Co-Expression of Multiple PAX Genes in Renal Cell Carcinoma (RCC) and Correlation of High PAX Expression with Favorable Clinical Outcome in RCC Patients
Abstract
:1. Introduction
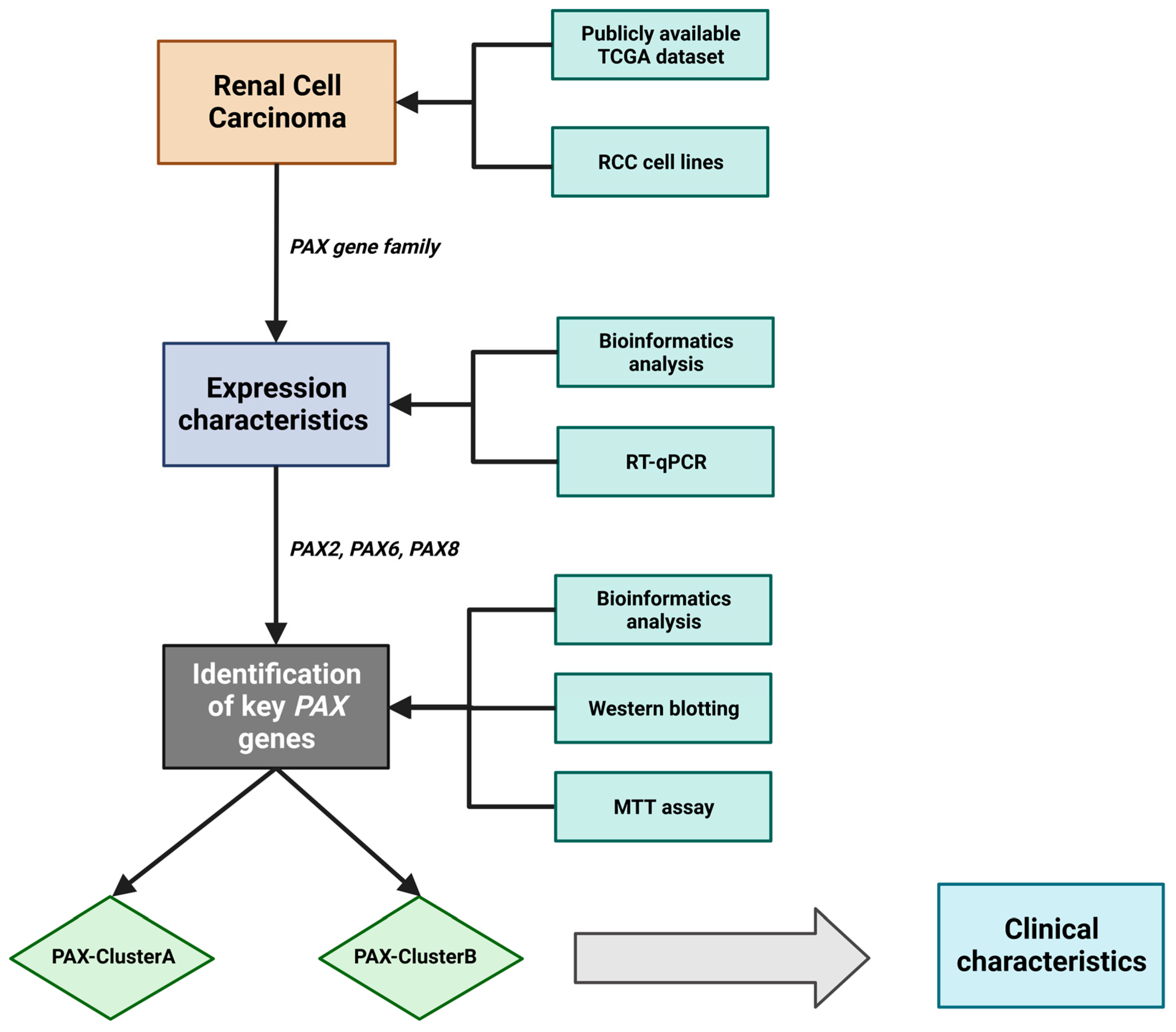
2. Results
2.1. Clinical Characteristics of RCC Patients in the TCGA Cohort
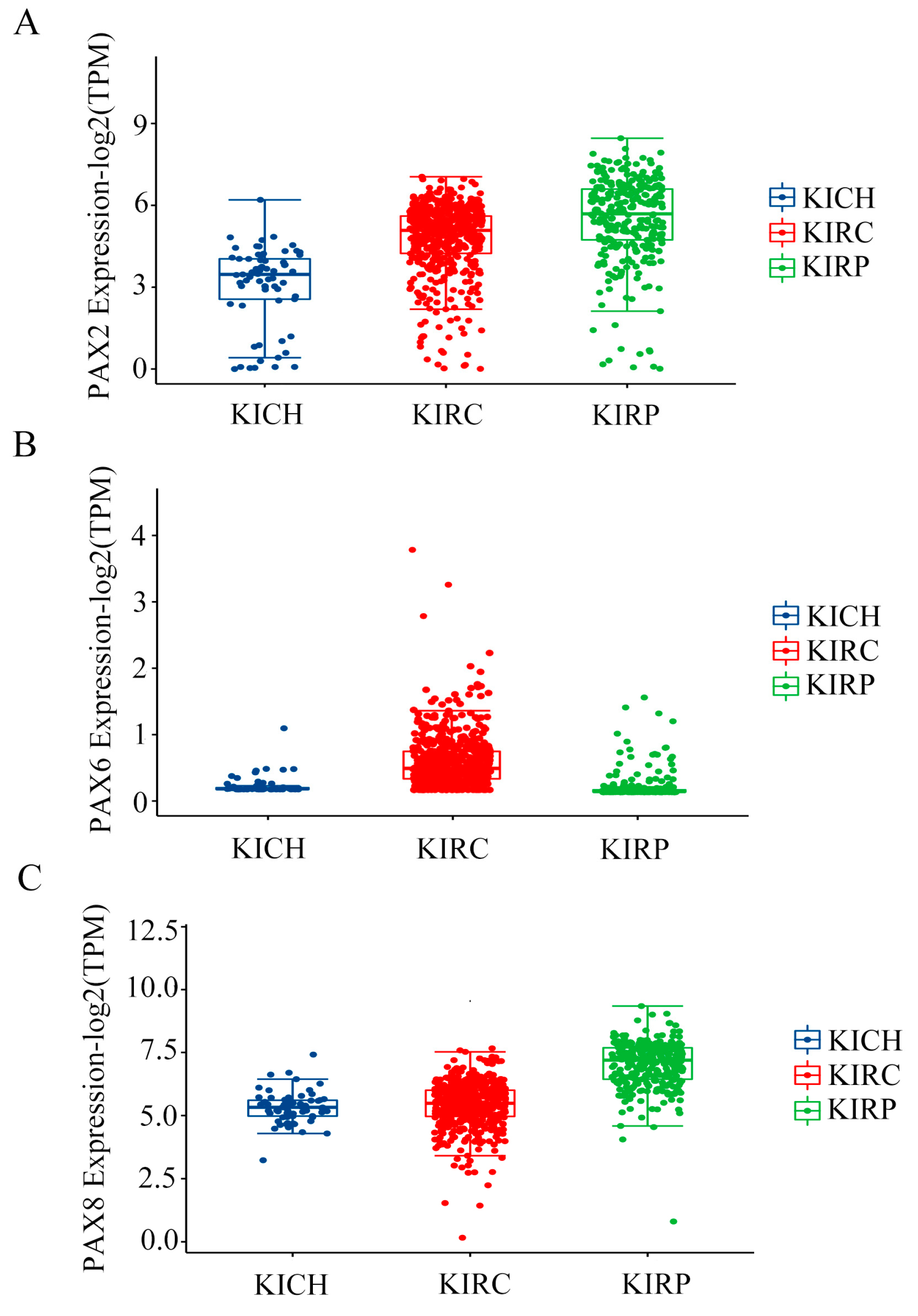
2.2. Multiple PAX Genes Are Expressed in Clear Cell (KIRC), Papillary (KIRP), and Chromophobe (KICH) Renal Cell Carcinoma
2.3. Analysis of PAX Gene Expression Levels in Human RCC Cell Lines
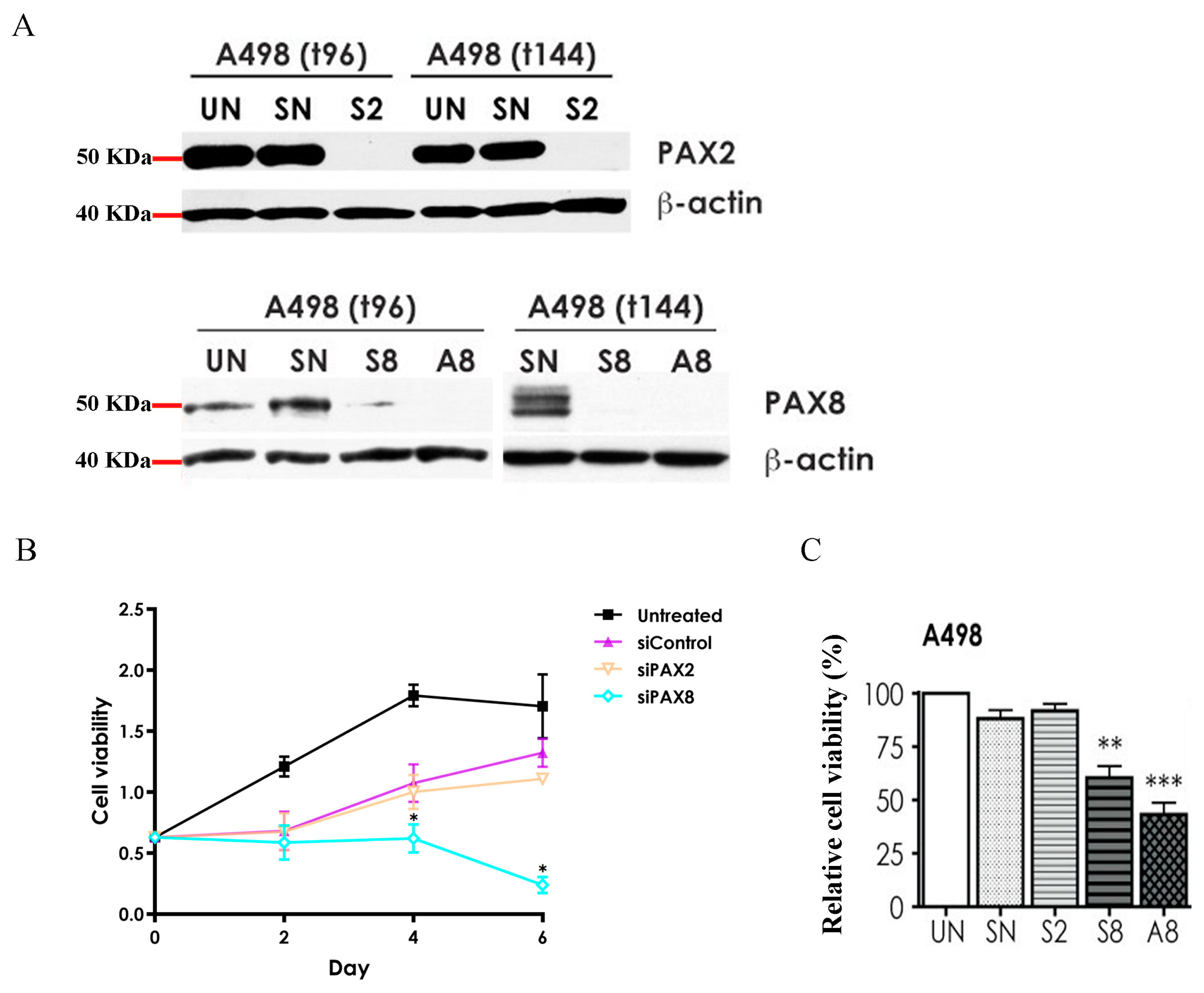
2.4. Effects of PAX2 or PAX8 Knockdown on A498 Cell Proliferation
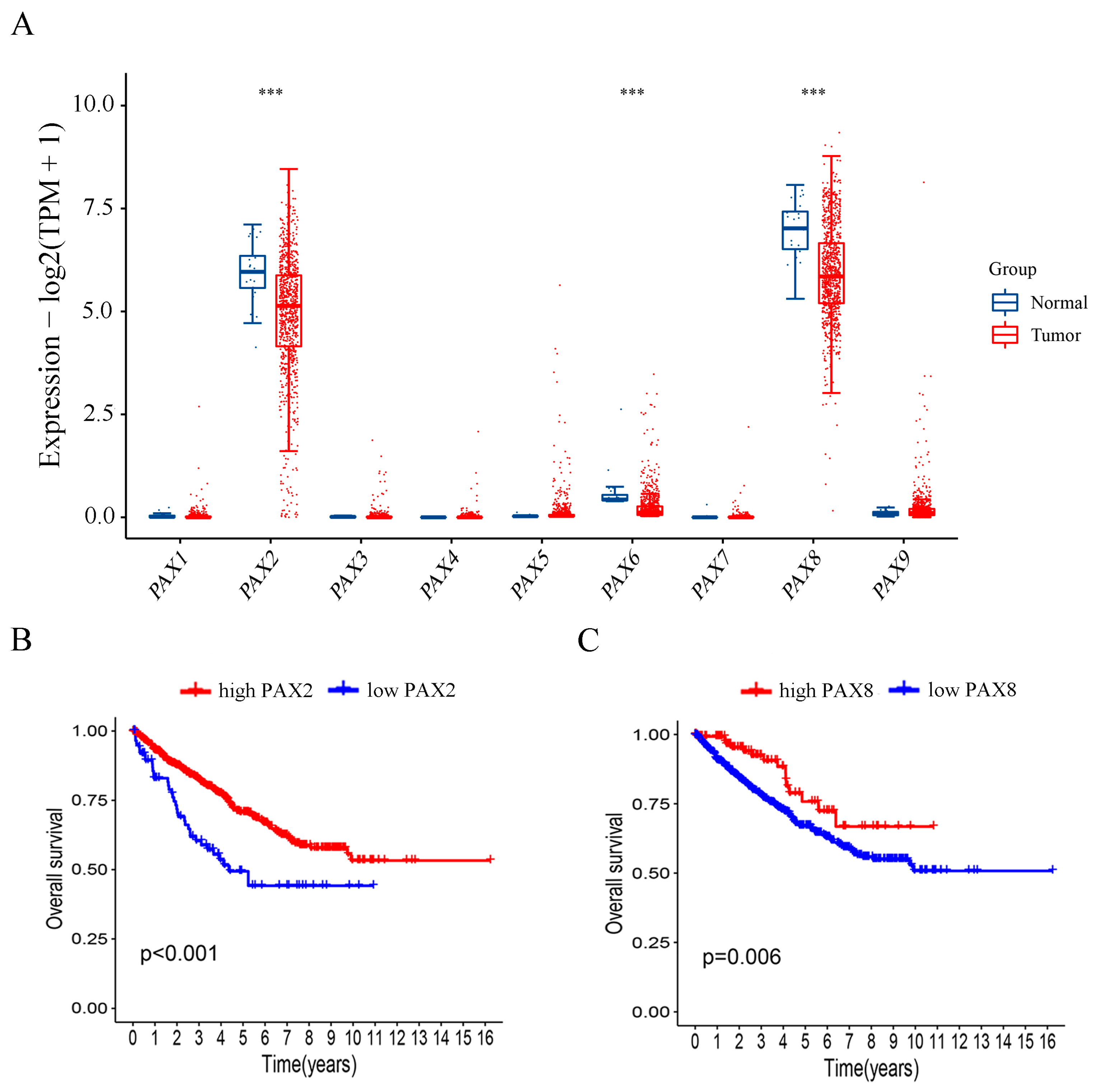
2.5. Investigation of PAX Gene Expression Levels in Relation to Publicly Available Clinical Data from RCC Patients
2.6. Patients with Higher PAX2 and PAX8 mRNA Expression Exhibited Better Overall Survival in RCC
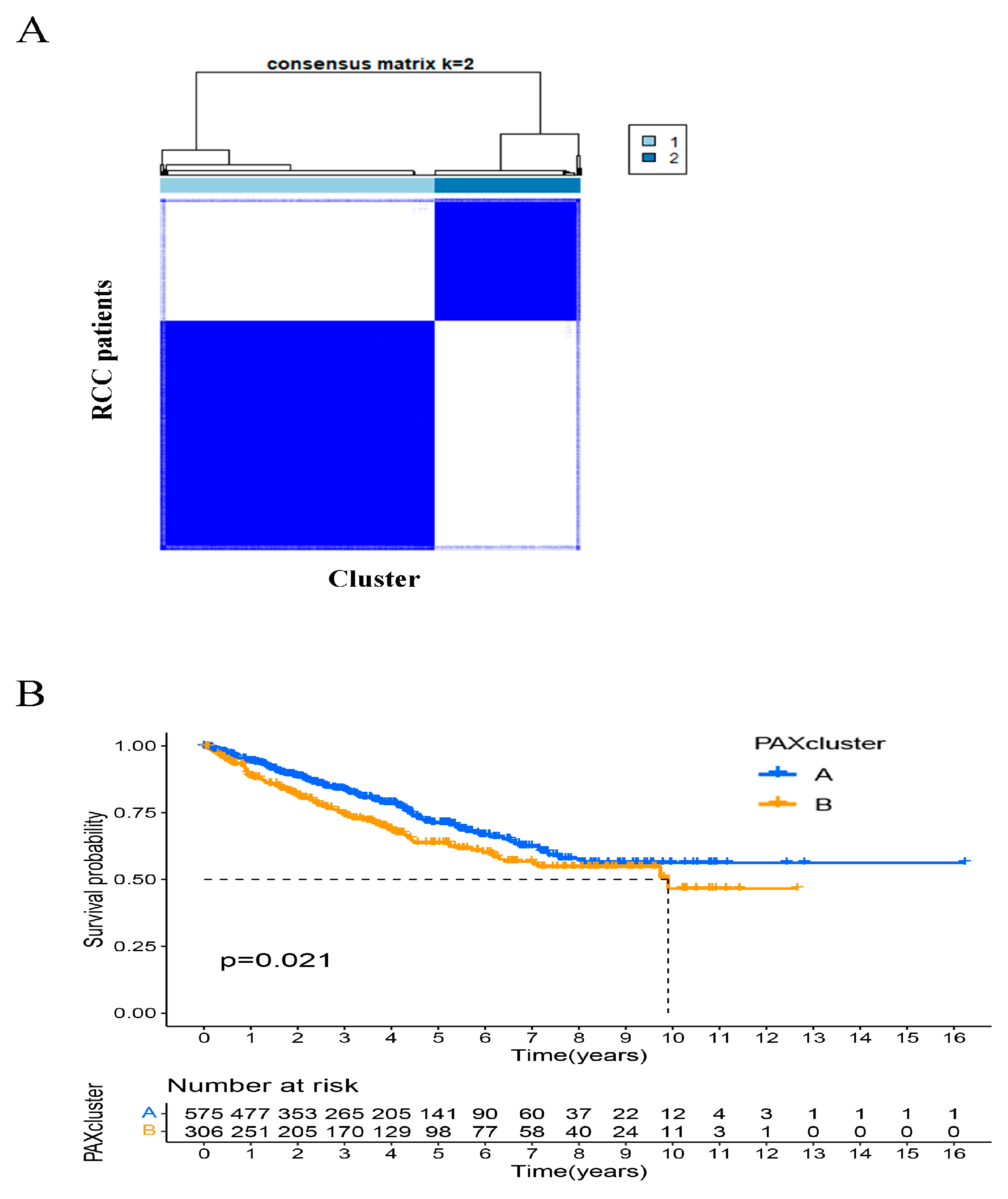
2.7. Identification of PAXcluster A and PAXcluster B Subgroups in RCC Tumors
2.8. Clinical Features Are Associated with PAXcluster A and PAXcluster B Expression Subtypes in RCC Patients
3. Discussion
4. Materials and Methods
4.1. TCGA Database Patient Selection
4.2. Analysis Using Gene Expression Profiling Interactive Analyses {GEPIA) Online Tools
4.3. Unsupervised Clustering Based on PAX Genes
4.4. Gene Set Variation Analysis (GSVA) and Functional Annotation
4.5. Cell Culture
4.6. Semi-Quantitative Reverse Transcription Real-Time PCR (RT-qPCR)
4.7. siRNA Transfection
4.8. Western Blotting
4.9. Measurement of Cell Proliferation: The MTT Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization International Agency for Research on Cancer. GLOBOCAN: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2022. Available online: https://gco.iarc.fr (accessed on 10 April 2023).
- Turajlic, S.; Swanton, C.; Boshoff, C. Kidney cancer: The next decade. J. Exp. Med. 2018, 215, 2477–2479. [Google Scholar] [CrossRef] [Green Version]
- Truong, L.D.; Shen, S.S. Immunohistochemical diagnosis of renal neoplasms. Arch. Pathol. Lab. Med. 2011, 135, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.R.; Humphrey, P.A.; Catena, J.R.; Narra, V.R.; Srigley, J.R.; Cortez, A.D.; Dalrymple, N.C.; Chintapalli, K.N. Common and un-common histologic subtypes of renal cell carcinoma: Imaging spectrum with pathologic correlation. Radiographics 2006, 26, 1795–1806; discussion 1806–1810. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhu, G. Predictive Biomarkers and Novel Targets in the Treatment of Metastatic Renal Cell Carcinoma. Curr. Med. Chem. 2021, 28, 5213–5227. [Google Scholar] [CrossRef]
- Ged, Y.; Voss, M.H. Novel emerging biomarkers to immunotherapy in kidney cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211059367. [Google Scholar] [CrossRef] [PubMed]
- Muratovska, A.; Zhou, C.; He, S.; Goodyer, P.; Eccles, M.R. Paired-Box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene 2003, 22, 7989–7997. [Google Scholar] [CrossRef] [Green Version]
- Li, C.G.; Eccles, M.R. PAX Genes in Cancer; Friends or Foes? Front. Genet. 2012, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Robson, E.J.; He, S.J.; Eccles, M.R. A PANorama of PAX genes in cancer and development. Nat. Rev. Cancer 2006, 6, 52–62. [Google Scholar] [CrossRef]
- Tong, G.X.; Melamed, J.; Mansukhani, M.; Memeo, L.; Hernandez, O.; Deng, F.M.; Chiriboga, L.; Waisman, J. PAX2: A reliable marker for nephrogenic adenoma. Mod. Pathol. 2006, 19, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozcan, A.; de la Roza, G.; Ro, J.Y.; Shen, S.S.; Truong, L.D. PAX2 and PAX8 expression in primary and metastatic renal tumors: A comprehensive comparison. Arch. Pathol. Lab. Med. 2012, 136, 1541–1551. [Google Scholar] [CrossRef] [Green Version]
- Hueber, P.A.; Waters, P.; Clark, P.; Eccles, M.; Goodyer, P. PAX2 inactivation enhances cisplatin-induced apoptosis in renal carcinoma cells. Kidney Int. 2006, 69, 1139–1145, Erratum in Kidney Int. 2006, 70, 1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, S.T.J.; Grimley, E.; Laszczyk, A.M.; Lee, P.H.; Patel, S.R.; Dressler, G.R. Identification of Pax protein inhibitors that suppress target gene expression and cancer cell proliferation. Cell Chem. Biol. 2022, 29, 412–422.e4. [Google Scholar] [CrossRef]
- Doberstein, K.; Pfeilschifter, J.; Gutwein, P. The transcription factor PAX2 regulates ADAM10 expression in renal cell carcinoma. Carcinogenesis 2011, 32, 1713–1723. [Google Scholar] [CrossRef] [Green Version]
- Singla, M.; Kumar, A.; Bal, A.; Sarkar, S.; Bhattacharyya, S. Epithelial to mesenchymal transition induces stem cell like phenotype in renal cell carcinoma cells. Cancer Cell Int. 2018, 18, 57. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Li, C.G.; Chantry, A.; Stayner, C.; Horsfield, J.; Eccles, M.R. SMAD proteins directly suppress PAX2 transcription downstream of transforming growth factor-beta 1 (TGF-β1) signalling in renal cell carcinoma. Oncotarget 2018, 9, 26852–26867. [Google Scholar] [CrossRef] [Green Version]
- Luu, V.D.; Boysen, G.; Struckmann, K.; Casagrande, S.; von Teichman, A.; Wild, P.J.; Sulser, T.; Schraml, P.; Moch, H. Loss of VHL and hypoxia provokes PAX2 up-regulation in clear cell renal cell carcinoma. Clin. Cancer Res. 2009, 15, 3297–3304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.A.; Hirosue, S.; Rodrigues, P.; Vojtasova, E.; Richardson, E.K.; Ge, J.; Syafruddin, S.E.; Speed, A.; Papachristou, E.K.; Baker, D.; et al. The renal lineage factor PAX8 controls oncogenic signalling in kidney cancer. Nature 2022, 606, 999–1006. [Google Scholar] [CrossRef]
- Bleu, M.; Gaulis, S.; Lopes, R.; Sprouffske, K.; Apfel, V.; Holwerda, S.; Pregnolato, M.; Yildiz, U.; Cordo’, V.; Dost, A.F.M.; et al. PAX8 activates metabolic genes via enhancer elements in Renal Cell Carcinoma. Nat. Commun. 2019, 10, 3739. [Google Scholar] [CrossRef] [Green Version]
- Li, C.G.; Nyman, J.E.; Braithwaite, A.W.; Eccles, M.R. PAX8 promotes tumor cell growth by transcriptionally regulating E2F1 and stabilizing RB protein. Oncogene 2011, 30, 4824–4834. [Google Scholar] [CrossRef] [Green Version]
- Lun, J.; Wang, Y.; Gao, Q.; Wang, Y.; Zhang, H.; Fang, J. PHD3 inhibits cell proliferation through hydroxylation of PAX2 at proline 9. Acta Biochim. Biophys. Sin. Shanghai 2022, 54, 708–715. [Google Scholar] [CrossRef]
- Song, H.; Kwan, S.Y.; Izaguirre, D.I.; Zu, Z.; Tsang, Y.T.; Tung, C.S.; King, E.R.; Mok, S.C.; Gershenson, D.M.; Wong, K.K. PAX2 Expression in Ovarian Cancer. Int. J. Mol. Sci. 2013, 14, 6090–6105. [Google Scholar] [CrossRef] [Green Version]
- Baumann Kubetzko, F.B.; Di Paolo, C.; Maag, C.; Meier, R.; Schäfer, B.W.; Betts, D.R.; Stahel, R.A.; Himmelmann, A. The PAX5 oncogene is expressed in N-type neuroblastoma cells and increases tumorigenicity of a S-type cell line. Carcinogenesis 2004, 25, 1839–1846. [Google Scholar] [CrossRef]
- Geeleher, P.; Cox, N.; Huang, R.S. pRRophetic: An R package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS ONE 2014, 9, e107468. [Google Scholar] [CrossRef] [Green Version]
- Lang, D.; Powell, S.K.; Plummer, R.S.; Young, K.P.; Ruggeri, B.A. PAX genes: Roles in development, pathophysiology, and cancer. Biochem. Pharmacol. 2007, 73, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Eccles, M.R.; Wallis, L.J.; Fidler, A.E.; Spurr, N.K.; Goodfellow, P.J.; Reeve, A.E. Expression of the PAX2 gene in human fetal kidney and Wilms’ tumor. Cell Growth Differ. 1992, 3, 279–289. [Google Scholar]
- Kaddour, H.; Coppola, E.; Di Nardo, A.A.; Le Poupon, C.; Mailly, P.; Wizenmann, A.; Volovitch, M.; Prochiantz, A.; Pierani, A. Extracellular Pax6 Regulates Tangential Cajal-Retzius Cell Migration in the Developing Mouse Neocortex. Cereb. Cortex. 2020, 30, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Harshman, L.A.; Brophy, P.D. PAX2 in human kidney malformations and disease. Pediatr. Nephrol. 2012, 27, 1265–1275. [Google Scholar] [CrossRef]
- Lee, S.; Jeon, H.G.; Kwak, C.; Kim, H.H.; Byun, S.S.; Lee, S.E.; Lee, E. Gender-specific clinicopathological features and survival in patients with renal cell carcinoma (RCC). BJU Int. 2012, 110, E28–E33. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Cecconi, F.; Bernier, G.; Andrejewski, N.; Kammandel, B.; Wagner, M.; Gruss, P. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development 2000, 127, 4325–4334. [Google Scholar] [CrossRef] [PubMed]
- Hans, S.; Liu, D.; Westerfield, M. Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development 2004, 131, 5091–5102. [Google Scholar] [CrossRef] [Green Version]
- Grote, D.; Souabni, A.; Busslinger, M.; Bouchard, M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 2006, 133, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Naylor, R.W.; Przepiorski, A.; Ren, Q.; Yu, J.; Davidson, A.J. HNF1β is essential for nephron segmentation during nephrogenesis. J. Am. Soc. Nephrol. 2013, 24, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessely, O.; Shankland, S.J. The proliferative and the antifibrotic side of PAX2 in tubular repair. Kidney Int. 2022, 102, 12–13. [Google Scholar] [CrossRef]
- Allison, S.J. PAX8: A candidate oncogene in RCC. Nat. Rev. Nephrol. 2019, 15, 662. [Google Scholar] [CrossRef] [PubMed]
- Hueber, P.A.; Iglesias, D.; Chu, L.L.; Eccles, M.; Goodyer, P. In vivo validation of PAX2 as a target for renal cancer therapy. Cancer Lett. 2008, 265, 148–155. [Google Scholar] [CrossRef]
- Roh, M.H.; Yassin, Y.; Miron, A.; Mehra, K.K.; Mehrad, M.; Monte, N.M.; Mutter, G.L.; Nucci, M.R.; Ning, G.; Mckeon, F.D.; et al. High-grade fimbrial-ovarian carcinomas are unified by altered p53, PTEN and PAX2 expression. Mod. Pathol. 2010, 23, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Modi, D.A.; Tagare, R.D.; Karthikeyan, S.; Russo, A.; Dean, M.; Davis, D.A.; Lantvit, D.D.; Burdette, J.E. PAX2 function, regulation and targeting in fallopian tube-derived high-grade serous ovarian cancer. Oncogene 2017, 36, 3015–3024. [Google Scholar] [CrossRef] [Green Version]
- Ueda, T.; Ito, S.; Shiraishi, T.; Kulkarni, P.; Ueno, A.; Nakagawa, H.; Kimura, Y.; Hongo, F.; Kamoi, K.; Kawauchi, A.; et al. Hyper-expression of PAX2 in human metastatic prostate tumors and its role as a cancer promoter in an in vitro invasion model. Prostate 2014, 73, 1403–1412, Erratum in Prostate 2014, 74, 450. [Google Scholar] [CrossRef]
- Frost, V.; Grocott, T.; Eccles, M.R.; Chantry, A. Self-regulated Pax gene expression and modulation by the TGFbeta superfamily. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Chen, Y.; Yang, H.; Cheng, W.; Zhang, S.; Liu, Y.; Liu, Q.; Liu, D.; Yang, G.; Li, K. Identification and Verification of Diagnostic Biomarkers for Glomerular Injury in Diabetic Nephropathy Based on Machine Learning Algorithms. Front. Endocrinol. 2022, 13, 876960. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Ye, L.; Xu, Y.; Sun, Q.; Gao, L.; Cai, J.; Ye, Z.; Tian, D.; Chen, Q. IRF2-ferroptosis related gene is associated with prognosis and EMT in gliomas. Transl. Oncol. 2022, 26, 101544. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, M.D.; Hayes, D.N. ConsensusClusterPlus: A class discovery tool with confidence assessments and item tracking. Bioinformatics 2010, 26, 1572–1573. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Yang, P.; Tang, S.; Zhu, Z.; Li, X.; Yang, Z.; Wu, R.; Tian, X.; Li, L. Classification of Lung Adenocarcinoma Based on Immune Checkpoint and Screening of Related Genes. J. Oncol. 2021, 2021, 5512325. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Murphy, D. Application of ggplot2 to Pharmacometric Graphics. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e79. [Google Scholar] [CrossRef]
- Liao, X.; Wang, W.; Yu, B.; Tan, S. Thrombospondin-2 acts as a bridge between tumor extracellular matrix and immune infiltration in pancreatic and stomach adenocarcinomas: An integrative pan-cancer analysis. Cancer Cell Int. 2022, 22, 213. [Google Scholar] [CrossRef]


| Clinical Features | KICH | KIRC | KIRP | |
|---|---|---|---|---|
| Status | Alive | 55 | 357 | 244 |
| Dead | 10 | 173 | 44 | |
| Age | Mean (SD) | 51.9 (14.1) | 60.6 (12.1) | 61.6 (11.9) |
| Median [MIN, MAX] | 50 [17, 86] | 61 [26, 90] | 61 [28, 88] | |
| Gender | Female | 26 | 186 | 76 |
| Male | 39 | 344 | 212 | |
| Race | Asian | 2 | 8 | 6 |
| Black | 4 | 56 | 60 | |
| White | 57 | 459 | 205 | |
| American Indian | 2 | |||
| pT_stage | T1 | 20 | 271 | 199 |
| T2 | 25 | 69 | 36 | |
| T3 | 18 | 179 | 47 | |
| T4 | 2 | 11 | 2 | |
| TX | 4 | |||
| pN_stage | N0 | 39 | 239 | 143 |
| N1 | 3 | 16 | 24 | |
| N2 | 2 | 3 | ||
| NX | 21 | 275 | 118 | |
| pM_stage | M0 | 50 | 440 | 205 |
| M1 | 2 | 80 | 9 | |
| MX | 13 | 10 | 74 | |
| pTNM_stage | I | 20 | 265 | 179 |
| II | 25 | 57 | 25 | |
| III | 14 | 123 | 51 | |
| IV | 6 | 82 | 15 |
| Target Gene | Forward Primer | Reverse Primer |
|---|---|---|
| PAX1 | ACCCCCGCAGTGAATGG | TGTACACGCCGTGCTGGTT |
| PAX2 | CCTGGCCACACCATTGTTC | TCACGTTTCCTCTTCTCACCAT |
| PAX3 | ACGCGGTCTGTGATCGAAACA | TCTCGCTTTCCTCTGCCTCCTT |
| PAX4 | CAGAGGCACTGGAGAAAGAGTTC | CCATTTGGCTCTTCTGTTGGA |
| PAX5 | GTGCCTGGGAGTGAGTTTTCC | GGCGGCAGCGCTATAATAGT |
| PAX6 | GAGGCTCAAATGCGACTTCAG | TGCTAGTCTTTCTCGGGCAAA |
| PAX7 | GGAAGAAAGAGGAGGAGGATGAG | CCAGCCGGTTCCCTTTGT |
| PAX8 | TGAGGGCGTCTGTGACAATG | CGGGACTCAGGGACTTGGT |
| PAX9 | AGTACGGTCAGGCACCAAATG | ATAACCAGAAGGAGCAGCACTGTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, C.G.; Almomani, S.N.; Hossain, S.M.; Eccles, M.R. Co-Expression of Multiple PAX Genes in Renal Cell Carcinoma (RCC) and Correlation of High PAX Expression with Favorable Clinical Outcome in RCC Patients. Int. J. Mol. Sci. 2023, 24, 11432. https://doi.org/10.3390/ijms241411432
Li L, Li CG, Almomani SN, Hossain SM, Eccles MR. Co-Expression of Multiple PAX Genes in Renal Cell Carcinoma (RCC) and Correlation of High PAX Expression with Favorable Clinical Outcome in RCC Patients. International Journal of Molecular Sciences. 2023; 24(14):11432. https://doi.org/10.3390/ijms241411432
Chicago/Turabian StyleLi, Lei, Caiyun G. Li, Suzan N. Almomani, Sultana Mehbuba Hossain, and Michael R. Eccles. 2023. "Co-Expression of Multiple PAX Genes in Renal Cell Carcinoma (RCC) and Correlation of High PAX Expression with Favorable Clinical Outcome in RCC Patients" International Journal of Molecular Sciences 24, no. 14: 11432. https://doi.org/10.3390/ijms241411432





