Dynamics of Polyamines, Proline, and Ethylene Metabolism under Increasing Cold in Winter Oilseed Rape
Abstract
:1. Introduction
2. Results
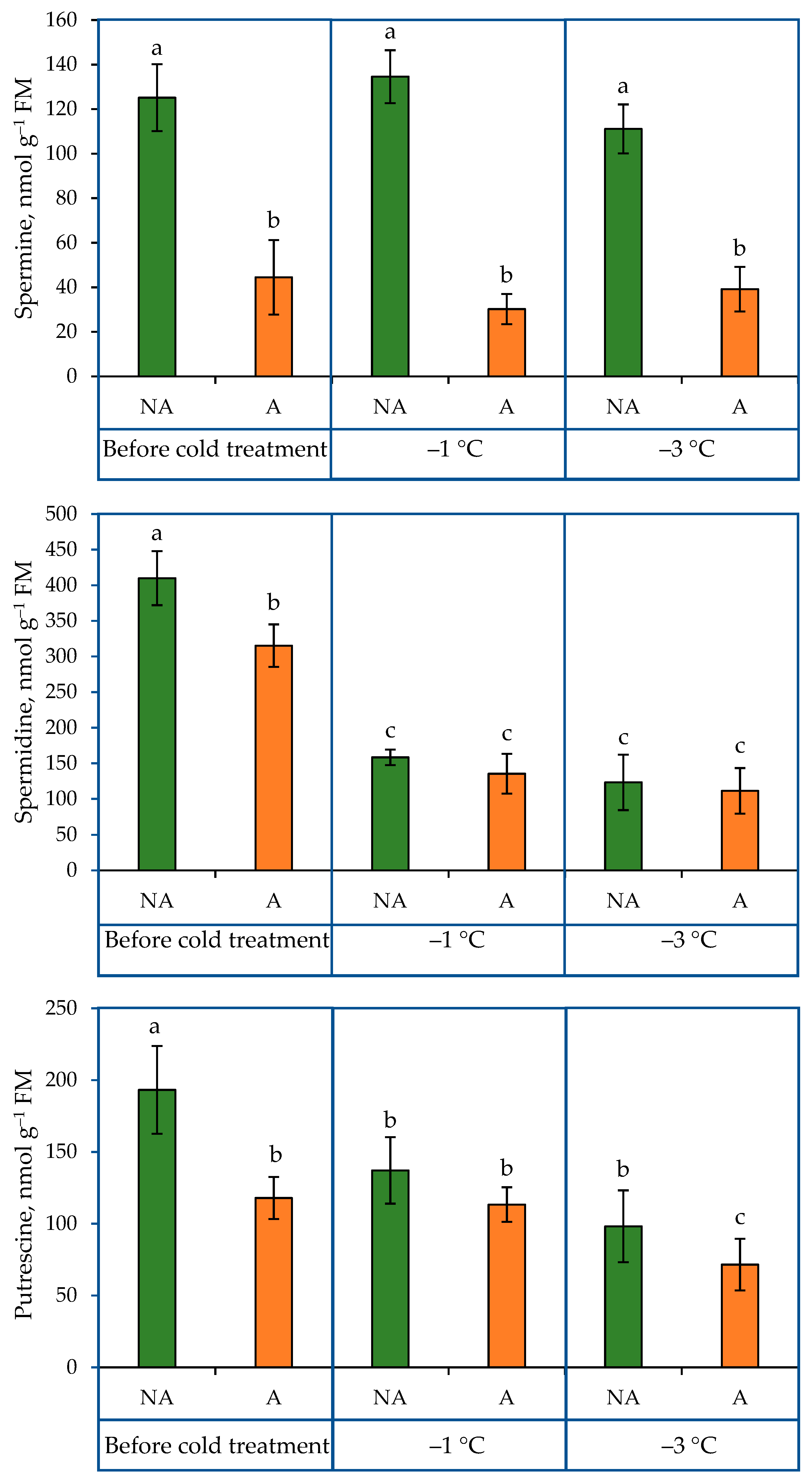
2.1. PA Content in the Leaves of Oilseed Rape Grown under Increasing Cold Conditions
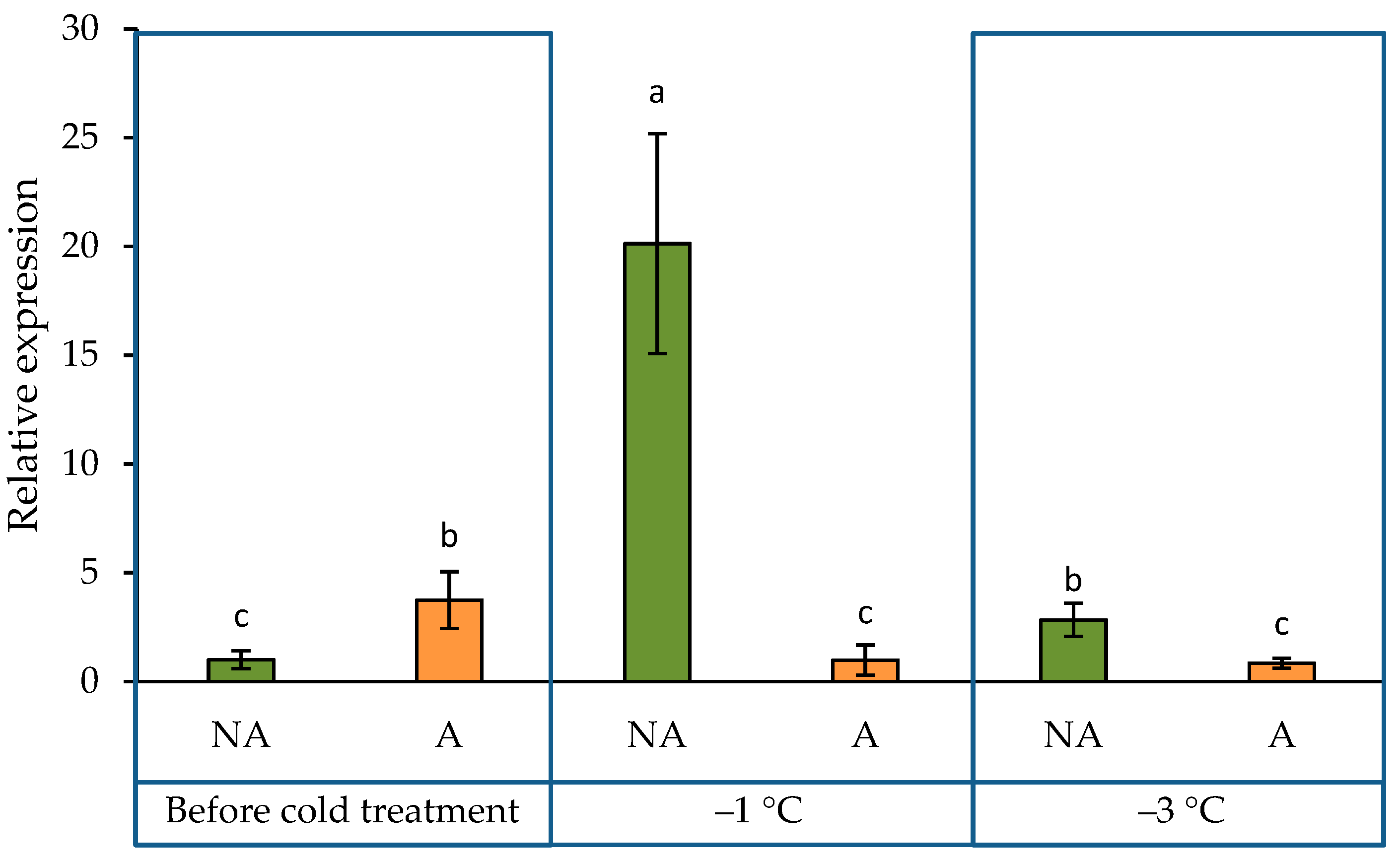
2.2. ADC2 Gene Expression Pattern in the Leaves of Oilseed Rape Grown under Increasing Cold Conditions
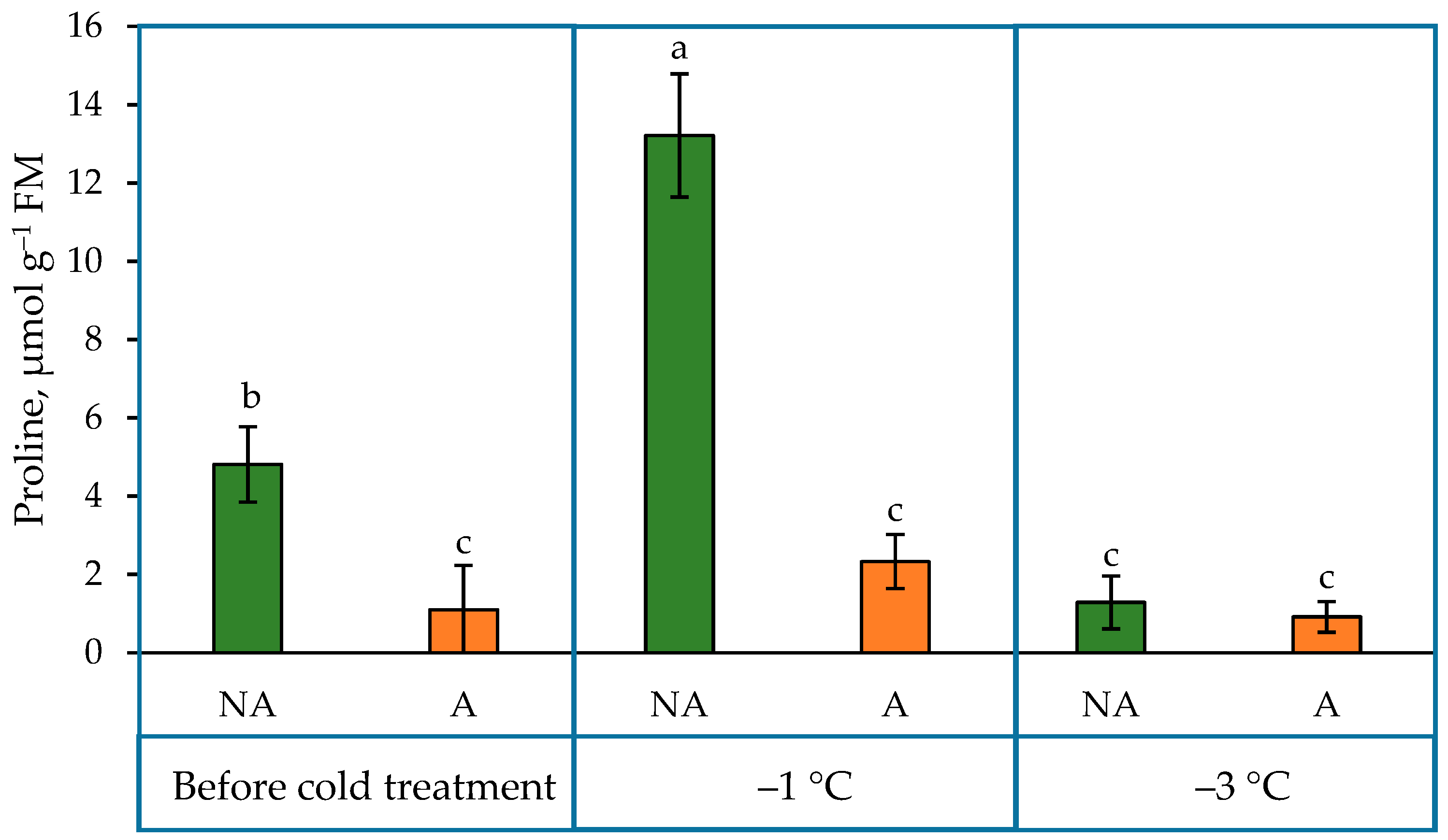
2.3. Proline Content in the Leaves of Oilseed Rape Grown under Increasing Cold Conditions
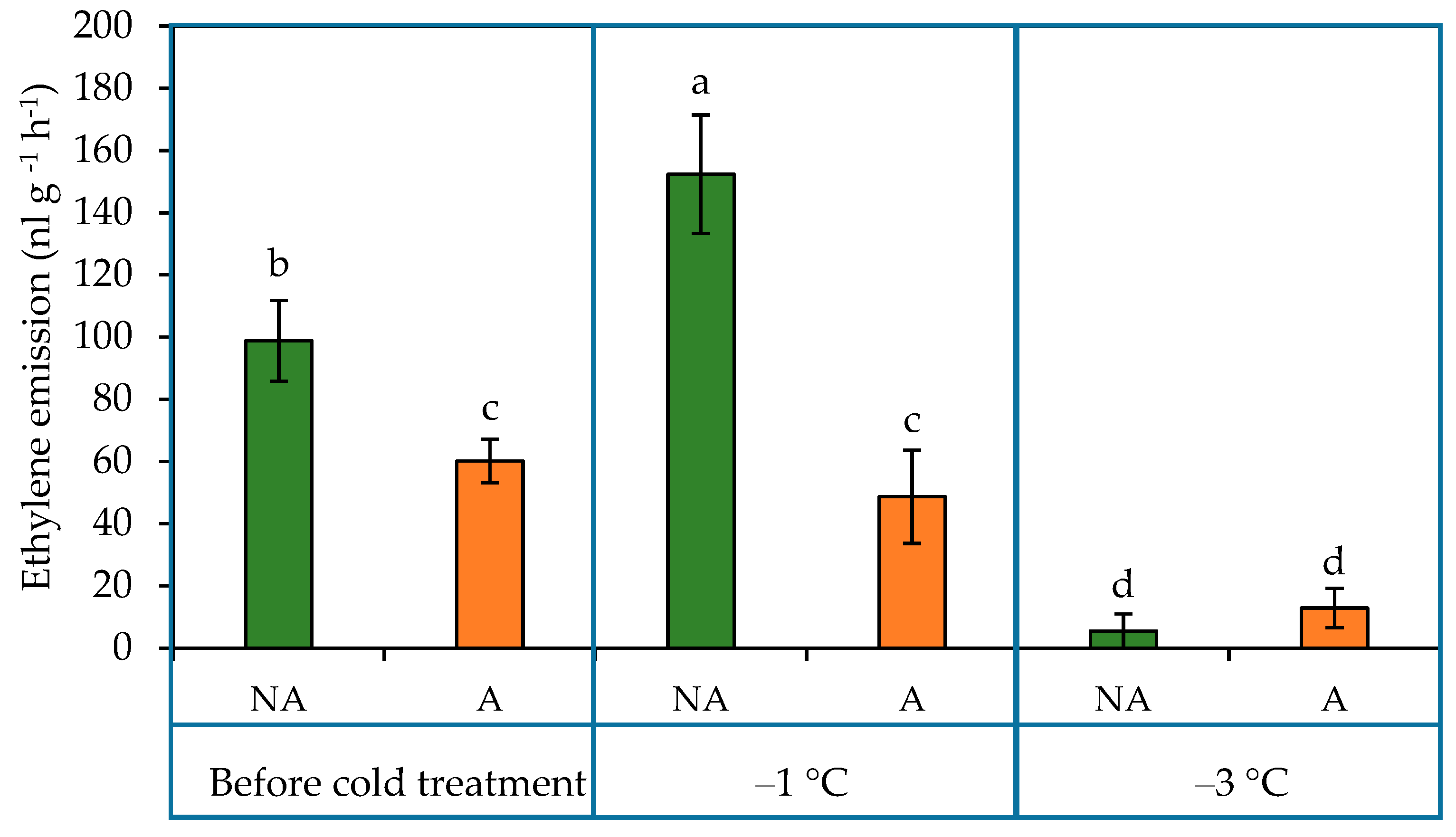
2.4. Ethylene Emission in the Leaves of Oilseed Rape Grown under Increasing Cold Conditions
3. Discussion
3.1. The Content of PAs
3.2. The Expression Pattern of PAs Biosynthetic Gene
3.3. The Content of Proline
3.4. The Level of Ethylene Emission
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Low-Temperature Treatments
4.3. Determination of PA Content
4.4. RT-qPCR
4.5. Proline Content Analysis
4.6. Ethylene Emission Analysis
4.7. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | acclimated |
| NA | non-acclimated |
| PAs | polyamines |
| ADC | arginine decarboxylase |
| SPDS | spermidine synthase |
| SAMDC | S-adenosylmethionine decarboxylase |
| ROS | reactive oxygen species |
| SAM | S-adenosylmethionine |
| HTB | histone superfamily protein |
| UBC | ubiquitin-conjugating enzyme |
References
- Upadhyay, H.; Sahoo, L.; Panda, S.K. Molecular physiology of osmotic stress in plants. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: New Delhi, India, 2013; pp. 179–192. [Google Scholar]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Snowdon, R.; Lühs, W.; Friedt, W. Oilseed Rape. In Genome Mapping and Molecular Breeding in Plants. Oilseeds; Kole, C., Ed.; Springer: New York, NY, USA, 2007; Volume 2, pp. 55–56. [Google Scholar]
- Raboanatahiry, N.; Li, H.; Yu, L.; Li, M. Rapeseed (Brassica napus): Processing, utilization, and genetic improvement. Agronomy 2021, 11, 1776. [Google Scholar] [CrossRef]
- Burbulis, N.; Kuprienė, R.; Blinstrubienė, A. The effect of de-acclimation and re-acclimation treatments on winter rapeseed cold resistance in vitro. Sodininkystė Daržininkystė 2008, 27, 233–240. [Google Scholar]
- Paulauskas, A.; Jodinskienė, M.; Griciuvienė, L.; Žukauskienė, J.; Petraitienė, E.; Brazauskienė, I. Morphological traits and genetic diversity of differently overwintered oilseed rape (Brassica napus L.) cultivars. Zemdirbyste-Agriculture 2013, 100, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Responses of plants to climate change: Metabolic changes during abiotic stress combination in plants. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef] [PubMed]
- Deveci, M.; Aksu, G. Effects of freezing temperatures that are applied to some vegetable seedlings from Brassicaceae family on the viability ratio in Thrace conditions. J. Environ. Prot. Ecol. 2010, 11, 76–82. [Google Scholar]
- Fiebelkorn, D.; Rahman, M. Development of a protocol for frost-tolerance evaluation in rapeseed/canola (Brassica napus L.). Crop J. 2016, 4, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Poor Oilseed Rape Yields. What Are the Causes? Available online: https://www.dsv-seeds.com/seeds.com/downloads/interesting-articles/oilseed-rape/Innovation-04-2016-POOR-OILSEED-RAPE-YIELDS-What-are-the-causes.pdf (accessed on 3 July 2023).
- Chinnusamy, V.; Ohta, M.; Kanrar, S.; Lee, B.; Hong, X.; Agarwal, M.; Zhu, J.K. ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054. [Google Scholar] [CrossRef] [Green Version]
- Janská, A.; Marsík, P.; Zelenková, S.; Ovesná, J. Cold stress and acclimation—What is important for metabolic adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef]
- Gusta, L.V.; Wisniewski, M. Understanding plant cold hardiness: An opinion. Physiol. Plant 2013, 147, 4–14. [Google Scholar] [CrossRef]
- Arora, R. Mechanism of freeze-thaw injury and recovery: A cool retrospective and warming up to new ideas. Plant Sci. 2018, 270, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Beillouin, D.; Schauberger, B.; Bastos, A.; Ciais, P.; Makowski, D. Impact of extreme weather conditions on European crop production in 2018. Philos. Trans. R. Soc. 2020, 375, 20190510. [Google Scholar] [CrossRef] [PubMed]
- Lamers, J.; Van Der Meer, T.; Testerink, C. How plants sense and respond to stressful environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrotra, S.; Verma, S.; Kumar, S.; Kumari, S.; Mishra, B.N. Transcriptional regulation and signalling of cold stress response in plants: An overview of current understanding. Environ. Exp. Bot. 2020, 180, 104243. [Google Scholar] [CrossRef]
- Tyagi, A.; Ali, S.; Ramakrishna, G.; Singh, A.; Park, S.; Mahmoudi, H.; Bae, H. Revisiting the Role of Polyamines in Plant Growth and Abiotic Stress Resilience: Mechanisms, Crosstalk, and Future Perspectives. J. Plant Growth Regul. 2022, 1–25. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Lechowska, K.; Wojtyla, Ł.; Quinet, M.; Kubala, S.; Lutts, S.; Garnczarska, M. Endogenous polyamines and ethylene biosynthesis in relation to germination of osmoprimed Brassica napus seeds under salt stress. Int. J. Mol. Sci. 2022, 23, 349. [Google Scholar] [CrossRef]
- Pal, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signalling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Bullock, D.A., Jr.; Alonso, J.M.; Stepanova, A.N. To Fight or to grow: The balancing role of ethylene in plant abiotic stress responses. Plants 2022, 11, 33. [Google Scholar] [CrossRef]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid responses of plants to temperature changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Banerjee, A.; Roychoudhury, A. Chapter 8. Polyamines ameliorate oxidative stress by regulating antioxidant systems and interacting with plant growth regulators. In Molecular Plant Abiotic Stress; Roychoudhury, A., Tripathi, D.K., Eds.; Wiley: Chichester, UK, 2019; pp. 135–143. [Google Scholar]
- Liu, W.; Yu, K.; He, T.; Li, F.; Zhang, D.; Liu, J. The low temperature induced physiological responses of Avena nuda L., a cold-tolerant plant species. Sci. World J. 2013, 2013, 658793. [Google Scholar]
- Do, P.T.; Degenkolbe, T.; Erban, A.; Heyer, A.G.; Kopka, J.; Köhl, K.I.; Hincha, D.K.; Zuther, E. Dissecting rice polyamine metabolism under controlled long-term drought stress. PLoS ONE 2013, 8, e60325. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, R.K.; Fatima, T.; Handa, A.K.; Mattoo, A.K. Polyamines and their biosynthesis/catabolism genes are differentially modulated in response to heat versus cold stress in tomato leaves (Solanum lycopersicum L.). Cells 2020, 9, 1749. [Google Scholar] [CrossRef]
- Yang, W.; Cai, T.; Ni, Y.; Li, Y.; Guo, J.; Peng, D.; Yang, D.; Yin, Y.; Wang, Z. Effects of exogenous abscisic acid and gibberellic acid on flling process and nitrogen metabolism characteristics in wheat grains. Aust. J. Crop Sci. 2013, 7, 58–65. [Google Scholar]
- Fariduddin, Q.; Varshney, P.; Yusuf, M.; Ahmad, A. Polyamines: Potent modulators of plant responses to stress. J. Plant Interact. 2012, 8, 1–16. [Google Scholar] [CrossRef]
- Yin, X.; Yang, Y.; Lv, Y.; Li, Y.; Yang, D.; Yue, Y.; Yang, Y. BrrICE1.1 is associated with putrescine synthesis through regulation of the arginine decarboxylase gene in freezing tolerance of turnip (Brassica rapa var. rapa). BMC Plant Biol. 2020, 20, 504. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Wang, H.; Tang, X.; Wang, H.; Shao, H.B. Proline accumulation and metabolism-related genes expression profiles in Kosteletzkya virginica seedlings under salt stress. Front. Plant Sci. 2015, 6, 792. [Google Scholar] [CrossRef] [Green Version]
- Gai, Z.; Liu, L.; Zhang, J.; Liu, J.; Cai, L. Effects of exogenous α-oxoglutarate on proline accumulation, ammonium assimilation and photosynthesis of soybean seedling (Glycine max (L.) Merr.) exposed to cold stress. Sci. Rep. 2020, 10, 17017. [Google Scholar] [CrossRef]
- Juurakko, C.L.; di Cenzo, G.C.; Walker, V.K. Cold acclimation and prospects for cold-resilient crops. Plant Stress 2021, 2, 100028. [Google Scholar] [CrossRef]
- Jankovska-Bortkevič, E.; Gavelienė, V.; Koryznienė, D.; Jankauskienė, J.; Mockevičiūtė, R.; Jurkonienė, S. Response of winter oilseed rape to imitated temperature fluctuations in autumn-winter period. Environ. Exp. Bot. 2019, 166, 10380. [Google Scholar] [CrossRef]
- Mattoo, A.; Avtar, K.H.; Fatima, H. Chapter 10. Polyamines in plants: Biosynthesis from arginine, and metabolic, physiological and stress-response roles. In Amino Acids in Higher Plants; D’Mello, J.P.F., Ed.; CABI: Wallingford, UK, 2015; pp. 177–194. [Google Scholar]
- Sun, X.; Li, X.; Zhu, J.; Huang, N.; Bian, X.; Li, H.; Wang, L.; Han, L. Polyamines and ethylene metabolism during cold acclimation in zoysiagrass (Zoysia Japonica Steud.). Acta Physiol. Plant 2020, 42, 138. [Google Scholar] [CrossRef]
- Liu, J.H.; Kitashiba, H.; Wang, J.; Ban, Y.; Moriguchi, T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol. 2007, 24, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, W.; Wang, M.; Zhang, H.Y.; Liu, J.H. The miR396b of Poncirus trifoliata functions in cold tolerance by regulating ACC oxidase gene expression and modulating ethylene–polyamine homeostasis. Plant Cell Physiol. 2016, 57, 1865–1878. [Google Scholar] [CrossRef] [Green Version]
- Weber, E.; Bleiholder, H.; Lancashire, P.D.; Langelüddecke, R.; Stauss, R.; Van der Boom, T.; Witzen-Berger, A. Growth stages of mono- and dicotyledonous plants. In BBCH Monograph, 2nd ed.; Meier, U., Ed.; Blackwells: Oxford, UK, 2018. [Google Scholar]
- Madhubala, R. Thin-layer chromatographic method for assaying polyamines. Methods Mol. Biol. 1998, 79, 131–136. [Google Scholar] [PubMed]
- Han, P.P.; Qin, L.; Li, Y.S.; Liao, X.S.; Xu, Z.X.; Hu, X.J.; Xie, L.H.; Yu, C.B.; Wu, Y.F.; Liao, X. Identification of suitable reference genes in leaves and roots of rapeseed (Brassica napus L) under different nutrient deficiencies. J. Integr. Agric. 2017, 16, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Liu, J.; Huang, S.; Guo, T.; Deng, L.; Hua, W. Selection and evaluation of novel reference genes for quantitative reverse transcription PCR (qRT-PCR) based on genome and transcriptome data in Brassica napus L. Gene 2014, 538, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.K.; Upadhyay, R.K.; Rudrabhatla, S. Abiotic stress in crops: Candidate genes, osmolytes, polyamines, and biotechnological intervention. In Elucidation of Abiotic Stress Signaling in Plants; Pandey, G.K., Ed.; Springer Science+Business Media: New York, NY, USA, 2015; pp. 415–437. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Child, R.D.; Chauvaux, N.; John, K.; Van Onckelen, H.A.; Ulvskov, P. Ethylene biosynthesis in oilseed rape pods in relation to pod shatter. J. Exp. Bot. 1998, 49, 829–838. [Google Scholar] [CrossRef]

| Nr. | Gene Name | Amplicon Size, bp | Primer Sequence | Oligomer Name | Tm, °C |
|---|---|---|---|---|---|
| 1. | Histone superfamily protein | 142 | TATTCGAGAAGCTCGCCCAG | HTB1-F | 62 |
| TTGGTTCCTTCAGAGACGGC | HTB1-R | 62 | |||
| 2. | Ubiquitin-conjugating enzyme 21 | 176 | TATCCTCTGCAGCCTCCTCA | UBC21-F | 62 |
| CTGTCTGCCTCAGGATGAGC | UBC21-R | 64 |
| Gene Name | Amplicon Size, bp | Primer Sequence | Oligomer Name | Tm, °C |
|---|---|---|---|---|
| Arginine decarboxylase | 240 | TTGTATGCTTGACTGTCTCCAG | BnADC2Pe-F | 64 |
| ACAGCTTCAGCGTACTCCTC | BnADC2Pe-R | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankovska-Bortkevič, E.; Jurkonienė, S.; Gavelienė, V.; Šveikauskas, V.; Mockevičiūtė, R.; Vaseva, I.; Todorova, D.; Žižytė-Eidetienė, M.; Šneideris, D.; Prakas, P. Dynamics of Polyamines, Proline, and Ethylene Metabolism under Increasing Cold in Winter Oilseed Rape. Int. J. Mol. Sci. 2023, 24, 11402. https://doi.org/10.3390/ijms241411402
Jankovska-Bortkevič E, Jurkonienė S, Gavelienė V, Šveikauskas V, Mockevičiūtė R, Vaseva I, Todorova D, Žižytė-Eidetienė M, Šneideris D, Prakas P. Dynamics of Polyamines, Proline, and Ethylene Metabolism under Increasing Cold in Winter Oilseed Rape. International Journal of Molecular Sciences. 2023; 24(14):11402. https://doi.org/10.3390/ijms241411402
Chicago/Turabian StyleJankovska-Bortkevič, Elžbieta, Sigita Jurkonienė, Virgilija Gavelienė, Vaidevutis Šveikauskas, Rima Mockevičiūtė, Irina Vaseva, Dessislava Todorova, Marija Žižytė-Eidetienė, Donatas Šneideris, and Petras Prakas. 2023. "Dynamics of Polyamines, Proline, and Ethylene Metabolism under Increasing Cold in Winter Oilseed Rape" International Journal of Molecular Sciences 24, no. 14: 11402. https://doi.org/10.3390/ijms241411402
APA StyleJankovska-Bortkevič, E., Jurkonienė, S., Gavelienė, V., Šveikauskas, V., Mockevičiūtė, R., Vaseva, I., Todorova, D., Žižytė-Eidetienė, M., Šneideris, D., & Prakas, P. (2023). Dynamics of Polyamines, Proline, and Ethylene Metabolism under Increasing Cold in Winter Oilseed Rape. International Journal of Molecular Sciences, 24(14), 11402. https://doi.org/10.3390/ijms241411402










