Nitazoxanide Inhibits the Bifunctional Enzyme GlG6PD::6PGL of Giardia lamblia: Biochemical and In Silico Characterization of a New Druggable Target
Abstract
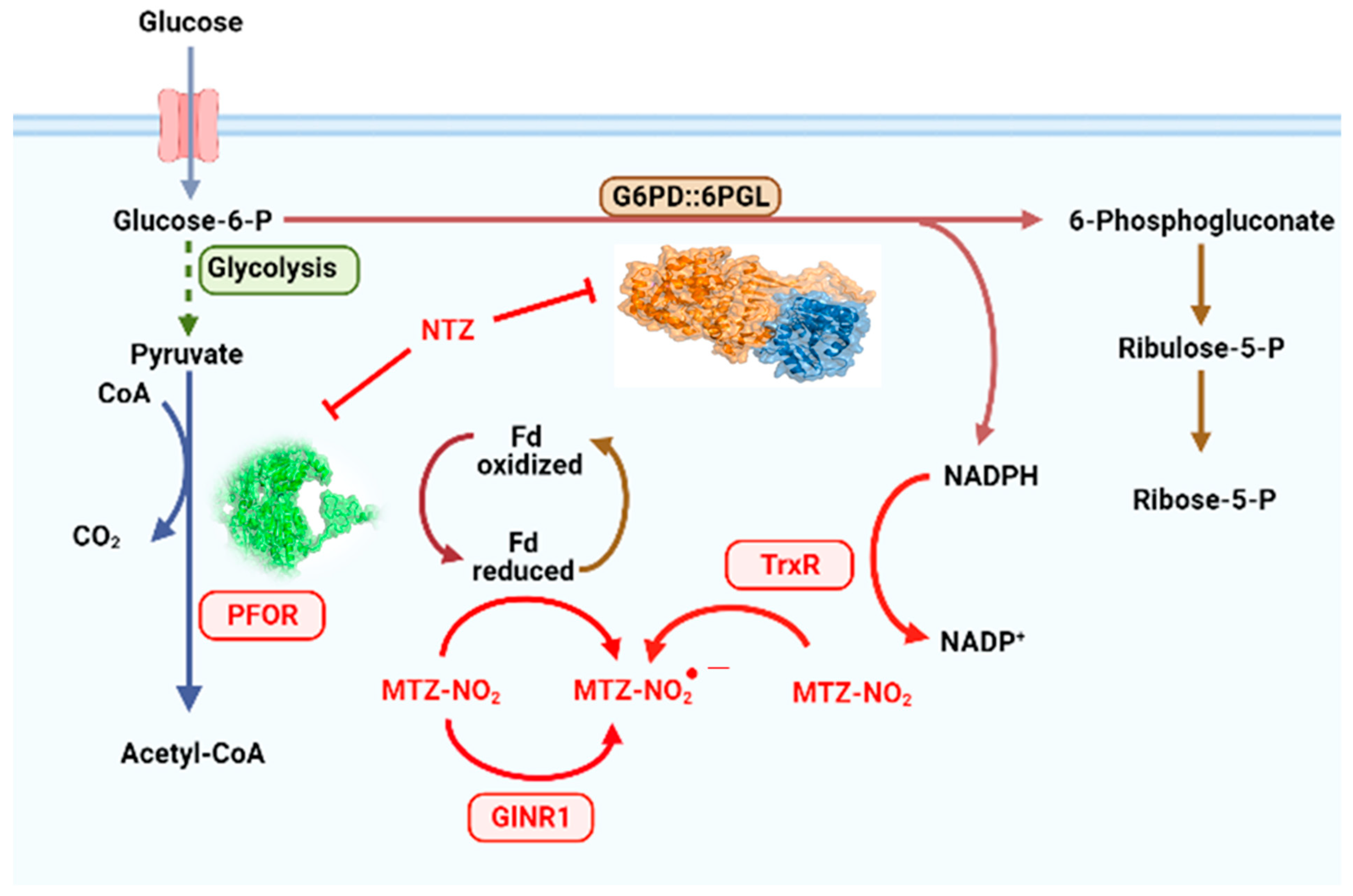
1. Introduction
2. Results and Discussion
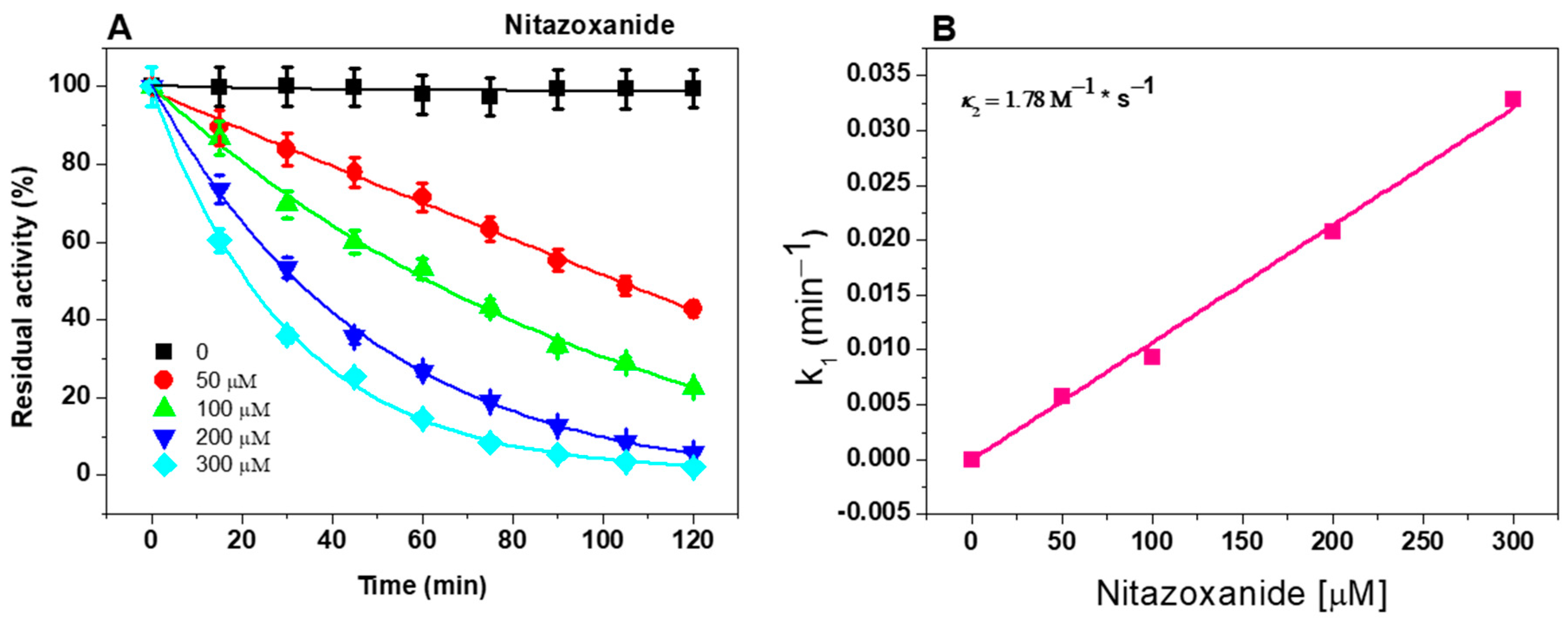
2.1. In Vitro Analysis of Fused GlG6PD::6PGL Inactivation with Antigiardial Drugs
2.2. Second-Order Rate Constant (k2) of NTZ
2.3. Intrinsic and Extrinsic Fluorescence Assays
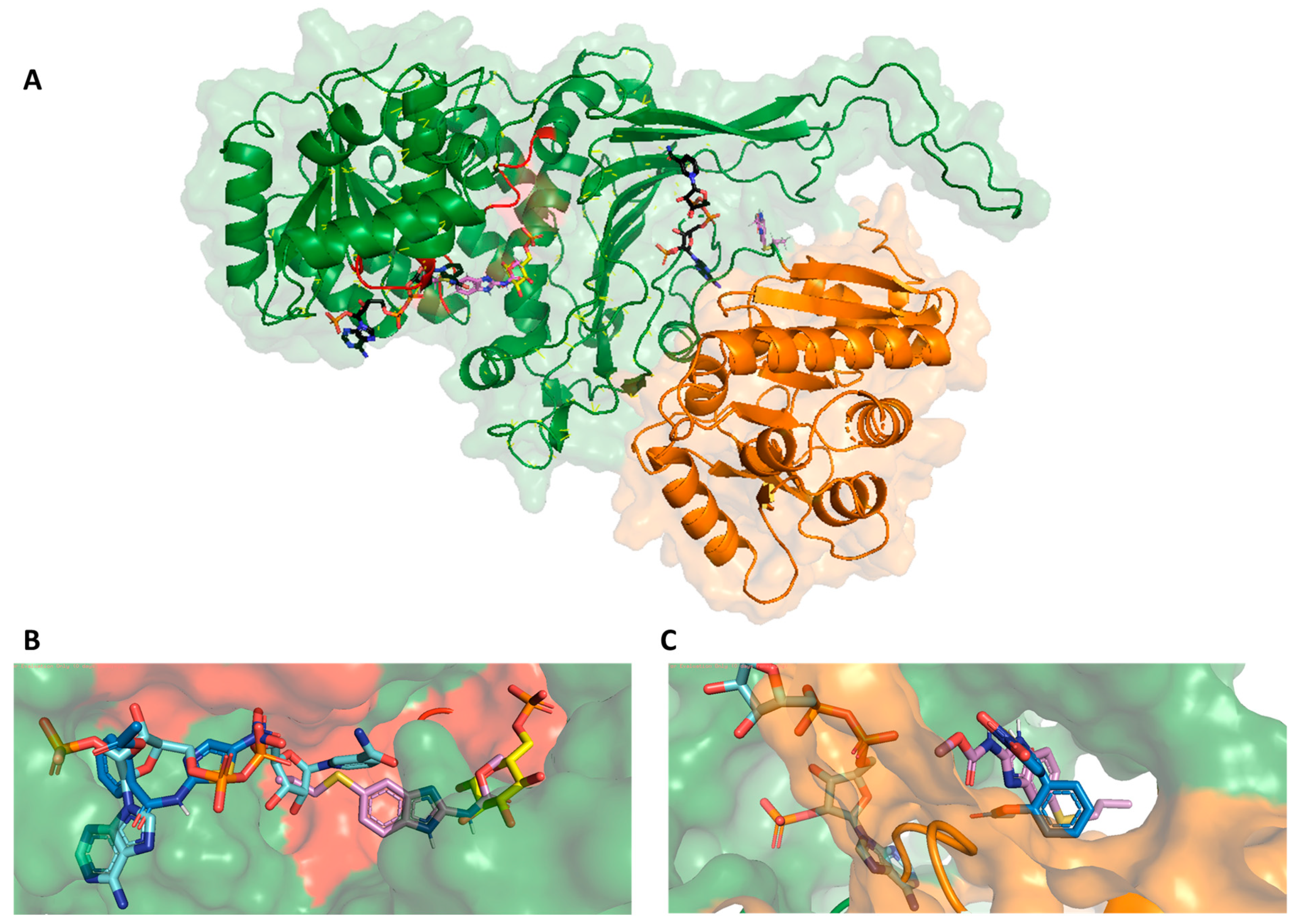
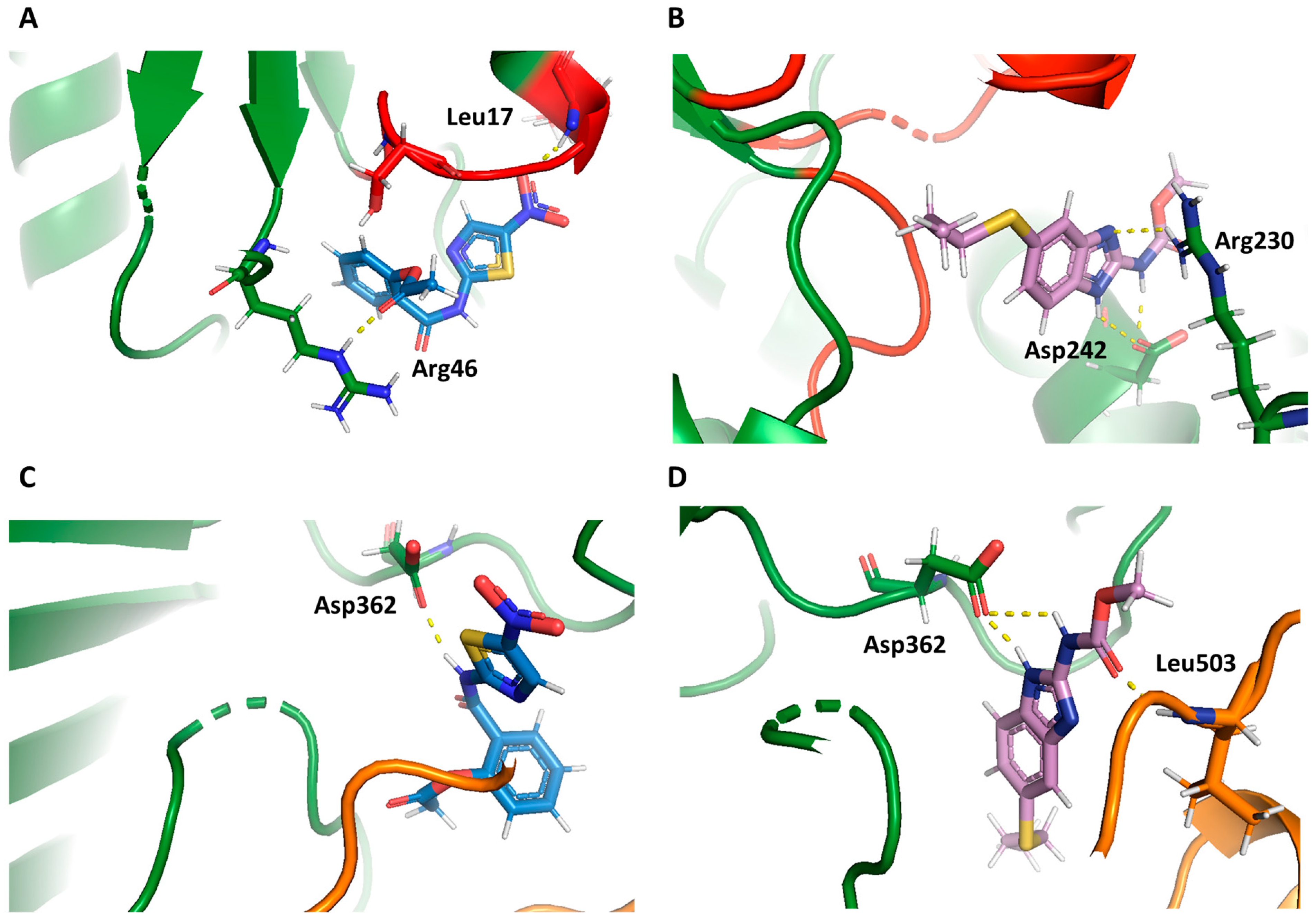
2.4. Molecular Docking Study
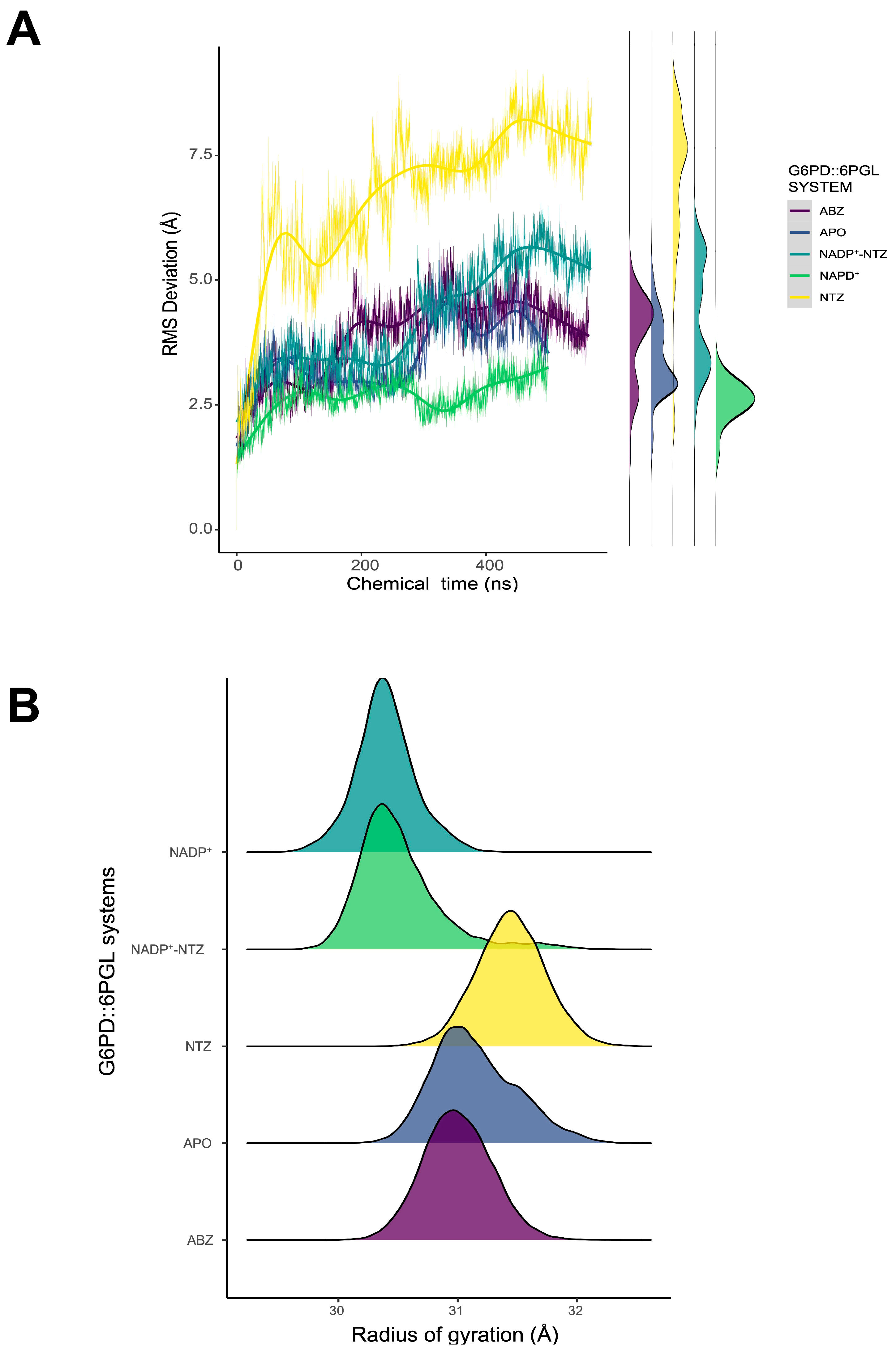
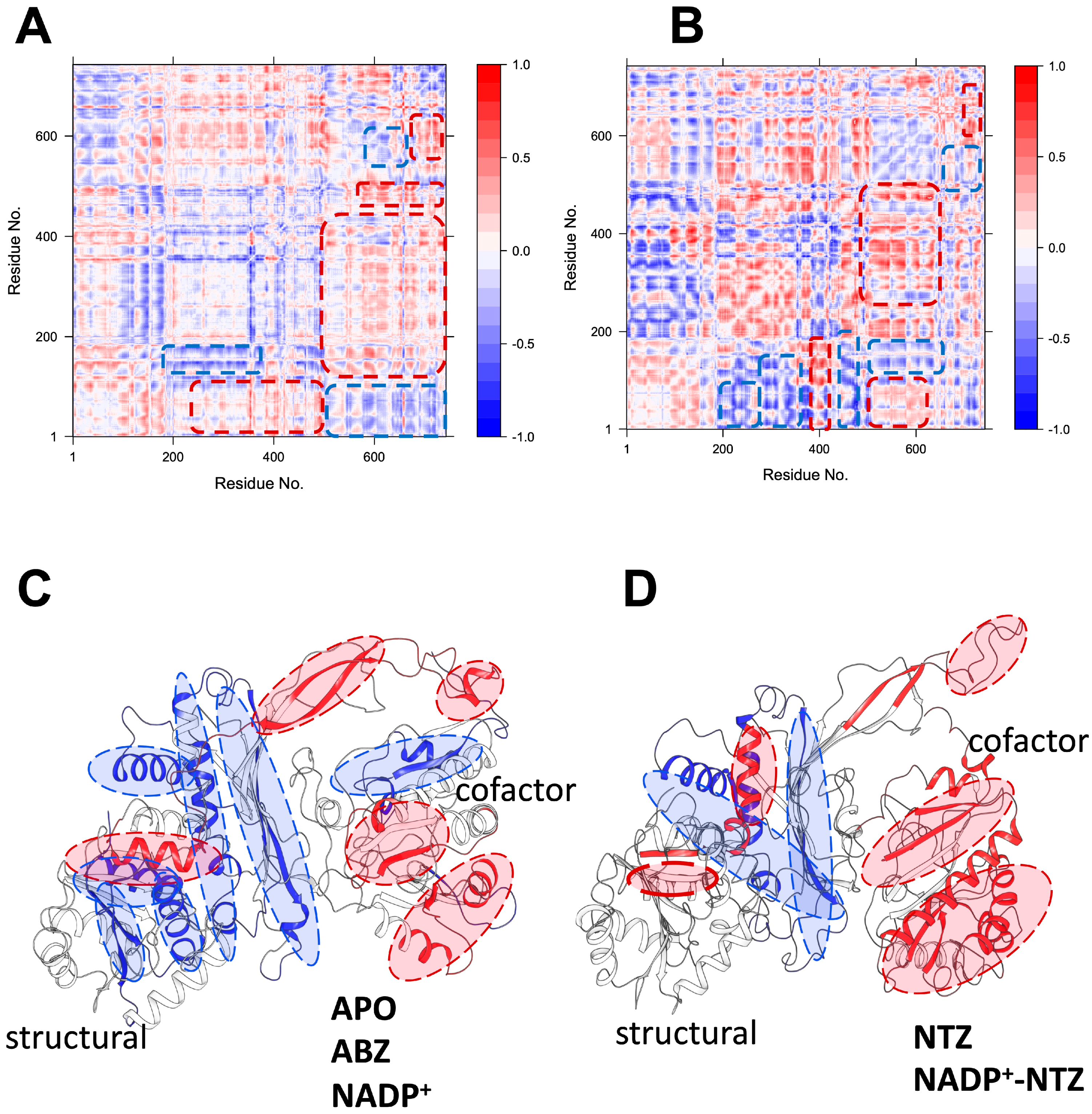
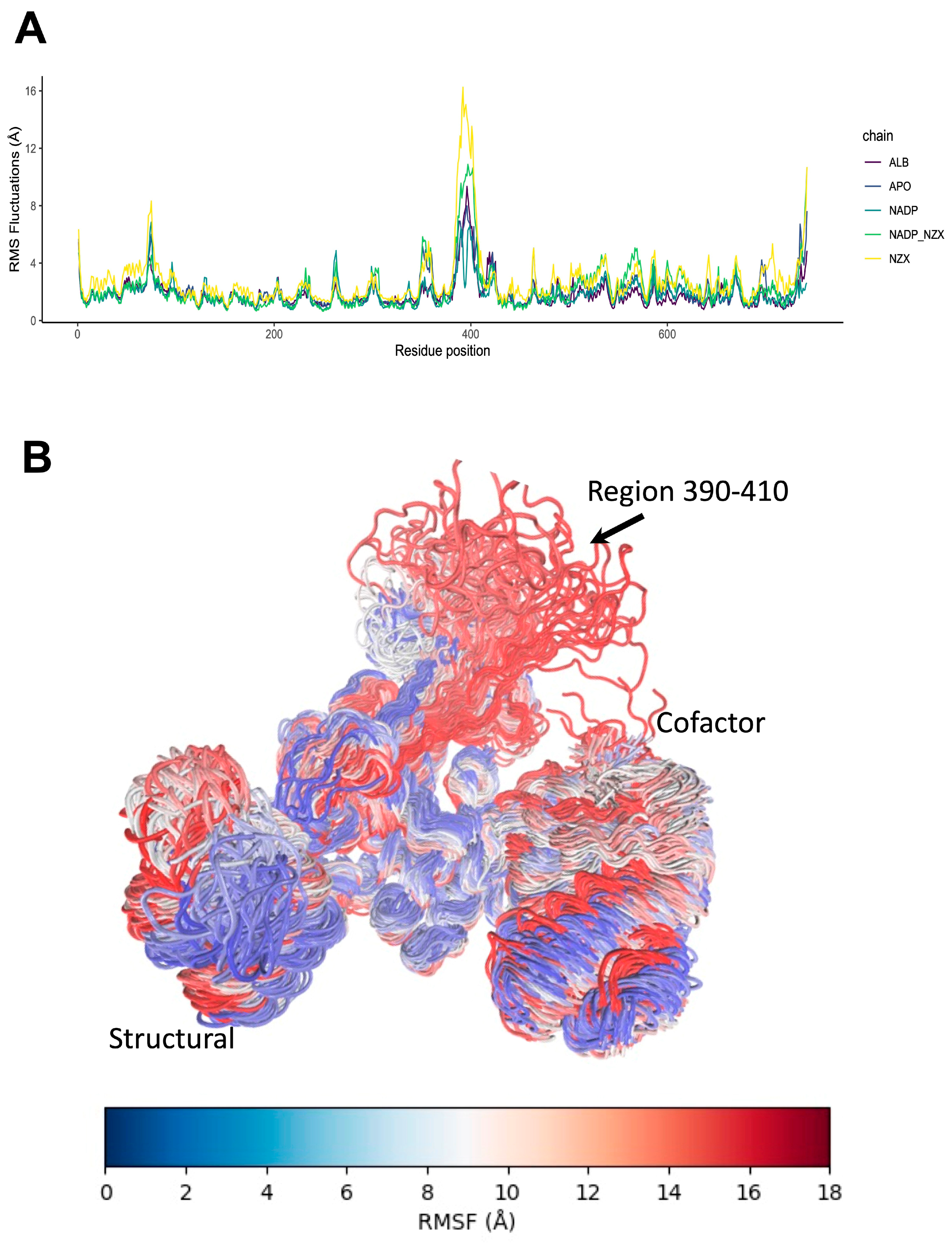
2.5. Dynamic Behavior of GlG6PD::6PGL in Complex with Antigiardial Drugs
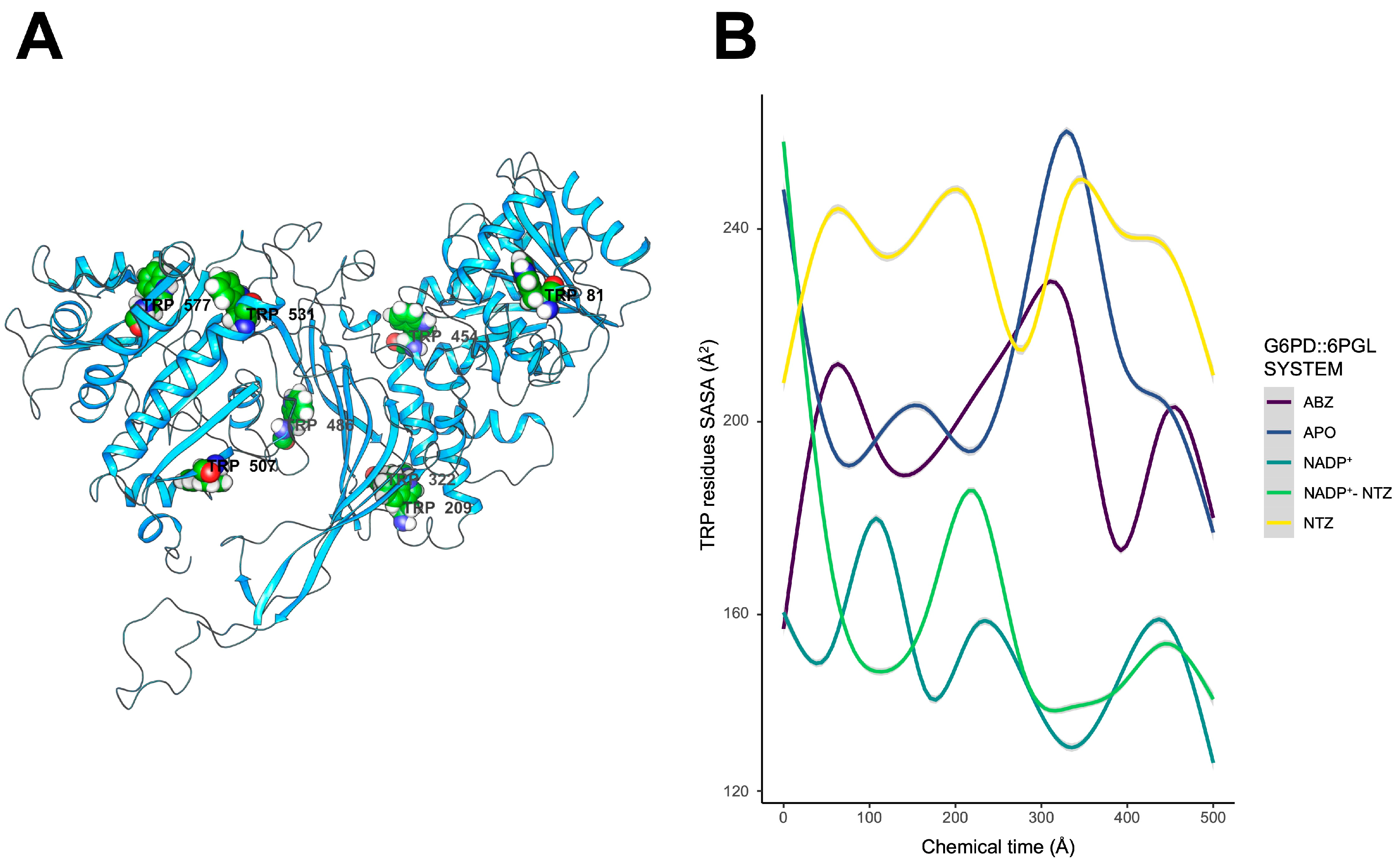
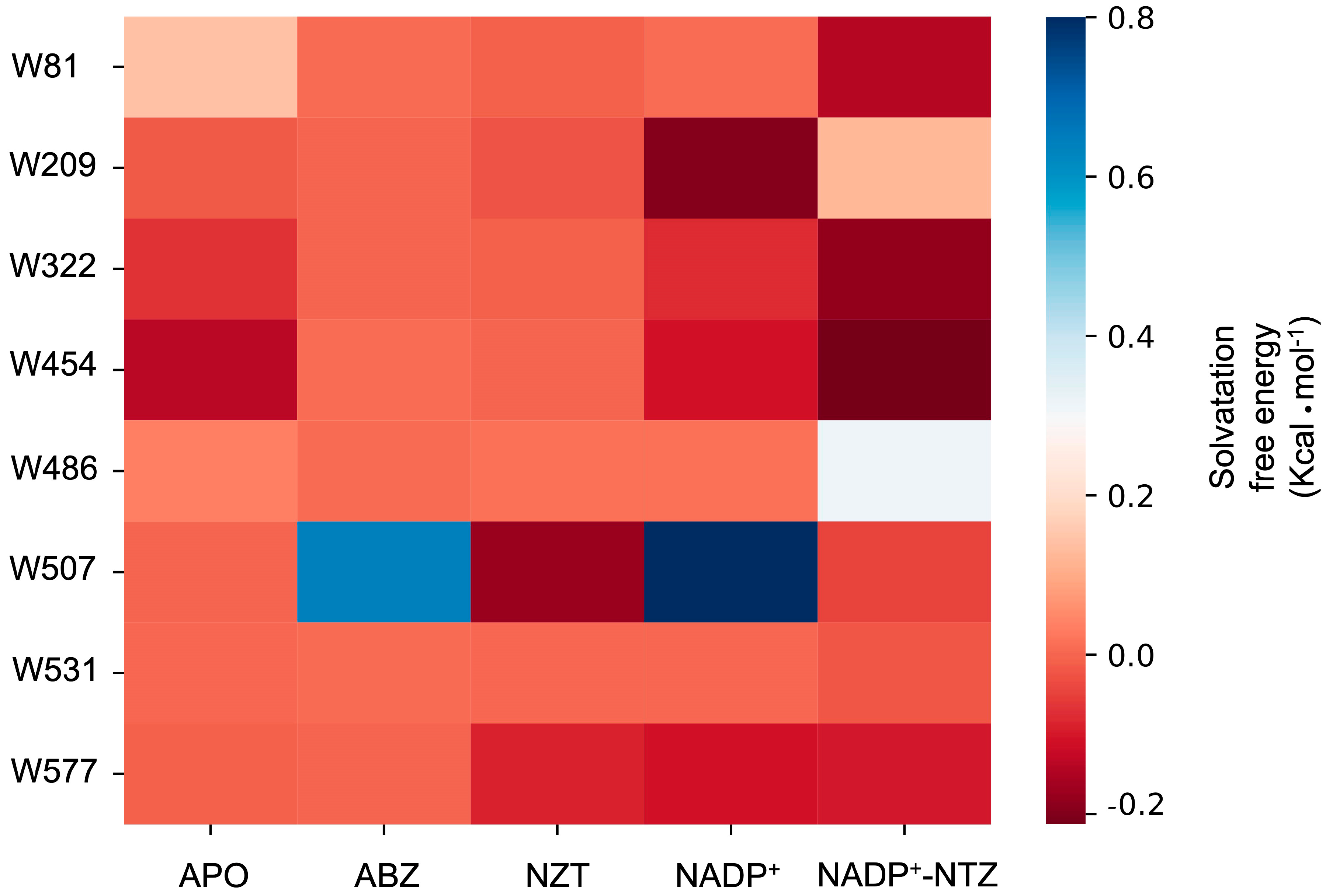
2.6. Exposure of Tryptophan Residues as a Result of Changes in Molecular Motions
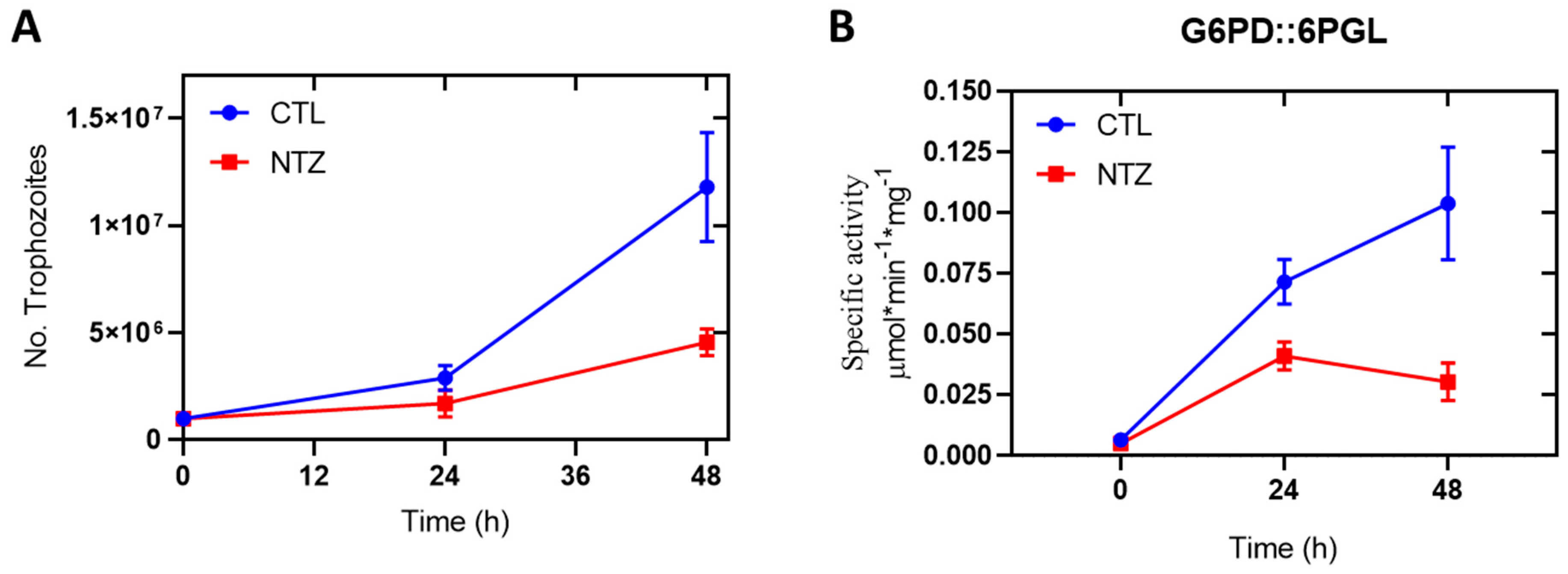
2.7. Enzyme Activity of Cellular GlG6PD::6PGL
3. Materials and Methods
3.1. Chemicals
3.2. Purification of the Recombinant Protein GlG6PD::6PGL
3.3. Inactivation Assays of Fused G6PD::6PGL by NTZ
3.3.1. In Vitro Analysis of Fused GlG6PD::6PGL Inactivation with Antigiardial Drugs
3.3.2. Second-Order Rate Constant (k2) of NTZ
3.4. Structural Analysis by Intrinsic and Extrinsic Fluorescence Assays
3.5. Molecular Docking Study
3.6. Molecular Dynamics Simulations of GlG6PD::6PGL
3.7. Trajectory Analysis
3.8. Enzyme Activity of Cellular GlG6PD::6PGL
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, L.M.; Saravolatz, L.D. Nitazoxanide: A new thiazolide antiparasitic agent. Clin. Infect. Dis. 2005, 40, 1173–1180. [Google Scholar] [CrossRef]
- Broekhuysen, J.; Stockis, A.; Lins, R.L.; De Graeve, J.; Rossignol, J.F. Nitazoxanide: Pharmacokinetics and metabolism in man. Int. J. Clin. Pharmacol. Ther. 2000, 38, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, A.; Mueller, J.; Esposito, M. Nitazoxanide, a broad-spectrum thiazolide anti-infective agent for the treatment of gastrointestinal infections. Expert Opin. Pharmacother. 2006, 7, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Gilles, H.M.; Hoffman, P.S. Treatment of intestinal parasitic infections: A review of nitazoxanide. Trends Parasitol. 2002, 18, 95–97. [Google Scholar] [CrossRef]
- White, C.A., Jr. Nitazoxanide: A new broad spectrum antiparasitic agent. Expert. Rev. Anti Infect. Ther. 2004, 2, 43–49. [Google Scholar] [CrossRef]
- Dubreuil, L.; Houcke, I.; Mouton, Y.; Rossignol, J.F. In vitro evaluation of activities of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrob. Agents Chemother. 1996, 40, 2266–2270. [Google Scholar] [CrossRef]
- Mégraud, F.; Occhialini, A.; Rossignol, J.F. Nitazoxanide, a potential drug for eradication of Helicobacter pylori with no cross-resistance to metronidazole. Antimicrob. Agents Chemother. 1998, 42, 2836–2840. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.F. Nitazoxanide: A first-in-class broad-spectrum antiviral agent. Antivir. Res. 2014, 110, 94–103. [Google Scholar] [CrossRef]
- Abuelazm, M.; Ghanem, A.; Awad, A.K.; Farahat, R.A.; Labieb, F.; Katamesh, B.E.; Abdelazeem, B. The Effect of Nitazoxanide on the Clinical Outcomes in Patients with COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Drug Investig. 2022, 42, 1031–1047. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Rühle, G.; Müller, N.; Rossignol, J.F.; Hemphill, A. In vitro effects of thiazolides on Giardia lamblia WB clone C6 cultured axenically and in coculture with Caco2 cells. Antimicrob. Agents Chemother. 2006, 50, 162–170. [Google Scholar] [CrossRef]
- de Carvalho, L.P.; Darby, C.M.; Rhee, K.Y.; Nathan, C. Nitazoxanide Disrupts Membrane Potential and Intrabacterial pH Homeostasis of Mycobacterium tuberculosis. ACS Med. Chem. Lett. 2011, 2, 849–854. [Google Scholar] [CrossRef]
- Leitsch, D.; Burgess, A.G.; Dunn, L.A.; Krauer, K.G.; Tan, K.; Duchêne, M.; Upcroft, P.; Eckmann, L.; Upcroft, J.A. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J. Antimicrob. Chemother. 2011, 66, 1756–1765. [Google Scholar] [CrossRef]
- Townson, S.M.; Hanson, G.R.; Upcroft, J.A.; Upcroft, P. A purified ferredoxin from Giardia duodenalis. Eur. J. Biochem. 1994, 220, 439–446. [Google Scholar] [CrossRef]
- Townson, S.M.; Upcroft, J.A.; Upcroft, P. Characterisation and purification of pyruvate:ferredoxin oxidoreductase from Giardia duodenalis. Mol. Biochem. Parasitol. 1996, 79, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Sisson, G.; Jeong, J.Y.; Goodwin, A.; Bryden, L.; Rossler, N.; Lim-Morrison, S.; Raudonikiene, A.; Berg, D.E.; Hoffman, P.S. Metronidazole activation is mutagenic and causes DNA fragmentation in Helicobacter pylori and in Escherichia coli containing a cloned H. pylori RdxA+ (Nitroreductase) gene. J. Bacteriol. 2000, 182, 5091–5096. [Google Scholar] [CrossRef]
- Müller, J.; Wastling, J.; Sanderson, S.; Müller, N.; Hemphill, A. A novel Giardia lamblia nitroreductase, GlNR1, interacts with nitazoxanide and other thiazolides. Antimicrob. Agents Chemother. 2007, 51, 1979–1986. [Google Scholar] [CrossRef]
- Müller, J.; Rout, S.; Leitsch, D.; Vaithilingam, J.; Hehl, A.; Müller, N. Comparative characterisation of two nitroreductases from Giardia lamblia as potential activators of nitro compounds. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 37–43. [Google Scholar] [CrossRef]
- Nillius, D.; Müller, J.; Müller, N. Nitroreductase (GlNR1) increases susceptibility of Giardia lamblia and Escherichia coli to nitro drugs. J. Antimicrob. Chemother. 2011, 66, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Sterk, M.; Hemphill, A.; Müller, N. Characterization of Giardia lamblia WB C6 clones resistant to nitazoxanide and to metronidazole. J. Antimicrob. Chemother. 2007, 60, 280–287. [Google Scholar] [CrossRef]
- Müller, J.; Naguleswaran, A.; Müller, N.; Hemphill, A. Neospora caninum: Functional inhibition of protein disulfide isomerase by the broad-spectrum anti-parasitic drug nitazoxanide and other thiazolides. Exp. Parasitol. 2008, 118, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Schildknecht, P.; Müller, N. Metabolism of nitro drugs metronidazole and nitazoxanide in Giardia lamblia: Characterization of a novel nitroreductase (GlNR2). J. Antimicrob. Chemother. 2013, 68, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.S.; Sisson, G.; Croxen, M.A.; Welch, K.; Harman, W.D.; Cremades, N.; Morash, M.G. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob. Agents Chemother. 2007, 51, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Morales-Luna, L.; Hernández-Ochoa, B.; Martínez-Rosas, V.; Navarrete-Vázquez, G.; Ortega-Cuellar, D.; Rufino-González, Y.; González-Valdez, A.; Arreguin-Espinosa, R.; Franco-Vásquez, A.M.; Pérez de la Cruz, V.; et al. Giardia lamblia G6PD::6PGL Fused Protein Inhibitors Decrease Trophozoite Viability: A New Alternative against Giardiasis. Int. J. Mol. Sci. 2022, 23, 14358. [Google Scholar] [CrossRef]
- Navarrete-Vázquez, G.; Chávez-Silva, F.; Colín-Lozano, B.; Estrada-Soto, S.; Hidalgo-Figueroa, S.; Guerrero-Álvarez, J.; Méndez, S.T.; Reyes-Vivas, H.; Oria-Hernández, J.; Canul-Canché, J.; et al. Synthesis of nitro(benzo)thiazole acetamides and in vitro antiprotozoal effect against amitochondriate parasites Giardia intestinalis and Trichomonas vaginalis. Bioorg Med. Chem. 2015, 23, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Kalisiak, J.; Korthals, K.; Lauwaet, T.; Cheung, D.Y.; Lozano, R.; Cobo, E.R.; Upcroft, P.; Upcroft, J.A.; Berg, D.E.; et al. Expanded therapeutic potential in activity space of next-generation 5-nitroimidazole antimicrobials with broad structural diversity. Proc. Natl. Acad. Sci. USA 2013, 110, 17564–17569. [Google Scholar] [CrossRef]
- Nava-Zuazo, C.; Chávez-Silva, F.; Moo-Puc, R.; Chan-Bacab, M.J.; Ortega-Morales, B.O.; Moreno-Díaz, H.; Díaz-Coutiño, D.; Hernández-Núñez, E.; Navarrete-Vázquez, G. 2-acylamino-5-nitro-1,3-thiazoles: Preparation and in vitro bioevaluation against four neglected protozoan parasites. Bioorg Med. Chem. 2014, 22, 1626. [Google Scholar] [CrossRef]
- Sisson, G.; Goodwin, A.; Raudonikiene, A.; Hughes, N.J.; Mukhopadhyay, A.K.; Berg, D.E.; Hoffman, P.S. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob. Agents Chemother. 2002, 46, 2116–2123. [Google Scholar] [CrossRef]
- Ballard, T.E.; Wang, X.; Olekhnovich, I.; Koerner, T.; Seymour, C.; Hoffman, P.S.; Macdonald, T.L. Biological activity of modified and exchanged 2-amino-5-nitrothiazole amide analogues of nitazoxanide. Bioorg. Med. Chem. Lett. 2010, 20, 3537–3539. [Google Scholar] [CrossRef]
- Pankuch, G.A.; Appelbaum, P.C. Activities of tizoxanide and nitazoxanide compared to those of five other thiazolides and three other agents against anaerobic species. Antimicrob. Agents Chemother. 2006, 50, 1112–1117. [Google Scholar] [CrossRef]
- Scior, T.; Lozano-Aponte, J.; Ajmani, S.; Hernández-Montero, E.; Chávez-Silva, F.; Hernández-Núñez, E.; Moo-Puc, R.; Fraguela-Collar, A.; Navarrete-Vázquez, G. Antiprotozoal Nitazoxanide Derivatives: Synthesis, Bioassays and QSAR Study Combined with Docking for Mechanistic Insight. Curr. Comput. Aided Drug Des. 2015, 11, 21–31. [Google Scholar] [CrossRef]
- Colín-Lozano, B.; León-Rivera, I.; Chan-Bacab, M.J.; Ortega-Morales, B.O.; Moo-Puc, R.; López-Guerrero, V.; Hernández-Núñez, E.; Argüello-Garcia, R.; Scior, T.; Barbosa-Cabrera, E.; et al. Synthesis, in vitro and in vivo giardicidal activity of nitrothiazole-NSAID chimeras displaying broad antiprotozoal spectrum. Bioorg. Med. Chem. Lett. 2017, 27, 3490–3494. [Google Scholar] [CrossRef]
- Krátký, M.; Vinsová, J. Salicylanilide ester prodrugs as potential antimicrobial agents—A review. Curr. Pharm. Des. 2011, 17, 3494–3505. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, S.A.; Dror, R.O. Molecular Dynamics Simulation for All. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Dror, R.O.; Dirks, R.M.; Grossman, J.P.; Xu, H.; Shaw, D.E. Biomolecular Simulation: A Computational Microscope for Molecular Biology. Annu. Rev. Biophys. 2012, 41, 429–452. [Google Scholar] [CrossRef]
- Morales-Luna, L.; González-Valdez, A.; Sixto-López, Y.; Correa-Basurto, J.; Hernández-Ochoa, B.; Cárdenas-Rodríguez, N.; Castillo-Rodríguez, R.A.; Ortega-Cuellar, D.; Arreguin-Espinosa, R.; Pérez de la Cruz, V.; et al. Identification of the NADP+ Structural Binding Site and Coenzyme Effect on the Fused G6PD::6PGL Protein from Giardia lamblia. Biomolecules 2020, 10, 46. [Google Scholar] [CrossRef]
- Miao, Y.; Feher, V.A.; McCammon, J.A. Gaussian Accelerated Molecular Dynamics: Unconstrained Enhanced Sampling and Free Energy Calculation. J. Chem. Theory Comput. 2015, 11, 3584–3595. [Google Scholar] [CrossRef] [PubMed]
- Ghisaidoobe, A.B.T.; Chung, S.J. Intrinsic Tryptophan Fluorescence in the Detection and Analysis of Proteins: A Focus on Förster Resonance Energy Transfer Techniques. Int. J. Mol. Sci. 2014, 15, 22518–22538. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, D.A.; Chaudhuri, A.; Haldar, S.; Chattopadhyay, A. Exploring tryptophan dynamics in acid-induced molten globule state of bovine α-lactalbumin: A wavelength-selective fluorescence approach. Eur. Biophys. J. 2010, 39, 1453–1463. [Google Scholar] [CrossRef]
- Rahman, S.J.; Kaur, P. Conformational changes in a multidrug resistance ABC transporter DrrAB: Fluorescence-based approaches to study substrate binding. Arch. Biochem. Biophys. 2018, 658, 31–45. [Google Scholar] [CrossRef]
- Cedillo-Rivera, R.; Chávez, B.; González-Robles, A.; Tapia, A.; Yépez-Mulia, L. In vitro effect of nitazoxanide against Entamoeba histolytica, Giardia intestinalis and Trichomonas vaginalis trophozoites. J. Eukaryot. Microbiol. 2002, 49, 201–208. [Google Scholar] [CrossRef]
- Leitsch, D.; Schlosser, S.; Burgess, A.; Duchêne, M. Nitroimidazole drugs vary in their mode of action in the human parasite Giardia lamblia. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 166–170. [Google Scholar] [CrossRef]
- Thomas, D.C. The phagocyte respiratory burst: Historical perspectives and recent advances. Immunol. Lett. 2017, 192, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Miele, A.E.; Ardini, M.; Boumis, G.; Saccoccia, F.; Bellelli, A. Typical 2-Cys peroxiredoxins in human parasites: Several physiological roles for a potential chemotherapy target. Mol. Biochem. Parasitol. 2016, 206, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Mastronicola, D.; Testa, F.; Forte, E.; Bordi, E.; Pucillo, L.P.; Sarti, P.; Giuffrè, A. Flavohemoglobin and nitric oxide detoxification in the human protozoan parasite Giardia intestinalis. Biochem. Biophys. Res. Commun. 2010, 399, 654–658. [Google Scholar] [CrossRef]
- Di Matteo, A.; Scandurra, F.M.; Testa, F.; Forte, E.; Sarti, P.; Brunori, M.; Giuffrè, A. The O2-scavenging flavodiiron protein in the human parasite Giardia intestinalis. J. Biol. Chem. 2008, 283, 4061–4068. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ramírez-Nava, E.J.; Hernández-Ochoa, B.; Navarrete-Vázquez, G.; Arreguín-Espinosa, R.; Ortega-Cuellar, D.; González-Valdez, A.; Martínez-Rosas, V.; Morales-Luna, L.; Martínez-Miranda, J.; Sierra-Palacios, E.; et al. Novel inhibitors of human glucose-6-phosphate dehydrogenase (HsG6PD) affect the activity and stability of the protein. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129828. [Google Scholar] [CrossRef]
- Martínez-Rosas, V.; Hernández-Ochoa, B.; Navarrete-Vázquez, G.; Martínez-Conde, C.; Gómez-Chávez, F.; Morales-Luna, L.; González-Valdez, A.; Arreguin-Espinosa, R.; Enríquez-Flores, S.; Pérez de la Cruz, V.; et al. Kinetic and Molecular Docking Studies to Determine the Effect of Inhibitors on the Activity and Structure of Fused G6PD::6PGL Protein from Trichomonas vaginalis. Molecules 2022, 27, 1174. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ochoa, B.; Martínez-Rosas, V.; Morales-Luna, L.; Calderón-Jaimes, E.; Rocha-Ramírez, L.M.; Ortega-Cuellar, D.; Rufino-González, Y.; González-Valdez, A.; Arreguin-Espinosa, R.; Enríquez-Flores, S.; et al. Pyridyl Methylsulfinyl Benzimidazole Derivatives as Promising Agents against Giardia lamblia and Trichomonas vaginalis. Molecules 2022, 27, 8902. [Google Scholar] [CrossRef]
- Gómez-Manzo, S.; Terrón-Hernández, J.; De la Mora-De la Mora, I.; González-Valdez, A.; Marcial-Quino, J.; García-Torres, I.; Vanoye-Carlo, A.; López-Velázquez, G.; Hernández-Alcántara, G.; Oria-Hernández, J.; et al. The stability of G6PD is affected by mutations with different clinical phenotypes. Int. J. Mol. Sci. 2014, 15, 21179–21201. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 1758–2946. [Google Scholar] [CrossRef] [PubMed]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.H.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Salomon-Ferrer, R.; Götz, A.W.; Poole, D.; Le Grand, S.; Walker, R.C. Routine Microsecond Molecular Dynamics Simulations with AMBER on GPUs. 2. Explicit Solvent Particle Mesh Ewald. J. Chem. Theory Comput. 2013, 9, 3878–3888. [Google Scholar] [CrossRef]
- Götz, A.W.; Williamson, M.J.; Xu, D.; Poole, D.; Le Grand, S.; Walker, R.C. Routine Microsecond Molecular Dynamics Simulations with AMBER on GPUs. 1. Generalized Born. J. Chem. Theory Comput. 2012, 8, 1542–1555. [Google Scholar] [CrossRef]
- Case, D.A.; Belfon, K.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Giambasu, G.; et al. AMBER 20; In Journal of Computational Chemistry; University of California: San Francisco, CA, USA, 2020; Available online: https://ambermd.org/doc12/Amber20.pdf (accessed on 13 January 2023).
- Miao, Y.; McCammon, J.A. Unconstrained enhanced sampling for free energy calculations of biomolecules: A review. Mol. Simul. 2016, 42, 1046–1055. [Google Scholar] [CrossRef]
- Miao, Y.; McCammon, J.A. Gaussian Accelerated Molecular Dynamics: Theory, Implementation, and Applications. Annu. Rep. Comput. Chem. 2017, 13, 231–278. [Google Scholar] [CrossRef]
- Miao, Y. Acceleration of biomolecular kinetics in Gaussian accelerated molecular dynamics. J. Chem. Phys. 2018, 149, 072308. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.J.; Rodrigues, A.P.C.; ElSawy, K.M.; McCammon, J.A.; Caves, L.S.D. Bio3d: An R package for the comparative analysis of protein structures. Bioinformatics 2006, 22, 2695–2696. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rosas, V.; Hernández-Ochoa, B.; Morales-Luna, L.; Ortega-Cuellar, D.; González-Valdez, A.; Arreguin-Espinosa, R.; Rufino-González, Y.; Calderón-Jaimes, E.; Castillo-Rodríguez, R.A.; Wong-Baeza, C.; et al. Nitazoxanide Inhibits the Bifunctional Enzyme GlG6PD::6PGL of Giardia lamblia: Biochemical and In Silico Characterization of a New Druggable Target. Int. J. Mol. Sci. 2023, 24, 11516. https://doi.org/10.3390/ijms241411516
Martínez-Rosas V, Hernández-Ochoa B, Morales-Luna L, Ortega-Cuellar D, González-Valdez A, Arreguin-Espinosa R, Rufino-González Y, Calderón-Jaimes E, Castillo-Rodríguez RA, Wong-Baeza C, et al. Nitazoxanide Inhibits the Bifunctional Enzyme GlG6PD::6PGL of Giardia lamblia: Biochemical and In Silico Characterization of a New Druggable Target. International Journal of Molecular Sciences. 2023; 24(14):11516. https://doi.org/10.3390/ijms241411516
Chicago/Turabian StyleMartínez-Rosas, Víctor, Beatriz Hernández-Ochoa, Laura Morales-Luna, Daniel Ortega-Cuellar, Abigail González-Valdez, Roberto Arreguin-Espinosa, Yadira Rufino-González, Ernesto Calderón-Jaimes, Rosa Angélica Castillo-Rodríguez, Carlos Wong-Baeza, and et al. 2023. "Nitazoxanide Inhibits the Bifunctional Enzyme GlG6PD::6PGL of Giardia lamblia: Biochemical and In Silico Characterization of a New Druggable Target" International Journal of Molecular Sciences 24, no. 14: 11516. https://doi.org/10.3390/ijms241411516
APA StyleMartínez-Rosas, V., Hernández-Ochoa, B., Morales-Luna, L., Ortega-Cuellar, D., González-Valdez, A., Arreguin-Espinosa, R., Rufino-González, Y., Calderón-Jaimes, E., Castillo-Rodríguez, R. A., Wong-Baeza, C., Baeza-Ramírez, I., Pérez de la Cruz, V., Vidal-Limón, A., & Gómez-Manzo, S. (2023). Nitazoxanide Inhibits the Bifunctional Enzyme GlG6PD::6PGL of Giardia lamblia: Biochemical and In Silico Characterization of a New Druggable Target. International Journal of Molecular Sciences, 24(14), 11516. https://doi.org/10.3390/ijms241411516







