Derivation of Human Extraembryonic Mesoderm-like Cells from Primitive Endoderm
Abstract
1. Introduction
2. Results
2.1. Evolutionary and Molecular Characterization of Human ExM
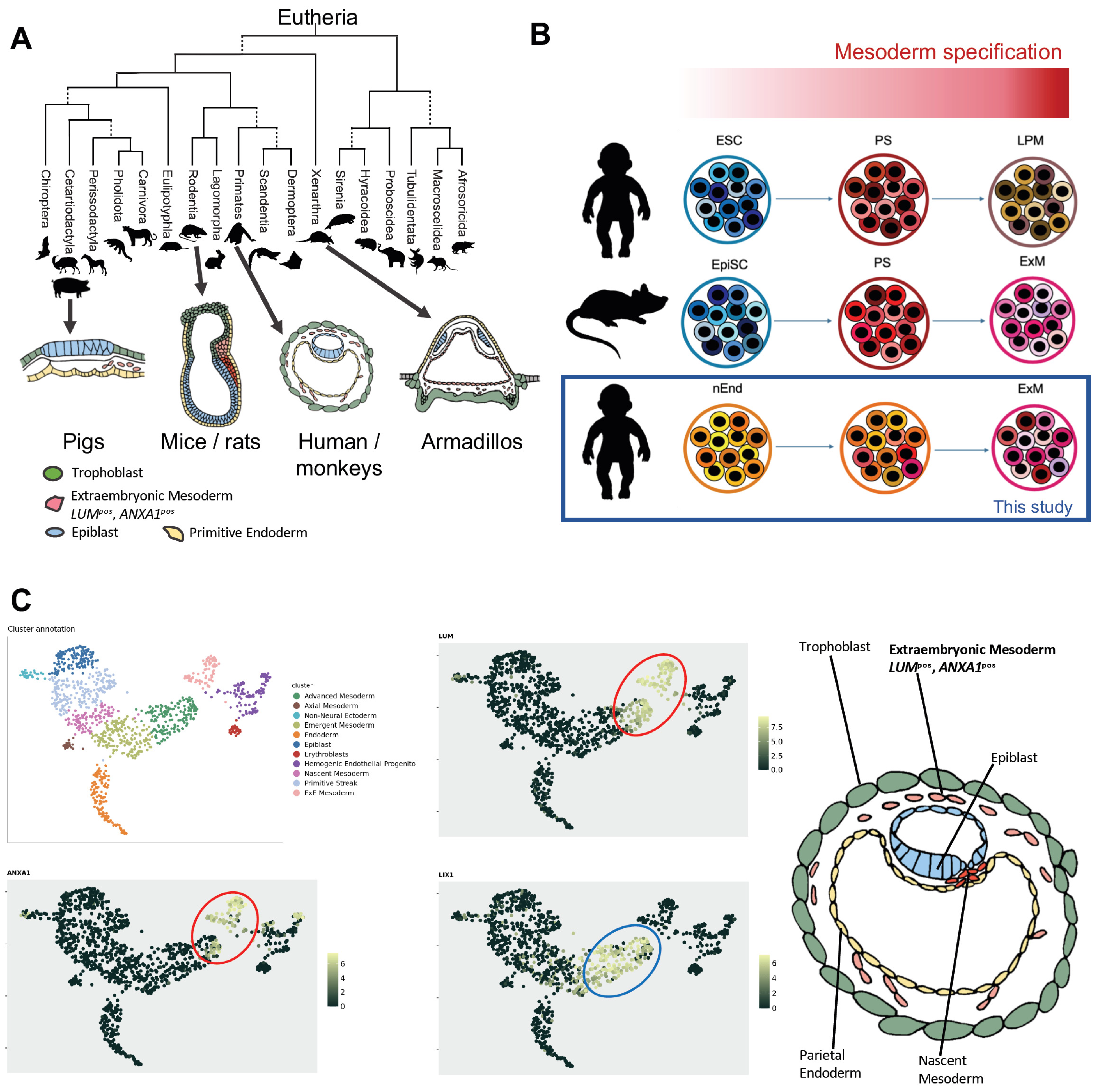
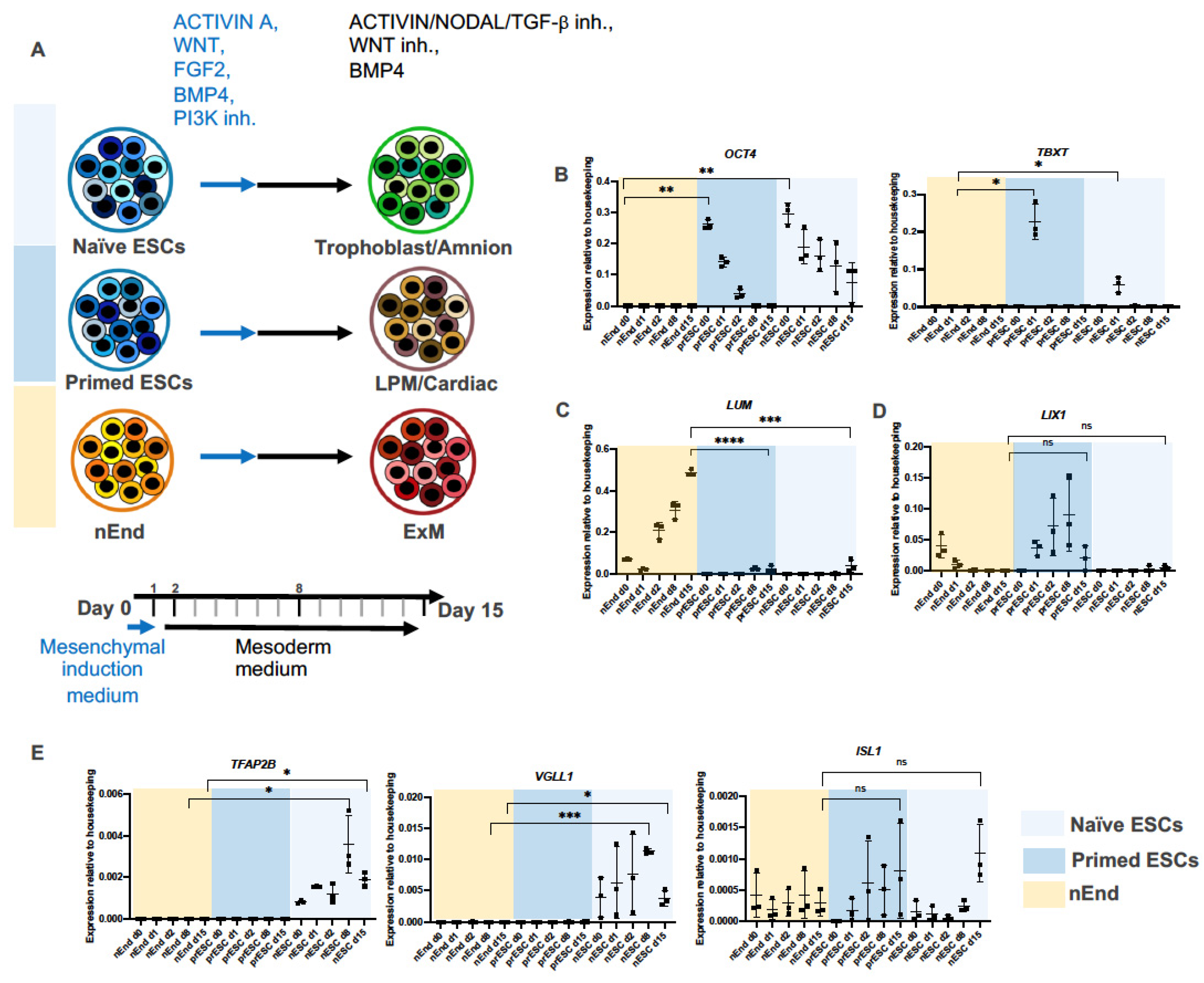
2.2. ExM Genes Can Be Efficiently Induced from nEnd Cells by Mesoderm Induction Factors
2.3. Bulk RNAseq Reveals Gene Expression Pattern Corresponding to Gradual ExM Specification
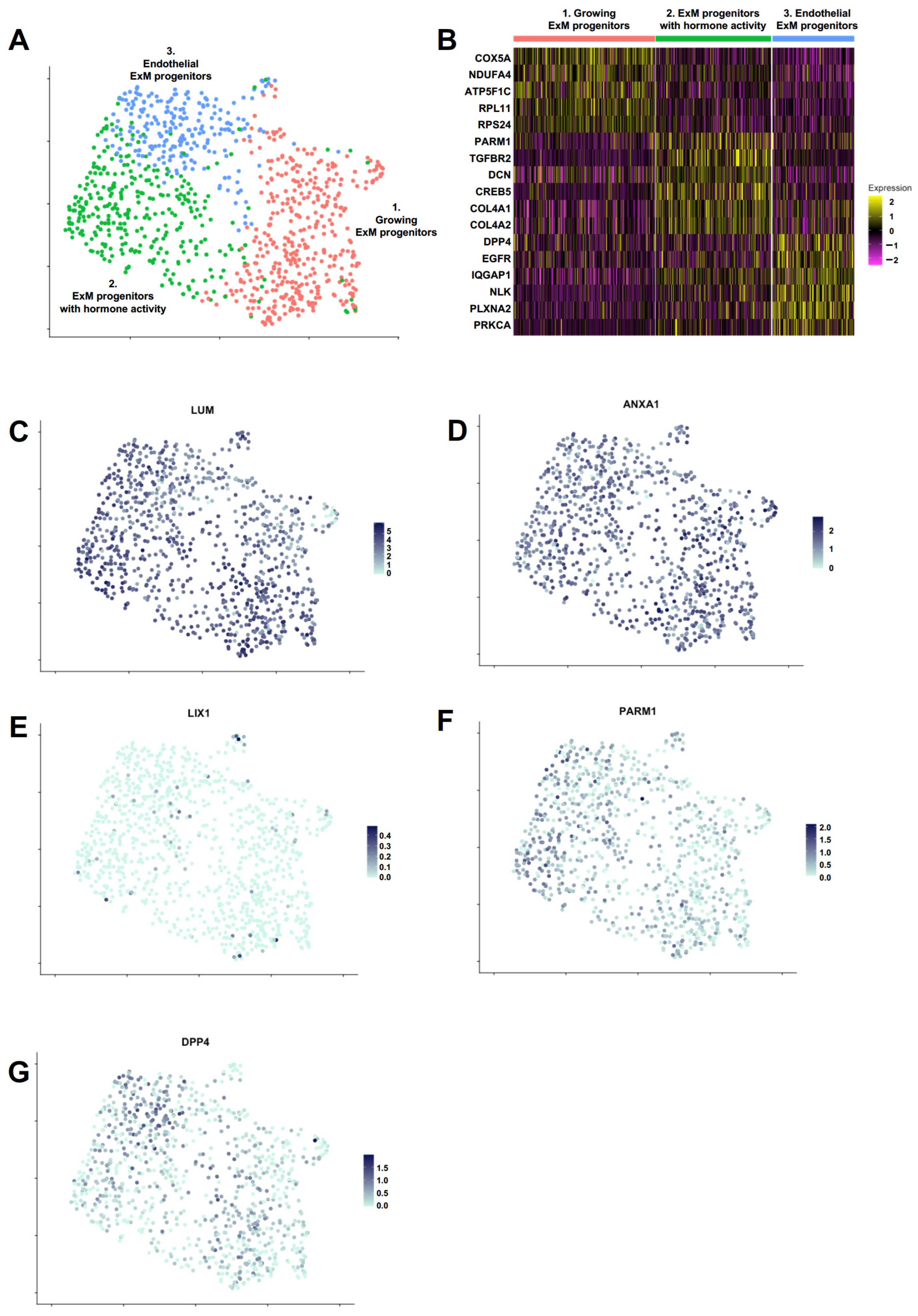
2.4. Single-Cell RNA Sequencing Reveals Distinct Cell Populations within PrxM
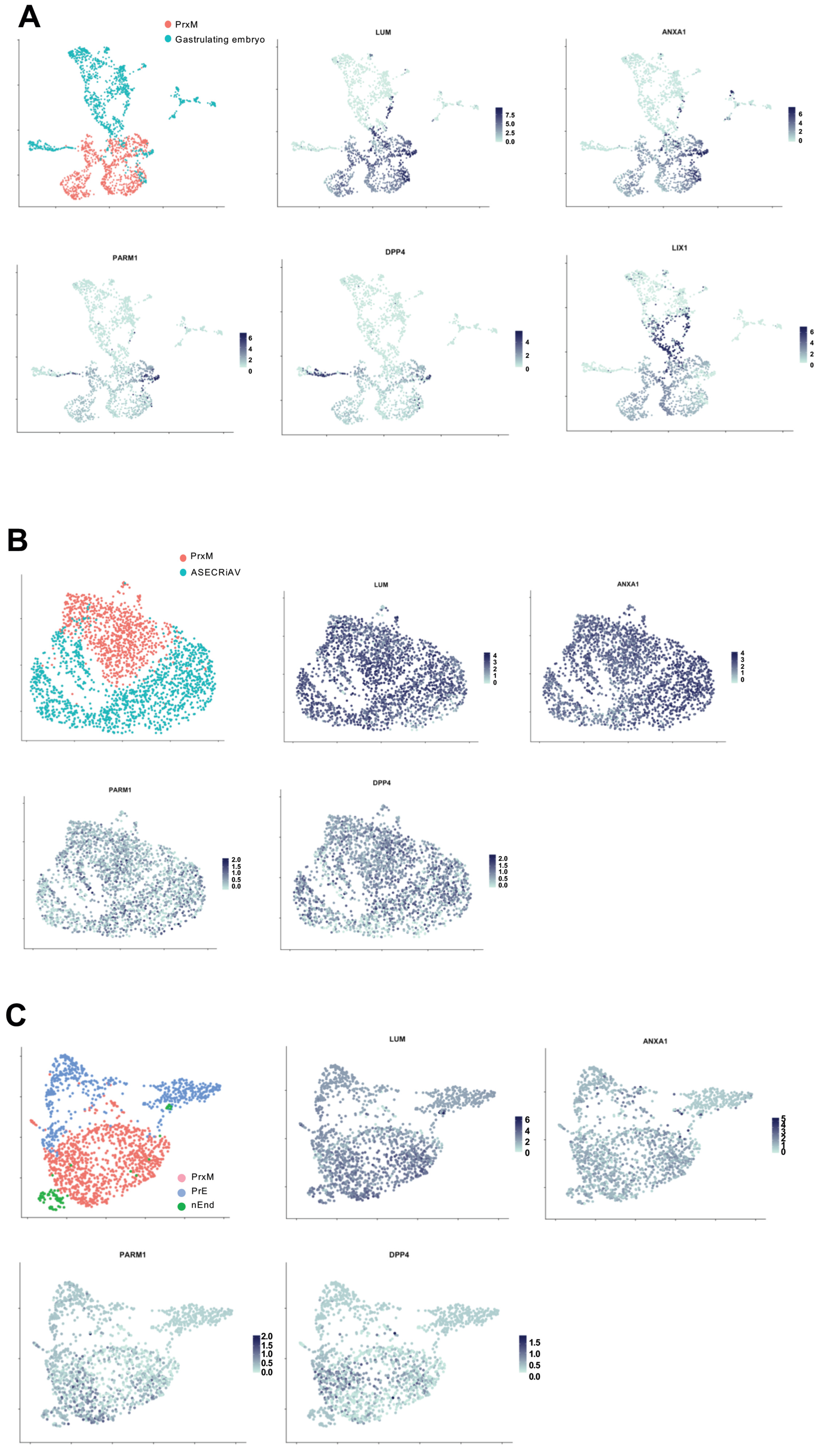
2.5. PrxM Shares Cell Populations with Gastrulating Human Embryo
2.6. PrxM Shows Angiogenesis Capacity on Soft Matrix
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Differentiation
4.3. RNA Extraction
4.4. RT-qPCR
4.5. Live-Cell Imaging of Beating Mesoderm
4.6. Bulk RNAseq
4.7. Single-Cell RNAseq
4.8. Angiogenesis Assay
4.9. GO Term Analyses
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jauniaux, E.; Burton, G.J. Pathophysiology of histological changes in early pregnancy loss. Placenta 2005, 26, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J.; Tam, P.P.L. Early human embryonic development: Blastocyst formation to gastrulation. Dev. Cell 2022, 57, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Avila-Gonzalez, D.; Portillo, W.; Garcia-Lopez, G.; Molina-Hernandez, A.; Diaz-Martinez, N.E.; Diaz, N.F. Unraveling the Spatiotemporal Human Pluripotency in Embryonic Development. Front. Cell Dev. Biol. 2021, 9, 676998. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Boroviak, T.E. Origin and function of the yolk sac in primate embryogenesis. Nat. Commun. 2020, 11, 3760. [Google Scholar] [CrossRef] [PubMed]
- Palis, J.; Robertson, S.; Kennedy, M.; Wall, C.; Keller, G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 1999, 126, 5073–5084. [Google Scholar] [CrossRef] [PubMed]
- Palis, J.; Yoder, M.C. Yolk-sac hematopoiesis: The first blood cells of mouse and man. Exp. Hematol. 2001, 29, 927–936. [Google Scholar] [CrossRef]
- Muench, M.O.; Kapidzic, M.; Gormley, M.; Gutierrez, A.G.; Ponder, K.L.; Fomin, M.E.; Beyer, A.I.; Stolp, H.; Qi, Z.; Fisher, S.J.; et al. The human chorion contains definitive hematopoietic stem cells from the fifteenth week of gestation. Development 2017, 144, 1399–1411. [Google Scholar]
- Meraviglia, V.; Vecellio, M.; Grasselli, A.; Baccarin, M.; Farsetti, A.; Capogrossi, M.C.; Pompilio, G.; Coviello, D.A.; Gaetano, C.; Di Segni, M.; et al. Human chorionic villus mesenchymal stromal cells reveal strong endothelial conversion properties. Differentiation 2012, 83, 260–270. [Google Scholar] [CrossRef]
- Carter, A.M. Unique Aspects of Human Placentation. Int. J. Mol. Sci. 2021, 22, 8099. [Google Scholar] [CrossRef]
- Pijuan-Sala, B.; Griffiths, J.A.; Guibentif, C.; Hiscock, T.W.; Jawaid, W.; Calero-Nieto, F.J.; Mulas, C.; Ibarra-Soria, X.; Tyser, R.C.V.; Ho, D.L.L.; et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature 2019, 566, 490–495. [Google Scholar] [CrossRef]
- Tam, P.P.; Loebel, D.A. Gene function in mouse embryogenesis: Get set for gastrulation. Nat. Rev. Genet. 2007, 8, 368–381. [Google Scholar] [CrossRef]
- Cavaliere, G. A 14-day limit for bioethics: The debate over human embryo research. BMC Med. Ethics 2017, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Appleby, J.B.; Bredenoord, A.L. Should the 14-day rule for embryo research become the 28-day rule? EMBO Mol. Med. 2018, 10, e9437. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Heemskerk, I.; Gumucio, D.L. Opening the black box: Stem cell-based modeling of human post-implantation development. J. Cell Biol. 2019, 218, 410–421. [Google Scholar] [CrossRef]
- Zhai, J.; Xiao, Z.; Wang, Y.; Wang, H. Human embryonic development: From peri-implantation to gastrulation. Trends Cell Biol. 2022, 32, 18–29. [Google Scholar] [CrossRef]
- Shahbazi, M.N.; Jedrusik, A.; Vuoristo, S.; Recher, G.; Hupalowska, A.; Bolton, V.; Fogarty, N.N.M.; Campbell, A.; Devito, L.; Ilic, D.; et al. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 2016, 18, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Deglincerti, A.; Croft, G.F.; Pietila, L.N.; Zernicka-Goetz, M.; Siggia, E.D.; Brivanlou, A.H. Self-organization of the in vitro attached human embryo. Nature 2016, 533, 251–254. [Google Scholar] [CrossRef]
- Chapman, M.S.; Ranzoni, A.M.; Myers, B.; Williams, N.; Coorens, T.H.H.; Mitchell, E.; Butler, T.; Dawson, K.J.; Hooks, Y.; Moore, L.; et al. Lineage tracing of human development through somatic mutations. Nature 2021, 595, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Tyser, R.C.V.; Mahammadov, E.; Nakanoh, S.; Vallier, L.; Scialdone, A.; Srinivas, S. Single-cell transcriptomic characterization of a gastrulating human embryo. Nature 2021, 600, 285–289. [Google Scholar] [CrossRef]
- Warmflash, A.; Sorre, B.; Etoc, F.; Siggia, E.D.; Brivanlou, A.H. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods 2014, 11, 847–854. [Google Scholar] [CrossRef]
- Rivron, N.C.; Frias-Aldeguer, J.; Vrij, E.J.; Boisset, J.C.; Korving, J.; Vivie, J.; Truckenmuller, R.K.; van Oudenaarden, A.; van Blitterswijk, C.A.; Geijsen, N. Blastocyst-like structures generated solely from stem cells. Nature 2018, 557, 106–111. [Google Scholar] [CrossRef]
- Moris, N.; Anlas, K.; van den Brink, S.C.; Alemany, A.; Schroder, J.; Ghimire, S.; Balayo, T.; van Oudenaarden, A.; Martinez Arias, A. An in vitro model of early anteroposterior organization during human development. Nature 2020, 582, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, S.C.; Baillie-Johnson, P.; Balayo, T.; Hadjantonakis, A.K.; Nowotschin, S.; Turner, D.A.; Martinez Arias, A. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development 2014, 141, 4231–4242. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, H.; Javali, A.; Khoei, H.H.; Sommer, T.M.; Sestini, G.; Novatchkova, M.; Scholte Op Reimer, Y.; Castel, G.; Bruneau, A.; Maenhoudt, N.; et al. Human blastoids model blastocyst development and implantation. Nature 2022, 601, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.; Siggia, E.D.; Brivanlou, A.H. In vitro attachment and symmetry breaking of a human embryo model assembled from primed embryonic stem cells. Cell Stem Cell 2022, 29, 962–972.e4. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, S. Ultrastructure in human implantation: Transmission and scanning electron microscopy. Baillieres Clin. Obstet. Gynaecol. 1991, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.C.; King, B.F. Formation and differentiation of extraembryonic mesoderm in the rhesus monkey. Am. J. Anat. 1988, 181, 327–340. [Google Scholar] [CrossRef]
- Linneberg-Agerholm, M.; Wong, Y.F.; Romero Herrera, J.A.; Monteiro, R.S.; Anderson, K.G.V.; Brickman, J.M. Naive human pluripotent stem cells respond to Wnt, Nodal and LIF signalling to produce expandable naive extra-embryonic endoderm. Development 2019, 146, dev180620. [Google Scholar] [CrossRef]
- Valadão, L.M.D.S.H.; Moreira da Silva, F. Bovine Embryonic Development to Implantation; IntechOpen: London, UK, 2018. [Google Scholar]
- Guillomot, M.; Turbe, A.; Hue, I.; Renard, J.P. Staging of ovine embryos and expression of the T-box genes Brachyury and Eomesodermin around gastrulation. Reproduction 2004, 127, 491–501. [Google Scholar] [CrossRef]
- Tveden-Nyborg, P.; Peura, T.T.; Hartwich, K.M.; Walker, S.K.; Maddox-Hyttel, P. Morphological characterization of pre- and peri-implantation in vitro cultured, somatic cell nuclear transfer and in vivo derived ovine embryos. Reproduction 2005, 130, 681–694. [Google Scholar] [CrossRef]
- Flechon, J.E.; Degrouard, J.; Flechon, B. Gastrulation events in the prestreak pig embryo: Ultrastructure and cell markers. Genesis 2004, 38, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Gaivao, M.M.; Rambags, B.P.; Stout, T.A. Gastrulation and the establishment of the three germ layers in the early horse conceptus. Theriogenology 2014, 82, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.C. Implantation in the nine-banded armadillo: How does a single blastocyst form four embryos? Placenta 2002, 23, 71–85. [Google Scholar] [CrossRef]
- Mac Auley, A.; Werb, Z.; Mirkes, P.E. Characterization of the unusually rapid cell cycles during rat gastrulation. Development 1993, 117, 873–883. [Google Scholar] [CrossRef]
- Viebahn, C.; Stortz, C.; Mitchell, S.A.; Blum, M. Low proliferative and high migratory activity in the area of Brachyury expressing mesoderm progenitor cells in the gastrulating rabbit embryo. Development 2002, 129, 2355–2365. [Google Scholar] [CrossRef]
- Richter, A.; Valdimarsdottir, L.; Hrafnkelsdottir, H.E.; Runarsson, J.F.; Omarsdottir, A.R.; Ward-van Oostwaard, D.; Mummery, C.; Valdimarsdottir, G. BMP4 promotes EMT and mesodermal commitment in human embryonic stem cells via SLUG and MSX2. Stem Cells 2014, 32, 636–648. [Google Scholar] [CrossRef]
- Loh, K.M.; Chen, A.; Koh, P.W.; Deng, T.Z.; Sinha, R.; Tsai, J.M.; Barkal, A.A.; Shen, K.Y.; Jain, R.; Morganti, R.M.; et al. Mapping the Pairwise Choices Leading from Pluripotency to Human Bone, Heart, and Other Mesoderm Cell Types. Cell 2016, 166, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Drozd, A.M.; Mariani, L.; Guo, X.; Goitea, V.; Menezes, N.A.; Ferretti, E. Progesterone Receptor Modulates Extraembryonic Mesoderm and Cardiac Progenitor Specification during Mouse Gastrulation. Int. J. Mol. Sci. 2022, 23, 10307. [Google Scholar] [CrossRef]
- Grover, J.; Chen, X.N.; Korenberg, J.R.; Roughley, P.J. The human lumican gene. Organization, chromosomal location, and expression in articular cartilage. J. Biol. Chem. 1995, 270, 21942–21949. [Google Scholar] [CrossRef]
- Liu, H.; He, J.; Wu, Y.; Du, Y.; Jiang, Y.; Chen, C.; Yu, Z.; Zhong, J.; Wang, Z.; Cheng, C.; et al. Endothelial Regulation by Exogenous Annexin A1 in Inflammatory Response and BBB Integrity Following Traumatic Brain Injury. Front. Neurosci. 2021, 15, 627110. [Google Scholar] [CrossRef] [PubMed]
- Han, P.F.; Che, X.D.; Li, H.Z.; Gao, Y.Y.; Wei, X.C.; Li, P.C. Annexin A1 involved in the regulation of inflammation and cell signaling pathways. Chin. J. Traumatol. 2020, 23, 96–101. [Google Scholar] [CrossRef]
- Swindell, E.C.; Moeller, C.; Thaller, C.; Eichele, G. Cloning and expression analysis of chicken Lix1, a founding member of a novel gene family. Mech. Dev. 2001, 109, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.S.; Stanhope, M.J.; Madsen, O.; de Jong, W.W. Molecules consolidate the placental mammal tree. Trends Ecol. Evol. 2004, 19, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Boroviak, T.; Nichols, J. Primate embryogenesis predicts the hallmarks of human naive pluripotency. Development 2017, 144, 175–186. [Google Scholar] [CrossRef]
- Huang, X.; Park, K.M.; Gontarz, P.; Zhang, B.; Pan, J.; McKenzie, Z.; Fischer, L.A.; Dong, C.; Dietmann, S.; Xing, X.; et al. OCT4 cooperates with distinct ATP-dependent chromatin remodelers in naive and primed pluripotent states in human. Nat. Commun. 2021, 12, 5123. [Google Scholar] [CrossRef]
- Theunissen, T.W.; Powell, B.E.; Wang, H.; Mitalipova, M.; Faddah, D.A.; Reddy, J.; Fan, Z.P.; Maetzel, D.; Ganz, K.; Shi, L.; et al. Systematic Identification of Culture Conditions for Induction and Maintenance of Naive Human Pluripotency. Cell Stem Cell 2014, 15, 524–526. [Google Scholar] [CrossRef]
- Shao, Y.; Taniguchi, K.; Gurdziel, K.; Townshend, R.F.; Xue, X.; Yong, K.M.A.; Sang, J.; Spence, J.R.; Gumucio, D.L.; Fu, J. Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat. Mater 2017, 16, 419–425. [Google Scholar] [CrossRef]
- Soncin, F.; Khater, M.; To, C.; Pizzo, D.; Farah, O.; Wakeland, A.; Arul Nambi Rajan, K.; Nelson, K.K.; Chang, C.W.; Moretto-Zita, M.; et al. Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development. Development 2018, 145, dev156273. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Goedel, A.; Kang, Y.; Si, C.; Chu, C.; Zheng, Y.; Chen, Z.; Gruber, P.J.; Xiao, Y.; Zhou, C.; et al. Amnion signals are essential for mesoderm formation in primates. Nat. Commun. 2021, 12, 5126. [Google Scholar] [CrossRef]
- Rostovskaya, M.; Andrews, S.; Reik, W.; Rugg-Gunn, P.J. Amniogenesis occurs in two independent waves in primates. Cell Stem Cell 2022, 29, 744–759.e6. [Google Scholar] [CrossRef]
- Chien, M.H.; Lin, Y.W.; Wen, Y.C.; Yang, Y.C.; Hsiao, M.; Chang, J.L.; Huang, H.C.; Lee, W.J. Targeting the SPOCK1-snail/slug axis-mediated epithelial-to-mesenchymal transition by apigenin contributes to repression of prostate cancer metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 246. [Google Scholar] [CrossRef]
- Alshargabi, R.; Sano, T.; Yamashita, A.; Takano, A.; Sanada, T.; Iwashita, M.; Shinjo, T.; Fukuda, T.; Sanui, T.; Kishida, S.; et al. SPOCK1 is a novel inducer of epithelial to mesenchymal transition in drug-induced gingival overgrowth. Sci. Rep. 2020, 10, 9785. [Google Scholar] [CrossRef] [PubMed]
- Fenouille, N.; Tichet, M.; Dufies, M.; Pottier, A.; Mogha, A.; Soo, J.K.; Rocchi, S.; Mallavialle, A.; Galibert, M.D.; Khammari, A.; et al. The epithelial-mesenchymal transition (EMT) regulatory factor SLUG (SNAI2) is a downstream target of SPARC and AKT in promoting melanoma cell invasion. PLoS ONE 2012, 7, e40378. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Lei, H.; Zhang, S.; Peng, Y.; Fu, C.; Shu, G.; Yin, G. Non-canonical signaling pathway of SNAI2 induces EMT in ovarian cancer cells by suppressing miR-222-3p transcription and upregulating PDCD10. Theranostics 2020, 10, 5895–5913. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, X.; Ni, J.; Zhao, R.; Shao, S.; Lu, S.; Han, W.; Yu, L. Effect of ISM1 on the Immune Microenvironment and Epithelial-Mesenchymal Transition in Colorectal Cancer. Front. Cell Dev. Biol. 2021, 9, 681240. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.; Srivastava, J.; Madson, N.; Wittmann, T.; Barber, D.L. Dynamic actin remodeling during epithelial-mesenchymal transition depends on increased moesin expression. Mol. Biol. Cell 2011, 22, 4750–4764. [Google Scholar] [CrossRef] [PubMed]
- Martin-Villar, E.; Megias, D.; Castel, S.; Yurrita, M.M.; Vilaro, S.; Quintanilla, M. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J. Cell Sci. 2006, 119 Pt 21, 4541–4553. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Hou, L.; Kang, H.; Chang, W.; Liu, Y.; Zhang, Y.; Ding, Y. NR2F2 plays a major role in insulin-induced epithelial-mesenchymal transition in breast cancer cells. BMC Cancer 2020, 20, 626. [Google Scholar] [CrossRef]
- Mauri, F.; Schepkens, C.; Lapouge, G.; Drogat, B.; Song, Y.; Pastushenko, I.; Rorive, S.; Blondeau, J.; Golstein, S.; Bareche, Y.; et al. NR2F2 controls malignant squamous cell carcinoma state by promoting stemness and invasion and repressing differentiation. Nat. Cancer 2021, 2, 1152–1169. [Google Scholar] [CrossRef]
- Kveiborg, M.; Albrechtsen, R.; Couchman, J.R.; Wewer, U.M. Cellular roles of ADAM12 in health and disease. Int. J. Biochem. Cell Biol. 2008, 40, 1685–1702. [Google Scholar] [CrossRef]
- Zoppi, N.; Gardella, R.; De Paepe, A.; Barlati, S.; Colombi, M. Human fibroblasts with mutations in COL5A1 and COL3A1 genes do not organize collagens and fibronectin in the extracellular matrix, down-regulate alpha2beta1 integrin, and recruit alphavbeta3 Instead of alpha5beta1 integrin. J. Biol. Chem. 2004, 279, 18157–18168. [Google Scholar] [CrossRef]
- Dobreva, M.P.; Lhoest, L.; Pereira, P.N.; Umans, L.; Camus, A.; Chuva de Sousa Lopes, S.M.; Zwijsen, A. Periostin as a biomarker of the amniotic membrane. Stem Cells Int. 2012, 2012, 987185. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, F.; Ho, J.; Stanford, W.L.; Fischer, K.D.; Schuh, A.C.; Schwartz, L.; Bernstein, A.; Rossant, J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell 1997, 89, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Chu, A.; Jefferson, W.N.; Casero, D.; Sudhakar, D.; Khurana, N.; Hogue, C.P.; Aryasomayajula, C.; Patel, P.; Sullivan, P.; et al. Epithelial membrane protein 2 (EMP2) deficiency alters placental angiogenesis, mimicking features of human placental insufficiency. J. Pathol. 2017, 242, 246–259. [Google Scholar] [CrossRef]
- O’Leary, H.; Ou, X.; Broxmeyer, H.E. The role of dipeptidyl peptidase 4 in hematopoiesis and transplantation. Curr. Opin. Hematol. 2013, 20, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, J.; Li, X.; Tian, R.; Shang, K.; Dong, X.; Cao, B. Angiogenesis is promoted by exosomal DPP4 derived from 5-fluorouracil-resistant colon cancer cells. Cancer Lett. 2021, 497, 190–201. [Google Scholar] [CrossRef]
- Tsogtbaatar, E.; Landin, C.; Minter-Dykhouse, K.; Folmes, C.D.L. Energy Metabolism Regulates Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 87. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Q.; Zhu, Y.; Chen, J.; Li, W. Ribosomes: An Exciting Avenue in Stem Cell Research. Stem Cells Int. 2020, 2020, 8863539. [Google Scholar] [CrossRef] [PubMed]
- Gabut, M.; Bourdelais, F.; Durand, S. Ribosome and Translational Control in Stem Cells. Cells 2020, 9, 497. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.; Sun, Y.; Li, Y.; Wang, P.; Shi, L.; Ni, A.; Zong, Y.; Zhao, J.; Bian, S.; et al. Genetic Basis of Sexual Maturation Heterosis: Insights from Ovary lncRNA and mRNA Repertoire in Chicken. Front. Endocrinol. 2022, 13, 951534. [Google Scholar] [CrossRef]
- Wen, S.; Ai, W.; Alim, Z.; Boehm, U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc. Natl. Acad. Sci. USA 2010, 107, 16372–16377. [Google Scholar] [CrossRef]
- Chou, C.S.; Beristain, A.G.; MacCalman, C.D.; Leung, P.C. Cellular localization of gonadotropin-releasing hormone (GnRH) I and GnRH II in first-trimester human placenta and decidua. J. Clin. Endocrinol. Metab. 2004, 89, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, C.S.; Karsenty, G.; Karaplis, A.C.; Kovacs, C.S. Parathyroid hormone regulates fetal-placental mineral homeostasis. J. Bone Miner. Res. 2010, 25, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Einspanier, A.; Lieder, K.; Husen, B.; Ebert, K.; Lier, S.; Einspanier, R.; Unemori, E.; Kemper, M. Relaxin supports implantation and early pregnancy in the marmoset monkey. Ann. N. Y. Acad. Sci. 2009, 1160, 140–146. [Google Scholar] [CrossRef]
- Nowak, M.; Gram, A.; Boos, A.; Aslan, S.; Ay, S.S.; Onyay, F.; Kowalewski, M.P. Functional implications of the utero-placental relaxin (RLN) system in the dog throughout pregnancy and at term. Reproduction 2017, 154, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Lackner, A.I.; Dietrich, B.; Kunihs, V.; Haslinger, P.; Meinhardt, G.; Maxian, T.; Saleh, L.; Fiala, C.; Pollheimer, J.; et al. Transforming growth factor-beta signaling governs the differentiation program of extravillous trophoblasts in the developing human placenta. Proc. Natl. Acad. Sci. USA 2022, 119, e2120667119. [Google Scholar] [CrossRef]
- Chuva de Sousa Lopes, S.M.; Alexdottir, M.S.; Valdimarsdottir, G. The TGFbeta Family in Human Placental Development at the Fetal-Maternal Interface. Biomolecules 2020, 10, 453. [Google Scholar] [CrossRef]
- Leach, L.; Lammiman, M.J.; Babawale, M.O.; Hobson, S.A.; Bromilou, B.; Lovat, S.; Simmonds, M.J. Molecular organization of tight and adherens junctions in the human placental vascular tree. Placenta 2000, 21, 547–557. [Google Scholar] [CrossRef]
- Knofler, M.; Pollheimer, J. Human placental trophoblast invasion and differentiation: A particular focus on Wnt signaling. Front. Genet. 2013, 4, 190. [Google Scholar] [CrossRef]
- Pham, T.X.A.; Panda, A.; Kagawa, H.; To, S.K.; Ertekin, C.; Georgolopoulos, G.; van Knippenberg, S.; Allsop, R.N.; Bruneau, A.; Chui, J.S.; et al. Modeling human extraembryonic mesoderm cells using naive pluripotent stem cells. Cell Stem Cell 2022, 29, 1346–1365.e10. [Google Scholar] [CrossRef]
- Staton, C.A.; Reed, M.W.; Brown, N.J. A critical analysis of current in vitro and in vivo angiogenesis assays. Int. J. Exp. Pathol. 2009, 90, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Francescone, R.A., 3rd; Faibish, M.; Shao, R. A Matrigel-based tube formation assay to assess the vasculogenic activity of tumor cells. J. Vis. Exp. 2011, 55, e3040. [Google Scholar]
- Liu, Y.; Petreaca, M.; Martins-Green, M. Cell and molecular mechanisms of insulin-induced angiogenesis. J. Cell. Mol. Med. 2009, 13, 4492–4504. [Google Scholar] [CrossRef]
- Escudero, C.A.; Herlitz, K.; Troncoso, F.; Guevara, K.; Acurio, J.; Aguayo, C.; Godoy, A.S.; Gonzalez, M. Pro-angiogenic Role of Insulin: From Physiology to Pathology. Front. Physiol. 2017, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, A.; Gruber, D.C.; Pisarsky, L.; Heck, C.; Kunita, A.; Yilmaz, M.; Meyer-Schaller, N.; Cornille, K.; Hopfer, U.; Bentires-Alj, M.; et al. VEGF-mediated angiogenesis links EMT-induced cancer stemness to tumor initiation. Cancer Res. 2014, 74, 1566–1575. [Google Scholar] [CrossRef]
- Fan, Y.L.; Zheng, M.; Tang, Y.L.; Liang, X.H. A new perspective of vasculogenic mimicry: EMT and cancer stem cells (Review). Oncol. Lett. 2013, 6, 1174–1180. [Google Scholar] [CrossRef]
- Zheng, Y.; Xue, X.; Shao, Y.; Wang, S.; Esfahani, S.N.; Li, Z.; Muncie, J.M.; Lakins, J.N.; Weaver, V.M.; Gumucio, D.L.; et al. Controlled modelling of human epiblast and amnion development using stem cells. Nature 2019, 573, 421–425. [Google Scholar] [CrossRef]
- Bernardo, A.S.; Faial, T.; Gardner, L.; Niakan, K.K.; Ortmann, D.; Senner, C.E.; Callery, E.M.; Trotter, M.W.; Hemberger, M.; Smith, J.C.; et al. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell 2011, 9, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.R.; Lee, J.H.; Kim, K.H.; Ban, S.; Kim, V.J.; Kim, J.Y.; Lee, J.Y.; Sun, W. Spatiotemporal contribution of neuromesodermal progenitor-derived neural cells in the elongation of developing mouse spinal cord. Life Sci. 2021, 282, 119393. [Google Scholar] [CrossRef]
- Xu, R.H.; Chen, X.; Li, D.S.; Li, R.; Addicks, G.C.; Glennon, C.; Zwaka, T.P.; Thomson, J.A. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 2002, 20, 1261–1264. [Google Scholar] [CrossRef]
- Papuchova, H.; Latos, P.A. Transcription factor networks in trophoblast development. Cell. Mol. Life Sci. 2022, 79, 337. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Nishihara, A.; Mishima, K.; Yamashita, J.; Shimizu, K.; Miyazawa, K.; Nishikawa, S.; Miyazono, K. TGF-beta receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J. Cell Biol. 2003, 163, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Xie, Y.L.; Gao, Y.X.; Cheng, T.; Wang, Z.Z. The balance of positive and negative effects of TGF-beta signaling regulates the development of hematopoietic and endothelial progenitors in human pluripotent stem cells. Stem Cells Dev. 2013, 22, 2765–2776. [Google Scholar] [CrossRef] [PubMed]
- Blank, U.; Karlsson, S. TGF-beta signaling in the control of hematopoietic stem cells. Blood 2015, 125, 3542–3550. [Google Scholar] [CrossRef]
- Vaidya, A.; Kale, V.P. TGF-beta signaling and its role in the regulation of hematopoietic stem cells. Syst. Synth. Biol. 2015, 9, 1–10. [Google Scholar] [CrossRef]
- Sun, Z.; Cai, S.; Zabkiewicz, C.; Liu, C.; Ye, L. Bone morphogenetic proteins mediate crosstalk between cancer cells and the tumour microenvironment at primary tumours and metastases (Review). Int. J. Oncol. 2020, 56, 1335–1351. [Google Scholar] [CrossRef]
- Guo, G.; von Meyenn, F.; Rostovskaya, M.; Clarke, J.; Dietmann, S.; Baker, D.; Sahakyan, A.; Myers, S.; Bertone, P.; Reik, W.; et al. Epigenetic resetting of human pluripotency. Development 2017, 144, 2748–2763. [Google Scholar] [CrossRef]
- Ying, Q.L.; Smith, A.G. Defined conditions for neural commitment and differentiation. Methods Enzymol. 2003, 365, 327–341. [Google Scholar]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Zheng, G.X.; Terry, J.M.; Belgrader, P.; Ryvkin, P.; Bent, Z.W.; Wilson, R.; Ziraldo, S.B.; Wheeler, T.D.; McDermott, G.P.; Zhu, J.; et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 2017, 8, 14049. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T.; Butler, A.; Hoffman, P.; Hafemeister, C.; Papalexi, E.; Mauck, W.M., 3rd; Hao, Y.; Stoeckius, M.; Smibert, P.; Satija, R. Comprehensive Integration of Single-Cell Data. Cell 2019, 177, 1888–1902.e21. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farkas, K.; Ferretti, E. Derivation of Human Extraembryonic Mesoderm-like Cells from Primitive Endoderm. Int. J. Mol. Sci. 2023, 24, 11366. https://doi.org/10.3390/ijms241411366
Farkas K, Ferretti E. Derivation of Human Extraembryonic Mesoderm-like Cells from Primitive Endoderm. International Journal of Molecular Sciences. 2023; 24(14):11366. https://doi.org/10.3390/ijms241411366
Chicago/Turabian StyleFarkas, Karin, and Elisabetta Ferretti. 2023. "Derivation of Human Extraembryonic Mesoderm-like Cells from Primitive Endoderm" International Journal of Molecular Sciences 24, no. 14: 11366. https://doi.org/10.3390/ijms241411366
APA StyleFarkas, K., & Ferretti, E. (2023). Derivation of Human Extraembryonic Mesoderm-like Cells from Primitive Endoderm. International Journal of Molecular Sciences, 24(14), 11366. https://doi.org/10.3390/ijms241411366





