Realizing Eco-Friendly Water-Resistant Sodium-Alginate-Based Films Blended with a Polyphenolic Aqueous Extract from Grape Pomace Waste for Potential Food Packaging Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical and Chemical Characterization of SA + GPWW Blended Films
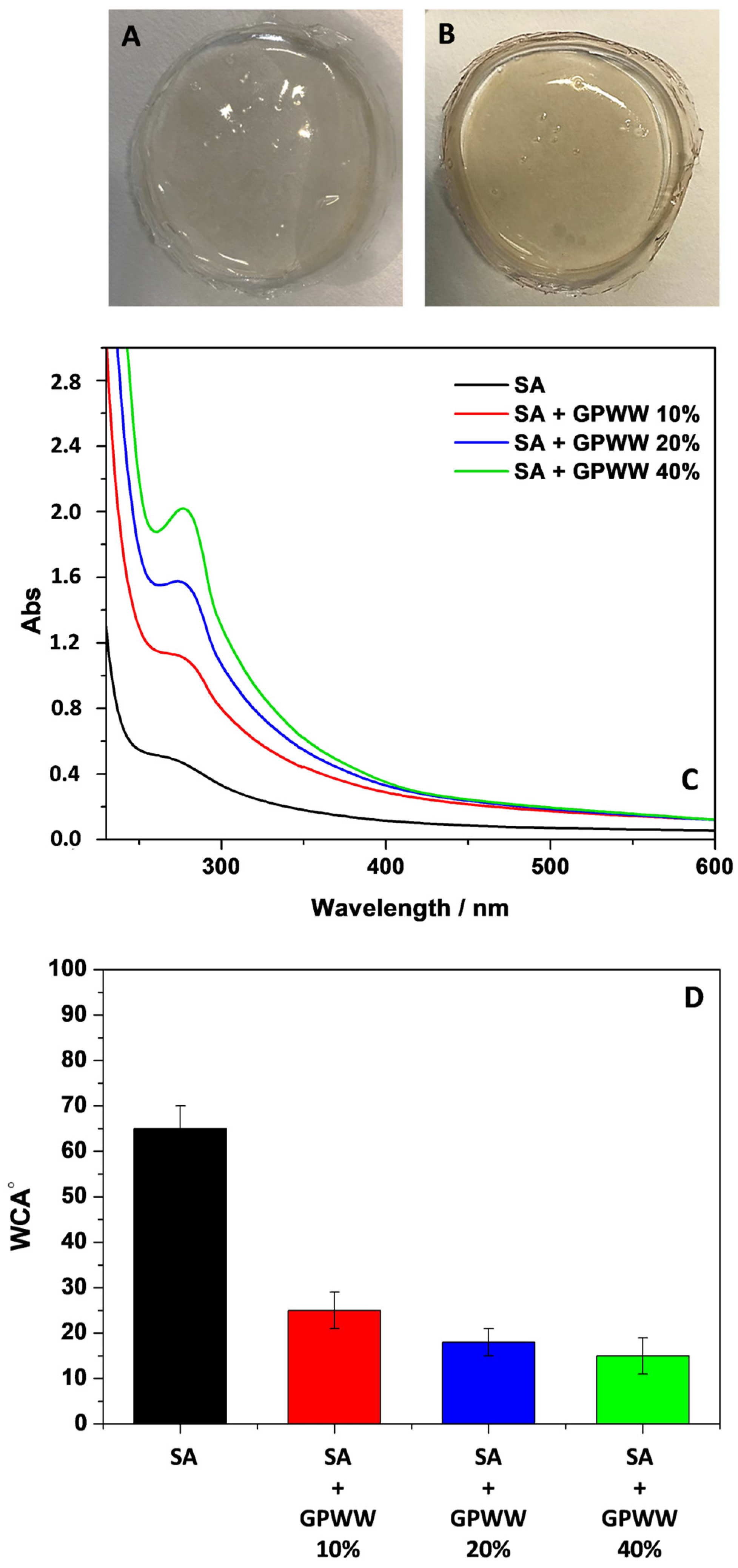
2.1.1. An Overview
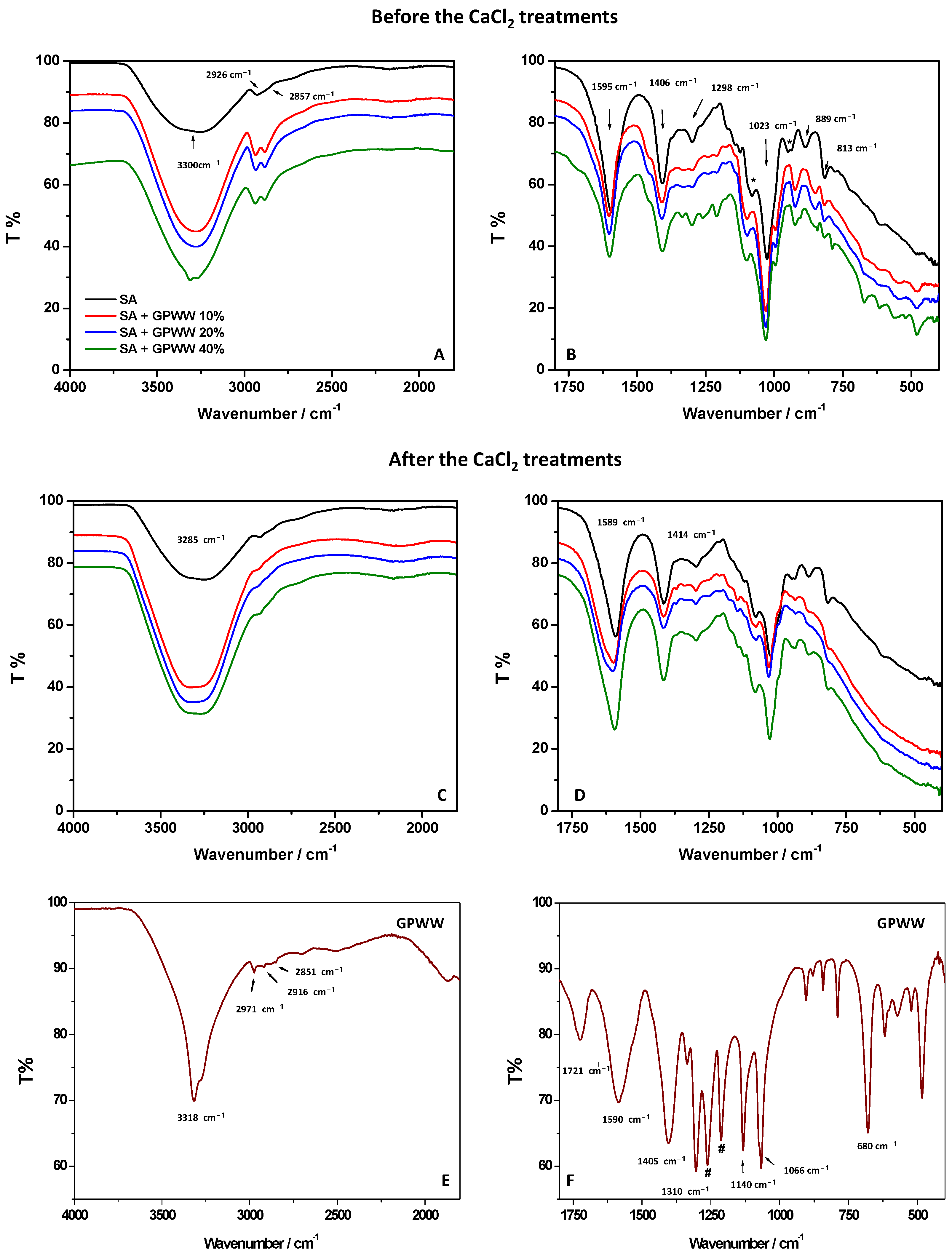
2.1.2. UV-Vis and ATR-FTIR Measurements
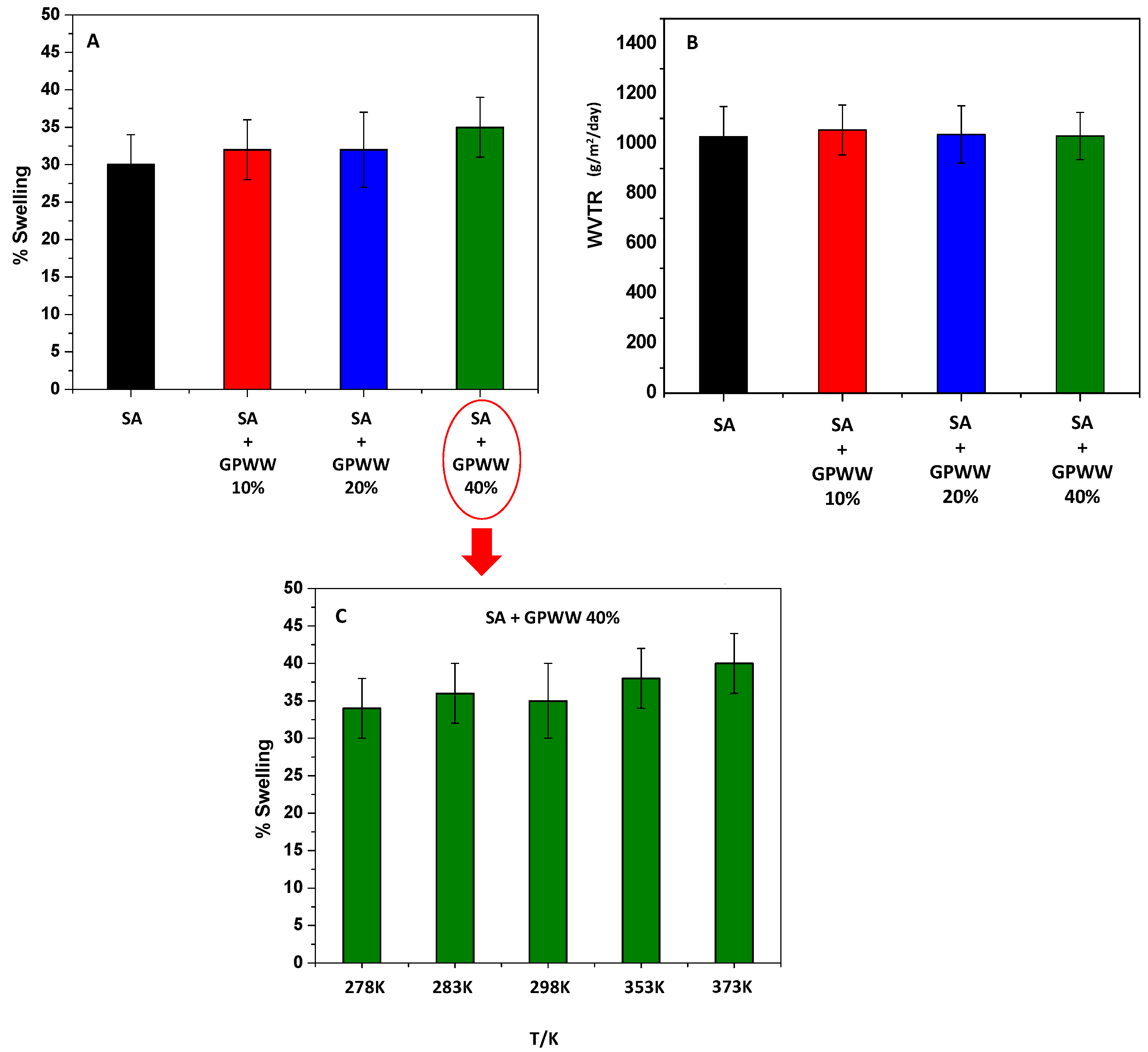
2.2. Swelling Measurements
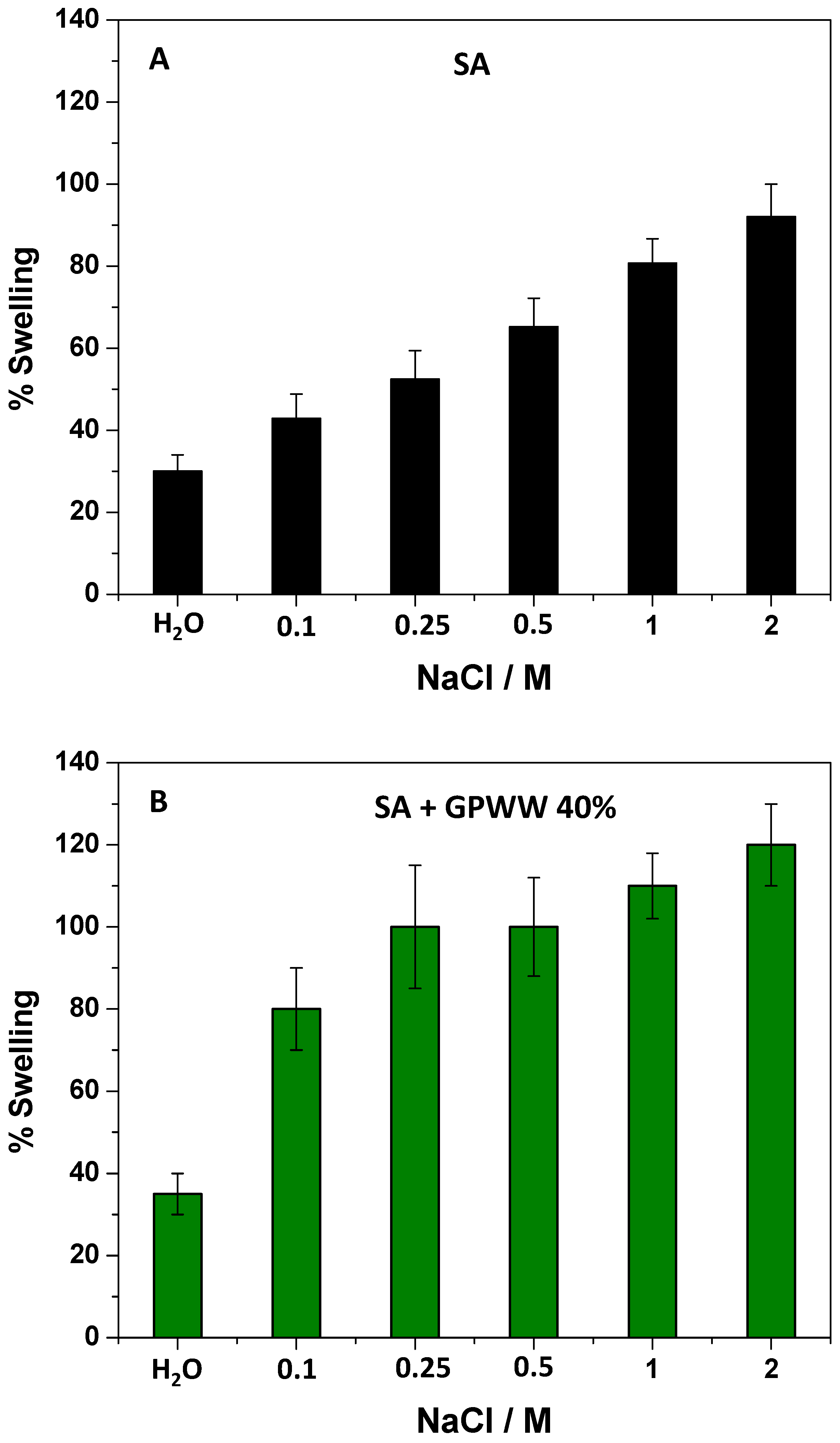
Effect of pH and Ionic Strength on the Degree of Swelling
2.3. Antioxidant Features and the Photostability of SA and SA + GPWW Films
2.3.1. Antioxidant Activity
2.3.2. UV-Light Protective Role of GPWW in SA + GPWW Composite Films
3. Materials and Methods
3.1. Chemicals
3.1.1. Reagents for the Preparation of SA and SA + GPWW Films
3.1.2. Reagents and Standards for HPLC Analysis
3.2. Grape Pomace Wastes
GPWW Obtained from Grape Pomace Waste
3.3. HPLC-MS/MS Analysis
3.4. Design and Preparation of SA and SA + GPWW Films
3.5. UV-Visible Measurements
3.6. ATR-FTIR Spectroscopic Measurements
3.7. Swelling Measurements
3.8. Determination of ABTS-Scavenging Activity
3.9. Photostability of SA and SA + GPWW Composite Films
3.10. Water Contact Angle (WCA) Measurements
3.11. Water Vapor Transmission Rate (WVTR) Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baghi, F.; Gharsallaoui, A.; Dumas, E.; Ghnimi, S. Advancements in Biodegradable Active Films for Food Packaging: Effects of Nano/Microcapsule Incorporation. Foods 2022, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Oliver-Simancas, R.; Castangia, I.; Rodríguez-García, A.M.; Alañón, M.E. Comprehensive review of natural based hydrogels as an upcoming trend for food packing. Food Hydrocoll. 2023, 135, 108124. [Google Scholar] [CrossRef]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Recent advances and future prospects of cellulose, starch, chitosan, polylactic acid and polyhydroxyalkanoates for sustainable food packaging applications. Int. J. Biol. Macromol. 2022, 221, 163–182. [Google Scholar] [CrossRef]
- Foschi, E.; Bonoli, A. The Commitment of Packaging Industry in the Framework of the European Strategy for Plastics in a Circular Economy. Adm. Sci. 2019, 9, 18. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Kontominas, M.G. Use of Alginates as Food Packaging Materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita, R.; Gutt, G.; Amariei, S. The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Signorile, R.; Fini, P.; Cecone, C.; Matencio, A.; Trotta, F.; Cosma, P. Cyclodextrin nanosponges as adsorbent material to remove hazardous pollutants from water: The case of ciprofloxacin. Chem. Eng. J. 2021, 411, 128514. [Google Scholar] [CrossRef]
- Rizzi, V.; Fini, P.; Fanelli, F.; Placido, T.; Semeraro, P.; Sibillano, T.; Fraix, A.; Sortino, S.; Agostiano, A.; Giannini, C.; et al. Molecular interactions, characterization and photoactivity of Chlorophyll a/chitosan/2-HP-β-cyclodextrin composite films as functional and active surfaces for ROS production. Food Hydrocoll. 2016, 58, 98–112. [Google Scholar] [CrossRef]
- Rizzi, V.; Fini, P.; Semeraro, P.; Cosma, P. Detailed investigation of ROS arisen from chlorophyll a/Chitosan based-biofilm. Colloids Surf. B Biointerfaces 2016, 142, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Yang, X.; Yao, L.; Hu, Z.; Li, H.; Xu, X.; Lu, J. Potential Food and Nutraceutical Applications of Alginate: A Review. Mar. Drugs 2022, 20, 564. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Uthappa, U.T.; Altalhi, T.; Jung, H.-Y.; Han, S.S.; Kurkuri, M.D. Alginate based polymeric systems for drug delivery, antibacterial/microbial, and wound dressing applications. Mater. Today Commun. 2022, 33, 104813. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Liyanapathiranage, A.; Makehelwala, M.; Dassanayake, R.S.; Manamperi, A.; Merah, O.; Mani, S.; Koduru, J.R.; Madhujith, T. Algal polysaccharides: Structure, preparation and applications in food packaging. Food Chem. 2022, 405, 134903. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of Crosslinking with Calcium Ions on the Physical Properties of Alginate Films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.W.; Lee, H.Y.; Heng, P.W.S. Mechanisms of external and internal gelation and their impact on the functions of alginate as a coat and delivery system. Carbohydr. Polym. 2006, 63, 176–187. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; He, J.; Huang, Y. A new insight to the effect of calcium concentration on gelation process and physical properties of alginate films. J. Mater. Sci. 2016, 51, 5791–5801. [Google Scholar] [CrossRef]
- Rhim, J.-W. Physical and mechanical properties of water resistant sodium alginate films. LWT Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. B Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Laurenzana, A.; Scavone, F.; Frediani, E.; Fibbi, G.; Fanelli, F.; Sibillano, T.; Giannini, C.; Fini, P.; et al. The “End Life” of the Grape Pomace Waste Become the New Beginning: The Development of a Virtuous Cycle for the Green Synthesis of Gold Nanoparticles and Removal of Emerging Contaminants from Water. Antioxidants 2022, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Sameen, D.E.; Zeng, Y.; Li, S.; Qin, W.; Liu, Y. An overview of tea polyphenols as bioactive agents for food packaging applications. LWT 2022, 167, 113845. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Shahabi, N.; Ghanbarzadeh, M.D.; Ghorbani, M. Development of Antimicrobial Active Food Packaging Film Based on Gelatin/Dialdehyde Quince Seed Gum Incorporated with Apple Peel Polyphenols. Food Bioprocess Technol. 2022, 15, 693–705. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.-W.; Cao, J.; Jiang, W. Tea polyphenols (TP): A promising natural additive for the manufacture of multifunctional active food packaging films. Crit. Rev. Food Sci. Nutr. 2023, 63, 288–301. [Google Scholar] [CrossRef]
- Yin, W.; Qiu, C.; Ji, H.; Li, X.; Sang, S.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Recent advances in biomolecule-based films and coatings for active and smart food packaging applications. Food Biosci. 2023, 52, 102378. [Google Scholar] [CrossRef]
- Saurabh, C.K.; Gupta, S.; Variyar, P.S. Development of guar gum based active packaging films using grape pomace. J. Food Sci. Technol. 2018, 55, 1982–1992. [Google Scholar] [CrossRef]
- Hyun-Joo, L.; Jae-Joon, L.; Myung-Ok, J.; Jung-Seok, C.; Ji-Taek, J.; Yang-Il, C.; Jin-Kyu, L. Meat Quality and Storage Characteristics of Pork Loin Marinated in Grape Pomace. Korean J. Food Sci. Anim. Resour. 2017, 37, 726–734. [Google Scholar] [CrossRef]
- Kaczmarek, B. Improving Sodium Alginate Films Properties by Phenolic Acid Addition. Materials 2020, 13, 2895. [Google Scholar] [CrossRef]
- Gorrasi, G.; Viscusi, G.; Gerardi, C.; Lamberti, E.; Giovinazzo, G. Physicochemical and Antioxidant Properties of White (Fiano cv) and Red (Negroamaro cv) Grape Pomace Skin Based Films. J. Polym. Environ. 2022, 30, 3609–3621. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.-W.; Lotfali, E.; Bagheri, R.; Pircheraghi, G. Alginate-based multifunctional films incorporated with sulfur quantum dots for active packaging applications. Colloids Surf. B Biointerfaces 2022, 215, 112519. [Google Scholar] [CrossRef]
- Cheng, H.; Cao, J.; Liu, W.; Mei, J.; Xie, J. Characterization of Sodium Alginate-Locust Bean Gum Films Reinforced with Daphnetin Emulsions for the Development of Active Packaging. Polymers 2022, 14, 731. [Google Scholar] [CrossRef]
- Biao, Y.; Yuxuan, C.; Qi, T.; Ziqi, Y.; Yourong, Z.; McClements, D.J.; Chongjiang, C. Enhanced performance and functionality of active edible films by incorporating tea polyphenols into thin calcium alginate hydrogels. Food Hydrocoll. 2019, 97, 105197. [Google Scholar] [CrossRef]
- de Farias, N.S.; Silva, B.; de Oliveira Costa, A.C.; Müller, C.M.O. Alginate based antioxidant films with yerba mate (Ilex paraguariensis St. Hil.): Characterization and kinetics of phenolic compounds release. Food Packag. Shelf Life 2021, 28, 100548. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.; Yang, S.; Zeng, J.; Wu, Z. Sodium Alginate-Based Green Packaging Films Functionalized by Guava Leaf Extracts and Their Bioactivities. Materials 2019, 12, 2923. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Acquaticci, L.; Xiao, J.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. A new HPLC-MS/MS method for the simultaneous determination of 36 polyphenols in blueberry, strawberry and their commercial products and determination of antioxidant activity. Food Chem. 2022, 367, 130743. [Google Scholar] [CrossRef]
- Hadi, A.; Nawab, A.; Alam, F.; Zehra, K. Sustainable food packaging films based on alginate and aloe vera. Polym. Eng. Sci. 2022, 62, 2111–2118. [Google Scholar] [CrossRef]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Azucena Castro-Yobal, M.; Contreras-Oliva, A.; Saucedo-Rivalcoba, V.; Rivera-Armenta, J.L.; Hernández-Ramírez, G.; Salinas-Ruiz, J.; Herrera-Corredor, A. Evaluation of physicochemical properties of film-based alginate for food packing applications. e-Polymers 2021, 21, 82–95. [Google Scholar] [CrossRef]
- Matei, E.; Gaidau, C.; Râpă, M.; Stefan, L.M.; Ditu, L.-M.; Predescu, A.M.; Stanca, M.; Pantilimon, M.C.; Berechet, M.D.; Predescu, C.; et al. Sustainable Coated Nanostructures Based on Alginate and Electrospun Collagen Loaded with Antimicrobial Agents. Coatings 2021, 11, 121. [Google Scholar] [CrossRef]
- Tam, S.K.; Dusseault, J.; Polizu, S.; Ménard, M.; Hallé, J.-P.; Yahia, L. Physicochemical model of alginate–poly-l-lysine microcapsules defined at the micrometric/nanometric scale using ATR-FTIR, XPS, and ToF-SIMS. Biomaterials 2005, 26, 6950–6961. [Google Scholar] [CrossRef]
- Kondaveeti, S.; de Assis Bueno, P.V.; Carmona-Ribeiro, A.M.; Esposito, F.; Lincopan, N.; Sierakowski, M.R.; Petri, D.F.S. Microbicidal gentamicin-alginate hydrogels. Carbohydr. Polym. 2018, 186, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Essifi, K.; Lakrat, M.; Berraaouan, D.; Fauconnier, M.-L.; El Bachiri, A.; Tahani, A. Optimization of gallic acid encapsulation in calcium alginate microbeads using Box-Behnken Experimental Design. Polym. Bull. 2021, 78, 5789–5814. [Google Scholar] [CrossRef]
- Roger, S.; Talbot, D.; Bee, A. Preparation and effect of Ca2+ on water solubility, particle release and swelling properties of magnetic alginate films. J. Magn. Magn. Mater. 2006, 305, 221–227. [Google Scholar] [CrossRef]
- bt Ibrahim, S.F.; Mohd Azam, N.A.N.; Mat Amin, K.A. Sodium alginate film: The effect of crosslinker on physical and mechanical properties. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012063. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Kirar, N. Swelling and drug release behavior of calcium alginate/poly (sodium acrylate) hydrogel beads. Des. Monomers Polym. 2016, 19, 89–98. [Google Scholar] [CrossRef]
- Huang, M.-H.; Yang, M.-C. Swelling and biocompatibility of sodium alginate/poly(γ-glutamic acid) hydrogels. Polym. Adv. Technol. 2010, 21, 561–567. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, C.; Jin, X. Influence of K+ and Na+ ions on the degradation of wet-spun alginate fibers for tissue engineering. J. Appl. Polym. Sci. 2017, 134, 44396. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Romita, R.; Agostiano, A.; Nuzzo, S.; Cosma, P. Commercial bentonite clay as low-cost and recyclable “natural” adsorbent for the Carbendazim removal/recover from water: Overview on the adsorption process and preliminary photodegradation considerations. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125060. [Google Scholar] [CrossRef]
- Topuz, F.; Henke, A.; Richtering, W.; Groll, J. Magnesium ions and alginate do form hydrogels: A rheological study. Soft Matter 2012, 8, 4877–4881. [Google Scholar] [CrossRef]
- Kelishomi, Z.H.; Goliaei, B.; Mahdavi, H.; Nikoofar, A.; Rahimi, M.; Moosavi-Movahedi, A.A.; Mamashli, F.; Bigdeli, B. Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food Chem. 2016, 196, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, S. Do the polyphenolic compounds from natural products can protect the skin from ultraviolet rays? Results Chem. 2022, 4, 100428. [Google Scholar] [CrossRef]
- Bekbolet, M. Light Effects on Food. J. Food Prot. 1990, 53, 430–440. [Google Scholar] [CrossRef]
- Duncan, S.E.; Chang, H.-H. Chapter Two—Implications of Light Energy on Food Quality and Packaging Selection. Adv. Food Nutr. Res. 2012, 67, 25–73. [Google Scholar]
- Cheng, H.; Chen, L.; McClements, D.J.; Xu, H.; Long, J.; Zhao, J.; Xu, Z.; Meng, M.; Jin, Z. Recent advances in the application of nanotechnology to create antioxidant active food packaging materials. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Lam, W.-H.; Chong, M.N.; Horri, B.A.; Tey, B.-T.; Chan, E.-S. Physicochemical stability of calcium alginate beads immobilizing TiO2 nanoparticles for removal of cationic dye under UV irradiation. J. Appl. Polym. Sci. 2017, 134, 45002. [Google Scholar] [CrossRef]

| No. | Compound | GPWW (mg/L) |
|---|---|---|
| Phenolic acids | ||
| 1 | Gallic acid | 26.57 |
| 2 | Neochlorogenic acid | n.d. |
| 3 | Chlorogenic acid | n.d. |
| 4 | p-Hydroxybenzoic acid | 0.61 |
| 5 | 3-Hydroxybenzoic acid | n.d. |
| 6 | Caffeic acid | 0.46 |
| 7 | Vanillic acid | 19.67 |
| 8 | Syringic acid | 3.46 |
| 9 | p-Coumaric acid | 0.37 |
| 10 | Ferulic acid | 0.02 |
| 11 | 3,5-Dicaffeoylquinic acid | 0.01 |
| 12 | Ellagic acid | 3.54 |
| Flavonoids | ||
| (A) Anthocyanins | ||
| 13 | Delphinidin 3,5 diglucoside | 0.19 |
| 14 | Delphinidin3-galactoside | 1.91 |
| 15 | Cyanidin-3-glucoside | 0.87 |
| 16 | Petunidin-3-glucoside | 2.61 |
| 17 | Pelargonidin-3-rutinoside | n.d. |
| 18 | Pelargonidin-3-glucoside | 0.002 |
| 19 | Malvidin-3-galactoside | 16.76 |
| (B) Flavonols | ||
| 20 | Rutin | 0.05 |
| 21 | Isoquercitrin | 0.23 |
| 22 | Quercitrin | 0.01 |
| 23 | Myricetin | 0.54 |
| 24 | Kaempferol-3-glucoside | 0.02 |
| 25 | Quercetin | 6.92 |
| 26 | Isorhamnetin | 0.05 |
| 27 | Hyperoside | 0.42 |
| 28 | Kaempferol | 0.50 |
| (C) Flavan-3-ols | ||
| 29 | Catechin | 27.10 |
| 30 | Epicatechin | 9.93 |
| 31 | Procyanidin B2 | 26.64 |
| 32 | Procyanidin A2 | 0.17 |
| (D) Dihydrochalcones | ||
| 33 | Phloridzin | 0.09 |
| 34 | Phloretin | 0.003 |
| (E) Flavanones | ||
| 35 | Hesperidin | n.d. |
| 36 | Naringin | n.d. |
| Stilbenes | ||
| 37 | Resveratrol | n.d. |
| Non-phenolic acids | ||
| 38 | Trans-cinnamic acid | 0.04 |
| Total phenolic content | 149.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubitosa, J.; Rizzi, V.; Marasciulo, C.; Maggi, F.; Caprioli, G.; Mustafa, A.M.; Fini, P.; De Vietro, N.; Aresta, A.M.; Cosma, P. Realizing Eco-Friendly Water-Resistant Sodium-Alginate-Based Films Blended with a Polyphenolic Aqueous Extract from Grape Pomace Waste for Potential Food Packaging Applications. Int. J. Mol. Sci. 2023, 24, 11462. https://doi.org/10.3390/ijms241411462
Gubitosa J, Rizzi V, Marasciulo C, Maggi F, Caprioli G, Mustafa AM, Fini P, De Vietro N, Aresta AM, Cosma P. Realizing Eco-Friendly Water-Resistant Sodium-Alginate-Based Films Blended with a Polyphenolic Aqueous Extract from Grape Pomace Waste for Potential Food Packaging Applications. International Journal of Molecular Sciences. 2023; 24(14):11462. https://doi.org/10.3390/ijms241411462
Chicago/Turabian StyleGubitosa, Jennifer, Vito Rizzi, Cosma Marasciulo, Filippo Maggi, Giovanni Caprioli, Ahmed M. Mustafa, Paola Fini, Nicoletta De Vietro, Antonella Maria Aresta, and Pinalysa Cosma. 2023. "Realizing Eco-Friendly Water-Resistant Sodium-Alginate-Based Films Blended with a Polyphenolic Aqueous Extract from Grape Pomace Waste for Potential Food Packaging Applications" International Journal of Molecular Sciences 24, no. 14: 11462. https://doi.org/10.3390/ijms241411462
APA StyleGubitosa, J., Rizzi, V., Marasciulo, C., Maggi, F., Caprioli, G., Mustafa, A. M., Fini, P., De Vietro, N., Aresta, A. M., & Cosma, P. (2023). Realizing Eco-Friendly Water-Resistant Sodium-Alginate-Based Films Blended with a Polyphenolic Aqueous Extract from Grape Pomace Waste for Potential Food Packaging Applications. International Journal of Molecular Sciences, 24(14), 11462. https://doi.org/10.3390/ijms241411462















