, M.; Le, T.; Brekken, R.; McDonald, D.M. Age-Related Changes in Vascular Endothelial Growth Factor Dependency and Angiopoietin-1-Induced Plasticity of Adult Blood Vessels. Circ. Res. 2004, 94, 984–992. [Google Scholar] [CrossRef]
- Lecce, L.; Lam, Y.T.; Lindsay, L.A.; Yuen, S.C.; Simpson, P.J.L.; Handelsman, D.J.; Ng, M.K.C. Aging Impairs VEGF-Mediated, Androgen-Dependent Regulation of Angiogenesis. Mol. Endocrinol. 2014, 28, 1487–1501. [Google Scholar] [CrossRef]
- Kohn, J.C.; Lampi, M.C.; Reinhart-King, C.A. Age-related vascular stiffening: Causes and consequences. Front. Genet. 2015, 6, 112. [Google Scholar] [CrossRef]
- Dessalles, C.A.; Leclech, C.; Castagnino, A.; Barakat, A.I. Integration of substrate- and flow-derived stresses in endothelial cell mechanobiology. Commun. Biol. 2021, 4, 764. [Google Scholar] [CrossRef]
- Bastounis, E.E.; Yeh, Y.T.; Theriot, J.A. Subendothelial stiffness alters endothelial cell traction force generation while exerting a minimal effect on the transcriptome. Sci. Rep. 2019, 9, 18209. [Google Scholar] [CrossRef]
- Spang, M.T.; Middleton, R.; Diaz, M.; Hunter, J.; Mesfin, J.; Banka, A.; Sullivan, H.; Wang, R.; Lazerson, T.S.; Bhatia, S.; et al. Intravascularly infused extracellular matrix as a biomaterial for targeting and treating inflamed tissues. Nat. Biomed. Eng. 2022, 7, 94–109. [Google Scholar] [CrossRef]
- Souilhol, C.; Serbanovic-Canic, J.; Fragiadaki, M.; Chico, T.J.; Ridger, V.; Roddie, H.; Evans, P.C. Endothelial responses to shear stress in atherosclerosis: A novel role for developmental genes. Nat. Rev. Cardiol. 2019, 17, 52–63. [Google Scholar] [CrossRef]
- White, C.R.; Haidekker, M.; Bao, X.; Frangos, J.A. Temporal Gradients in Shear, but Not Spatial Gradients, Stimulate Endothelial Cell Proliferation. Circulation 2001, 103, 2508–2513. [Google Scholar] [CrossRef]
- Lin, K.; Hsu, P.-P.; Chen, B.P.; Yuan, S.; Usami, S.; Shyy, J.Y.-J.; Li, Y.-S.; Chien, S. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc. Natl. Acad. Sci. USA 2000, 97, 9385–9389. [Google Scholar] [CrossRef]
- Warboys, C.M.; De Luca, A.; Amini, N.; Luong, L.; Duckles, H.; Hsiao, S.; White, A.; Biswas, S.; Khamis, R.; Chong, C.K.; et al. Disturbed flow promotes endothelial senescence via a p53-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 985–995. [Google Scholar] [CrossRef]
- Li, Y.S.J.; Haga, J.H.; Chien, S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 2005, 38, 1949–1971. [Google Scholar] [CrossRef]
- Weinbaum, S.; Tzeghai, G.; Ganatos, P. Effect of cell turnover and leaky junctions on arterial macromolecular transport. Am. J. Physiol. Circ. Physiol. 1985, 248, H945–H960. [Google Scholar] [CrossRef]
- Ye, M.; Sanchez, H.M.; Hultz, M.; Yang, Z.; Bogorad, M.; Wong, A.D.; Searson, P.C. Brain microvascular endothelial cells resist elongation due to curvature and shear stress. Sci. Rep. 2014, 4, 4681. [Google Scholar] [CrossRef]
- Ostrowski, M.A.; Huang, N.F.; Walker, T.W.; Verwijlen, T.; Poplawski, C.; Khoo, A.S.; Cooke, J.P.; Fuller, G.G.; Dunn, A.R. Microvascular Endothelial Cells Migrate Upstream and Align Against the Shear Stress Field Created by Impinging Flow. Biophys. J. 2014, 106, 366–374. [Google Scholar] [CrossRef]
- Dolan, J.M.; Meng, H.; Singh, S.; Paluch, R.; Kolega, J. High Fluid Shear Stress and Spatial Shear Stress Gradients Affect Endothelial Proliferation, Survival, and Alignment. Ann. Biomed. Eng. 2011, 39, 1620. [Google Scholar] [CrossRef]
- Kondrychyn, I.; Kelly, D.J.; Carretero, N.T.; Nomori, A.; Kato, K.; Chong, J.; Nakajima, H.; Okuda, S.; Mochizuki, N.; Phng, L.K. Marcksl1 modulates endothelial cell mechanoresponse to haemodynamic forces to control blood vessel shape and size. Nat. Commun. 2020, 11, 5476. [Google Scholar] [CrossRef]
- Chala, N.; Moimas, S.; Giampietro, C.; Zhang, X.; Zambelli, T.; Exarchos, V.; Nazari-Shafti, T.Z.; Poulikakos, D.; Ferrari, A. Mechanical Fingerprint of Senescence in Endothelial Cells. Nano Lett. 2021, 21, 4911–4920. [Google Scholar] [CrossRef]
- Cheng, C.; Van Haperen, R.; De Waard, M.; Van Damme, L.C.A.; Tempel, D.; Hanemaaijer, L.; Van Cappellen, G.W.A.; Bos, J.; Slager, C.J.; Duncker, D.J.; et al. Shear stress affects the intracellular distribution of eNOS: Direct demonstration by a novel in vivo technique. Blood 2005, 106, 3691–3698. [Google Scholar] [CrossRef]
- Sena, C.M.; Leandro, A.; Azul, L.; Seiça, R.; Perry, G. Vascular oxidative stress: Impact and therapeutic approaches. Front. Physiol. 2018, 9, 1668. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Matsui-Hirai, H.; Miyazaki-Akita, A.; Fukatsu, A.; Funami, J.; Ding, Q.F.; Kamalanathan, S.; Nation, Y.; Ignarro, L.J.; Iguchi, A. Endothelial cellular senescence is inhibited by nitric oxide: Implications in atherosclerosis associated with menopause and diabetes. Proc. Natl. Acad. Sci. USA 2006, 103, 17018–17023. [Google Scholar] [CrossRef] [PubMed]
- De Moudt, S.; Hendrickx, J.O.; De Meyer, G.R.Y.; Martinet, W.; Fransen, P. Basal Vascular Smooth Muscle Cell Tone in eNOS Knockout Mice Can Be Reversed by Cyclic Stretch and Is Independent of Age. Front. Physiol. 2022, 13, 882527. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Wang, G.; Moore, J.; Girouard, H.; Zhou, P.; Anrather, J.; Iadecola, C. The key role of transient receptor potential melastatin-2 channels in amyloid-β-induced neurovascular dysfunction. Nat. Commun. 2014, 5, 5318. [Google Scholar] [CrossRef]
- Sriram, K.; Laughlin, J.G.; Rangamani, P.; Tartakovsky, D.M. Shear-Induced Nitric Oxide Production by Endothelial Cells. Biophys. J. 2016, 111, 208–221. [Google Scholar] [CrossRef]
- Davis, M.E.; Grumbach, I.M.; Fukai, T.; Cutchins, A.; Harrison, D.G. Shear stress regulates endothelial nitric-oxide synthase promoter activity through nuclear factor kappaB binding. J. Biol. Chem. 2004, 279, 163–168. [Google Scholar] [CrossRef]
- Ghimire, K.; Zaric, J.; Alday-Parejo, B.; Seebach, J.; Bousquenaud, M.; Stalin, J.; Bieler, G.; Schnittler, H.J.; Rüegg, C. MAGI1 Mediates eNOS Activation and NO Production in Endothelial Cells in Response to Fluid Shear Stress. Cells 2019, 8, 388. [Google Scholar] [CrossRef]
- Welford, S.M.; Giaccia, A.J. Hypoxia and Senescence: The impact of oxygenation on tumor suppression. Mol. Cancer Res. 2011, 9, 538. [Google Scholar] [CrossRef]
- Donato, A.J.; Eskurza, I.; Silver, A.E.; Levy, A.S.; Pierce, G.L.; Gates, P.E.; Seals, D.R. Direct evidence of endothelial oxidative stress with aging in humans: Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ. Res. 2007, 100, 1659–1666. [Google Scholar] [CrossRef]
- Yang, W.; Hekimi, S. A Mitochondrial Superoxide Signal Triggers Increased Longevity in Caenorhabditis elegans. PLoS Biol. 2010, 8, 1000556. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tucsek, Z.; Sosnowska, D.; Toth, P.; Gautam, T.; Podlutsky, A.; Csiszar, A.; Losonczy, G.; Valcarcel-Ares, M.N.; Sonntag, W.E.; et al. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Kurz, D.J.; Decary, S.; Hong, Y.; Trivier, E.; Akhmedov, A.; Erusalimsky, J.D. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 2004, 117, 2417–2426. [Google Scholar] [CrossRef]
- Kirova, D.G.; Judasova, K.; Vorhauser, J.; Zerjatke, T.; Leung, J.K.; Glauche, I.; Mansfeld, J. A ROS-dependent mechanism promotes CDK2 phosphorylation to drive progression through S phase. Dev. Cell 2022, 57, 1712–1727.e9. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, S.; Samper, E.; Krtolica, A.; Goldstein, J.; Melov, S.; Campisi, J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 2003, 5, 741–747. [Google Scholar] [CrossRef]
- Saito, H.; Hammond, A.T.; Moses, R.E. The effect of low oxygen tension on the in vitro-replicative life span of human diploid fibroblast cells and their transformed derivatives. Exp. Cell Res. 1995, 217, 272–279. [Google Scholar] [CrossRef]
- Packer, L.; Fuehr, K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature 1977, 267, 423–425. [Google Scholar] [CrossRef]
- Epel, E.S. The geroscience agenda: Toxic stress, hormetic stress, and the rate of aging. Ageing Res. Rev. 2020, 63, 101167. [Google Scholar] [CrossRef]
- Franke, K.; Gassmann, M.; Wielockx, B. Erythrocytosis: The HIF pathway in control. Blood 2013, 122, 1122–1128. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 83, 83–92. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, H.; Zhang, M.; He, Y.; Li, X.; Xu, Y.; Liu, X. Hypoxia Induced Changes of Exosome Cargo and Subsequent Biological Effects. Front. Immunol. 2022, 13, 1140. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, Y.; Dong, L.; Zhang, Y.; Zhang, Y.; Wang, C.; Zhang, Q.; Yang, S.; Cao, L.; Zhang, X.; et al. Hypoxic exosomes facilitate angiogenesis and metastasis in esophageal squamous cell carcinoma through altering the phenotype and transcriptome of endothelial cells. J. Exp. Clin. Cancer Res. 2019, 38, 389. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, D.; Liu, N.; Xiong, W.; Huang, H.; Li, Y.; Ma, Z.; Zhao, H.; Chen, P.; Qi, X.; et al. Reciprocal Regulation between the Circadian Clock and Hypoxia Signaling at the Genome Level in Mammals. Cell Metab. 2017, 25, 73–85. [Google Scholar] [CrossRef]
- Sipilä, J.O.T.; Ruuskanen, J.O.; Rautava, P.; Kytö, V. Changes in ischemic stroke occurrence following daylight saving time transitions. Sleep Med. 2016, 27–28, 20–24. [Google Scholar] [CrossRef]
- Adamovich, Y.; Ladeuix, B.; Golik, M.; Koeners, M.P.; Asher, G. Rhythmic Oxygen Levels Reset Circadian Clocks through HIF1α. Cell Metab. 2017, 25, 93–101. [Google Scholar] [CrossRef]
- Tzoneva, R.; Georgieva, I.; Ivanova, N.; Uzunova, V.; Nenchovska, Z.; Apostolova, S.; Stoyanova, T.; Tchekalarova, J. The Role of Melatonin on Behavioral Changes and Concomitant Oxidative Stress in icvA β 1-42 Rat Model with Pinealectomy. Int. J. Mol. Sci. 2021, 22, 12763. [Google Scholar] [CrossRef]
- Guzy, R.D.; Hoyos, B.; Robin, E.; Chen, H.; Liu, L.; Mansfield, K.D.; Simon, M.C.; Hammerling, U.; Schumacker, P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005, 1, 401–408. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, J.; Miao, Y.; Zhang, Q. The effect of extracellular vesicles on the regulation of mitochondria under hypoxia. Cell Death Dis. 2021, 12, 358. [Google Scholar] [CrossRef]
- Semba, H.; Takeda, N.; Isagawa, T.; Sugiura, Y.; Honda, K.; Wake, M.; Miyazawa, H.; Yamaguchi, Y.; Miura, M.; Jenkins, D.M.R.; et al. HIF-1α-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat. Commun. 2016, 7, 11635. [Google Scholar] [CrossRef]
- Peek, C.B.; Levine, D.C.; Cedernaes, J.; Taguchi, A.; Kobayashi, Y.; Tsai, S.J.; Bonar, N.A.; McNulty, M.R.; Ramsey, K.M.; Bass, J. Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab. 2017, 25, 86–92. [Google Scholar] [CrossRef]
- Walton, Z.E.; Patel, C.H.; Brooks, R.C.; Yu, Y.; Ibrahim-Hashim, A.; Riddle, M.; Porcu, A.; Jiang, T.; Ecker, B.L.; Tameire, F.; et al. Acid Suspends the Circadian Clock in Hypoxia through Inhibition of mTOR. Cell 2018, 174, 72–87.e32. [Google Scholar] [CrossRef] [PubMed]
- Jazvinšćak Jembrek, M.; Hof, P.R.; Šimić, G. Ceramides in Alzheimer’s Disease: Key Mediators of Neuronal Apoptosis Induced by Oxidative Stress and A β Accumulation. Oxid. Med. Cell. Longev. 2015, 2015, 346783. [Google Scholar] [CrossRef] [PubMed]
- Leontieva, O.V.; Natarajan, V.; Demidenko, Z.N.; Burdelya, L.G.; Gudkov, A.V.; Blagosklonny, M.V. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc. Natl. Acad. Sci. USA 2012, 109, 13314–13318. [Google Scholar] [CrossRef] [PubMed]
- Khor, E.S.; Wong, P.F. The roles of MTOR and miRNAs in endothelial cell senescence. Biogerontology 2020, 21, 517–530. [Google Scholar] [CrossRef]
- van Vliet, T.; Varela-Eirin, M.; Wang, B.; Borghesan, M.; Brandenburg, S.M.; Franzin, R.; Evangelou, K.; Seelen, M.; Gorgoulis, V.; Demaria, M. Physiological hypoxia restrains the senescence-associated secretory phenotype via AMPK-mediated mTOR suppression. Mol. Cell 2021, 81, 2041–2052.e6. [Google Scholar] [CrossRef]
- Tramutola, A.; Triplett, J.C.; Di Domenico, F.; Niedowicz, D.M.; Murphy, M.P.; Coccia, R.; Perluigi, M.; Allan Butterfield, D. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): Analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J. Neurochem. 2015, 133, 739–749. [Google Scholar] [CrossRef]
- Klimova, T.A.; Bell, E.L.; Shroff, E.H.; Weinberg, F.D.; Snyder, C.M.; Dimri, G.P.; Schumacker, P.T.; Scott Budinger, G.R.; Chandel, N.S. Hyperoxia-induced premature senescence requires p53 and pRb, but not mitochondrial matrix ROS. FASEB J. 2009, 23, 783–794. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Conboy, 2005, Nature, Rejuvenecimento celular e nicho.pdf. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Villeda, S.A.; Plambeck, K.E.; Middeldorp, J.; Castellano, J.M.; Mosher, K.I.; Luo, J.; Smith, L.K.; Bieri, G.; Lin, K.; Berdnik, D.; et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014, 20, 659–663. [Google Scholar] [CrossRef]
- Jeon, O.H.; Mehdipour, M.; Gil, T.H.; Kang, M.; Aguirre, N.W.; Robinson, Z.R.; Kato, C.; Etienne, J.; Lee, H.G.; Alimirah, F.; et al. Systemic induction of senescence in young mice after single heterochronic blood exchange. Nat. Metab. 2022, 4, 995–1006. [Google Scholar] [CrossRef]
- Oh, C.; Koh, D.; Jeon, H.B.; Kim, K.M. The Role of Extracellular Vesicles in Senescence. Mol. Cells 2022, 45, 603–609. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Kuriyama, N.; Yoshioka, Y.; Kikuchi, S.; Azuma, N.; Ochiya, T. Extracellular Vesicles Are Key Regulators of Tumor Neovasculature. Front. Cell Dev. Biol. 2020, 8, 611039. [Google Scholar] [CrossRef]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C.; et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef]
- Riquelme, J.A.; Takov, K.; Santiago-Fernández, C.; Rossello, X.; Lavandero, S.; Yellon, D.M.; Davidson, S.M. Increased production of functional small extracellular vesicles in senescent endothelial cells. J. Cell. Mol. Med. 2020, 24, 4871–4876. [Google Scholar] [CrossRef]
- Choi, E.J.; Kil, I.S.; Cho, E.G. Extracellular Vesicles Derived from Senescent Fibroblasts Attenuate the Dermal Effect on Keratinocyte Differentiation. Int. J. Mol. Sci. 2020, 21, 1022. [Google Scholar] [CrossRef]
- Taraboletti, G.; D’Ascenzo, S.; Borsotti, P.; Giavazzi, R.; Pavan, A.; Dolo, V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 2002, 160, 673–680. [Google Scholar] [CrossRef]
- Alexander, R.A.; Prager, G.W.; Mihaly-Bison, J.; Uhrin, P.; Sunzenauer, S.; Binder, B.R.; Schütz, G.J.; Freissmuth, M.; Breuss, J.M. VEGF-induced endothelial cell migration requires urokinase receptor (uPAR)-dependent integrin redistribution. Cardiovasc. Res. 2012, 94, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Uhrin, P. VEGF-initiated angiogenesis and the uPA/uPAR system. Cell Adh. Migr. 2012, 6, 535. [Google Scholar] [CrossRef] [PubMed]
- Herkenne, S.; Paques, C.; Nivelles, O.; Lion, M.; Bajou, K.; Pollenus, T.; Fontaine, M.; Carmeliet, P.; Martial, J.A.; Nguyen, N.Q.N.; et al. The interaction of uPAR with VEGFR2 promotes VEGF-induced angiogenesis. Sci. Signal. 2015, 8, ra117. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Heier, P.C.; Mihaly-Bison, J.; Priglinger, U.; Binder, B.R.; Prager, G.W. Density enhanced phosphatase-1 down-regulates urokinase receptor surface expression in confluent endothelial cells. Blood 2011, 117, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.E.; Rai, R.; Khan, S.S.; Eren, M.; Ghosh, A.K. PAI-1 is a Marker and a Mediator of Senescence. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1446. [Google Scholar] [CrossRef]
- van Balkom, B.W.M.; de Jong, O.G.; Smits, M.; Brummelman, J.; den Ouden, K.; de Bree, P.M.; van Eijndhoven, M.A.J.; Pegtel, D.M.; Stoorvogel, W.; Würdinger, T.; et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 2013, 121, 3997–4006. [Google Scholar] [CrossRef]
- Ramakrishnan, D.P.; Hajj-Ali, R.A.; Chen, Y.; Silverstein, R.L. Extracellular vesicles activate a CD36-dependent signaling pathway to inhibit microvascular endothelial cell migration and tube formation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 534–544. [Google Scholar] [CrossRef]
- Mezentsev, A.; Merks, R.M.H.; O’Riordan, E.; Chen, J.; Mendelev, N.; Goligorsky, M.S.; Brodsky, S.V. Endothelial microparticles affect angiogenesis in vitro: Role of oxidative stress. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, 1106–1114. [Google Scholar] [CrossRef]
- Lu, Q.; Qin, H.; Tan, H.; Wei, C.; Yang, X.; He, J.; Liang, W.; Li, J. Senescence Osteoblast-Derived Exosome-Mediated miR-139-5p Regulates Endothelial Cell Functions. Biomed Res. Int. 2021, 2021, 5576023. [Google Scholar] [CrossRef]
- Wong, P.F.; Tong, K.L.; Jamal, J.; Khor, E.S.; Lai, S.L.; Mustafa, M.R. Senescent HUVECs-secreted exosomes trigger endothelial barrier dysfunction in young endothelial cells. Excli J. 2019, 18, 764–776. [Google Scholar] [CrossRef]
- Lacroix, R.; Sabatier, F.; Mialhe, A.; Basire, A.; Pannell, R.; Borghi, H.; Robert, S.; Lamy, E.; Plawinski, L.; Camoin-Jau, L.; et al. Activation of plasminogen into plasmin at the surface of endothelial microparticles: A mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood 2007, 110, 2432–2439. [Google Scholar] [CrossRef]
- Ou, Z.J.; Chang, F.J.; Luo, D.; Liao, X.L.; Wang, Z.P.; Zhang, X.; Xu, Y.Q.; Ou, J.S. Endothelium-derived microparticles inhibit angiogenesis in the heart and enhance the inhibitory effects of hypercholesterolemia on angiogenesis. Am. J. Physiol. Endocrinol. Metab. 2011, 300, 661–668. [Google Scholar] [CrossRef]
- Li, Y.; Bax, C.; Patel, J.; Vazquez, T.; Ravishankar, A.; Bashir, M.M.; Grinnell, M.; Diaz, D.; Werth, V.P. Plasma-derived DNA containing-extracellular vesicles induce STING-mediated proinflammatory responses in dermatomyositis. Theranostics 2021, 11, 7144–7158. [Google Scholar] [CrossRef]
- Yu, H.; Liao, K.; Hu, Y.; Lv, D.; Luo, M.; Liu, Q.; Huang, L.; Luo, S. Role of the cGAS-STING Pathway in Aging-related Endothelial Dysfunction. Aging Dis. 2022, 13, 1901. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, J.H.; Lee, S.W.; Ji, H.D.; Jung, J.A.; Yoon, K.W.; Lee, J.I.; Won, K.S.; Song, B.I.; Kim, H.W. The effect of chronic cerebral hypoperfusion on the pathology of Alzheimer’s disease: A positron emission tomography study in rats. Sci. Rep. 2019, 9, 14102. [Google Scholar] [CrossRef]
- Qiu, L.; Ng, G.; Tan, E.K.; Liao, P.; Kandiah, N.; Zeng, L. Chronic cerebral hypoperfusion enhances Tau hyperphosphorylation and reduces autophagy in Alzheimer’s disease mice. Sci. Rep. 2016, 6, 23964. [Google Scholar] [CrossRef]
- Babcock, K.R.; Page, J.S.; Fallon, J.R.; Webb, A.E. Adult hippocampal neurogenesis in aging and Alzheimer’s disease. Stem Cell Rep. 2021, 16, 681–693. [Google Scholar] [CrossRef]
- Cameron, D.J.; Galvin, C.; Alkam, T.; Sidhu, H.; Ellison, J.; Luna, S.; Ethell, D.W. Alzheimer’s-Related Peptide Amyloid-b Plays a Conserved Role in Angiogenesis. PLoS ONE 2012, 7, e39598. [Google Scholar] [CrossRef]
- Bennett, R.E.; Robbins, A.B.; Hu, M.; Cao, X.; Betensky, R.A.; Clark, T.; Das, S.; Hyman, B.T. Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E1289–E1298. [Google Scholar] [CrossRef]
- Alvarez-Vergara, M.I.; Rosales-Nieves, A.E.; March-Diaz, R.; Rodriguez-Perinan, G.; Lara-Ureña, N.; Ortega-de San Luis, C.; Sanchez-Garcia, M.A.; Martin-Bornez, M.; Gómez-Gálvez, P.; Vicente-Munuera, P.; et al. Non-productive angiogenesis disassembles Aß plaque-associated blood vessels. Nat. Commun. 2021, 12, 3098. [Google Scholar] [CrossRef]
- Tarkowski, E.; Issa, R.; Sjögren, M.; Wallin, A.; Blennow, K.; Tarkowski, A.; Kumar, P. Increased intrathecal levels of the angiogenic factors VEGF and TGF-β in Alzheimer’s disease and vascular dementia. Neurobiol. Aging 2002, 23, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Angom, R.S.; Wang, Y.; Wang, E.; Pal, K.; Bhattacharya, S.; Watzlawik, J.O.; Rosenberry, T.L.; Das, P.; Mukhopadhyay, D. VEGF receptor-1 modulates amyloid β 1-42 oligomer-induced senescence in brain endothelial cells. FASEB J. 2019, 33, 4626. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsova, E.; Klingner, M.; Sorger, D.; Sabri, O.; Großmann, U.; Steinbach, J.; Scheunemann, M.; Schliebs, R. Developmental and amyloid plaque-related changes in cerebral cortical capillaries in transgenic Tg2576 Alzheimer mice. Int. J. Dev. Neurosci. 2006, 24, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Religa, P.; Cao, R.; Religa, D.; Xue, Y.; Bogdanovic, N.; Westaway, D.; Marti, H.H.; Winblad, B.; Cao, Y. VEGF significantly restores impaired memory behavior in Alzheimer’s mice by improvement of vascular survival. Sci. Rep. 2013, 3, srep02053. [Google Scholar] [CrossRef]
- Jullienne, A.; Quan, R.; Szu, J.I.; Trinh, M.V.; Behringer, E.J.; Obenaus, A. Progressive Vascular Abnormalities in the Aging 3xTg-AD Mouse Model of Alzheimer’s Disease. Biomedicines 2022, 10, 1967. [Google Scholar] [CrossRef]
- Steinman, J.; Sun, H.S.; Feng, Z.P. Microvascular Alterations in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 14, 472. [Google Scholar] [CrossRef]
- Rahbarghazi, A.; Siahkouhian, M.; Rahbarghazi, R.; Ahmadi, M.; Bolboli, L.; Keyhanmanesh, R.; Mahdipour, M.; Rajabi, H. Role of melatonin in the angiogenesis potential; highlights on the cardiovascular disease. J. Inflamm. 2021, 18, 4. [Google Scholar] [CrossRef]
- Goradel, N.H.; Asghari, M.H.; Moloudizargari, M.; Negahdari, B.; Haghi-Aminjan, H.; Abdollahi, M. Melatonin as an angiogenesis inhibitor to combat cancer: Mechanistic evidence. Toxicol. Appl. Pharmacol. 2017, 335, 56–63. [Google Scholar] [CrossRef]
- Lobov, I.B.; Renard, R.A.; Papadopoulos, N.; Gale, N.W.; Thurston, G.; Yancopoulos, G.D.; Wiegand, S.J. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. USA 2007, 104, 3219–3224. [Google Scholar] [CrossRef]
- Das, A.; Huang, G.X.; Bonkowski, M.S.; Longchamp, A.; Li, C.; Schultz, M.B.; Kim, L.J.; Osborne, B.; Joshi, S.; Lu, Y.; et al. Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2018, 173, 74–89.e20. [Google Scholar] [CrossRef]
- Biron, K.E.; Dickstein, D.L.; Gopaul, R.; Fenninger, F.; Jefferies, W.A. Cessation of Neoangiogenesis in Alzheimer’s Disease Follows Amyloid-beta Immunization. Sci. Rep. 2013, 3, 1354. [Google Scholar] [CrossRef]
- Lau, S.; Cao, H.; Fu, A.K.Y.; Ip, N.Y. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2020, 117, 25800–25809. [Google Scholar] [CrossRef]
- Laulagnier, K.; Javalet, C.; Hemming, F.J.; Chivet, M.; Lachenal, G.; Blot, B.; Chatellard, C.; Sadoul, R. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell. Mol. Life Sci. 2018, 75, 757–773. [Google Scholar] [CrossRef]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjö, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41. [Google Scholar] [CrossRef]
- Vaz, M.; Martins, T.S.; Henriques, A.G. Extracellular vesicles in the study of Alzheimer’s and Parkinson’s diseases: Methodologies applied from cells to biofluids. J. Neurochem. 2022, 163, 266–309. [Google Scholar] [CrossRef]
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome determinants of physiological aging and age-related neurodegenerative diseases. Front. Aging Neurosci. 2019, 11, 232. [Google Scholar] [CrossRef]
- Aulston, B.; Liu, Q.; Mante, M.; Florio, J.; Rissman, R.A.; Yuan, S.H. Extracellular Vesicles Isolated from Familial Alzheimer’s Disease Neuronal Cultures Induce Aberrant Tau Phosphorylation in the Wild-Type Mouse Brain. J. Alzheimers. Dis. 2019, 72, 575–585. [Google Scholar] [CrossRef]
- Gallart-Palau, X.; Guo, X.; Serra, A.; Sze, S.K. Alzheimer’s disease progression characterized by alterations in the molecular profiles and biogenesis of brain extracellular vesicles. Alzheimer’s Res. Ther. 2020, 12, 54. [Google Scholar] [CrossRef]
- Falker, C.; Hartmann, A.; Guett, I.; Dohler, F.; Altmeppen, H.; Betzel, C.; Schubert, R.; Thurm, D.; Wegwitz, F.; Joshi, P.; et al. Exosomal cellular prion protein drives fibrillization of amyloid beta and counteracts amyloid beta-mediated neurotoxicity. J. Neurochem. 2016, 137, 88–100. [Google Scholar] [CrossRef]
- Gabrielli, M.; Tozzi, F.; Verderio, C.; Origlia, N. Emerging Roles of Extracellular Vesicles in Alzheimer’s Disease: Focus on Synaptic Dysfunction and Vesicle–Neuron Interaction. Cells 2022, 12, 63. [Google Scholar] [CrossRef]
- Lee, S.; Mankhong, S.; Kang, J.H. Extracellular Vesicle as a Source of Alzheimer’s Biomarkers: Opportunities and Challenges. Int. J. Mol. Sci. 2019, 20, 1728. [Google Scholar] [CrossRef]
- Quiroz-Baez, R.; Hernández-Ortega, K.; Martínez-Martínez, E. Insights Into the Proteomic Profiling of Extracellular Vesicles for the Identification of Early Biomarkers of Neurodegeneration. Front. Neurol. 2020, 11, 1636. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Palau, X.; Serra, A.; Hase, Y.; Tan, C.F.; Chen, C.P.; Kalaria, R.N.; Sze, S.K. Brain-derived and circulating vesicle profiles indicate neurovascular unit dysfunction in early Alzheimer’s disease. Brain Pathol. 2019, 29, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Sagare, A.P.; Bell, R.D.; Zlokovic, B.V. Neurovascular Dysfunction and Faulty Amyloid β-Peptide Clearance in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a011452. [Google Scholar] [CrossRef] [PubMed]
- Custodia, A.; Ouro, A.; Romaus-Sanjurjo, D.; Pías-Peleteiro, J.M.; de Vries, H.E.; Castillo, J.; Sobrino, T. Endothelial Progenitor Cells and Vascular Alterations in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 13, 811210. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Yoder, M.C. Human endothelial progenitor cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006692. [Google Scholar] [CrossRef]
- Bauman, E.; Granja, P.L.; Barrias, C.C. Fetal bovine serum-free culture of endothelial progenitor cells-progress and challenges. J. Tissue Eng. Regen. Med. 2018, 12, 1567–1578. [Google Scholar] [CrossRef]
- Hur, J.; Yoon, C.H.; Kim, H.S.; Choi, J.H.; Kang, H.J.; Hwang, K.K.; Oh, B.H.; Lee, M.M.; Park, Y.B. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 288–293. [Google Scholar] [CrossRef]
- Lee, S.T.; Chu, K.; Jung, K.H.; Park, H.K.; Kim, D.H.; Bahn, J.J.; Kim, J.H.; Oh, M.J.; Lee, S.K.; Kim, M.; et al. Reduced circulating angiogenic cells in Alzheimer disease. Neurology 2009, 72, 1858–1863. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Y.; Liu, L.; Sun, N.; Zhang, M.; Zhang, J. Endothelial progenitor cells with Alzheimer’s disease. Chin. Med. J. 2011, 124, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, X.; Liu, M.; Wei, L.; Gu, X.; Song, H.; Chen, Q.; Xu, J. Study of quantity and function of endothelial progenitor cells in peripheral blood of patients with Alzheimer’s disease. J. New Med. 2020, 51, 590. [Google Scholar] [CrossRef]
- Zhang, S.; Zhi, Y.; Li, F.; Huang, S.; Gao, H.; Han, Z.; Ge, X.; Li, D.; Chen, F.; Kong, X.; et al. Transplantation of in vitro cultured endothelial progenitor cells repairs the blood-brain barrier and improves cognitive function of APP/PS1 transgenic AD mice. J. Neurol. Sci. 2018, 387, 6–15. [Google Scholar] [CrossRef]
- Heller, L.; Thinard, R.; Chevalier, M.; Arpag, S.; Jing, Y.; Greferath, R.; Heller, R.; Nicolau, C. Secretion of proteins and antibody fragments from transiently transfected endothelial progenitor cells. J. Cell. Mol. Med. 2020, 24, 8772–8778. [Google Scholar] [CrossRef] [PubMed]
- Viboolvorakul, S.; Patumraj, S. Exercise training could improve age-related changes in cerebral blood flow and capillary vascularity through the upregulation of VEGF and eNOS. Biomed Res. Int. 2014, 2014, 230791. [Google Scholar] [CrossRef]
- Soto, I.; Graham, L.C.; Richter, H.J.; Simeone, S.N.; Radell, J.E.; Grabowska, W. APOE Stabilization by Exercise Prevents Aging Neurovascular Dysfunction and Complement Induction. PLoS Biol. 2015, 13, 1002279. [Google Scholar] [CrossRef]
- Archer, T. Physical exercise alleviates debilities of normal aging and Alzheimer Õ s disease. Acta Neurol. Scand. 2011, 123, 221–238. [Google Scholar] [CrossRef]
- Morland, C.; Andersson, K.A.; Haugen, Ø.P.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef]
- Ding, Y.; Li, J.; Zhou, Y.; Rafols, J.A.; Clark, J.C.; Ding, Y. Cerebral Angiogenesis and Expression of Angiogenic Factors in Aging Rats after Exercise. Curr. Neurovascular Res. 2006, 3, 15–23. [Google Scholar] [CrossRef]
- Frühbeis, C.; Helmig, S.; Tug, S.; Simon, P.; Krämer-Albers, E.M. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J. Extracell. Vesicles 2015, 4, 28239. [Google Scholar] [CrossRef]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Möbius, W.; Simon, P.; Krämer-Albers, E.M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell. Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A.; Mccarthy, J.J. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2021, 11, 604274. [Google Scholar] [CrossRef]
- Boerman, E.M.; Everhart, J.E.; Segal, S.S. Advanced age decreases local calcium signaling in endothelium of mouse mesenteric arteries in vivo. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H1091–H1096. [Google Scholar] [CrossRef]
- Kumar, V.B.S.; Viji, R.I.; Kiran, M.S.; Sudhakaran, P.R. Endothelial cell response to lactate: Implication of PAR modification of VEGF. J. Cell. Physiol. 2007, 211, 477–485. [Google Scholar] [CrossRef]
- Porporato, P.E.; Payen, V.L.; De Saedeleer, C.J.; Préat, V.; Thissen, J.P.; Feron, O.; Sonveaux, P. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis 2012, 15, 581–592. [Google Scholar] [CrossRef]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Fasting and cancer: Molecular mechanisms and clinical application. Nat. Rev. Cancer 2018, 18, 707–719. [Google Scholar] [CrossRef]
- Ewald, C.Y.; Castillo-Quan, J.I.; Blackwell, T.K. Untangling longevity, dauer, and healthspan in Caenorhabditis elegans insulin/IGF-1-signalling. Gerontology 2018, 64, 96. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; McMahon, A.C.; Ballard, J.W.O.; Ruohonen, K.; Wu, L.E.; Cogger, V.C.; Warren, A.; Huang, X.; Pichaud, N.; Melvin, R.G.; et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014, 19, 418–430. [Google Scholar] [CrossRef]
- Rajapakse, A.G.; Yepuri, G.; Carvas, J.M.; Stein, S.; Matter, C.M.; Scerri, I.; Ruffieux, J.; Montani, J.P.; Ming, X.F.; Yang, Z. Hyperactive S6K1 Mediates Oxidative Stress and Endothelial Dysfunction in Aging: Inhibition by Resveratrol. PLoS ONE 2011, 6, e19237. [Google Scholar] [CrossRef]
- Donato, A.J.; Walker, A.E.; Magerko, K.A.; Bramwell, R.C.; Black, A.D.; Henson, G.D.; Lawson, B.R.; Lesniewski, L.A.; Seals, D.R. Life-Long Caloric Restriction Reduces Oxidative Stress and Preserves Nitric Oxide Bioavailability and Function in Arteries of Old Mice. Aging Cell 2013, 12, 772. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Seals, D.R. Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J. Physiol. 2016, 594, 7177–7195. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Shi, Y.; Zhang, C.; Zheng, Y.; Wang, Y.; Zhao, B.; Zeng, Y.; Liu, X.; Liu, J. Antioxidants inhibit cell senescence and preserve stemness of adipose tissue-derived stem cells by reducing ROS generation during long-term in vitro expansion. Stem Cell Res. Ther. 2019, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Sovernigo, T.C.; Adona, P.R.; Monzani, P.S.; Guemra, S.; Barros, F.D.A.; Lopes, F.G.; Leal, C.L.V. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod. Domest. Anim. 2017, 52, 561–569. [Google Scholar] [CrossRef]
- Guillot, E.; Lemay, A.; Allouche, M.; Vitorino Silva, S.; Coppola, H.; Sabatier, F.; Dignat-George, F.; Sarre, A.; Peyter, A.C.; Simoncini, S.; et al. Resveratrol Reverses Endothelial Colony-Forming Cell Dysfunction in Adulthood in a Rat Model of Intrauterine Growth Restriction. Int. J. Mol. Sci. 2023, 24, 9747. [Google Scholar] [CrossRef]
| Type of WSS | Cell Line | Cell Response | Reference |
|---|---|---|---|
| Sudden, temporal, 10 dyne/cm2 | HUVECs | ↑ proliferation | [74] |
| Steady, uniform | HUVECs | no effect | [74] |
| Linear, physiological, 12 dyne/cm2 * | BAECs | ↓ proliferation | [75] |
| Gradient, <68 dyne/cm2 | HMVECs | migrate against flow; orient perpendicularly at highest WSS | [80] |
| Linear, high, 284 dyne/cm2 Gradient, positive, 150–170 dyne/cm2 | BAECs ** | ↓ alignment; ↑ proliferation; ↑ apoptosis | [81] |
| Linear, low, 30 dyne/cm2 Gradient, negative, 170–150 dyne/cm2 | BAECs | ↑ alignment; ↓ proliferation; ↓ apoptosis | [81] |
| AD Model | Blood Vessels | Protein Expression | References |
|---|---|---|---|
| Aβ monomers in HUVEC and zebrafish | ↑ capillary density | - | [151] |
| Tau overexpressing mice; 15 months old | ↑ capillary density; ↑ angiogenesis; ↑ BBB permeability; ↓ CBF | ↑ VEGF; ↑ uPAR; ↑ MMP-9; ↑ PAI-1 | [152] |
| AD patients | − | ↑ VEGF; ↑ TGF-β | [153] |
| HMVECs + Aβ monomers | ↓ angiogenesis | ↑ VEGFR1- ↑ senescence | [154] |
| APP-PSEN1/+ mice | ↑ non-productive angiogenesis; ↓ capillary density around plaques | ↑ VEGF | [155] |
| Tg2576 mice | ↓ capillary density around plaques | ↓ GLUT1 | [156] |
| AD patients; APP695 mice | ↓ capillary density | VEGF supplementation improved cognitive function | [157] |
| 3xTG-AD mice | ↑ capillary density; ↓ junction density | − | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, I.; Tchekalarova, J.; Iliev, D.; Tzoneva, R. Endothelial Senescence and Its Impact on Angiogenesis in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 11344. https://doi.org/10.3390/ijms241411344
Georgieva I, Tchekalarova J, Iliev D, Tzoneva R. Endothelial Senescence and Its Impact on Angiogenesis in Alzheimer’s Disease. International Journal of Molecular Sciences. 2023; 24(14):11344. https://doi.org/10.3390/ijms241411344
Chicago/Turabian StyleGeorgieva, Irina, Jana Tchekalarova, Dimitar Iliev, and Rumiana Tzoneva. 2023. "Endothelial Senescence and Its Impact on Angiogenesis in Alzheimer’s Disease" International Journal of Molecular Sciences 24, no. 14: 11344. https://doi.org/10.3390/ijms241411344
APA StyleGeorgieva, I., Tchekalarova, J., Iliev, D., & Tzoneva, R. (2023). Endothelial Senescence and Its Impact on Angiogenesis in Alzheimer’s Disease. International Journal of Molecular Sciences, 24(14), 11344. https://doi.org/10.3390/ijms241411344








