Leptomeningeal Metastases in Melanoma Patients: An Update on and Future Perspectives for Diagnosis and Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Anatomical Structure
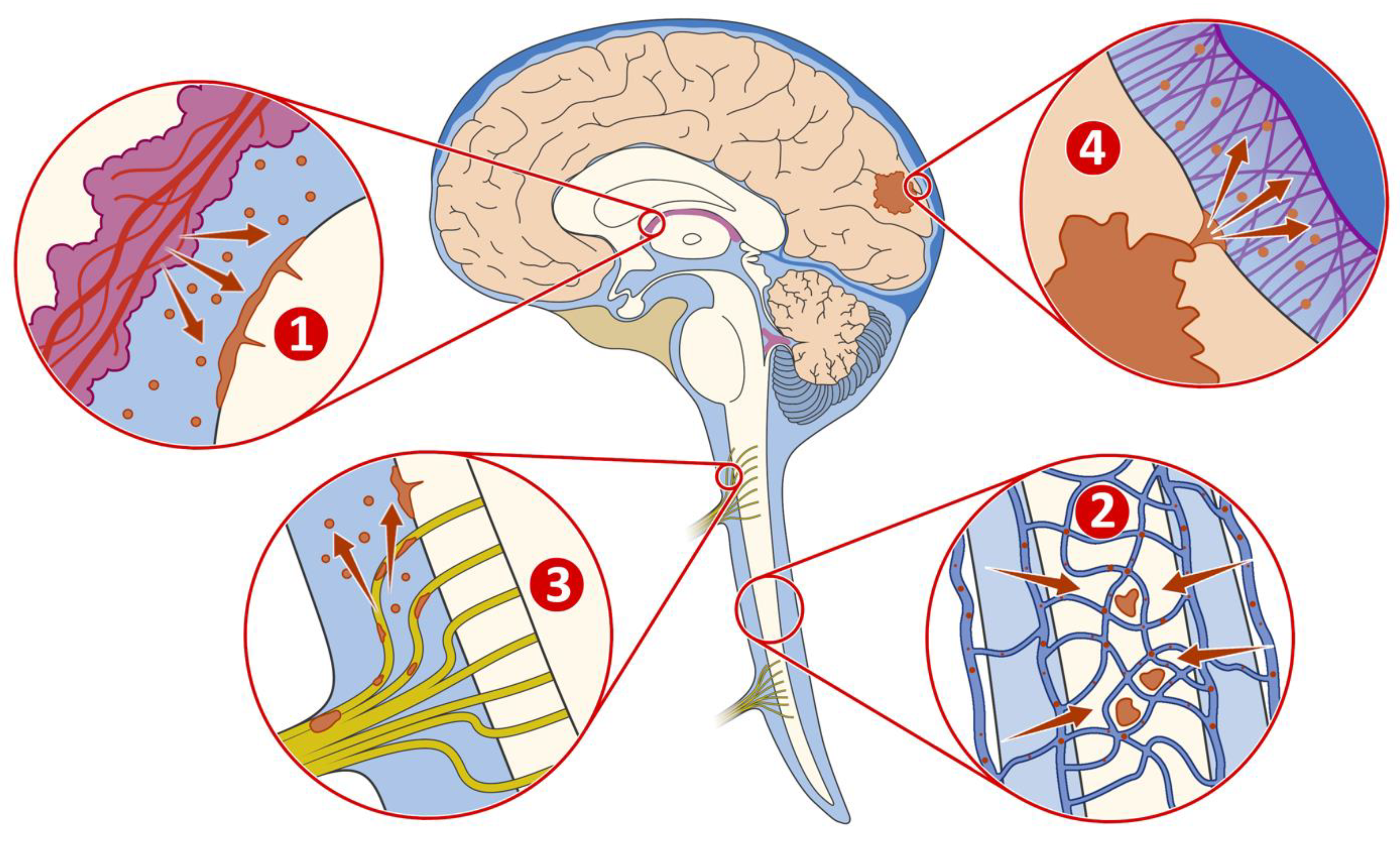
4. Tumour Microenvironment
5. Diagnosis
5.1. Current Standard in Diagnostics
5.1.1. Symptoms
5.1.2. CSF
5.1.3. MRI
- -
- In MM, high-risk patients (stage IIC and higher) should undergo imaging every six months for the first three years after diagnosis, according to the German guidelines [38]. This interval should be shortened in the presence of locoregional or distant metastases.
- -
- In breast cancer, brain imaging should not be routinely performed in all asymptomatic patients at initial diagnosis of metastases or during disease surveillance [37]. In some subtypes (asymptomatic HER2-positive breast cancer or triple negative breast cancer), brain metastases are more common at the initial diagnosis of metastases. This may justify subtype-specific brain imaging in asymptomatic patients with metastatic breast cancer.
- -
- Patients with small cell lung cancer should receive prophylactic cranial irradiation (PCI) if they are in remission after completing chemo-radiotherapy [36]. In patients who have not received PCI, the ESMO guidelines recommend regular brain MRI [39,40]. However, the use of PCI does not appear to have any effect on the development of LMD [41].
- -
- After successful curative therapy, imaging is not recommended for the detection of brain metastases in clinically normal patients with non-small cell lung cancer (NSCLC), as there are currently no clinical data on outcomes [36]. However, advanced NSCLC has a very high metastatic potential: In stage III, in addition to the relatively high risk of locoregional recurrence and the risk of developing distant metastases, there is also a high risk of developing brain metastases. In addition to systemic metastases outside the CNS, stage III patients have a cumulative risk of up to 50% of developing brain metastases at five years [42,43].
5.2. Novel Perspectives in Diagnostics
5.2.1. Circulating Tumour Cells (CTCs)
5.2.2. Cell-Free Tumour DNA (ctDNA)
5.2.3. Cell-Free RNA (cfRNA)
6. Therapy
- Possible reasons for this still poor survival could include:
- Diagnosis remains challenging, as outlined above.
- At the time of LMD diagnosis, most patients have been exposed to various drugs, specifically ICIs and targeted therapies. LMD cells might represent a subpopulation of resistant cells in a “sheltered” TME.
- In contrast to parenchymal metastasis, local tumour control with stereotactic radiotherapy (RTx) is often not possible due to the distribution of LMD.
- Studies suggest a reprogramming of the LMD TME with a dysfunctional T cell landscape, making systemic therapy less effective [25].
- While we are seeing an increase in clinical trials for patients with brain metastases from various tumour types, dedicated clinical trials for LMD patients are largely absent.
- LMD often leads to rapid decline and significant morbidity, often resulting in the recommendation of supportive care only.
6.1. Systemic Therapy
6.1.1. Chemotherapy
6.1.2. Immunotherapy
6.1.3. Targeted Therapy
6.2. Intrathecal Therapy
6.2.1. Chemotherapy
6.2.2. Interleukin-2
6.2.3. Immunotherapy
6.3. Radiotherapy (RTx)
6.4. Novel Perspectives in Treatment
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cohen, J.V.; Tawbi, H.; Margolin, K.A.; Amravadi, R.; Bosenberg, M.; Brastianos, P.K.; Chiang, V.L.; de Groot, J.; Glitza, I.C.; Herlyn, M.; et al. Melanoma Central Nervous System Metastases: Current Approaches, Challenges, and Opportunities. Pigment. Cell Melanoma Res. 2016, 29, 627–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, S.D.; Bindal, S.; Bassett, R.L.; Haydu, L.E.; McCutcheon, I.E.; Heimberger, A.B.; Li, J.; O’Brien, B.J.; Guha-Thakurta, N.; Tetzlaff, M.T.; et al. Predictors of Survival in Metastatic Melanoma Patients with Leptomeningeal Disease (LMD). J. Neurooncol. 2019, 142, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Leal, T.; Chang, J.E.; Mehta, M.; Robins, H.I. Leptomeningeal Metastasis: Challenges in Diagnosis and Treatment. Curr. Cancer Ther. Rev. 2011, 7, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Chorti, E.; Kebir, S.; Ahmed, M.S.; Keyvani, K.; Umutlu, L.; Kanaki, T.; Zaremba, A.; Reinboldt-Jockenhoefer, F.; Knispel, S.; Gratsias, E.; et al. Leptomeningeal Disease from Melanoma-Poor Prognosis despite New Therapeutic Modalities. Eur. J. Cancer 2021, 148, 395–404. [Google Scholar] [CrossRef]
- Glitza, I.C.; Phillips, S.; Brown, C.; Haymaker, C.L.; Bassett, R.L.; Lee, J.J.; Rohlfs, M.L.; Richard, J.; Iqbal, M.; John, I.; et al. Single-Center Phase I/Ib Study of Concurrent Intrathecal (IT) and Intravenous (IV) Nivolumab (N) for Metastatic Melanoma (MM) Patients (Pts) with Leptomeningeal Disease (LMD). J. Clin. Oncol. 2020, 38, 10008. [Google Scholar] [CrossRef]
- Kokkoris, C.P. Leptomeningeal Carcinomatosis. How Does Cancer Reach the Pia-Arachnoid? Cancer 1983, 51, 154–160. [Google Scholar] [CrossRef]
- Glover, R.L.; Brook, A.L.; Welch, M.R. Teaching NeuroImages: Leptomeningeal Lung Carcinoma. Neurology 2014, 82, e183–e184. [Google Scholar] [CrossRef] [Green Version]
- Boyle, R.; Thomas, M.; Adams, J.H. Diffuse Involvement of the Leptomeninges by Tumour—A Clinical and Pathological Study of 63 Cases. Postgrad. Med. J. 1980, 56, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Kim, S.; Joo, J.; Shin, K.H.; Gwak, H.-S.; Lee, S.H. Incidence and Risk Factors for Leptomeningeal Carcinomatosis in Breast Cancer Patients with Parenchymal Brain Metastases. J. Korean Neurosurg. Soc. 2012, 52, 193–199. [Google Scholar] [CrossRef]
- Khaled, M.L.; Tarhini, A.A.; Forsyth, P.A.; Smalley, I.; Piña, Y. Leptomeningeal Disease (LMD) in Patients with Melanoma Metastases. Cancers 2023, 15, 1884. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, G.; Wang, Y.; Yuan, T.; Gao, Y.; Dong, L. Leptomeningeal Metastases from a Primary Central Nervous System Melanoma: A Case Report and Literature Review. World J. Surg. Oncol. 2014, 12, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.-Y.; Hsieh, K.L.-C.; Tsang, Y.-M.; Cheung, W.-K.; Hsieh, C.-H. Primary Leptomeningeal Melanoma. J. Clin. Neurosci. 2014, 21, 1051–1052. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, L.M.; Posner, J.B. Neurologic Complications of Cancer; Contemporary Neurology Series; Oxford University Press: Oxford, UK, 2008; ISBN 978-0-19-971055-3. [Google Scholar]
- Remsik, J.; Chi, Y.; Tong, X.; Sener, U.; Derderian, C.; Park, A.; Saadeh, F.; Bale, T.; Boire, A. Leptomeningeal Metastatic Cells Adopt Two Phenotypic States. Cancer Rep. 2022, 5, e1236. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, F.; Boire, A. Leptomeningeal Disease and the Role of Intrathecal Therapy. In Central Nervous System Metastases: Diagnosis and Treatment; Ramakrishna, R., Magge, R.S., Baaj, A.A., Knisely, J.P.S., Eds.; Springer: Cham, Switzerland, 2020; pp. 169–186. ISBN 978-3-030-42958-4. [Google Scholar]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The Human Tumor Microbiome Is Composed of Tumor Type–Specific Intracellular Bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Junttila, M.R.; de Sauvage, F.J. Influence of Tumour Micro-Environment Heterogeneity on Therapeutic Response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef]
- Zhao, K.; Hu, Y. Microbiome Harbored within Tumors: A New Chance to Revisit Our Understanding of Cancer Pathogenesis and Treatment. Signal Transduct. Target. Ther. 2020, 5, 1–3. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun. Signal 2020, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Fischer, G.M.; Jalali, A.; Kircher, D.A.; Lee, W.-C.; McQuade, J.L.; Haydu, L.E.; Joon, A.Y.; Reuben, A.; de Macedo, M.P.; Carapeto, F.C.L.; et al. Molecular Profiling Reveals Unique Immune and Metabolic Features of Melanoma Brain Metastases. Cancer Discov. 2019, 9, 628–645. [Google Scholar] [CrossRef] [Green Version]
- Giridharan, N.; Glitza Oliva, I.C.; O’Brien, B.J.; Parker Kerrigan, B.C.; Heimberger, A.B.; Ferguson, S.D. Targeting the Tumor Microenvironment in Brain Metastasis. Neurosurg. Clin. N. Am. 2020, 31, 641–649. [Google Scholar] [CrossRef]
- Spector, R.; Robert Snodgrass, S.; Johanson, C.E. A Balanced View of the Cerebrospinal Fluid Composition and Functions: Focus on Adult Humans. Exp. Neurol. 2015, 273, 57–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boire, A.; Zou, Y.; Shieh, J.; Macalinao, D.G.; Pentsova, E.; Massagué, J. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell 2017, 168, 1101–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smalley, I.; Chen, Z.; Phadke, M.; Li, J.; Yu, X.; Wyatt, C.; Evernden, B.; Messina, J.L.; Sarnaik, A.; Sondak, V.K.; et al. Single-Cell Characterization of the Immune Microenvironment of Melanoma Brain and Leptomeningeal Metastases. Clin. Cancer Res. 2021, 27, 4109–4125. [Google Scholar] [CrossRef] [PubMed]
- Smalley, I.; Law, V.; Wyatt, C.; Evernden, B.; Fang, B.; Koomen, J.M.; Welsh, E.A.; Macaulay, R.J.B.; Forsyth, P.A.; Smalley, K.S.M. Proteomic Analysis of CSF from Patients with Leptomeningeal Melanoma Metastases Identifies Signatures Associated with Disease Progression and Therapeutic Resistance. Clin. Cancer Res. 2020, 26, 2163–2175. [Google Scholar] [CrossRef] [Green Version]
- Law, V.; Chen, Z.; Vena, F.; Smalley, I.; Macaulay, R.; Evernden, B.R.; Tran, N.; Pina, Y.; Puskas, J.; Caceres, G.; et al. A Preclinical Model of Patient-Derived Cerebrospinal Fluid Circulating Tumor Cells for Experimental Therapeutics in Leptomeningeal Disease from Melanoma. Neuro Oncol. 2022, 24, 1673–1686. [Google Scholar] [CrossRef]
- Le Rhun, E.; Taillibert, S.; Chamberlain, M.C. Carcinomatous Meningitis: Leptomeningeal Metastases in Solid Tumors. Surg. Neurol. Int. 2013, 4, S265–S288. [Google Scholar] [CrossRef]
- Nayak, L.; Fleisher, M.; Gonzalez-Espinoza, R.; Lin, O.; Panageas, K.; Reiner, A.; Liu, C.-M.; Deangelis, L.M.; Omuro, A. Rare Cell Capture Technology for the Diagnosis of Leptomeningeal Metastasis in Solid Tumors. Neurology 2013, 80, 1598–1605. [Google Scholar] [CrossRef] [Green Version]
- Subirá, D.; Serrano, C.; Castañón, S.; Gonzalo, R.; Illán, J.; Pardo, J.; Martínez-García, M.; Millastre, E.; Aparisi, F.; Navarro, M.; et al. Role of Flow Cytometry Immunophenotyping in the Diagnosis of Leptomeningeal Carcinomatosis. Neuro Oncol. 2012, 14, 43–52. [Google Scholar] [CrossRef]
- Subirá, D.; Simó, M.; Illán, J.; Serrano, C.; Castañón, S.; Gonzalo, R.; Granizo, J.J.; Martínez-García, M.; Navarro, M.; Pardo, J.; et al. Diagnostic and Prognostic Significance of Flow Cytometry Immunophenotyping in Patients with Leptomeningeal Carcinomatosis. Clin. Exp. Metastasis 2015, 32, 383–391. [Google Scholar] [CrossRef]
- Lee, J.S.; Melisko, M.E.; Magbanua, M.J.M.; Kablanian, A.T.; Scott, J.H.; Rugo, H.S.; Park, J.W. Detection of Cerebrospinal Fluid Tumor Cells and Its Clinical Relevance in Leptomeningeal Metastasis of Breast Cancer. Breast Cancer Res. Treat. 2015, 154, 339–349. [Google Scholar] [CrossRef]
- Wasserstrom, W.R.; Glass, J.P.; Posner, J.B. Diagnosis and Treatment of Leptomeningeal Metastases from Solid Tumors: Experience with 90 Patients. Cancer 1982, 49, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Cagney, D.N.; Lamba, N.; Sinha, S.; Catalano, P.J.; Bi, W.L.; Alexander, B.M.; Aizer, A.A. Association of Neurosurgical Resection With Development of Pachymeningeal Seeding in Patients With Brain Metastases. JAMA Oncol. 2019, 5, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Glitza, I.C.; Smalley, K.S.M.; Brastianos, P.K.; Davies, M.A.; McCutcheon, I.; Liu, J.K.C.; Ahmed, K.A.; Arrington, J.A.; Evernden, B.R.; Smalley, I.; et al. Leptomeningeal Disease in Melanoma Patients: An Update to Treatment, Challenges, and Future Directions. Pigment. Cell. Melanoma Res. 2020, 33, 527–541. [Google Scholar] [CrossRef] [Green Version]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Prävention, Diagnostik, Therapie Und Nachsorge Des Lungenkarzinoms, Lang-Version 1.0, 2018, AWMF-Registernummer: 020/007OL. Available online: http://Leitlinienprogramm-on-Kologie.de/Lungenkarzinom.98.0.Html (accessed on 10 July 2023).
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF):Diagnostik, Therapie Und Nachsorge Des Melanoms, Langversion 3.3, 2020, AWMF Registernummer: 032/024OL. Available online: Http://Www.Leitlinienprogramm-Onkologie.de/Leitlinien/Melanom/ (accessed on 31 August 2021).
- Dingemans, A.-M.C.; Früh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up☆. Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef]
- Takahashi, T.; Yamanaka, T.; Seto, T.; Harada, H.; Nokihara, H.; Saka, H.; Nishio, M.; Kaneda, H.; Takayama, K.; Ishimoto, O.; et al. Prophylactic Cranial Irradiation versus Observation in Patients with Extensive-Disease Small-Cell Lung Cancer: A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 663–671. [Google Scholar] [CrossRef]
- Rosen, S.T.; Makuch, R.W.; Lichter, A.S.; Ihde, D.C.; Matthews, M.J.; Minna, J.D.; Glatstein, E.; Bunn, P.A. Role of Prophylactic Cranial Irradiation in Prevention of Central Nervous System Metastases in Small Cell Lung Cancer: Potential Benefit Restricted to Patients with Complete Response. Am. J. Med. 1983, 74, 615–624. [Google Scholar] [CrossRef]
- Bajard, A.; Westeel, V.; Dubiez, A.; Jacoulet, P.; Pernet, D.; Dalphin, J.C.; Depierre, A. Multivariate Analysis of Factors Predictive of Brain Metastases in Localised Non-Small Cell Lung Carcinoma. Lung Cancer 2004, 45, 317–323. [Google Scholar] [CrossRef]
- Gaspar, L.E.; Chansky, K.; Albain, K.S.; Vallieres, E.; Rusch, V.; Crowley, J.J.; Livingston, R.B.; Gandara, D.R. Time from Treatment to Subsequent Diagnosis of Brain Metastases in Stage III Non-Small-Cell Lung Cancer: A Retrospective Review by the Southwest Oncology Group. J. Clin. Oncol. 2005, 23, 2955–2961. [Google Scholar] [CrossRef]
- Boire, A.; Brandsma, D.; Brastianos, P.K.; Le Rhun, E.; Ahluwalia, M.; Junck, L.; Glantz, M.; Groves, M.D.; Lee, E.Q.; Lin, N.; et al. Liquid Biopsy in Central Nervous System Metastases: A RANO Review and Proposals for Clinical Applications. Neuro Oncol. 2019, 21, 571–584. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Fleisher, M.; Rosenblum, M.; Lin, O.; Boire, A.; Briggs, S.; Bensman, Y.; Hurtado, B.; Shagabayeva, L.; DeAngelis, L.M.; et al. Cerebrospinal Fluid Circulating Tumor Cells: A Novel Tool to Diagnose Leptomeningeal Metastases from Epithelial Tumors. Neuro Oncol. 2017, 19, 1248–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Rhun, E.; Tu, Q.; De Carvalho Bittencourt, M.; Farre, I.; Mortier, L.; Cai, H.; Kohler, C.; Faure, G.C. Detection and Quantification of CSF Malignant Cells by the CellSearch Technology in Patients with Melanoma Leptomeningeal Metastasis. Med. Oncol. 2013, 30, 538. [Google Scholar] [CrossRef] [PubMed]
- Campoli, M.R.; Chang, C.-C.; Kageshita, T.; Wang, X.; McCarthy, J.B.; Ferrone, S. Human High Molecular Weight-Melanoma-Associated Antigen (HMW-MAA): A Melanoma Cell Surface Chondroitin Sulfate Proteoglycan (MSCP) with Biological and Clinical Significance. Crit. Rev. Immunol. 2004, 24, 267–296. [Google Scholar] [CrossRef]
- Diaz, M.; Singh, P.; Kotchetkov, I.S.; Skakodub, A.; Meng, A.; Tamer, C.; Young, R.J.; Reiner, A.S.; Panageas, K.S.; Ramanathan, L.V.; et al. Quantitative Assessment of Circulating Tumor Cells in Cerebrospinal Fluid as a Clinical Tool to Predict Survival in Leptomeningeal Metastases. J. Neurooncol. 2022, 157, 81–90. [Google Scholar] [CrossRef]
- van Bussel, M.T.J.; Pluim, D.; Bol, M.; Beijnen, J.H.; Schellens, J.H.M.; Brandsma, D. EpCAM-Based Assays for Epithelial Tumor Cell Detection in Cerebrospinal Fluid. J. Neurooncol. 2018, 137, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pellerino, A.; Brastianos, P.K.; Rudà, R.; Soffietti, R. Leptomeningeal Metastases from Solid Tumors: Recent Advances in Diagnosis and Molecular Approaches. Cancers 2021, 13, 2888. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.-A.; Koo, G.-B.; Han, H.; Sohn, J.; Choi, W.; Kim, S.-I.; Jung, H.-I.; Kim, Y.-S. Epithelial-to-Mesenchymal Transition Leads to Loss of EpCAM and Different Physical Properties in Circulating Tumor Cells from Metastatic Breast Cancer. Oncotarget 2016, 7, 24677–24687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spisák, S.; Solymosi, N.; Ittzés, P.; Bodor, A.; Kondor, D.; Vattay, G.; Barták, B.K.; Sipos, F.; Galamb, O.; Tulassay, Z.; et al. Complete Genes May Pass from Food to Human Blood. PLoS ONE 2013, 8, e69805. [Google Scholar] [CrossRef] [Green Version]
- Telekes, A.; Horváth, A. The Role of Cell-Free DNA in Cancer Treatment Decision Making. Cancers 2022, 14, 6115. [Google Scholar] [CrossRef]
- Ferguson, S.D.; Fomchenko, E.I.; Guerrieri, R.A.; Glitza Oliva, I.C. Challenges and Advances in Diagnosis and Treatment of Leptomeningeal Disease (LMD). Front. Oncol. 2021, 11, 800053. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Siravegna, G. How to Use Liquid Biopsies to Treat Patients with Cancer. ESMO Open. 2021, 6, 100060. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [Green Version]
- De Mattos-Arruda, L.; Mayor, R.; Ng, C.K.Y.; Weigelt, B.; Martínez-Ricarte, F.; Torrejon, D.; Oliveira, M.; Arias, A.; Raventos, C.; Tang, J.; et al. Cerebrospinal Fluid-Derived Circulating Tumour DNA Better Represents the Genomic Alterations of Brain Tumours than Plasma. Nat. Commun. 2015, 6, 8839. [Google Scholar] [CrossRef] [Green Version]
- Ying, S.; Ke, H.; Ding, Y.; Liu, Y.; Tang, X.; Yang, D.; Li, M.; Liu, J.; Yu, B.; Xiang, J.; et al. Unique Genomic Profiles Obtained from Cerebrospinal Fluid Cell-Free DNA of Non-Small Cell Lung Cancer Patients with Leptomeningeal Metastases. Cancer Biol. Ther. 2019, 20, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Ballester, L.Y.; Glitza Oliva, I.C.; Douse, D.Y.; Chen, M.M.; Lan, C.; Haydu, L.E.; Huse, J.T.; Roy-Chowdhuri, S.; Luthra, R.; Wistuba, I.I.; et al. Evaluating Circulating Tumor DNA From the Cerebrospinal Fluid of Patients With Melanoma and Leptomeningeal Disease. J. Neuropathol. Exp. Neurol. 2018, 77, 628–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janku, F.; Huang, H.J.; Claes, B.; Falchook, G.S.; Fu, S.; Hong, D.; Ramzanali, N.M.; Nitti, G.; Cabrilo, G.; Tsimberidou, A.M.; et al. BRAF Mutation Testing in Cell-Free DNA from the Plasma of Patients with Advanced Cancers Using a Rapid, Automated Molecular Diagnostics System. Mol. Cancer Ther. 2016, 15, 1397–1404. [Google Scholar] [CrossRef] [Green Version]
- Carvajal, R.D.; Butler, M.O.; Shoushtari, A.N.; Hassel, J.C.; Ikeguchi, A.; Hernandez-Aya, L.; Nathan, P.; Hamid, O.; Piulats, J.M.; Rioth, M.; et al. Clinical and Molecular Response to Tebentafusp in Previously Treated Patients with Metastatic Uveal Melanoma: A Phase 2 Trial. Nat. Med. 2022, 28, 2364–2373. [Google Scholar] [CrossRef]
- Wijetunga, N.A.; Goglia, A.G.; Weinhold, N.; Berger, M.F.; Cislo, M.; Higginson, D.S.; Chabot, K.; Osman, A.M.; Schaff, L.; Pentsova, E.; et al. Dynamic Mutational Landscape of Cerebrospinal Fluid Circulating Tumor DNA and Predictors of Survival after Proton Craniospinal Irradiation for Leptomeningeal Metastases. Clin. Cancer Res. 2023, 29, 775–783. [Google Scholar] [CrossRef]
- White, M.D.; Klein, R.H.; Shaw, B.; Kim, A.; Subramanian, M.; Mora, J.L.; Giobbie-Hurder, A.; Nagabhushan, D.; Jain, A.; Singh, M.; et al. Detection of Leptomeningeal Disease Using Cell-Free DNA From Cerebrospinal Fluid. JAMA Netw. Open. 2021, 4, e2120040. [Google Scholar] [CrossRef]
- Tosevska, A.; Morselli, M.; Basak, S.K.; Avila, L.; Mehta, P.; Wang, M.B.; Srivatsan, E.S.; Pellegrini, M. Cell-Free RNA as a Novel Biomarker for Response to Therapy in Head & Neck Cancer. Front. Oncol. 2022, 12, 869108. [Google Scholar] [CrossRef]
- de Unamuno Bustos, B.; Murria Estal, R.; Pérez Simó, G.; de Juan Jimenez, I.; Escutia Muñoz, B.; Rodríguez Serna, M.; Alegre de Miquel, V.; Llavador Ros, M.; Ballester Sánchez, R.; Nagore Enguídanos, E.; et al. Towards Personalized Medicine in Melanoma: Implementation of a Clinical Next-Generation Sequencing Panel. Sci. Rep. 2017, 7, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, L.J.; Höwner, A.; Griewank, K.; Lueong, S.S.; von Neuhoff, N.; Horn, P.A.; Sucker, A.; Paschen, A.; Livingstone, E.; Ugurel, S.; et al. Circulating Cell-Free Messenger RNA Enables Non-Invasive Pan-Tumour Monitoring of Melanoma Therapy Independent of the Mutational Genotype. Clin. Transl. Med. 2022, 12, e1090. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Polyak, D.; Lamsam, L.; Connolly, I.D.; Johnson, E.; Khoeur, L.K.; Andersen, S.; Granucci, M.; Stanley, G.; Liu, B.; et al. Comprehensive RNA Analysis of CSF Reveals a Role for CEACAM6 in Lung Cancer Leptomeningeal Metastases. NPJ Precis. Oncol. 2021, 5, 90. [Google Scholar] [CrossRef]
- Steininger, J.; Gellrich, F.F.; Schulz, A.; Westphal, D.; Beissert, S.; Meier, F. Systemic Therapy of Metastatic Melanoma: On the Road to Cure. Cancers 2021, 13, 1430. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Forsyth, P.A.; Hodi, F.S.; Algazi, A.P.; Hamid, O.; Lao, C.D.; Moschos, S.J.; Atkins, M.B.; Lewis, K.; Postow, M.A.; et al. Long-Term Outcomes of Patients with Active Melanoma Brain Metastases Treated with Combination Nivolumab plus Ipilimumab (CheckMate 204): Final Results of an Open-Label, Multicentre, Phase 2 Study. Lancet Oncol. 2021, 22, 1692–1704. [Google Scholar] [CrossRef]
- Le Rhun, E.; Weller, M.; Brandsma, D.; Van den Bent, M.; de Azambuja, E.; Henriksson, R.; Boulanger, T.; Peters, S.; Watts, C.; Wick, W.; et al. EANO-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up of Patients with Leptomeningeal Metastasis from Solid Tumours. Ann. Oncol. 2017, 28, iv84–iv99. [Google Scholar] [CrossRef]
- Rudnicka, H.; Niwińska, A.; Murawska, M. Breast Cancer Leptomeningeal Metastasis—The Role of Multimodality Treatment. J. Neurooncol. 2007, 84, 57–62. [Google Scholar] [CrossRef]
- Gauthier, H.; Guilhaume, M.N.; Bidard, F.C.; Pierga, J.Y.; Girre, V.; Cottu, P.H.; Laurence, V.; Livartowski, A.; Mignot, L.; Diéras, V. Survival of Breast Cancer Patients with Meningeal Carcinomatosis. Ann. Oncol. 2010, 21, 2183–2187. [Google Scholar] [CrossRef]
- Lee, S.; Ahn, H.K.; Park, Y.H.; Nam, D.H.; Lee, J.I.; Park, W.; Choi, D.H.; Huh, S.J.; Park, K.T.; Ahn, J.S.; et al. Leptomeningeal Metastases from Breast Cancer: Intrinsic Subtypes May Affect Unique Clinical Manifestations. Breast Cancer Res. Treat. 2011, 129, 809–817. [Google Scholar] [CrossRef]
- de Azevedo, C.R.A.S.; Cruz, M.R.S.; Chinen, L.T.D.; Peres, S.V.; Peterlevitz, M.A.; de Azevedo Pereira, A.E.; Fanelli, M.F.; Gimenes, D.L. Meningeal Carcinomatosis in Breast Cancer: Prognostic Factors and Outcome. J. Neurooncol. 2011, 104, 565–572. [Google Scholar] [CrossRef]
- Lara-Medina, F.; Crismatt, A.; Villarreal-Garza, C.; Alvarado-Miranda, A.; Flores-Hernández, L.; González-Pinedo, M.; Gamboa-Vignolle, C.; Ruiz-González, J.D.S.; Arrieta, O. Clinical Features and Prognostic Factors in Patients with Carcinomatous Meningitis Secondary to Breast Cancer. Breast J. 2012, 18, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Livi, L.; Saieva, C.; Franceschini, D.; Marrazzo, L.; Greto, D.; Scotti, V.; Scoccianti, S.; Paiar, F.; Bordi, L.; et al. Prognostic Factors and Clinical Features in Patients with Leptominengeal Metastases from Breast Cancer: A Single Center Experience. J. Chemother. 2012, 24, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Niwińska, A.; Rudnicka, H.; Murawska, M. Breast Cancer Leptomeningeal Metastasis: Propensity of Breast Cancer Subtypes for Leptomeninges and the Analysis of Factors Influencing Survival. Med. Oncol. 2013, 30, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yust-Katz, S.; Garciarena, P.; Liu, D.; Yuan, Y.; Ibrahim, N.; Yerushalmi, R.; Penas-Prado, M.; Groves, M.D. Breast Cancer and Leptomeningeal Disease (LMD): Hormone Receptor Status Influences Time to Development of LMD and Survival from LMD Diagnosis. J. Neurooncol. 2013, 114, 229–235. [Google Scholar] [CrossRef]
- Le Rhun, E.; Taillibert, S.; Zairi, F.; Kotecki, N.; Devos, P.; Mailliez, A.; Servent, V.; Vanlemmens, L.; Vennin, P.; Boulanger, T.; et al. A Retrospective Case Series of 103 Consecutive Patients with Leptomeningeal Metastasis and Breast Cancer. J. Neurooncol. 2013, 113, 83–92. [Google Scholar] [CrossRef]
- Morris, P.G.; Reiner, A.S.; Szenberg, O.R.; Clarke, J.L.; Panageas, K.S.; Perez, H.R.; Kris, M.G.; Chan, T.A.; DeAngelis, L.M.; Omuro, A.M. Leptomeningeal Metastasis from Non-Small Cell Lung Cancer: Survival and the Impact of Whole Brain Radiotherapy. J. Thorac. Oncol. 2012, 7, 382–385. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Kim, Y.J.; Lee, J.-O.; Lee, K.-W.; Kim, J.H.; Bang, S.-M.; Chung, J.-H.; Kim, J.S.; Lee, J.S. Clinical Outcomes of Leptomeningeal Metastasis in Patients with Non-Small Cell Lung Cancer in the Modern Chemotherapy Era. Lung Cancer 2012, 76, 387–392. [Google Scholar] [CrossRef]
- Gwak, H.-S.; Joo, J.; Kim, S.; Yoo, H.; Shin, S.H.; Han, J.-Y.; Kim, H.T.; Lee, J.S.; Lee, S.H. Analysis of Treatment Outcomes of Intraventricular Chemotherapy in 105 Patients for Leptomeningeal Carcinomatosis from Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Lee, J.-I.; Nam, D.-H.; Ahn, Y.C.; Han, J.H.; Sun, J.-M.; Ahn, J.S.; Park, K.; Ahn, M.-J. Leptomeningeal Carcinomatosis in Non-Small-Cell Lung Cancer Patients: Impact on Survival and Correlated Prognostic Factors. J. Thorac. Oncol. 2013, 8, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Riess, J.W.; Nagpal, S.; Iv, M.; Zeineh, M.; Gubens, M.A.; Ramchandran, K.; Neal, J.W.; Wakelee, H.A. Prolonged Survival of Patients with Non-Small-Cell Lung Cancer with Leptomeningeal Carcinomatosis in the Modern Treatment Era. Clin. Lung Cancer 2014, 15, 202–206. [Google Scholar] [CrossRef]
- Kuiper, J.L.; Hendriks, L.E.; van der Wekken, A.J.; de Langen, A.J.; Bahce, I.; Thunnissen, E.; Heideman, D.A.M.; Berk, Y.; Buijs, E.J.M.; Speel, E.-J.M.; et al. Treatment and Survival of Patients with EGFR-Mutated Non-Small Cell Lung Cancer and Leptomeningeal Metastasis: A Retrospective Cohort Analysis. Lung Cancer 2015, 89, 255–261. [Google Scholar] [CrossRef]
- Harstad, L.; Hess, K.R.; Groves, M.D. Prognostic Factors and Outcomes in Patients with Leptomeningeal Melanomatosis. Neuro Oncol. 2008, 10, 1010–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geukes Foppen, M.H.; Brandsma, D.; Blank, C.U.; van Thienen, J.V.; Haanen, J.B.; Boogerd, W. Targeted Treatment and Immunotherapy in Leptomeningeal Metastases from Melanoma. Ann. Oncol. 2016, 27, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, H.; Yakisich, J.S. Overcoming the Blood-Brain Barrier for Chemotherapy: Limitations, Challenges and Rising Problems. Anticancer Agents Med. Chem. 2014, 14, 1085–1093. [Google Scholar] [CrossRef]
- Segura, P.P.; Gil, M.; Balañá, C.; Chacón, I.; Langa, J.M.; Martín, M.; Bruna, J. Phase II Trial of Temozolomide for Leptomeningeal Metastases in Patients with Solid Tumors. J. Neurooncol. 2012, 109, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.; Andreou, T.; Scott, K.J.; Williams, J.; Rippaus, N.; Brownlie, R.J.; Ilett, E.J.; Salmond, R.J.; Melcher, A.; Lorger, M. Anti-PD-1/Anti-CTLA-4 Efficacy in Melanoma Brain Metastases Depends on Extracranial Disease and Augmentation of CD8+ T Cell Trafficking. Proc. Natl. Acad. Sci. USA 2018, 115, E1540–E1549. [Google Scholar] [CrossRef] [Green Version]
- Van Bussel, M.T.J.; Beijnen, J.H.; Brandsma, D. Intracranial Antitumor Responses of Nivolumab and Ipilimumab: A Pharmacodynamic and Pharmacokinetic Perspective, a Scoping Systematic Review. BMC Cancer 2019, 19, 519. [Google Scholar] [CrossRef] [Green Version]
- Benarroch, E.E. Choroid Plexus--CSF System: Recent Developments and Clinical Correlations. Neurology 2016, 86, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Pluim, D.; Ros, W.; van Bussel, M.T.J.; Brandsma, D.; Beijnen, J.H.; Schellens, J.H.M. Enzyme Linked Immunosorbent Assay for the Quantification of Nivolumab and Pembrolizumab in Human Serum and Cerebrospinal Fluid. J. Pharm. Biomed. Anal. 2019, 164, 128–134. [Google Scholar] [CrossRef]
- Stemmler, H.-J.; Schmitt, M.; Willems, A.; Bernhard, H.; Harbeck, N.; Heinemann, V. Ratio of Trastuzumab Levels in Serum and Cerebrospinal Fluid Is Altered in HER2-Positive Breast Cancer Patients with Brain Metastases and Impairment of Blood-Brain Barrier. Anticancer Drugs 2007, 18, 23–28. [Google Scholar] [CrossRef]
- Prakadan, S.M.; Alvarez-Breckenridge, C.A.; Markson, S.C.; Kim, A.E.; Klein, R.H.; Nayyar, N.; Navia, A.W.; Kuter, B.M.; Kolb, K.E.; Bihun, I.; et al. Genomic and Transcriptomic Correlates of Immunotherapy Response within the Tumor Microenvironment of Leptomeningeal Metastases. Nat. Commun. 2021, 12, 5955. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Atkinson, V.; Lo, S.; Guminski, A.D.; Sandhu, S.K.; Brown, M.P.; Gonzalez, M.; Scolyer, R.A.; Emmett, L.; McArthur, G.A.; et al. Five-Year Overall Survival from the Anti-PD1 Brain Collaboration (ABC Study): Randomized Phase 2 Study of Nivolumab (Nivo) or Nivo+ipilimumab (Ipi) in Patients (Pts) with Melanoma Brain Metastases (Mets). J. Clin. Oncol. 2021, 39, 9508. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Strickland, M.R.; Lee, E.Q.; Wang, N.; Cohen, J.V.; Chukwueke, U.; Forst, D.A.; Eichler, A.; Overmoyer, B.; Lin, N.U.; et al. Phase II Study of Ipilimumab and Nivolumab in Leptomeningeal Carcinomatosis. Nat. Commun. 2021, 12, 5954. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Lee, E.Q.; Cohen, J.V.; Tolaney, S.M.; Lin, N.U.; Wang, N.; Chukwueke, U.; White, M.D.; Nayyar, N.; Kim, A.; et al. Single-Arm, Open-Label Phase 2 Trial of Pembrolizumab in Patients with Leptomeningeal Carcinomatosis. Nat. Med. 2020, 26, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Schreck, K.C.; Fu, W.; Hu, C.; Carvajal-Gonzalez, A.; Connolly, R.M.; Santa-Maria, C.A.; Lipson, E.J.; Holdhoff, M.; Forde, P.M.; et al. Pembrolizumab for Patients with Leptomeningeal Metastasis from Solid Tumors: Efficacy, Safety, and Cerebrospinal Fluid Biomarkers. J. Immunother. Cancer 2021, 9, e002473. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Mehta, U.N.; Dsouza, L.H.; Guadagnolo, B.A.; Sanders, D.L.; Kim, K.B. Long-Term Stabilization of Leptomeningeal Disease with Whole-Brain Radiation Therapy in a Patient with Metastatic Melanoma Treated with Vemurafenib: A Case Report. Melanoma Res. 2013, 23, 175–178. [Google Scholar] [CrossRef]
- Kim, D.W.; Barcena, E.; Mehta, U.N.; Rohlfs, M.L.; Kumar, A.J.; Penas-Prado, M.; Kim, K.B. Prolonged Survival of a Patient with Metastatic Leptomeningeal Melanoma Treated with BRAF Inhibition-Based Therapy: A Case Report. BMC Cancer 2015, 15, 400. [Google Scholar] [CrossRef] [Green Version]
- Glitza, I.C.; Ferguson, S.D.; Guha-Thakurta, N. Rapid Resolution of Leptomeningeal Disease with Targeted Therapy in a Metastatic Melanoma Patient. J. Neurooncol. 2017, 133, 663–665. [Google Scholar] [CrossRef]
- Papadatos-Pastos, D.; Januszewski, A.; Dalgleish, A. Revisiting the Role of Systemic Therapies in Patients with Metastatic Melanoma to the CNS. Expert. Rev. Anticancer Ther. 2013, 13, 559–567. [Google Scholar] [CrossRef]
- Mittapalli, R.K.; Vaidhyanathan, S.; Dudek, A.Z.; Elmquist, W.F. Mechanisms Limiting Distribution of the Threonine-Protein Kinase B-RaF(V600E) Inhibitor Dabrafenib to the Brain: Implications for the Treatment of Melanoma Brain Metastases. J. Pharmacol. Exp. Ther. 2013, 344, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, S.; Hartz, A.M.S.; Elmquist, W.F.; Bauer, B. Breast Cancer Resistance Protein and P-Glycoprotein in Brain Cancer: Two Gatekeepers Team Up. Curr. Pharm. Des. 2011, 17, 2793–2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-P.; Ambudkar, S.V. The Pharmacological Impact of ATP-Binding Cassette Drug Transporters on Vemurafenib-Based Therapy. Acta Pharm. Sin. B 2014, 4, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Choo, E.F.; Ly, J.; Chan, J.; Shahidi-Latham, S.K.; Messick, K.; Plise, E.; Quiason, C.M.; Yang, L. Role of P-Glycoprotein on the Brain Penetration and Brain Pharmacodynamic Activity of the MEK Inhibitor Cobimetinib. Mol. Pharm. 2014, 11, 4199–4207. [Google Scholar] [CrossRef]
- Mittapalli, R.K.; Vaidhyanathan, S.; Sane, R.; Elmquist, W.F. Impact of P-Glycoprotein (ABCB1) and Breast Cancer Resistance Protein (ABCG2) on the Brain Distribution of a Novel BRAF Inhibitor: Vemurafenib (PLX4032). J. Pharmacol. Exp. Ther. 2012, 342, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Durmus, S.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Oral Availability and Brain Penetration of the B-RAFV600E Inhibitor Vemurafenib Can Be Enhanced by the P-GLYCOprotein (ABCB1) and Breast Cancer Resistance Protein (ABCG2) Inhibitor Elacridar. Mol. Pharm. 2012, 9, 3236–3245. [Google Scholar] [CrossRef]
- Gampa, G.; Vaidhyanathan, S.; Resman, B.-W.; Parrish, K.E.; Markovic, S.N.; Sarkaria, J.N.; Elmquist, W.F. Challenges in the Delivery of Therapies to Melanoma Brain Metastases. Curr. Pharmacol. Rep. 2016, 2, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Sakji-Dupré, L.; Le Rhun, E.; Templier, C.; Desmedt, E.; Blanchet, B.; Mortier, L. Cerebrospinal Fluid Concentrations of Vemurafenib in Patients Treated for Brain Metastatic BRAF-V600 Mutated Melanoma. Melanoma Res. 2015, 25, 302–305. [Google Scholar] [CrossRef]
- MD Anderson Cancer Center. Phase II Study of Binimetinib With Encorafenib in Patients with Metastatic Melanoma and CNS Metastases; MD Anderson Cancer Center: Houston, TX, USA, 2023. [Google Scholar]
- Pfizer. A Two-Part, Phase 1a/B, Open-Label, Multicenter Trial Evaluating Pharmacokinetics, Safety and Efficacy of pf-07284890 (Arry 461) in Participants with Braf V600 Mutant Solid Tumors with and without Brain Involvement; Pfizer: New York, NY, USA, 2023. [Google Scholar]
- Wichmann, J.; Rynn, C.; Friess, T.; Petrig-Schaffland, J.; Kornacker, M.; Handl, C.; Emmenegger, J.; Eckmann, J.; Herting, F.; Frances, N.; et al. Preclinical Characterization of a Next-Generation Brain Permeable, Paradox Breaker BRAF Inhibitor. Clin. Cancer Res. 2022, 28, 770–780. [Google Scholar] [CrossRef]
- Pape, E.; Desmedt, E.; Zairi, F.; Baranzelli, M.-C.; Dziwniel, V.; Dubois, F.; Bonneterre, J.; Mortier, L.; Le Rhun, E. Leptomeningeal Metastasis in Melanoma: A Prospective Clinical Study of Nine Patients. In Vivo 2012, 26, 1079–1086. [Google Scholar] [PubMed]
- Glitza, I.C.; Rohlfs, M.; Guha-Thakurta, N.; Bassett, R.L.; Bernatchez, C.; Diab, A.; Woodman, S.E.; Yee, C.; Amaria, R.N.; Patel, S.P.; et al. Retrospective Review of Metastatic Melanoma Patients with Leptomeningeal Disease Treated with Intrathecal Interleukin-2. ESMO Open 2018, 3, e000283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glitza Oliva, I.C.; Ferguson, S.D.; Bassett, R.; Foster, A.P.; John, I.; Hennegan, T.D.; Rohlfs, M.; Richard, J.; Iqbal, M.; Dett, T.; et al. Concurrent Intrathecal and Intravenous Nivolumab in Leptomeningeal Disease: Phase 1 Trial Interim Results. Nat. Med. 2023, 29, 898–905. [Google Scholar] [CrossRef]
- Le Rhun, E.; Preusser, M.; van den Bent, M.; Andratschke, N.; Weller, M. How We Treat Patients with Leptomeningeal Metastases. ESMO Open 2019, 4, e000507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, P.D.; Pugh, S.; Laack, N.N.; Wefel, J.S.; Khuntia, D.; Meyers, C.; Choucair, A.; Fox, S.; Suh, J.H.; Roberge, D.; et al. Memantine for the Prevention of Cognitive Dysfunction in Patients Receiving Whole-Brain Radiotherapy: A Randomized, Double-Blind, Placebo-Controlled Trial. Neuro Oncol. 2013, 15, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Pugh, S.L.; Tome, W.A.; Caine, C.; Corn, B.; Kanner, A.; Rowley, H.; Kundapur, V.; DeNittis, A.; Greenspoon, J.N.; et al. Preservation of Memory with Conformal Avoidance of the Hippocampal Neural Stem-Cell Compartment during Whole-Brain Radiotherapy for Brain Metastases (RTOG 0933): A Phase II Multi-Institutional Trial. J. Clin. Oncol. 2014, 32, 3810–3816. [Google Scholar] [CrossRef]
- Yang, J.T.; Wijetunga, N.A.; Pentsova, E.; Wolden, S.; Young, R.J.; Correa, D.; Zhang, Z.; Zheng, J.; Steckler, A.; Bucwinska, W.; et al. Randomized Phase II Trial of Proton Craniospinal Irradiation Versus Photon Involved-Field Radiotherapy for Patients With Solid Tumor Leptomeningeal Metastasis. J. Clin. Oncol. 2022, 40, 3858–3867. [Google Scholar] [CrossRef]
- Yang, T.J.; Wijetunga, N.A.; Yamada, J.; Wolden, S.; Mehallow, M.; Goldman, D.A.; Zhang, Z.; Young, R.J.; Kris, M.G.; Yu, H.A.; et al. Clinical Trial of Proton Craniospinal Irradiation for Leptomeningeal Metastases. Neuro Oncol. 2021, 23, 134–143. [Google Scholar] [CrossRef] [PubMed]
- University of Aarhus. Phase II Study of Proton Cranio-Spinal Irradiation for Leptomeningeal Metastasis; University of Aarhus: Aarhus, Denmark, 2023. [Google Scholar]
- University of Zurich. Intrathecal Administration of Anti-PD1/Anti-CTLA-4 in Combination with Systemic Combination of Anti-PD1/Anti-CTLA-4 in Patients with NSCLC without Oncogenic Driver Mutation or Melanoma and Newly Diagnosed Leptomeningeal Metastasis: A Multicentric Phase I Study; University of Zurich: Zurich, Swizterland, 2022. [Google Scholar]
- Mall, H. Phase II Trial of Pembrolizumab and Lenvatinib for Leptomeningeal Metastases; Dana-Farber Cancer Institute: Boston, MA, USA, 2021. [Google Scholar]
- Merck Sharp & Dohme LLC. A Phase 3 Randomized, Placebo-Controlled Trial to Evaluate the Safety and Efficacy of Pembrolizumab (MK-3475) and Lenvatinib (E7080/MK-7902) versus Pembrolizumab Alone as First-Line Intervention in Participants with Advanced Melanoma (LEAP-003); Merck Sharp & Dohme LLC: Rahway, NJ, USA, 2023. [Google Scholar]
- Merck Sharp & Dohme LLC. A Multicenter, Open-Label, Phase 2 Trial to Assess the Efficacy and Safety of Lenvatinib (E7080/MK-7902) in Combination with Pembrolizumab (MK-3475) in Participants with Advanced Melanoma Previously Exposed to an Anti-PD-1/L1 Agent (LEAP-004); Merck Sharp & Dohme LLC: Rahway, NJ, USA, 2022. [Google Scholar]
- Arance, A.; de la Cruz-Merino, L.; Petrella, T.M.; Jamal, R.; Ny, L.; Carneiro, A.; Berrocal, A.; Márquez-Rodas, I.; Spreafico, A.; Atkinson, V.; et al. Phase II LEAP-004 Study of Lenvatinib Plus Pembrolizumab for Melanoma with Confirmed Progression on a Programmed Cell Death Protein-1 or Programmed Death Ligand 1 Inhibitor Given as Monotherapy or in Combination. J. Clin. Oncol. 2023, 41, 75–85. [Google Scholar] [CrossRef]
- SWOG Cancer Research Network. A Randomized Phase 2 Trial of Encorafenib + Binimetinib + Nivolumab vs Ipilimumab + Nivolumab in BRAF-V600 Mutant Melanoma with Brain Metastases; SWOG Cancer Research Network: San Antonio, TX, USA, 2023. [Google Scholar]
- Gampa, G.; Kim, M.; Cook-Rostie, N.; Laramy, J.K.; Sarkaria, J.N.; Paradiso, L.; DePalatis, L.; Elmquist, W.F. Brain Distribution of a Novel MEK Inhibitor E6201: Implications in the Treatment of Melanoma Brain Metastases. Drug Metab. Dispos. 2018, 46, 658–666. [Google Scholar] [CrossRef]
- Babiker, H.M.; Byron, S.A.; Hendricks, W.P.D.; Elmquist, W.F.; Gampa, G.; Vondrak, J.; Aldrich, J.; Cuyugan, L.; Adkins, J.; De Luca, V.; et al. E6201, an Intravenous MEK1 Inhibitor, Achieves an Exceptional Response in BRAF V600E-Mutated Metastatic Malignant Melanoma with Brain Metastases. Investig. New Drugs 2019, 37, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic. Phase 1 Study of E6201 Plus Dabrafenib for the Treatment of Central Nervous System (CNS) Metastases from BRAF V600-Mutated Metastatic Melanoma; Mayo Clinic: Rochester, MN, USA, 2023. [Google Scholar]
- Plus Therapeutics. A Dual Phase 1/2, Investigator Initiated Study to Determine the Maximum Tolerated Dose, Safety, and Efficacy of 186Rhenium Nanoliposomes (186RNL) in Recurrent Glioma (CTRC# 12-02); Plus Therapeutics: Austin, TX, USA, 2022. [Google Scholar]
- Guerra-Garcia, M.; Balinda, H.; Bao, A.; Garcia, M.; Gilbert, A.; Phillips, W.; Floyd, J.; Brenner, A. LMD-14. Preclinical Safety and Activity of Intraventricular Rhenium-186 Nanoliposome (186RNL) for Leptomeningeal Metastases. Neurooncol. Adv. 2021, 3, iii10. [Google Scholar] [CrossRef]
- Istituto Clinico Humanitas. Prospective Double Arm. Randomized Trial for Patients with Multiple Brain Metastasis and/or Leptomeningeal Carcinomatosis: Comparison of WBRT Alone and WBRT Plus Silibinin (Sillbrain); Istituto Clinico Humanitas: Rozzano, Italy, 2023. [Google Scholar]
- Verdura, S.; Cuyàs, E.; Ruiz-Torres, V.; Micol, V.; Joven, J.; Bosch-Barrera, J.; Menendez, J.A. Lung Cancer Management with Silibinin: A Historical and Translational Perspective. Pharmaceuticals 2021, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- Priego, N.; Zhu, L.; Monteiro, C.; Mulders, M.; Wasilewski, D.; Bindeman, W.; Doglio, L.; Martínez, L.; Martínez-Saez, E.; Ramón Cajal, S.; et al. STAT3 Labels a Subpopulation of Reactive Astrocytes Required for Brain Metastasis. Nat. Med. 2018, 24, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z. An Open-Label, Randomized, Multicenter Trial of Intrathecal-Pemetrexed Combined with Concurrent Involved-Field Radiotherapy and Intrathecal-Pemetrexed Alone in Patients with Leptomeningeal Metastasis from Solid Tumors; Clinicaltrials.gov: Bethesda, MA, USA, 2022. [Google Scholar]
- Fan, C.; Zhao, Q.; Li, L.; Shen, W.; Du, Y.; Teng, C.; Gao, F.; Song, X.; Jiang, Q.; Huang, D.; et al. Efficacy and Safety of Intrathecal Pemetrexed Combined With Dexamethasone for Treating Tyrosine Kinase Inhibitor-Failed Leptomeningeal Metastases From EGFR-Mutant NSCLC-a Prospective, Open-Label, Single-Arm Phase 1/2 Clinical Trial (Unique Identifier: ChiCTR1800016615). J. Thorac. Oncol. 2021, 16, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Remsik, J.; Kiseliovas, V.; Derderian, C.; Sener, U.; Alghader, M.; Saadeh, F.; Nikishina, K.; Bale, T.; Iacobuzio-Donahue, C.; et al. Cancer Cells Deploy Lipocalin-2 to Collect Limiting Iron in Leptomeningeal Metastasis. Science 2020, 369, 276–282. [Google Scholar] [CrossRef]
- Memorial Sloan Kettering Cancer Center. A Phase 1a/1b Trial of Intrathecal Deferoxamine for Leptomeningeal Metastases; Memorial Sloan Kettering Cancer Center: New York, NY, USA, 2022. [Google Scholar]


| Title | CTI | Phase | P | Design | Disease | Intervention | Country |
|---|---|---|---|---|---|---|---|
| Intrathecal Double Checkpoint Inhibition (IT-IO) | NCT05598853 | 1 | 26 | NR, MC | NSCLC and melanoma | IT/SYS nivolumab + ipilimumab | Switzerland |
| Intrathecal Application of PD1 Antibody in Metastatic Solid Tumors With Leptomeningeal Disease (IT-PD1/NOA 26) (IT-PD1) | NCT05112549 | 1 | 46 | NR, MC | Solid tumours | Part 1: Dose escalation of IT nivolumab in 4 cohorts Part 2: Dose expansion of IT nivolumab | Germany |
| Pembrolizumab And Lenvatinib In Leptomeningeal Metastases | NCT04729348 | 2 | 19 | NR, MC | Solid tumours | Pembrolizumab + lenvatinib | USA |
| Binimetinib and Encorafenib for the Treatment of Metastatic Melanoma and Central Nervous System Metastases | NCT05026983 | 2 | 35 | NR, SC | Melanoma | Binimetinib + encorafenib high dose | USA |
| A Study to Compare the Administration of Encorafenib + Binimetinib + Nivolumab Versus Ipilimumab + Nivolumab in BRAF-V600 Mutant Melanoma with Brain Metastases | NCT04511013 | 2 | 112 | R, MC | Melanoma | Arm A: Encorafenib, binimetinib + nivolumab Arm B: Ipilimumab + nivolumab | USA |
| E6201 and Dabrafenib for the Treatment of Central Nervous System Metastases from BRAF V600 Mutated Metastatic Melanoma | NCT05388877 | 1 | 18 | NR, MC | Melanoma | MEK-1/MEKK-1 inhibitor E6201 + dabrafenib | USA |
| Proton Cranio-spinal Irradiation for Leptomeningeal Metastasis (CSI ProLong) | NCT05746754 | 2 | 50 | NR, SC | Solid tumours and haematological cancer | Proton radiotherapy with 30 Gy in 10 fractions to the entire craniospinal axis | Denmark |
| Intraventricular Administration of Rhenium-186 NanoLiposome for Leptomeningeal Metastases (ReSPECT-LM) | NCT05034497 | 1 | 18 | NR, MC | LMD of any primary type | Single-dose Rhenium-186 NanoLiposome (186RNL) | USA |
| Prospective Double Arm Randomized Trial: WBRT Alone and WBRT Plus Silibinin | NCT05793489 | NA | 44 | R, SC | Solid tumours with brain metastases and/or LMD | Arm A: WBRT + silibinin Arm B: WBRT | Italy |
| Intra-pemetrexed Alone or Combined With Concurrent Radiotherapy for Leptomeningeal Metastasis | NCT05305885 | NA | 100 | R, MC | Solid tumours | Arm A: IT pemetrexed in combination with involved field RTX Arm B: IT pemetrexed monotherapy | China |
| A Study of Deferoxamine (DFO) in People With Leptomeningeal Metastasis | NCT05184816 | 1a/1b | 35 | NR, SC | 1a: Solid tumours 1b: NSCLC | Phase 1a: Dose escalation of IT deferoxamine (solid tumours) Phase 1b: Dose expansion (NSCLC) | USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steininger, J.; Gellrich, F.F.; Engellandt, K.; Meinhardt, M.; Westphal, D.; Beissert, S.; Meier, F.; Glitza Oliva, I.C. Leptomeningeal Metastases in Melanoma Patients: An Update on and Future Perspectives for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 11443. https://doi.org/10.3390/ijms241411443
Steininger J, Gellrich FF, Engellandt K, Meinhardt M, Westphal D, Beissert S, Meier F, Glitza Oliva IC. Leptomeningeal Metastases in Melanoma Patients: An Update on and Future Perspectives for Diagnosis and Treatment. International Journal of Molecular Sciences. 2023; 24(14):11443. https://doi.org/10.3390/ijms241411443
Chicago/Turabian StyleSteininger, Julian, Frank Friedrich Gellrich, Kay Engellandt, Matthias Meinhardt, Dana Westphal, Stefan Beissert, Friedegund Meier, and Isabella C. Glitza Oliva. 2023. "Leptomeningeal Metastases in Melanoma Patients: An Update on and Future Perspectives for Diagnosis and Treatment" International Journal of Molecular Sciences 24, no. 14: 11443. https://doi.org/10.3390/ijms241411443
APA StyleSteininger, J., Gellrich, F. F., Engellandt, K., Meinhardt, M., Westphal, D., Beissert, S., Meier, F., & Glitza Oliva, I. C. (2023). Leptomeningeal Metastases in Melanoma Patients: An Update on and Future Perspectives for Diagnosis and Treatment. International Journal of Molecular Sciences, 24(14), 11443. https://doi.org/10.3390/ijms241411443





