The UPR Maintains Proteostasis and the Viability and Function of Hippocampal Neurons in Adult Mice
Abstract
:1. Introduction
2. Results
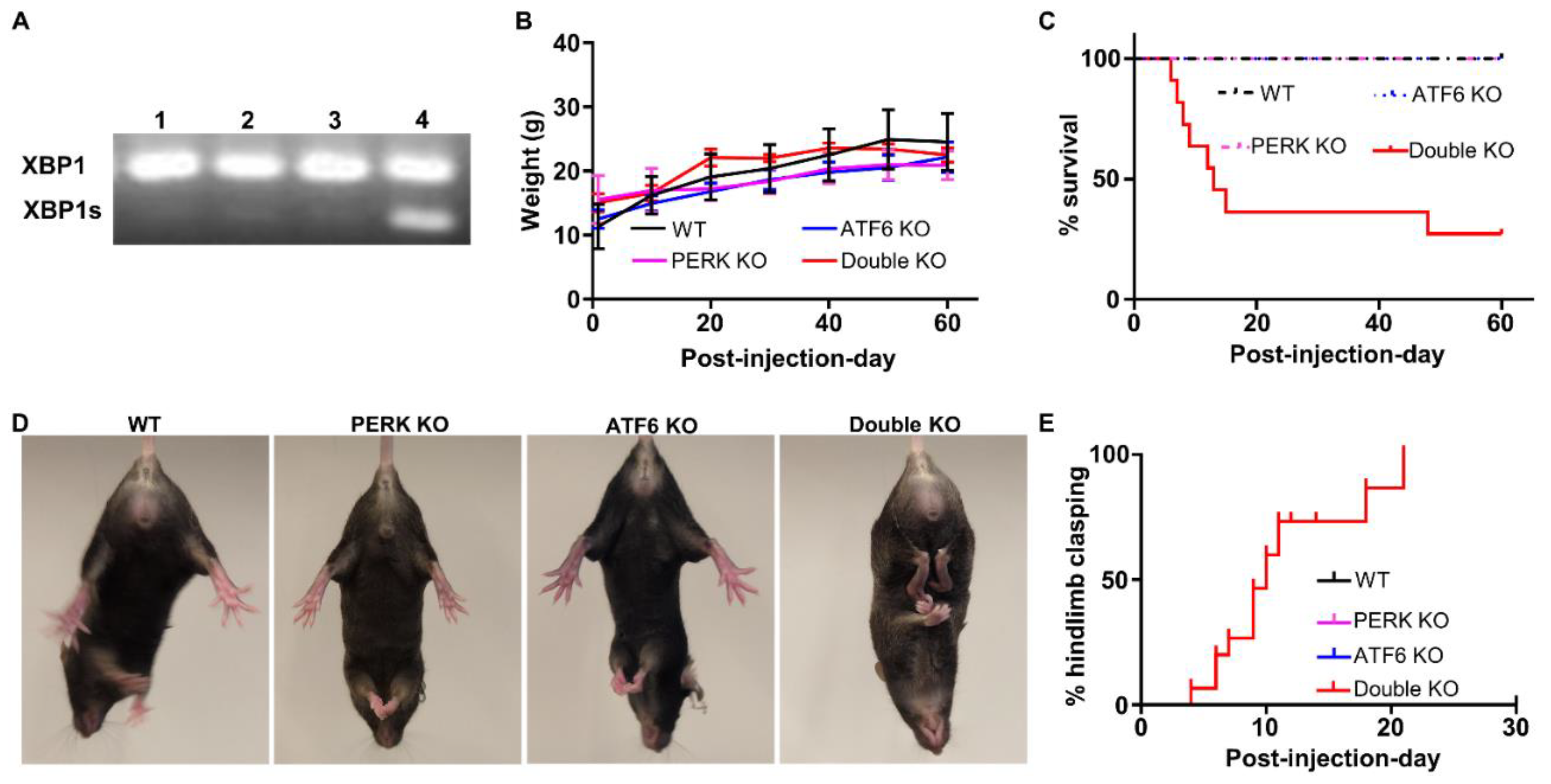
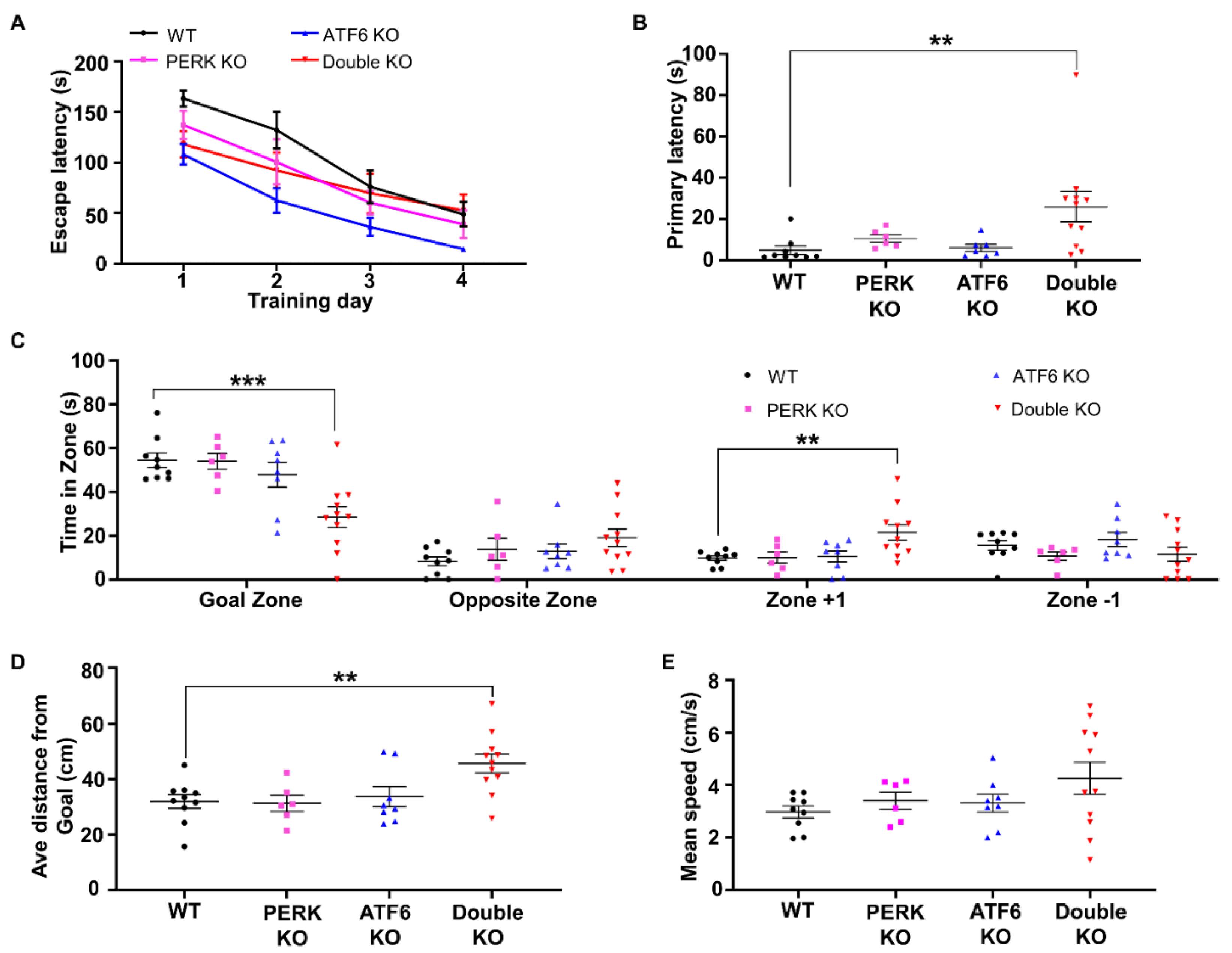
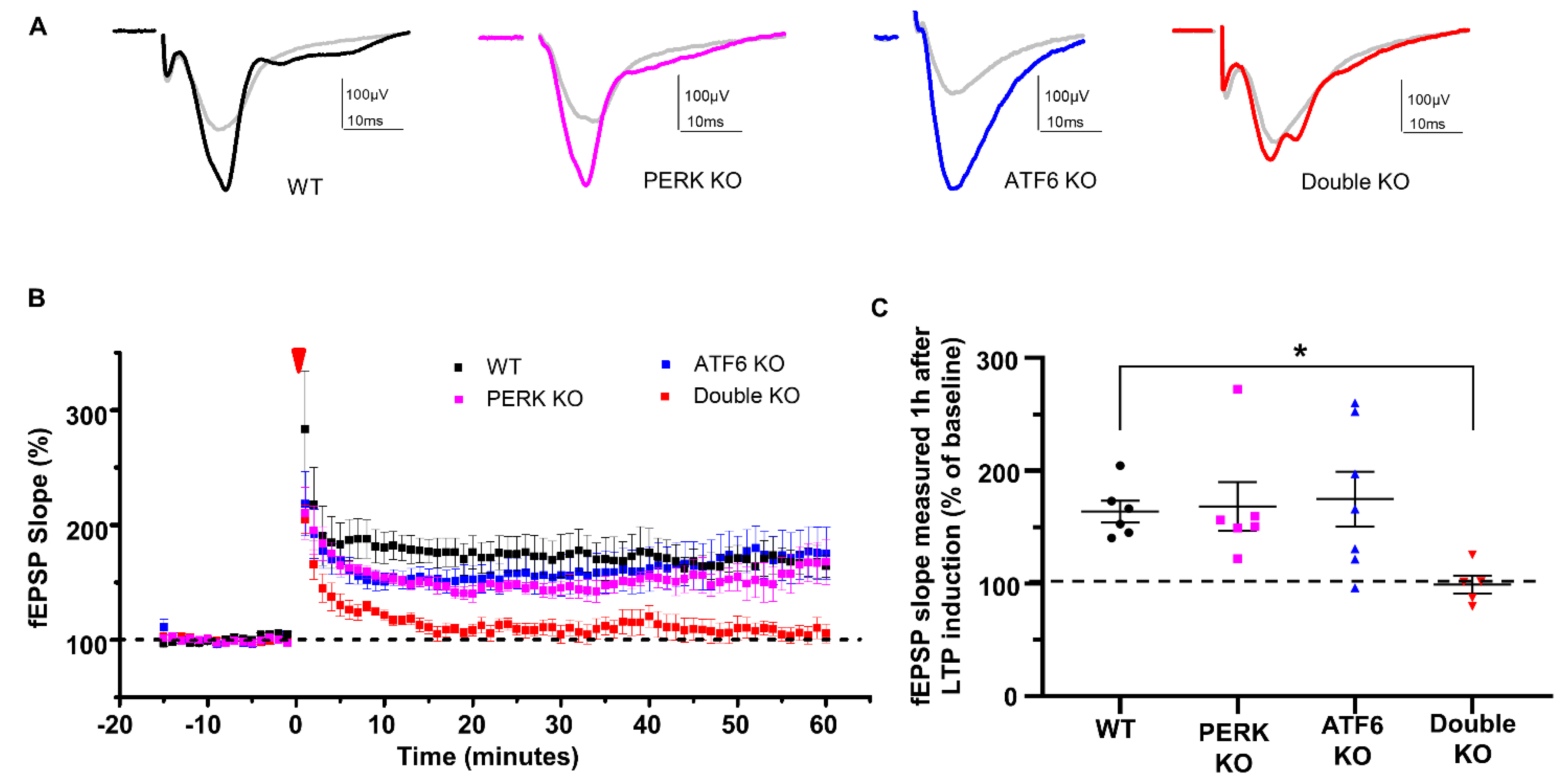
2.1. Inactivation of PERK and ATF6α in Neurons Led to Death and Memory Loss in Adult Mice
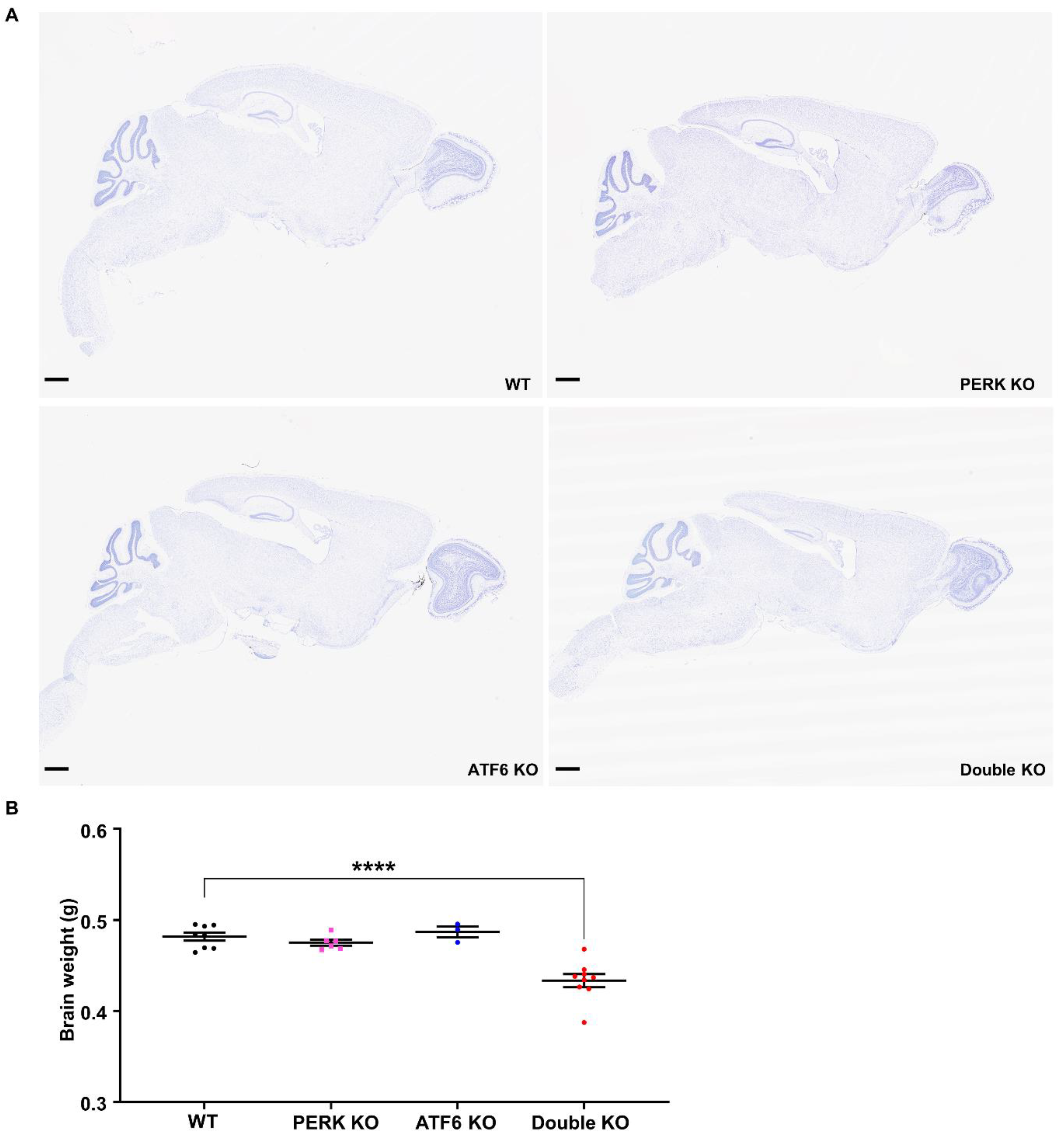
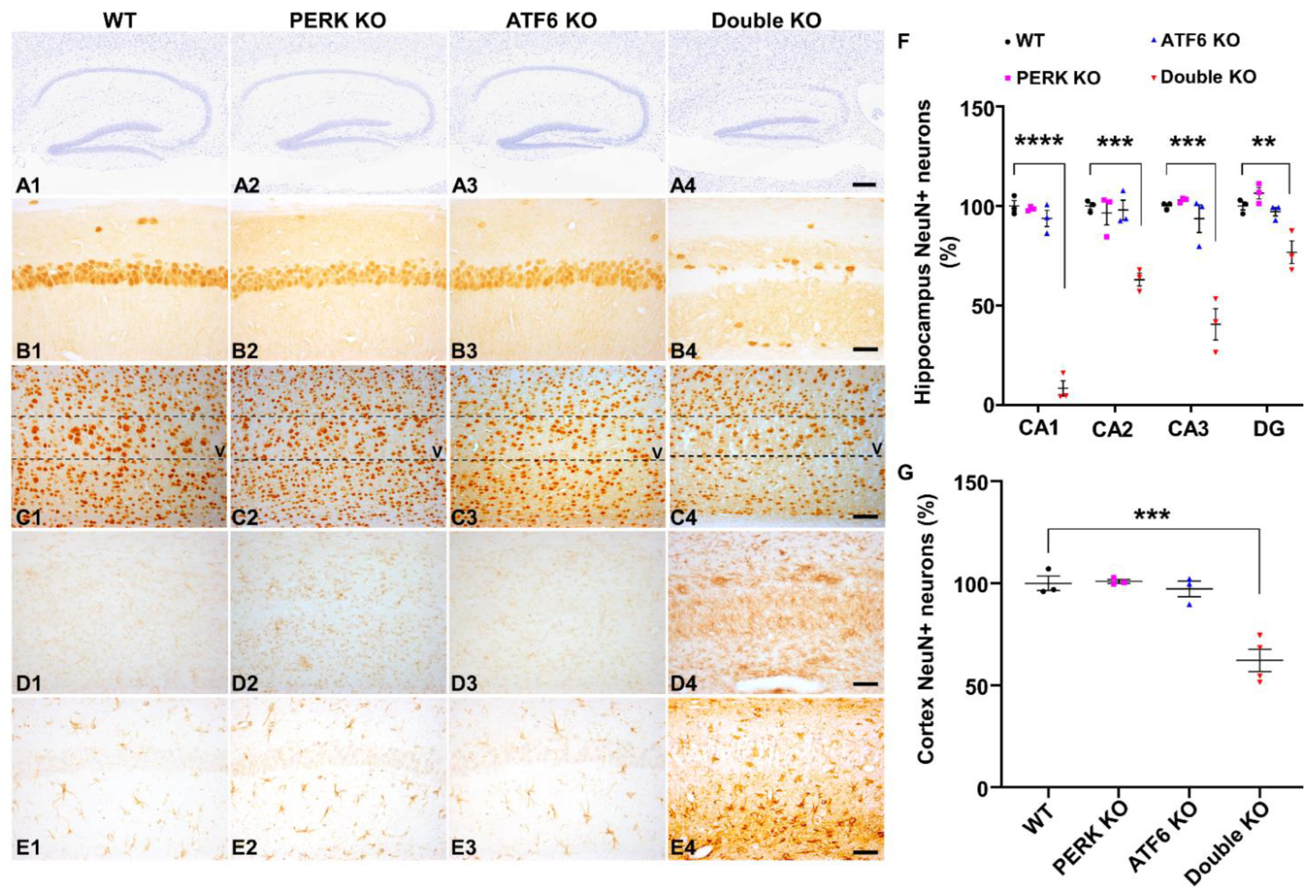
2.2. Inactivation of PERK and ATF6α in Neurons Led to Brain Atrophy and Hippocampal Degeneration in Adult Mice
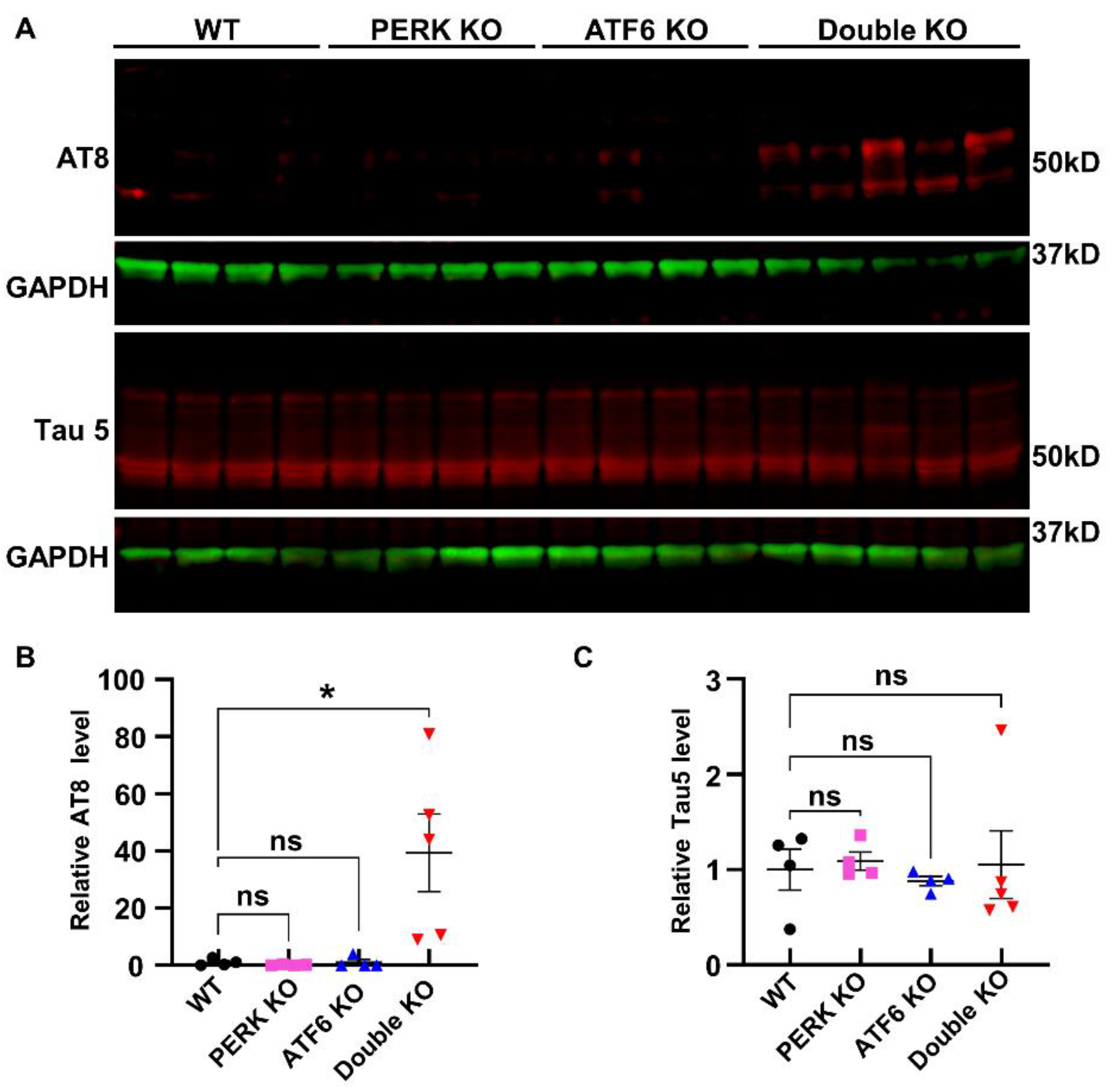
2.3. Inactivation of PERK and ATF6α in Neurons Led to Accumulation of p-tau and Aβ42 in the Hippocampus of Adult Mice
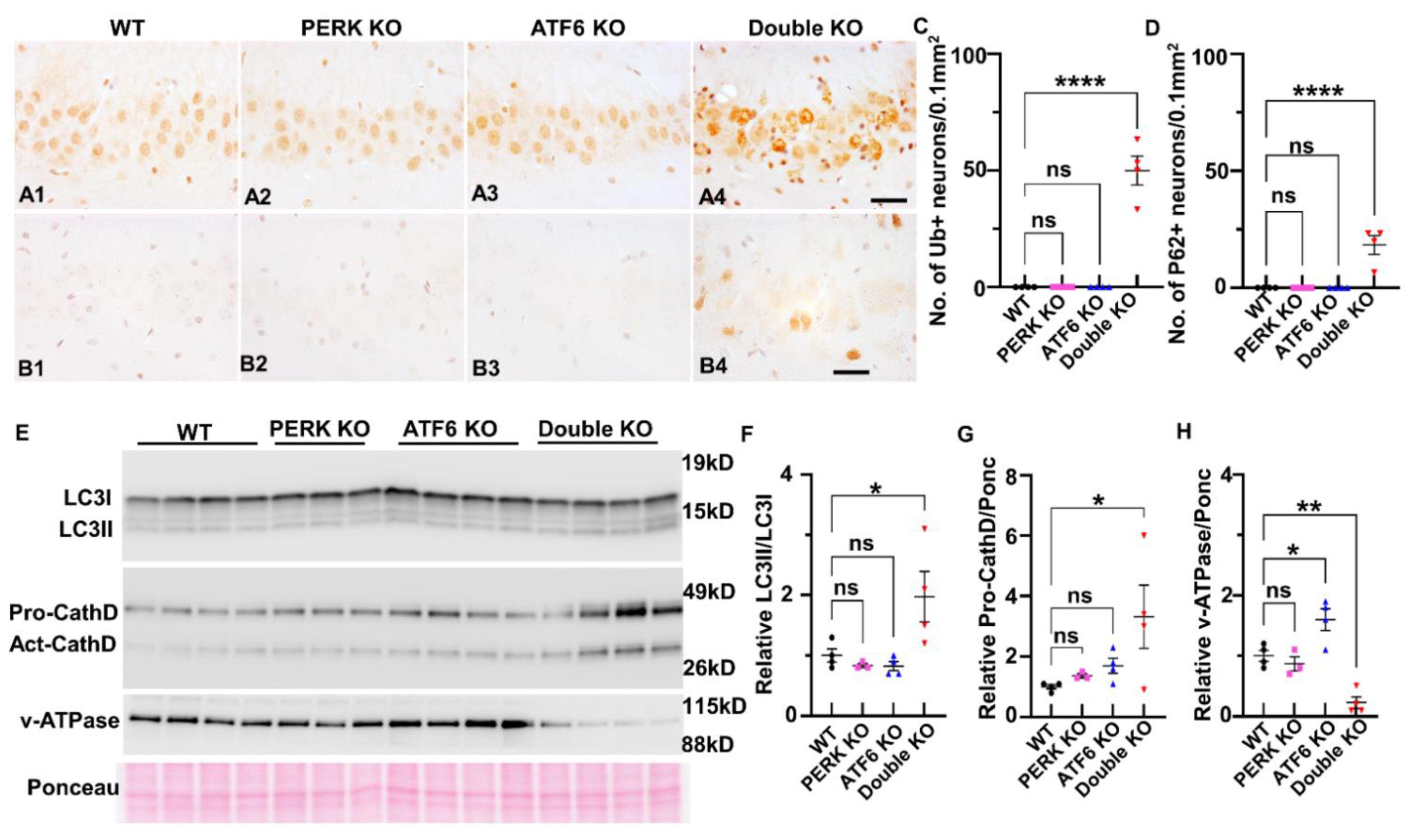
2.4. Inactivation of PERK and ATF6α Suppressed Lysosomal Function and Impaired Autophagic Flux in Hippocampal Neurons of Adult Mice
3. Discussion
4. Materials and Methods
4.1. Mice and Tamoxifen Treatment
4.2. Hindlimb Clasping Test
4.3. Immunohistochemistry (IHC) and Nissl Staining
4.4. Barnes Maze Learning and Memory Test
4.5. XBP1 Splicing Assay
4.6. Western Blot Analysis
4.7. Long-Term Potentiation (LTP) Experiment
4.8. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hetz, C.; Chevet, E.; Oakes, S.A. Proteostasis Control by the Unfolded Protein Response. Nat. Cell Biol. 2015, 17, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marciniak, S.J.; Ron, D. Endoplasmic Reticulum Stress Signaling in Disease. Physiol. Rev. 2006, 86, 1133–1149. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic Reticulum Stress Signalling-from Basic Mechanisms to Clinical Applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Kaufman, R.J. Protein Misfolding in the Endoplasmic Reticulum as a Conduit to Human Disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef]
- Hetz, C.; Mollereau, B. Disturbance of Endoplasmic Reticulum Proteostasis in Neurodegenerative Diseases. Nat. Rev. Neurosci. 2014, 15, 233–249. [Google Scholar] [CrossRef]
- Scheper, W.; Hoozemans, J.J.M. The Unfolded Protein Response in Neurodegenerative Diseases: A Neuropathological Perspective. Acta Neuropathol. 2015, 130, 315–331. [Google Scholar] [CrossRef] [Green Version]
- Hetz, C.; Saxena, S. ER Stress and the Unfolded Protein Response in Neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef]
- Ohno, M. PERK as a Hub of Multiple Pathogenic Pathways Leading to Memory Deficits and Neurodegeneration in Alzheimer’s Disease. Brain Res. Bull. 2018, 141, 72–78. [Google Scholar] [CrossRef]
- Hughes, D.; Mallucci, G.R. The Unfolded Protein Response in Neurodegenerative Disorders-Therapeutic Modulation of the PERK Pathway. FEBS J. 2019, 286, 342–355. [Google Scholar] [CrossRef] [Green Version]
- Ghemrawi, R.; Khair, M. Endoplasmic Reticulum Stress and Unfolded Protein Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 6127. [Google Scholar] [CrossRef]
- Naughton, M.; McMahon, J.; Healy, S.; FitzGerald, U. Profile of the Unfolded Protein Response in Rat Cerebellar Cortical Development. J. Comp. Neurol. 2019, 527, 2910–2924. [Google Scholar] [CrossRef]
- Martínez, G.; Khatiwada, S.; Costa-Mattioli, M.; Hetz, C. ER Proteostasis Control of Neuronal Physiology and Synaptic Function Regulation of Protein Synthesis in Neuronal Communication HHS Public Access. Trends Neurosci. 2018, 41, 610–624. [Google Scholar] [CrossRef]
- Ma, T.; Trinh, M.A.; Wexler, A.J.; Bourbon, C.; Gatti, E.; Pierre, P.; Cavener, D.R.; Klann, E. Suppression of EIF2α Kinases Alleviates Alzheimer’s Disease-Related Plasticity and Memory Deficits. Nat. Neurosci. 2013, 16, 1299–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, A.; Kamide, T.; Hashida, K.; Ta, H.M.; Inahata, Y.; Takarada-Iemata, M.; Hattori, T.; Mori, K.; Takahashi, R.; Matsuyama, T.; et al. Deletion of Atf6α Impairs Astroglial Activation and Enhances Neuronal Death Following Brain Ischemia in Mice. J. Neurochem. 2015, 132, 342–353. [Google Scholar] [CrossRef]
- Duran-Aniotz, C.; Cornejo, V.H.; Espinoza, S.; Ardiles, Á.O.; Medinas, D.B.; Salazar, C.; Foley, A.; Gajardo, I.; Thielen, P.; Iwawaki, T.; et al. IRE1 Signaling Exacerbates Alzheimer’s Disease Pathogenesis. Acta Neuropathol. 2017, 134, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.; Yue, Y.; Stanojlovic, M.; Wu, S.; Karsenty, G.; Lin, W. Neuron-Specific PERK Inactivation Exacerbates Neurodegeneration during Experimental Autoimmune Encephalomyelitis. JCI Insight 2019, 4, e124232. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.; Wu, S.; Nave, K.A.; Lin, W. The UPR Preserves Mature Oligodendrocyte Viability and Function in Adults by Regulating Autophagy of PLP. JCI Insight 2020, 5, e132364. [Google Scholar] [CrossRef] [Green Version]
- Holtzman, D.M.; Carrillo, M.C.; Hendrix, J.A.; Bain, L.J.; Catafau, A.M.; Gault, L.M.; Goedert, M.; Mandelkow, E.; Mandelkow, E.M.; Miller, D.S.; et al. Tau: From Research to Clinical Development. Alzheimers Dement. 2016, 12, 1033–1039. [Google Scholar] [CrossRef]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of Tau Protein in Health and Disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef] [Green Version]
- Strang, K.H.; Golde, T.E.; Giasson, B.I. MAPT Mutations, Tauopathy, and Mechanisms of Neurodegeneration. Lab. Investig. 2019, 99, 912–928. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Melhem, N.M.; Dickson, D.W.; Sleiman, P.M.A.; Wang, L.S.; Klei, L.; Rademakers, R.; De Silva, R.; Litvan, I.; Riley, D.E.; et al. Identification of Common Variants Influencing Risk of the Tauopathy Progressive Supranuclear Palsy. Nat. Genet. 2011, 43, 699–705. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Yu, J.T.; Miao, D.; Ma, X.Y.; Wang, H.F.; Wang, W.; Tan, L. An Exploratory Study on STX6, MOBP, MAPT, and EIF2AK3 and Late-Onset Alzheimer’s Disease. Neurobiol. Aging 2013, 34, 1519.e13. [Google Scholar] [CrossRef]
- Ferrari, R.; Ryten, M.; Simone, R.; Trabzuni, D.; Nicolaou, N.; Hondhamuni, G.; Ramasamy, A.; Vandrovcova, J.; Weale, M.E.; Lees, A.J.; et al. Assessment of Common Variability and Expression Quantitative Trait Loci for Genome-Wide Associations for Progressive Supranuclear Palsy. Neurobiol. Aging 2014, 35, 1514.e1–1514.e12. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.H.; Hiramatsu, N.; Liu, Q.; Sun, X.V.; Lenh, D.; Chan, P.; Chiang, K.; Koo, E.H.; Kao, A.W.; Litvan, I.; et al. Tauopathy-Associated PERK Alleles Are Functional Hypomorphs That Increase Neuronal Vulnerability to ER Stress. Hum. Mol. Genet. 2018, 27, 3951–3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruch, J.; Kurz, C.; Vasiljevic, A.; Nicolino, M.; Arzberger, T.; Höglinger, G.U. Early Neurodegeneration in the Brain of a Child Without Functional PKR-like Endoplasmic Reticulum Kinase. J. Neuropathol. Exp. Neurol. 2015, 74, 850–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avivar-Valderas, A.; Salas, E.; Bobrovnikova-Marjon, E.; Diehl, J.A.; Nagi, C.; Debnath, J.; Aguirre-Ghiso, J.A. PERK Integrates Autophagy and Oxidative Stress Responses to Promote Survival during Extracellular Matrix Detachment. Mol. Cell. Biol. 2011, 31, 3616–3629. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Tameire, F.; Koumenis, C. PERK-Ing up Autophagy during MYC-Induced Tumorigenesis. Autophagy 2013, 9, 612–614. [Google Scholar] [CrossRef] [Green Version]
- Martina, J.A.; Diab, H.I.; Brady, O.A.; Puertollano, R. TFEB and TFE3 Are Novel Components of the Integrated Stress Response. EMBO J. 2016, 35, 479–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Tan, J.; Miao, Y.; Zhang, Q. Crosstalk of ER Stress-Mediated Autophagy and ER-Phagy: Involvement of UPR and the Core Autophagy Machinery. J. Cell. Physiol. 2018, 233, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Lee, J.H.; Rubinsztein, D.C. Tau Degradation: The Ubiquitin-Proteasome System versus the Autophagy-Lysosome System. Prog. Neurobiol. 2013, 105, 49–59. [Google Scholar] [CrossRef]
- Menzies, F.M.; Fleming, A.; Caricasole, A.; Bento, C.F.; Andrews, S.P.; Ashkenazi, A.; Füllgrabe, J.; Jackson, A.; Jimenez Sanchez, M.; Karabiyik, C.; et al. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron 2017, 93, 1015–1034. [Google Scholar] [CrossRef] [Green Version]
- Ramesh Babu, J.; Lamar Seibenhener, M.; Peng, J.; Strom, A.L.; Kemppainen, R.; Cox, N.; Zhu, H.; Wooten, M.C.; Diaz-Meco, M.T.; Moscat, J.; et al. Genetic Inactivation of P62 Leads to Accumulation of Hyperphosphorylated Tau and Neurodegeneration. J. Neurochem. 2008, 106, 107–120. [Google Scholar] [CrossRef]
- Inoue, K.; Rispoli, J.; Kaphzan, H.; Klann, E.; Chen, E.I.; Kim, J.; Komatsu, M.; Abeliovich, A. Macroautophagy Deficiency Mediates Age-Dependent Neurodegeneration through a Phospho-Tau Pathway. Mol. Neurodegener. 2012, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Peric, A.; Annaert, W. Early Etiology of Alzheimer’s Disease: Tipping the Balance toward Autophagy or Endosomal Dysfunction? Acta Neuropathol. 2015, 129, 363–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, K.; Sato, T.; Matsui, T.; Sato, M.; Okada, T.; Yoshida, H.; Harada, A.; Mori, K. Transcriptional Induction of Mammalian ER Quality Control Proteins Is Mediated by Single or Combined Action of ATF6alpha and XBP1. Dev. Cell 2007, 13, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Stone, S.; Wu, S.; Jamison, S.; Durose, W.; Pallais, J.P.; Lin, W. Activating Transcription Factor 6α Deficiency Exacerbates Oligodendrocyte Death and Myelin Damage in Immune-Mediated Demyelinating Diseases. Glia 2018, 66, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Yamamoto, K.; Inoue, H.; Hikawa, R.; Nishi, K.; Mori, K.; Takahashi, R. The Endoplasmic Reticulum Stress Sensor, ATF6α, Protects against Neurotoxin-Induced Dopaminergic Neuronal Death. J. Biol. Chem. 2011, 286, 7947–7957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyenet, S.J.; Furrer, S.A.; Damian, V.M.; Baughan, T.D.; la Spada, A.R.; Garden, G.A. A Simple Composite Phenotype Scoring System for Evaluating Mouse Models of Cerebellar Ataxia. J. Vis. Exp. 2010, 39, e1787. [Google Scholar] [CrossRef] [Green Version]
- Fernagut, P.O.; Diguet, E.; Bioulac, B.; Tison, F. MPTP Potentiates 3-Nitropropionic Acid-Induced Striatal Damage in Mice: Reference to Striatonigral Degeneration. Exp. Neurol. 2004, 185, 47–62. [Google Scholar] [CrossRef]
- Lieu, C.A.; Chinta, S.J.; Rane, A.; Andersen, J.K. Age-Related Behavioral Phenotype of an Astrocytic Monoamine Oxidase-B Transgenic Mouse Model of Parkinson’s Disease. PLoS ONE 2013, 8, e54200. [Google Scholar] [CrossRef] [Green Version]
- Lalonde, R.; Strazielle, C. Brain Regions and Genes Affecting Limb-Clasping Responses. Brain Res. Rev. 2011, 67, 252–259. [Google Scholar] [CrossRef]
- Cahill, L.S.; Zhang, M.A.; Ramaglia, V.; Whetstone, H.; Sabbagh, M.P.; Yi, T.J.; Woo, L.; Przybycien, T.S.; Moshkova, M.; Zhao, F.L.; et al. Aged Hind-Limb Clasping Experimental Autoimmune Encephalomyelitis Models Aspects of the Neurodegenerative Process Seen in Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 22710–22720. [Google Scholar] [CrossRef]
- Lynch, M.A. Long-Term Potentiation and Memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar] [CrossRef]
- Teravskis, P.J.; Covelo, A.; Miller, E.C.; Singh, B.; Martell-Martínez, H.A.; Benneyworth, M.A.; Gallardo, C.; Oxnard, B.R.; Araque, A.; Lee, M.K.; et al. A53T Mutant Alpha-Synuclein Induces Tau-Dependent Postsynaptic Impairment Independently of Neurodegenerative Changes. J. Neurosci. 2018, 38, 9754–9767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Covelo, A.; Martell-Martínez, H.; Nanclares, C.; Sherman, M.A.; Okematti, E.; Meints, J.; Teravskis, P.J.; Gallardo, C.; Savonenko, A.V.; et al. Tau Is Required for Progressive Synaptic and Memory Deficits in a Transgenic Mouse Model of α-Synucleinopathy. Acta Neuropathol. 2019, 138, 551–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.S.; Yoo, W.H.; Chae, H.J. ER Stress and Autophagy. Curr. Mol. Med. 2015, 15, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Leli, N.M.; Koumenis, C.; Amaravadi, R.K. Regulation of Autophagy by Canonical and Non-Canonical ER Stress Responses. Semin. Cancer Biol. 2020, 66, 116–128. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.I.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of Autophagy in the Central Nervous System Causes Neurodegeneration in Mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; et al. Suppression of Basal Autophagy in Neural Cells Causes Neurodegenerative Disease in Mice. Nature 2006, 441, 885–889. [Google Scholar] [CrossRef]
- Lamark, T.; Svenning, S.; Johansen, T. Regulation of Selective Autophagy: The P62/SQSTM1 Paradigm. Essays Biochem. 2017, 61, 609–624. [Google Scholar] [CrossRef]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. P62 Links the Autophagy Pathway and the Ubiqutin-Proteasome System upon Ubiquitinated Protein Degradation. Cell. Mol. Biol. Lett. 2016, 21, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klionsky, D.J.; Abdalla, F.C.; Abeliovich, H.; Abraham, R.T.; Acevedo-Arozena, A.; Adeli, K.; Agholme, L.; Agnello, M.; Agostinis, P.; Aguirre-Ghiso, J.A.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy. Autophagy 2012, 8, 445–544. [Google Scholar] [CrossRef] [Green Version]
- Karim, M.R.; Kanazawa, T.; Daigaku, Y.; Fujimura, S.; Miotto, G.; Kadowaki, M. Cytosolic LC3 Ratio as a Sensitive Index of Macroautophagy in Isolated Rat Hepatocytes and H4-II-E Cells. Autophagy 2007, 3, 553–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, M.R.; Liao, E.E.; Kim, J.; Meints, J.; Martinez, H.M.; Pletnikova, O.; Troncoso, J.C.; Lee, M.K. α-Synucleinopathy Associated c-Abl Activation Causes P53-Dependent Autophagy Impairment. Mol. Neurodegener. 2020, 15, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidi, N.; Maurer, A.; Nieke, S.; Kalbacher, H. Cathepsin D: A Cellular Roadmap. Biochem. Biophys. Res. Commun. 2008, 376, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, C.; Follo, C.; Savino, M.; Melone, M.A.B.; Isidoro, C. The Role of Cathepsin D in the Pathogenesis of Human Neurodegenerative Disorders. Med. Res. Rev. 2016, 36, 845–870. [Google Scholar] [CrossRef]
- Mindell, J.A. Lysosomal Acidification Mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef] [Green Version]
- Colacurcio, D.J.; Nixon, R.A. Disorders of Lysosomal Acidification-The Emerging Role of v-ATPase in Aging and Neurodegenerative Disease. Ageing Res. Rev. 2016, 32, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Martínez, G.; Duran-Aniotz, C.; Cabral-Miranda, F.; Vivar, J.P.; Hetz, C. Endoplasmic Reticulum Proteostasis Impairment in Aging. Aging Cell 2017, 16, 615–623. [Google Scholar] [CrossRef]
- Amanullah, A.; Upadhyay, A.; Joshi, V.; Mishra, R.; Jana, N.R.; Mishra, A. Progressing Neurobiological Strategies against Proteostasis Failure: Challenges in Neurodegeneration. Prog. Neurobiol. 2017, 159, 1–38. [Google Scholar] [CrossRef]
- Klaips, C.L.; Jayaraj, G.G.; Hartl, F.U. Pathways of Cellular Proteostasis in Aging and Disease. J. Cell Biol. 2018, 217, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C. Aging and the UPR(ER). Brain Res. 2016, 1648, 588–593. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, A.; Cohen, R.A.; Porges, E.C.; Nissim, N.R.; Woods, A.J. Cognitive Aging and the Hippocampus in Older Adults. Front. Aging Neurosci. 2016, 8, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettio, L.E.B.; Rajendran, L.; Gil-Mohapel, J. The Effects of Aging in the Hippocampus and Cognitive Decline. Neurosci. Biobehav. Rev. 2017, 79, 66–86. [Google Scholar] [CrossRef]
- Bishop, N.A.; Lu, T.; Yankner, B.A. Neural Mechanisms of Ageing and Cognitive Decline. Nature 2010, 464, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Malik, B.R.; Maddison, D.C.; Smith, G.A.; Peters, O.M. Autophagic and Endo-Lysosomal Dysfunction in Neurodegenerative Disease. Mol. Brain 2019, 12, 100. [Google Scholar] [CrossRef]
- Toledano-Zaragoza, A.; Ledesma, M.D. Addressing Neurodegeneration in Lysosomal Storage Disorders: Advances in Niemann Pick Diseases. Neuropharmacology 2020, 171, 107851. [Google Scholar] [CrossRef]
- Ma, K.; Bin, N.R.; Shi, S.; Harada, H.; Wada, Y.; Wada, G.H.S.; Monnier, P.P.; Sugita, S.; Zhang, L. Observations From a Mouse Model of Forebrain Voa1 Knockout: Focus on Hippocampal Structure and Function. Front. Cell. Neurosci. 2019, 13, 484. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; McGrath, B.; Li, S.; Frank, A.; Zambito, F.; Reinert, J.; Gannon, M.; Ma, K.; McNaughton, K.; Cavener, D.R. The PERK Eukaryotic Initiation Factor 2 Alpha Kinase Is Required for the Development of the Skeletal System, Postnatal Growth, and the Function and Viability of the Pancreas. Mol. Cell. Biol. 2002, 22, 3864–3874. [Google Scholar] [CrossRef] [Green Version]
- Heimer-Mcginn, V.; Young, P. Efficient Inducible Pan-Neuronal Cre-Mediated Recombination in SLICK-H Transgenic Mice. Genesis 2011, 49, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Stanojlovic, M.; Pang, X.; Lin, Y.; Stone, S.; Cvetanovic, M.; Lin, W. Inhibition of Vascular Endothelial Growth Factor Receptor 2 Exacerbates Loss of Lower Motor Neurons and Axons during Experimental Autoimmune Encephalomyelitis. PLoS ONE 2016, 11, e0160158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, Y.; Stanojlovic, M.; Lin, Y.; Karsenty, G.; Lin, W. Oligodendrocyte-Specific ATF4 Inactivation Does Not Influence the Development of EAE. J. Neuroinflammation 2019, 16, 23. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Karim, M.R.; Covelo, A.; Yue, Y.; Lee, M.K.; Lin, W. The UPR Maintains Proteostasis and the Viability and Function of Hippocampal Neurons in Adult Mice. Int. J. Mol. Sci. 2023, 24, 11542. https://doi.org/10.3390/ijms241411542
Liu P, Karim MR, Covelo A, Yue Y, Lee MK, Lin W. The UPR Maintains Proteostasis and the Viability and Function of Hippocampal Neurons in Adult Mice. International Journal of Molecular Sciences. 2023; 24(14):11542. https://doi.org/10.3390/ijms241411542
Chicago/Turabian StyleLiu, Pingting, Md Razaul Karim, Ana Covelo, Yuan Yue, Michael K. Lee, and Wensheng Lin. 2023. "The UPR Maintains Proteostasis and the Viability and Function of Hippocampal Neurons in Adult Mice" International Journal of Molecular Sciences 24, no. 14: 11542. https://doi.org/10.3390/ijms241411542
APA StyleLiu, P., Karim, M. R., Covelo, A., Yue, Y., Lee, M. K., & Lin, W. (2023). The UPR Maintains Proteostasis and the Viability and Function of Hippocampal Neurons in Adult Mice. International Journal of Molecular Sciences, 24(14), 11542. https://doi.org/10.3390/ijms241411542






