Repositioning of Anti-Diabetic Drugs against Dementia: Insight from Molecular Perspectives to Clinical Trials
Abstract
1. Introduction
2. Insulin/Insulin-like Growth Factor 1 (IGF-1) Signaling in the Brain
2.1. The Source of Insulin and IGF-1 in the Brain
2.2. Neuroprotective Effect of Insulin and IGF-1
2.3. Impaired Insulin Signaling in the Brain
3. Involvement of Brain Insulin/IGF-1 Signaling in Dementia Pathologies
3.1. Alzheimer’s Diseases
3.2. Vascular Dementia
3.3. Lewy Body Dementia
3.4. Frontotemporal Dementia
4. Preclinical Evidence of Anti-Diabetic Drugs for Alzheimer’s Drug Repositioning
4.1. Insulin
4.2. Insulin Sensitizer
4.3. Insulin Secretagogues
4.4. Other Anti-Diabetic Drugs
| Classification | Drugs | Mechanisms of Action | Pharmacological Effects | References |
|---|---|---|---|---|
| Insulin | Intranasal insulin |
|
| [86,87,88,89,90,91,92] |
| Insulin Sensitizer | Metformin |
|
| [94,95,96,97,98] |
Thiazolidinediones
|
|
| [103,104,105,106,107] | |
| Insulin secretagogues | Glucagon-like peptide-1
|
|
| [110,111,112,113,114] |
Sulfonylurea
|
|
| [116,117,118] | |
| Other anti-diabetics | Sodium-glucose cotransporter-2
|
|
| [120,122] |
Amylin analogs
|
|
| [125,126] |
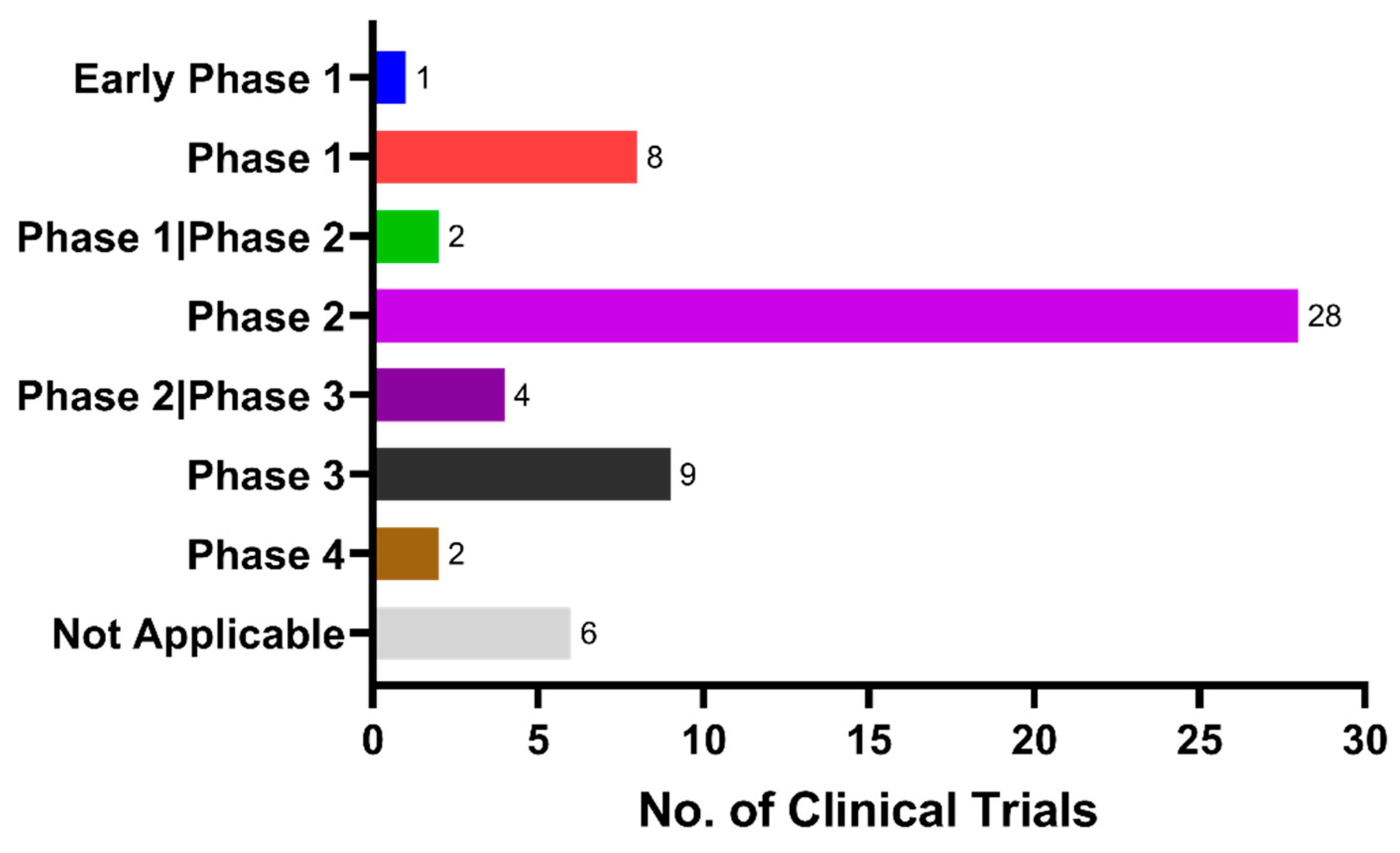
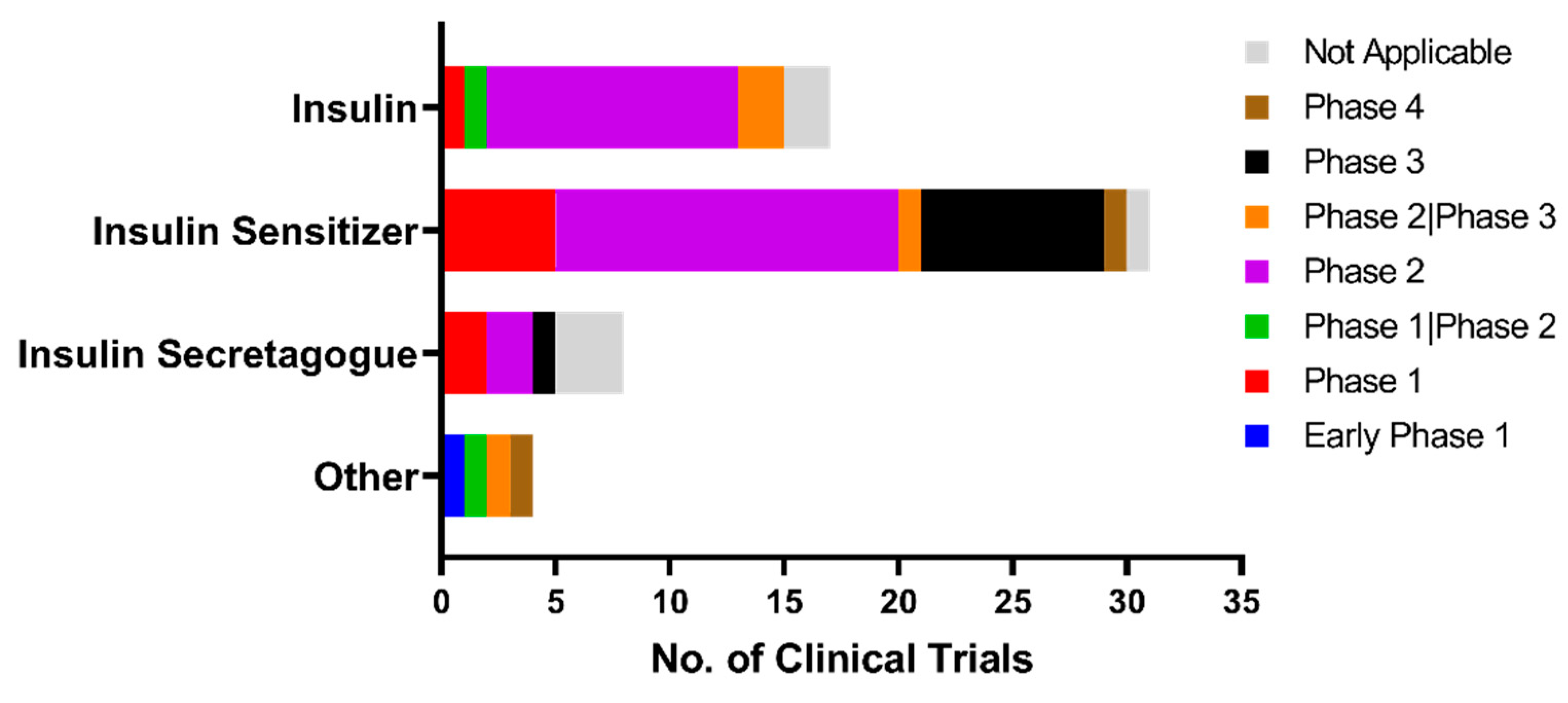
5. Current Status of Clinical Trials for Anti-Diabetic Repositioning as Anti-Dementia Drugs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corbett, A.; Pickett, J.; Burns, A.; Corcoran, J.; Dunnett, S.B.; Edison, P.; Hagan, J.J.; Holmes, C.; Jones, E.; Katona, C.; et al. Drug Repositioning for Alzheimer’s Disease. Nat. Publ. Group 2012, 11, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Milstein, J.L.; Ferris, H.A. The Brain as an Insulin-Sensitive Metabolic Organ. Mol. Metab. 2021, 52, 101234. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Rosa-Neto, P.; Morais, J.A.; Webster, C. World Alzheimer Report 2021: Journey Through the Diagnosis of Dementia; Alzheimer’s Disease International: London, UK, 2021. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaff, K.; Brayne, C. Potential for Primary Prevention of Alzheimer’s Disease: An Analysis of Population-Based Data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Mukadam, N.; Petersen, I.; Cooper, C. Mild Cognitive Impairment and Progression to Dementia in People with Diabetes, Prediabetes and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Soc. Psychiatr. Epidemiol. 2018, 53, 1149–1160. [Google Scholar] [CrossRef]
- Alexander, G.C.; Knopman, D.S.; Emerson, S.S.; Ovbiagele, B.; Kryscio, R.J.; Perlmutter, J.S.; Kesselheim, A.S. Revisiting FDA Approval of Aducanumab. N. Engl. J. Med. 2021, 385, 769–771. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The Antibody Aducanumab Reduces Aβ Plaques in Alzheimer’s Disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Larkin, H.D. Lecanemab Gains FDA Approval for Early Alzheimer Disease. JAMA 2023, 329, 363. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Hung, S.Y.; Fu, W.M. Drug Candidates in Clinical Trials for Alzheimer’s Disease. J. Biomed. Sci. 2017, 24, 47. [Google Scholar] [CrossRef]
- Fang, C.; Hernandez, P.; Liow, K.; Damiano, E.; Zetterberg, H.; Blennow, K.; Feng, D.; Chen, M.; Maccecchini, M. Buntanetap, a Novel Translational Inhibitor of Multiple Neurotoxic Proteins, Proves to Be Safe and Promising in Both Alzheimer’s and Parkinson’s Patients. J. Prev. Alzheimer’s Dis. 2023, 10, 25–33. [Google Scholar] [CrossRef]
- Gray, S.M.; Barrett, E.J. Insulin Transport into the Brain. Am. J. Physiol. Cell. Physiol. 2018, 315, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. The Source of Cerebral Insulin. Eur. J. Pharmacol. 2004, 490, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, T.; Piriz, J.; Duflot, S.; Fernandez, A.M.; Gaitan, G.; Gomez-Pinedo, U.; Verdugo, J.M.G.; Leroy, F.; Soya, H.; Nuñez, A.; et al. Neuronal Activity Drives Localized Blood-Brain-Barrier Transport of Serum Insulin-like Growth Factor-I into the CNS. Neuron 2010, 67, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Bondy, C.A.; Cheng, C.M. Signaling by Insulin-like Growth Factor 1 in Brain. Eur. J. Pharmacol. 2004, 490, 25–31. [Google Scholar] [CrossRef]
- Chernausek, S.D. Insulin-like Growth Factor-I (IGF-I) Production by Astroglial Cells: Regulation and Importance for Epidermal Growth Factor-Induced Cell Replication. J. Neurosci. Res. 1993, 34, 189–197. [Google Scholar] [CrossRef]
- Ye, P.; Popken, G.J.; Kemper, A.; McCarthy, K.; Popko, B.; D’Ercole, A.J. Astrocyte-Specific Overexpression of Insulin-like Growth Factor-I Promotes Brain Overgrowth and Glial Fibrillary Acidic Protein Expression. J. Neurosci. Res. 2004, 78, 472–484. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Costa-Besada, M.A.; Labandeira, C.M.; Villar-Cheda, B.; Rodríguez-Perez, A.I. Insulin-like Growth Factor-1 and Neuroinflammation. Front. Aging. Neurosci. 2017, 9, 365. [Google Scholar] [CrossRef]
- Terasaki, T.; Hosoya, K.I. The Blood-Brain Barrier Efflux Transporters as a Detoxifying System for the Brain. Adv. Drug Deliv. Rev. 1999, 36, 195–209. [Google Scholar] [CrossRef]
- Csajbók, É.A.; Tamás, G. Cerebral Cortex: A Target and Source of Insulin? Diabetologia 2016, 59, 1609–1615. [Google Scholar] [CrossRef]
- Devaskar, S.U.; Singh, B.S.; Carnaghi, L.R.; Rajakumar, P.A.; Giddings, S.J. Insulin II Gene Expression in Rat Central Nervous System. Regul. Pept. 1993, 48, 55–63. [Google Scholar] [CrossRef]
- Devaskar, S.U.; Giddings, S.J.; Rajakumar, P.A.; Carnaghi, L.R.; Menon, R.K.; Zahm, D.S. Insulin Gene Expression and Insulin Synthesis in Mammalian Neuronal Cells. J. Biol. Chem. 1994, 269, 8445–8454. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Salman, Z.; Quinlan, P.; Wallin, A.; Svensson, J. Patients with Alzheimer’s Disease Have Increased Levels of Insulin-like Growth Factor-I in Serum but Not in Cerebrospinal Fluid. J. Alzheimer’s Dis. 2020, 75, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Decoding Alzheimer’s Disease from Perturbed Cerebral Glucose Metabolism: Implications for Diagnostic and Therapeutic Strategies. Prog. Neurobiol. 2013, 108, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Hu, J.; Tsai, C.W.; Yue, M.; Melrose, H.L.; Kanekiyo, T.; Bu, G. Neuronal LRP1 Regulates Glucose Metabolism and Insulin Signaling in the Brain. J. Neurosci. 2015, 35, 5851–5859. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.; Pharaoh, G.A.; Marlin, M.C.; Masser, D.R.; Matsuzaki, S.; Wronowski, B.; Yeganeh, A.; Parks, E.E.; Premkumar, P.; Farley, J.A.; et al. Insulin-like Growth Factor Receptor Signaling Regulates Working Memory, Mitochondrial Metabolism, and Amyloid-β Uptake in Astrocytes. Mol. Metab. 2018, 9, 141–155. [Google Scholar] [CrossRef]
- Brabazon, F.; Bermudez, S.; Shaughness, M.; Khayrullina, G.; Byrnes, K.R. The Effects of Insulin on the Inflammatory Activity of BV2 Microglia. PLoS ONE 2018, 13, e0201878. [Google Scholar] [CrossRef]
- Spielman, L.J.; Bahniwal, M.; Little, J.P.; Walker, D.G.; Klegeris, A. Insulin Modulates In Vitro Secretion of Cytokines and Cytotoxins by Human Glial Cells. Curr. Alzheimer Res. 2015, 12, 684–693. [Google Scholar] [CrossRef]
- Suh, H.-S.; Zhao, M.-L.; Derico, L.; Choi, N.; Lee, S.C. Insulin-like Growth Factor 1 and 2 (IGF1, IGF2) Expression in Human Microglia: Differential Regulation by Inflammatory Mediators. J. Neuroinflamm. 2013, 10, 805. [Google Scholar] [CrossRef]
- Grinberg, Y.Y.; Dibbern, M.E.; Levasseur, V.A.; Kraig, R.P. Insulin-like Growth Factor-1 Abrogates Microglial Oxidative Stress and TNF-α Responses to Spreading Depression. J. Neurochem. 2013, 126, 662–672. [Google Scholar] [CrossRef]
- Zheng, W.H.; Quirion, R. Insulin-like Growth Factor-1 (IGF-1) Induces the Activation/Phosphorylation of Akt Kinase and CAMP Response Element-Binding Protein (CREB) by Activating Different Signaling Pathways in PC12 Cells. BMC Neurosci. 2006, 7, 51. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Li, D.; Li, M.; Wang, P.; Wen, J.; Liang, M.; Su, B.; Yin, Y. IGF-1 Alleviates NMDA-Induced Excitotoxicity in Cultured Hippocampal Neurons Against Autophagy via the NR2B/PI3K-AKT-MTOR Pathway. J. Cell. Physiol. 2014, 229, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, Y.; Weiss, S. Insulin-Like Growth Factor-I Is a Differentiation Factor for Postmitotic CNS Stem Cell-Derived Neuronal Precursors: Distinct Actions from Those of Brain-Derived Neurotrophic Factor. J. Neurosci. 1998, 18, 2118–2128. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.P.; Rosenblatt, K.P.; Kuljiš, R.O. Exercise-Induced Cognitive Plasticity, Implications for Mild Cognitive Impairment and Alzheimer’s Disease. Front. Neurol. 2011, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Ogundele, O.M.; Pardo, J.; Francis, J.; Goya, R.G.; Lee, C.C. A Putative Mechanism of Age-Related Synaptic Dysfunction Based on the Impact of IGF-1 Receptor Signaling on Synaptic CaMKIIα Phosphorylation. Front. Neuroanat. 2018, 12, 35. [Google Scholar] [CrossRef]
- Grillo, C.A.; Piroli, G.G.; Lawrence, R.C.; Wrighten, S.A.; Green, A.J.; Wilson, S.P.; Sakai, R.R.; Kelly, S.J.; Wilson, M.A.; Mott, D.D.; et al. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes 2015, 64, 3927–3936. [Google Scholar] [CrossRef]
- Solano, D.C.; Sironi, M.; Bonfini, C.; Solerte, S.B.; Govoni, S.; Racchi, M. Insulin Regulates Soluble Amyloid Precursor Protein Release via Phosphatidyl Inositol 3 Kinase-dependent Pathway. FASEB J. 2000, 14, 1015–1022. [Google Scholar] [CrossRef]
- Mattson, M.P. Cellular Actions of Amyloid Precursor Protein and Its Soluble and Fibrillogenic Derivatives. Physiol. Rev. 1997, 77, 1081–1132. [Google Scholar] [CrossRef]
- Dou, J.T.; Chen, M.; Dufour, F.; Alkon, D.L.; Zhao, W.Q. Insulin Receptor Signaling in Long-Term Memory Consolidation Following Spatial Learning. Learn. Mem. 2005, 12, 646–655. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Torres-Alemán, I. The Many Faces of Insulin-like Peptide Signalling in the Brain. Nat. Rev. Neurosci. 2012, 13, 225–239. [Google Scholar] [CrossRef]
- Talbot, K.; Wang, H.Y.; Kazi, H.; Han, L.Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated Brain Insulin Resistance in Alzheimer’s Disease Patients Is Associated with IGF-1 Resistance, IRS-1 Dysregulation, and Cognitive Decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef]
- Denver, P.; English, A.; McClean, P.L. Inflammation, Insulin Signaling and Cognitive Function in Aged APP/PS1 Mice. Brain Behav. Immun. 2018, 70, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, N.; Yan, F.; Jin, H.; Zhou, S.; Shi, J.; Jin, F. Diabetes Mellitus and Alzheimer’s Disease: GSK-3β as a Potential Link. Behav. Brain Res. 2018, 339, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Gratuze, M.; Julien, J.; Petry, F.R.; Morin, F.; Planel, E. Insulin Deprivation Induces PP2A Inhibition and Tau Hyperphosphorylation in HTau Mice, a Model of Alzheimer’s Disease-like Tau Pathology. Sci. Rep. 2017, 7, srep46359. [Google Scholar] [CrossRef] [PubMed]
- Guillot, F.; Kemppainen, S.; Lavasseur, G.; Miettinen, P.O.; Laroche, S.; Tanila, H.; Davis, S. Brain-Specific Basal and Novelty-Induced Alternations in PI3K-Akt and MAPK/ERK Signaling in a Middle-Aged AβPP/PS1 Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 51, 1157–1173. [Google Scholar] [CrossRef]
- Bloom, G.S.; Lazo, J.S.; Norambuena, A. Reduced Brain Insulin Signaling: A Seminal Process in Alzheimer’s Disease Pathogenesis. Neuropharmacology 2018, 136, 192–195. [Google Scholar] [CrossRef]
- Shieh, J.C.C.; Huang, P.T.; Lin, Y.F. Alzheimer’s Disease and Diabetes: Insulin Signaling as the Bridge Linking Two Pathologies. Mol. Neurobiol. 2020, 57, 1966–1977. [Google Scholar] [CrossRef]
- Son, S.M.; Song, H.; Byun, J.; Park, K.S.; Jang, H.C.; Park, Y.J.; Mook-Jung, I. Altered APP Processing in Insulin-Resistant Conditions Is Mediated by Autophagosome Accumulation via the Inhibition of Mammalian Target of Rapamycin Pathway. Diabetes 2012, 61, 3126–3138. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, W.; Xie, J.-W.; Wang, T.; Wang, S.-L.; Teng, W.-P.; Wang, Z.-Y. Insulin Deficiency Exacerbates Cerebral Amyloidosis and Behavioral Deficits in an Alzheimer Transgenic Mouse Model. Mol. Neurodegener. 2010, 5, 46. [Google Scholar] [CrossRef]
- Xie, L.; Helmerhorst, E.; Plewright, B.; Van Bronswijk, W.; Martins, R. Alzheimer’s Beta-Amyloid Peptides Compete for Insulin Binding to the Insulin Receptor. J. Neurosci. 2002, 22, 1–5. [Google Scholar] [CrossRef]
- Tang, W.J. Targeting Insulin-Degrading Enzyme to Treat Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2016, 27, 24–34. [Google Scholar] [CrossRef]
- Sousa, L.; Guarda, M.; Meneses, M.J.; Macedo, M.P.; Vicente Miranda, H. Insulin-Degrading Enzyme: An Ally against Metabolic and Neurodegenerative Diseases. J. Pathol. 2021, 255, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, D.; Huang, H.; Zhao, Y.; Zhou, H. Characteristics of Insulin-Degrading Enzyme in Alzheimer’s Disease: A Meta-Analysis. Curr. Alzheimer Res. 2018, 15, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.A.; Wijesekara, N.; Fraser, P.E.; de Felice, F.G. The Link between Tau and Insulin Signaling: Implications for Alzheimer’s Disease and Other Tauopathies. Front. Cell. Neurosci. 2019, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Moon, S.; Paik, J.H.; Shin, D.W.; Kim, L.S.; Park, C.S.; Ha, J.; Kang, J.H. Activation of the 5′-AMP-Activated Protein Kinase in the Cerebral Cortex of Young Senescence-Accelerated P8 Mice and Association with GSK3β- and PP2A-Dependent Inhibition of p-Tau396 Expression. J. Alzheimer’s Dis. 2015, 46, 249–259. [Google Scholar] [CrossRef]
- Sayas, C.L.; Ávila, J. GSK-3 and Tau: A Key Duet in Alzheimer’s Disease. Cells 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Lauretti, E.; Praticò, D. Caspase-3-Dependent Cleavage of Akt Modulates Tau Phosphorylation via GSK3β Kinase: Implications for Alzheimer’s Disease. Mol. Psychiatry 2017, 22, 1002–1008. [Google Scholar] [CrossRef]
- Javadpour, P.; Dargahi, L.; Ahmadiani, A.; Ghasemi, R. To Be or Not to Be: PP2A as a Dual Player in CNS Functions, Its Role in Neurodegeneration, and Its Interaction with Brain Insulin Signaling. Cell. Mol. Life Sci. 2019, 76, 2277–2297. [Google Scholar] [CrossRef]
- Sontag, J.-M.; Sontag, E. Protein Phosphatase 2A Dysfunction in Alzheimer’s Disease. Front. Mol. Neurosci. 2014, 7, 16. [Google Scholar] [CrossRef]
- Ponce-Lopez, T.; Hong, E.; Abascal-Díaz, M.; Meneses, A. Role of GSK3β and PP2A on Regulation of Tau Phosphorylation in Hippocampus and Memory Impairment in ICV-STZ Animal Model of Alzheimer’s Disease. Adv. Alzheimer Dis. 2017, 6, 13. [Google Scholar] [CrossRef]
- Chiu, I.M.; Chen, A.; Zheng, Y.; Kosaras, B.; Tsiftsoglou, S.A.; Vartanian, T.K.; Brown, R.H.B.; Carroll, M.C.; Day, B. T Lymphocytes Potentiate Endogenous Neuroprotective Inflammation in a Mouse Model of ALS. Proc. Natl. Acad. Sci. USA 2008, 105, 17913–17918. [Google Scholar] [CrossRef]
- Baik, S.H.; Kang, S.; Lee, W.; Choi, H.; Chung, S.; Kim, J.-I.; Mook-Jung, I. A Breakdown in Metabolic Reprogramming Causes Microglia Dysfunction in Alzheimer’s Disease. Cell. Metab. 2019, 30, 493–507. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G.; Gonçalves, R.A.; Ferreira, S.T. Impaired Insulin Signalling and Allostatic Load in Alzheimer Disease. Nat. Rev. Neurosci. 2022, 23, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Osborn, L.M.; Kamphuis, W.; Wadman, W.J.; Hol, E.M. Astrogliosis: An Integral Player in the Pathogenesis of Alzheimer’s Disease. Prog. Neurobiol. 2016, 144, 121–141. [Google Scholar] [CrossRef]
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskaine, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain-Special Emphasis on Pi3k-Akt Pathway. Front. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Ulland, T.K.; Song, W.M.; Huang, S.C.-C.; Ulrich, J.D.; Sergushichev, A.; Beatty, W.L.; Loboda, A.A.; Zhou, Y.; Cairns, N.J.; Kambal, A. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 2017, 170, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-Brain Barrier Breakdown in Alzheimer Disease and Other Neurodegenerative Disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Rhea, E.M.; Banks, W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019, 13, 521. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, D.H.; Song, J. HMGB1 Signaling Pathway in Diabetes-Related Dementia: Blood-Brain Barrier Breakdown, Brain Insulin Resistance, and Aβ Accumulation. Biomed. Pharmacother. 2022, 150, 112933. [Google Scholar] [CrossRef]
- Van Straaten, E.C.W.; Scheltens, P.; Knol, D.L.; Van Buchem, M.A.; Van Dijk, E.J.; Hofman, P.A.M.; Karas, G.; Kjartansson, O.; De Leeuw, F.E.; Prins, N.D.; et al. Operational Definitions for the NINDS-AIREN Criteria for Vascular Dementia an Interobserver Study. Stroke 2003, 34, 1907–1912. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Thomas, A. Vascular Dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chen, C.J.; Lin, S.Y.; Chuang, Y.H.; Sheu, W.H.H.; Tung, K.C. Hyperglycemia Is Associated with Enhanced Gluconeogenesis in a Rat Model of Permanent Cerebral Ischemia. Mol. Cell. Endocrinol. 2013, 367, 50–56. [Google Scholar] [CrossRef]
- Zong, W.; Zeng, X.; Chen, S.; Chen, L.; Zhou, L.; Wang, X.; Gao, Q.; Zeng, G.; Hu, K.; Ouyang, D. Ginsenoside Compound K Attenuates Cognitive Deficits in Vascular Dementia Rats by Reducing the Aβ Deposition. J. Pharmacol. Sci. 2019, 139, 223–230. [Google Scholar] [CrossRef]
- Gong, X.; Ma, M.; Fan, X.; Li, M.; Liu, Q.; Liu, X.; Xu, G. Down-Regulation of IGF-1/IGF-1R in Hippocampus of Rats with Vascular Dementia. Neurosci. Lett. 2012, 513, 20–24. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Capuano, A.W.; Wang, H.Y.; Schneider, J.A.; Kapasi, A.; Bennett, D.A.; Ahima, R.S.; Arnold, S.E. Brain Insulin Signaling and Cerebrovascular Disease in Human Postmortem Brain. Acta Neuropathol. Commun. 2021, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, P.; Horvath, A.; Nordlund, A.; Wallin, A.; Svensson, J. Low Serum Insulin-like Growth Factor-I (IGF-I) Level Is Associated with Increased Risk of Vascular Dementia. Psychoneuroendocrinology 2017, 86, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Dong, M.; de La Monte, S.M. Brain Insulin-like Growth Factor and Neurotrophin Resistance in Parkinson’s Disease and Dementia with Lewy Bodies: Potential Role of Manganese Neurotoxicity. J. Alzheimer’s Dis. 2009, 16, 585–599. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, Q.; Xu, J.; Sun, Q.; Qiao, Y.; Chen, W.; Wu, Y.; Wang, Y.; Xiao, Q.; Liu, J.; et al. Plasma Insulin-like Growth Factor 1 Is Associated with Cognitive Impairment in Parkinson’s Disease. Dement. Geriatr. Cogn. Disord. 2015, 39, 251–256. [Google Scholar] [CrossRef]
- Irwin, D.J.; Fedler, J.; Coffey, C.S.; Caspell-Garcia, C.; Kang, J.H.; Simuni, T.; Foroud, T.; Toga, A.W.; Tanner, C.M.; Kieburtz, K. Evolution of Alzheimer’s Disease Cerebrospinal Fluid Biomarkers in Early Parkinson’s Disease. Ann. Neurol. 2020, 88, 574–587. [Google Scholar] [CrossRef]
- Kang, J.-H.; Mollenhauer, B.; Coffey, C.S.; Toledo, J.B.; Weintraub, D.; Galasko, D.R.; Irwin, D.J.; Van Deerlin, V.; Chen-Plotkin, A.S.; Caspell-Garcia, C. CSF Biomarkers Associated with Disease Heterogeneity in Early Parkinson’s Disease: The Parkinson’s Progression Markers Initiative Study. Acta Neuropathol. 2016, 131, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Deleon, J.; Miller, B.L. Frontotemporal Dementia. In Handbook of Clinical Neurology, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 148, ISBN 9780444640765. [Google Scholar]
- Liou, C.J.; Tong, M.; Vonsattel, J.P.; de la Monte, S.M. Altered Brain Expression of Insulin and Insulin-Like Growth Factors in Frontotemporal Lobar Degeneration: Another Degenerative Disease Linked to Dysregulation of Insulin Metabolic Pathways. ASN Neuro. 2019, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.M.; MacMillan, M.; Bartley, L.; Halliday, G.M.; Kiernan, M.C.; Hodges, J.R.; Piguet, O. Systemic Metabolism in Frontotemporal Dementia. Neurology 2014, 83, 1812–1818. [Google Scholar] [CrossRef]
- Gaddam, M.; Singh, A.; Jain, N.; Avanthika, C.; Jhaveri, S.; De la Hoz, I.; Sanka, S.; Goli, S.R. A Comprehensive Review of Intranasal Insulin and Its Effect on the Cognitive Function of Diabetics. Cureus 2021, 13, e17219. [Google Scholar] [CrossRef]
- Salameh, T.S.; Bullock, K.M.; Hujoel, I.A.; Niehoff, M.L.; Wolden-Hanson, T.; Kim, J.; Morley, J.E.; Farr, S.A.; Banks, W.A. Central Nervous System Delivery of Intranasal Insulin: Mechanisms of Uptake and Effects on Cognition. J. Alzheimer’s Dis. 2015, 47, 715–728. [Google Scholar] [CrossRef]
- Mao, Y.F.; Guo, Z.; Zheng, T.; Jiang, Y.; Yan, Y.; Yin, X.; Chen, Y.; Zhang, B. Intranasal Insulin Alleviates Cognitive Deficits and Amyloid Pathology in Young Adult APPswe/PS1dE9 Mice. Aging Cell 2016, 15, 893–902. [Google Scholar] [CrossRef]
- Bazrgar, M.; Khodabakhsh, P.; Dargahi, L.; Mohagheghi, F.; Ahmadiani, A. MicroRNA Modulation Is a Potential Molecular Mechanism for Neuroprotective Effects of Intranasal Insulin Administration in Amyloid Βeta Oligomer Induced Alzheimer’s like Rat Model. Exp. Gerontol. 2022, 164, 111812. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, D.; Wang, Y.; Jiang, T.; Hu, S.; Zhang, M.; Yu, X.; Gongb, C.X. Intranasal Insulin Ameliorates Tau Hyperphosphorylation in a Rat Model of Type 2 Diabetes. J. Alzheimer’s Dis. 2013, 33, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Dai, C.; Liang, Z.; Run, X.; Iqbal, K.; Liu, F.; Gong, C.X. Intranasal Insulin Restores Insulin Signaling, Increases Synaptic Proteins, and Reduces Aβ Level and Microglia Activation in the Brains of 3xTg-AD Mice. Exp. Neurol. 2014, 261, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, Y.; Mao, Y.F.; Zheng, T.; Jiang, Y.; Yan, Y.; Yin, X.; Zhang, B. Long-Term Treatment with Intranasal Insulin Ameliorates Cognitive Impairment, Tau Hyperphosphorylation, and Microglial Activation in a Streptozotocin-Induced Alzheimer’s Rat Model. Sci. Rep. 2017, 7, srep45971. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Z.; Mao, Y.F.; Zheng, T.; Zhang, B. Intranasal Insulin Ameliorates Cerebral Hypometabolism, Neuronal Loss, and Astrogliosis in Streptozotocin-Induced Alzheimer’s Rat Model. Neurotox. Res. 2018, 33, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Sanati, M.; Aminyavari, S.; Afshari, A.R.; Sahebkar, A. Mechanistic Insight into the Role of Metformin in Alzheimer’s Disease. Life Sci. 2022, 291, 120299. [Google Scholar] [CrossRef]
- Kazkayasi, I.; Telli, G.; Nemutlu, E.; Uma, S. Intranasal Metformin Treatment Ameliorates Cognitive Functions via Insulin Signaling Pathway in ICV-STZ-Induced Mice Model of Alzheimer’s Disease. Life Sci. 2022, 299, 120538. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, J.; Sheng, W.; Zuo, Z. Metformin Attenuates Alzheimer’s Disease-like Neuropathology in Obese, Leptin-Resistant Mice. Pharmacol. Biochem. Behav. 2012, 101, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Hettich, M.M.; Matthes, F.; Ryan, D.P.; Griesche, N.; Schröder, S.; Dorn, S.; Krauß, S.; Ehninger, D. The Anti-Diabetic Drug Metformin Reduces BACE1 Protein Level by Interfering with the MID1 Complex. PLoS ONE 2014, 9, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, K.; Wang, R.; Liu, Y.; Kwak, Y.D.; Ma, T.; Thompson, R.C.; Zhao, Y.; Smith, L.; Gasparini, L.; et al. Antidiabetic Drug Metformin (GlucophageR) Increases Biogenesis of Alzheimer’s Amyloid Peptides via up-Regulating BACE1 Transcription. Proc. Natl. Acad. Sci. USA 2009, 106, 3907–3912. [Google Scholar] [CrossRef]
- Inoue, Y.; Masuda, T.; Misumi, Y.; Ando, Y.; Ueda, M. Metformin Attenuates Vascular Pathology by Increasing Expression of Insulin-Degrading Enzyme in a Mixed Model of Cerebral Amyloid Angiopathy and Type 2 Diabetes Mellitus. Neurosci. Lett. 2021, 762, 136136. [Google Scholar] [CrossRef]
- Imfeld, P.; Bodmer, M.; Jick, S.S.; Meier, C.R. Metformin, Other Antidiabetic Drugs, and Risk of Alzheimer’s Disease: A Population-Based Case-Control Study. J. Am. Geriatr. Soc. 2012, 60, 916–921. [Google Scholar] [CrossRef]
- Luo, A.; Ning, P.; Lu, H.; Huang, H.; Shen, Q.; Zhang, D.; Xu, F.; Yang, L.; Xu, Y. Association Between Metformin and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Clinical Observational Studies. J. Alzheimer’s Dis. 2022, 88, 1311–1323. [Google Scholar] [CrossRef]
- Ping, F.; Jiang, N.; Li, Y. Association between Metformin and Neurodegenerative Diseases of Observational Studies: Systematic Review and Meta-Analysis. BMJ Open Diabetes Res. Care 2020, 8, e001370. [Google Scholar] [CrossRef]
- Cardoso, S.; Moreira, P.I. Antidiabetic Drugs for Alzheimer’s and Parkinson’s Diseases: Repurposing Insulin, Metformin, and Thiazolidinediones. In International Review of Neurobiology, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 155, ISBN 9780128231210. [Google Scholar]
- Quan, Q.; Qian, Y.; Li, X.; Li, M. Pioglitazone Reduces β Amyloid Levels via Inhibition of PPARγ Phosphorylation in a Neuronal Model of Alzheimer’s Disease. Front. Aging Neurosci. 2019, 10, 178. [Google Scholar] [CrossRef]
- Escribano, L.; Simón, A.M.; Gimeno, E.; Cuadrado-Tejedor, M.; De Maturana, R.L.; García-Osta, A.; Ricobaraza, A.; Pérez-Mediavilla, A.; Del Río, J.; Frechilla, D. Rosiglitazone Rescues Memory Impairment in Alzheimer’s Transgenic Mice: Mechanisms Involving a Reduced Amyloid and Tau Pathology. Neuropsychopharmacology 2010, 35, 1593–1604. [Google Scholar] [CrossRef]
- Ma, L.; Shao, Z.; Wang, R.; Zhao, Z.; Dong, W.; Zhang, J.; Zhang, X.; Sheng, S.; Ji, Z.; Zhang, J. Rosiglitazone Improves Learning and Memory Ability in Rats with Type 2 Diabetes Through the Insulin Signaling Pathway. Am. J. Med. Sci. 2015, 350, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Kim, H.J.; Yang, A.H.; Kim, H.M.; Lee, B.W.; Kang, E.S.; Lee, H.C.; Cha, B.S. The Effect of Rosiglitazone on LRP1 Expression and Amyloid β Uptake in Human Brain Microvascular Endothelial Cells: A Possible Role of a Low-Dose Thiazolidinedione for Dementia Treatment. Int. J. Neuropsychopharmacol. 2012, 15, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Singh, N. Behavioral and Biochemical Investigations to Explore Pharmacological Potential of PPAR-Gamma Agonists in Vascular Dementia of Diabetic Rats. Pharmacol. Biochem. Behav. 2011, 100, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhuo, L.; Sun, Y.; Shen, P.; Lin, H.; Zhan, S. Thiazolidinedione Use Is Associated with Reduced Risk of Dementia in Patients with Type 2 Diabetes Mellitus: A Retrospective Cohort Study. J. Diabetes 2023, 15, 97–109. [Google Scholar] [CrossRef]
- Yassine, H.N.; Solomon, V.; Thakral, A.; Sheikh-Bahaei, N.; Chui, H.C.; Braskie, M.N.; Schneider, L.S.; Talbot, K. Brain Energy Failure in Dementia Syndromes: Opportunities and Challenges for Glucagon-like Peptide-1 Receptor Agonists. Alzheimer’s Dement. 2022, 18, 478–497. [Google Scholar] [CrossRef]
- King, M.R.; Anderson, N.J.; Deciu, M.; Guernsey, L.S.; Cundiff, M.; Hajizadeh, S.; Jolivalt, C.G. Insulin Deficiency, but Not Resistance, Exaggerates Cognitive Deficits in Transgenic Mice Expressing Human Amyloid and Tau Proteins. Reversal by Exendin-4 Treatment. J. Neurosci. Res. 2020, 98, 2357–2369. [Google Scholar] [CrossRef]
- Carranza-Naval, M.J.; del Marco, A.; Hierro-Bujalance, C.; Alves-Martinez, P.; Infante-Garcia, C.; Vargas-Soria, M.; Herrera, M.; Barba-Cordoba, B.; Atienza-Navarro, I.; Lubian-Lopez, S.; et al. Liraglutide Reduces Vascular Damage, Neuronal Loss, and Cognitive Impairment in a Mixed Murine Model of Alzheimer’s Disease and Type 2 Diabetes. Front. Aging Neurosci. 2021, 13, 741923. [Google Scholar] [CrossRef]
- Robinson, A.; Lubitz, I.; Atrakchi-Baranes, D.; Licht-Murava, A.; Katsel, P.; Leroith, D.; Liraz-Zaltsman, S.; Haroutunian, V.; Beeri, M.S. Combination of Insulin with a GLP1 Agonist Is Associated with Better Memory and Normal Expression of Insulin Receptor Pathway Genes in a Mouse Model of Alzheimer’s Disease. J. Mol. Neurosci. 2019, 67, 504–510. [Google Scholar] [CrossRef]
- McClean, P.L.; Hölscher, C. Liraglutide Can Reverse Memory Impairment, Synaptic Loss and Reduce Plaque Load in Aged APP/PS1 Mice, a Model of Alzheimer’s Disease. Neuropharmacology 2014, 76, 57–67. [Google Scholar] [CrossRef]
- Batista, A.F.; Forny-Germano, L.; Clarke, J.R.; Lyra e Silva, N.M.; Brito-Moreira, J.; Boehnke, S.E.; Winterborn, A.; Coe, B.C.; Lablans, A.; Vital, J.F.; et al. The Diabetes Drug Liraglutide Reverses Cognitive Impairment in Mice and Attenuates Insulin Receptor and Synaptic Pathology in a Non-Human Primate Model of Alzheimer’s Disease. J. Pathol. 2018, 245, 85–100. [Google Scholar] [CrossRef]
- Nørgaard, C.H.; Friedrich, S.; Hansen, C.T.; Gerds, T.; Ballard, C.; Møller, D.V.; Knudsen, L.B.; Kvist, K.; Zinman, B.; Holm, E.; et al. Treatment with Glucagon-like Peptide-1 Receptor Agonists and Incidence of Dementia: Data from Pooled Double-Blind Randomized Controlled Trials and Nationwide Disease and Prescription Registers. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12268. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.J.; Kim, N.; Gee, M.S.; Jeon, S.H.; Lee, D.; Do, J.; Ryu, J.S.; Lee, J.K. Glibenclamide Modulates Microglial Function and Attenuates Aβ Deposition in 5XFAD Mice. Eur. J. Pharmacol. 2020, 884, 173416. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.H.; Enayati, M.; Khabbaz Abkenar, F.; Ebrahimian, F.; Salari, A.A. Glibenclamide Mitigates Cognitive Impairment and Hippocampal Neuroinflammation in Rats with Type 2 Diabetes and Sporadic Alzheimer-like Disease. Behav. Brain Res. 2020, 379, 112359. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.H.; Bahari, B.; Salari, A.A. ATP-Sensitive Potassium-Channel Inhibitor Glibenclamide Attenuates HPA Axis Hyperactivity, Depression- and Anxiety-Related Symptoms in a Rat Model of Alzheimer’s Disease. Brain Res. Bull. 2018, 137, 265–276. [Google Scholar] [CrossRef]
- Newby, D.; Linden, A.B.; Fernandes, M.; Molero, Y.; Winchester, L.; Sproviero, W.; Ghose, U.; Li, Q.S.; Launer, L.J.; Duijn, C.M.V.; et al. Comparative Effect of Metformin versus Sulfonylureas with Dementia and Parkinson’s Disease Risk in US Patients over 50 with Type 2 Diabetes Mellitus. BMJ Open Diabetes Res. Care 2022, 10, e003036. [Google Scholar] [CrossRef]
- Piątkowska-Chmiel, I.; Herbet, M.; Gawrońska-Grzywacz, M.; Pawłowski, K.; Ostrowska-Leśko, M.; Dudka, J. Molecular and Neural Roles of Sodium-Glucose Cotransporter 2 Inhibitors in Alleviating Neurocognitive Impairment in Diabetic Mice. Psychopharmacology 2023, 240, 983–1000. [Google Scholar] [CrossRef]
- Siao, W.Z.; Lin, T.K.; Huang, J.Y.; Tsai, C.F.; Jong, G.P. The Association between Sodium-Glucose Cotransporter 2 Inhibitors and Incident Dementia: A Nationwide Population-Based Longitudinal Cohort Study. Diab. Vasc. Dis. Res. 2022, 19, 1–8. [Google Scholar] [CrossRef]
- Ibrahim, W.W.; Kamel, A.S.; Wahid, A.; Abdelkader, N.F. Dapagliflozin as an Autophagic Enhancer via LKB1/AMPK/SIRT1 Pathway in Ovariectomized/d-Galactose Alzheimer’s Rat Model. Inflammopharmacology 2022, 30, 2505–2520. [Google Scholar] [CrossRef]
- Corrigan, R.R.; Piontkivska, H.; Casadesus, G. Amylin Pharmacology in Alzheimer’s Disease Pathogenesis and Treatment. Curr. Neuropharmacol. 2021, 20, 1894–1907. [Google Scholar] [CrossRef]
- Fu, W.; Patel, A.; Kimura, R.; Soudy, R.; Jhamandas, J.H. Amylin Receptor: A Potential Therapeutic Target for Alzheimer’s Disease. Trends Mol. Med. 2017, 23, 709–720. [Google Scholar] [CrossRef]
- Adler, B.L.; Yarchoan, M.; Hwang, H.M.; Louneva, N.; Blair, J.A.; Palm, R.; Smith, M.A.; Lee, H.; Arnold, S.E.; Casadesus, G. Neuroprotective Effects of the Amylin Analogue Pramlintide on Alzheimer’s Disease Pathogenesis and Cognition. Neurobiol. Aging 2014, 35, 793–801. [Google Scholar] [CrossRef]
- Nassar, S.Z.; Badae, N.M.; Issa, Y.A. Effect of Amylin on Memory and Central Insulin Resistance in a Rat Model of Alzheimer’s Disease. Arch. Physiol. Biochem. 2020, 126, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Mietlicki-Baase, E.G. Amylin in Alzheimer’s Disease: Pathological Peptide or Potential Treatment? Neuropharmacology 2018, 136, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.B.; Tang, X.; Han, M.; Yang, J.; Simó, R. Impact of Antidiabetic Agents on Dementia Risk: A Bayesian Network Meta-Analysis. Metabolism 2020, 109, 154265. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Hallschmid, M.; Schmitz, K.; Schultes, B.; Ratter, F.; Fehm, H.L.; Born, J.; Kern, W. Intranasal Insulin Improves Memory in Humans: Superiority of Insulin Aspart. Neuropsychopharmacology 2007, 32, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Hallschmid, M.; Hatke, A.; Schultes, B.; Fehm, H.L.; Born, J.; Kern, W. Intranasal Insulin Improves Memory in Humans. Psychoneuroendocrinology 2004, 29, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal Insulin Therapy for Alzheimer Disease and Amnestic Mild Cognitive Impairment: A Pilot Clinical Trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef]
- Claxton, A.; Baker, L.D.; Wilkinson, C.W.; Trittschuh, E.H.; Chapman, D.; Watson, G.S.; Cholerton, B.; Plymate, S.R.; Arbuckle, M.; Craft, S. Sex and ApoE Genotype Differences in Treatment Response to Two Doses of Intranasal Insulin in Adults with Mild Cognitive Impairment or Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 35, 789–797. [Google Scholar] [CrossRef]
- Claxton, A.; Baker, L.D.; Hanson, A.; Trittschuh, E.H.; Cholerton, B.; Morgan, A.; Callaghan, M.; Arbuckle, M.; Behl, C.; Craft, S. Long-Acting Intranasal Insulin Detemir Improves Cognition for Adults with Mild Cognitive Impairment or Early-Stage Alzheimer’s Disease Dementia. J. Alzheimer’s Dis. 2015, 44, 897–906. [Google Scholar] [CrossRef]
- Craft, S.; Claxton, A.; Baker, L.D.; Hanson, A.J.; Cholerton, B.; Trittschuh, E.H.; Dahl, D.; Caulder, E.; Neth, B.; Montine, T.J.; et al. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J. Alzheimer’s Dis. 2017, 57, 1325–1334. [Google Scholar] [CrossRef]
- Craft, S.; Raman, R.; Chow, T.W.; Rafii, M.S.; Sun, C.K.; Rissman, R.A.; Donohue, M.C.; Brewer, J.B.; Jenkins, C.; Harless, K.; et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.M.; Mechanic-Hamilton, D.; Xie, S.X.; Combs, M.F.; Cappola, A.R.; Xie, L.; Detre, J.A.; Wolk, D.A.; Arnold, S.E. Effects of the Insulin Sensitizer Metformin in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2017, 31, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Perez, T.; Chang, H.; Mehta, P.; Steffener, J.; Pradabhan, G.; Ichise, M.; Manly, J.; Devanand, D.P.; Bagiella, E. Metformin in Amnestic Mild Cognitive Impairment: Results of a Pilot Randomized Placebo Controlled Clinical Trial. J. Alzheimer’s Dis. 2016, 51, 501–514. [Google Scholar] [CrossRef] [PubMed]
- De Jager, J.; Kooy, A.; Lehert, P.; Wulffelé, M.G.; Van Der Kolk, J.; Bets, D.; Verburg, J.; Donker, A.J.M.; Stehouwer, C.D.A. Long Term Treatment with Metformin in Patients with Type 2 Diabetes and Risk of Vitamin B-12 Deficiency: Randomised Placebo Controlled Trial. BMJ 2010, 340, 1177. [Google Scholar] [CrossRef]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-Term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Greaves, C.V.; Rohrer, J.D. An Update on Genetic Frontotemporal Dementia. J. Neurol. 2019, 266, 2075–2086. [Google Scholar] [CrossRef]
- Burns, D.K.; Alexander, R.C.; Welsh-Bohmer, K.A.; Culp, M.; Chiang, C.; O’Neil, J.; Evans, R.M.; Harrigan, P.; Plassman, B.L.; Burke, J.R.; et al. Safety and Efficacy of Pioglitazone for the Delay of Cognitive Impairment in People at Risk of Alzheimer’s Disease (TOMMORROW): A Prognostic Biomarker Study and a Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 2021, 20, 537–547. [Google Scholar] [CrossRef]
- Hildreth, K.L.; Van Pelt, R.E.; Moreau, K.L.; Grigsby, J.; Hoth, K.F.; Pelak, V.; Anderson, C.A.; Parnes, B.; Kittelson, J.; Wolfe, P.; et al. Effects of Pioglitazone or Exercise in Older Adults with Mild Cognitive Impairment and Insulin Resistance: A Pilot Study. Dement. Geriatr. Cogn. Dis. Extra 2015, 5, 51–63. [Google Scholar] [CrossRef]
- Watson, G.S.; Cholerton, B.A.; Reger, M.A.; Baker, L.D.; Plymate, S.R.; Asthana, S.; Fishel, M.A.; Kulstad, J.J.; Green, P.S.; Cook, D.G.; et al. Preserved Cognition in Patients With Early Alzheimer Disease and Amnestic Mild Cognitive Impairment During Treatment With Rosiglitazone. Am. J. Geriatr. Psychiatry 2005, 13, 950–958. [Google Scholar] [CrossRef]
- Harrington, C.; Sawchak, S.; Chiang, C.; Davies, J.; Donovan, C.; Saunders, A.M.; Irizarry, M.; Jeter, B.; Zvartau-Hind, M.; van Dyck, C.H.; et al. Rosiglitazone Does Not Improve Cognition or Global Function When Used as Adjunctive Therapy to AChE Inhibitors in Mild-to-Moderate Alzheimers Disease: Two Phase 3 Studies. Curr. Alzheimer Res. 2011, 8, 592–606. [Google Scholar] [CrossRef]
- Gold, M.; Alderton, C.; Zvartau-Hind, M.; Egginton, S.; Saunders, A.M.; Irizarry, M.; Craft, S.; Landreth, G.; Linnamägi, Ü.; Sawchak, S. Rosiglitazone Monotherapy in Mild-to-Moderate Alzheimer’s Disease: Results from a Randomized, Double-Blind, Placebo-Controlled Phase III Study. Dement. Geriatr. Cogn. Disord. 2010, 30, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Gejl, M.; Gjedde, A.; Egefjord, L.; Møller, A.; Hansen, S.B.; Vang, K.; Rodell, A.; Brændgaard, H.; Gottrup, H.; Schacht, A.; et al. In Alzheimer’s Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front. Aging Neurosci. 2016, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Femminella, G.D.; Frangou, E.; Love, S.B.; Busza, G.; Holmes, C.; Ritchie, C.; Lawrence, R.; McFarlane, B.; Tadros, G.; Ridha, B.H.; et al. Evaluating the Effects of the Novel GLP-1 Analogue Liraglutide in Alzheimer’s Disease: Study Protocol for a Randomised Controlled Trial (ELAD Study). Trials 2019, 20, 191. [Google Scholar] [CrossRef]
- Mullins, R.J.; Mustapic, M.; Chia, C.W.; Carlson, O.; Gulyani, S.; Tran, J.; Li, Y.; Mattson, M.P.; Resnick, S.; Egan, J.M.; et al. A Pilot Study of Exenatide Actions in Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Stomrud, E.; Minthon, L.; Zetterberg, H.; Blennow, K.; Hansson, O. Longitudinal Cerebrospinal Fluid Biomarker Measurements in Preclinical Sporadic Alzheimer’s Disease: A Prospective 9-Year Study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2015, 1, 403–411. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantik, K.E.K.; Kim, S.; Gu, B.; Moon, S.; Kwak, H.-B.; Park, D.-H.; Kang, J.-H. Repositioning of Anti-Diabetic Drugs against Dementia: Insight from Molecular Perspectives to Clinical Trials. Int. J. Mol. Sci. 2023, 24, 11450. https://doi.org/10.3390/ijms241411450
Mantik KEK, Kim S, Gu B, Moon S, Kwak H-B, Park D-H, Kang J-H. Repositioning of Anti-Diabetic Drugs against Dementia: Insight from Molecular Perspectives to Clinical Trials. International Journal of Molecular Sciences. 2023; 24(14):11450. https://doi.org/10.3390/ijms241411450
Chicago/Turabian StyleMantik, Keren Esther Kristina, Sujin Kim, Bonsang Gu, Sohee Moon, Hyo-Bum Kwak, Dong-Ho Park, and Ju-Hee Kang. 2023. "Repositioning of Anti-Diabetic Drugs against Dementia: Insight from Molecular Perspectives to Clinical Trials" International Journal of Molecular Sciences 24, no. 14: 11450. https://doi.org/10.3390/ijms241411450
APA StyleMantik, K. E. K., Kim, S., Gu, B., Moon, S., Kwak, H.-B., Park, D.-H., & Kang, J.-H. (2023). Repositioning of Anti-Diabetic Drugs against Dementia: Insight from Molecular Perspectives to Clinical Trials. International Journal of Molecular Sciences, 24(14), 11450. https://doi.org/10.3390/ijms241411450






