Combined Tumor-Based BRCA1/2 and TP53 Mutation Testing in Ovarian Cancer
Abstract
:1. Introduction
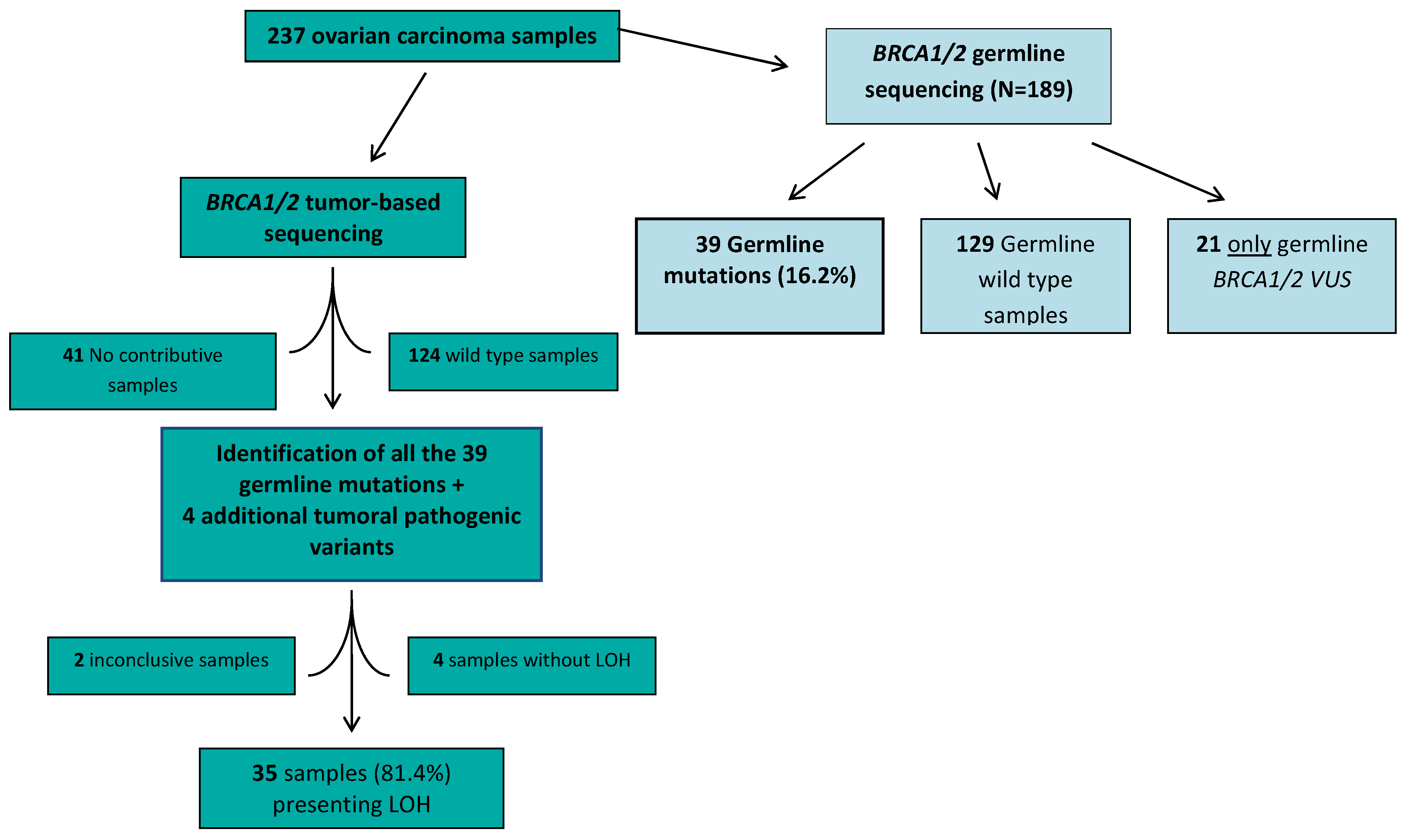
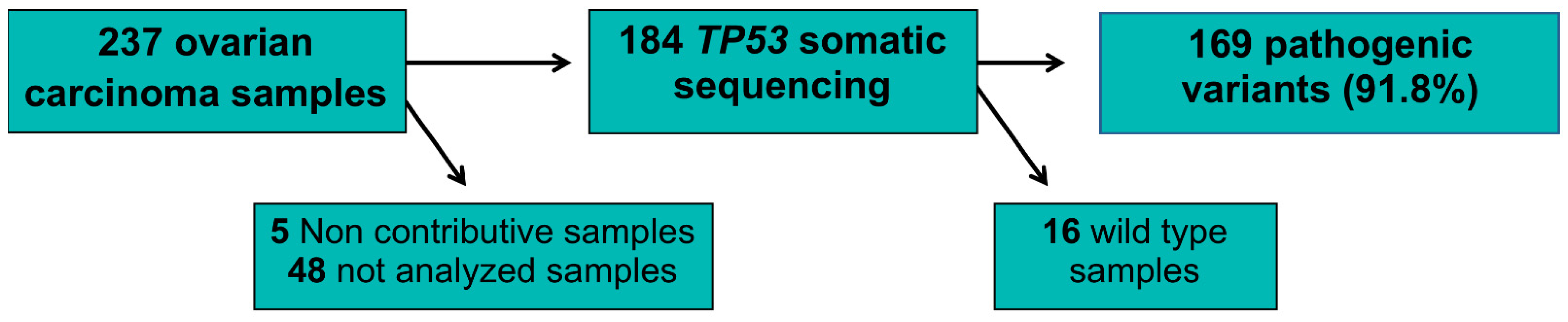
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Helleday, T.; Lo, J.; van Gent, D.C.; Engelward, B.P. DNA Double-Strand Break Repair: From Mechanistic Understanding to Cancer Treatment. DNA Repair 2007, 6, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Hauke, J.; Heitz, F.; Reuss, A.; Kommoss, S.; Marmé, F.; Heimbach, A.; Prieske, K.; Richters, L.; Burges, A.; et al. Prevalence of Deleterious Germline Variants in Risk Genes Including BRCA1/2 in Consecutive Ovarian Cancer Patients (AGO-TR-1). PLoS ONE 2017, 12, e0186043. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G. The Concept of Synthetic Lethality in the Context of Anticancer Therapy. Nat. Rev. Cancer 2005, 5, 689–698. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [Green Version]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib Maintenance Treatment for Recurrent Ovarian Carcinoma after Response to Platinum Therapy (ARIEL3): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Genome Atlas Research Network. Integrated Genomic Analyses of Ovarian Carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, B.A.; Lai, Z.; Hodgson, D.R.; Orr, M.C.M.; Hawryluk, M.; Sun, J.; Yelensky, R.; Spencer, S.K.; Robertson, J.D.; Ho, T.W.; et al. Biological and Clinical Evidence for Somatic Mutations in BRCA1 and BRCA2 as Predictive Markers for Olaparib Response in High-Grade Serous Ovarian Cancers in the Maintenance Setting. Oncotarget 2017, 8, 43653–43661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.N.; Wubbenhorst, B.; Wenz, B.M.; De Sloover, D.; Pluta, J.; Emery, L.; Barrett, A.; Kraya, A.A.; Anastopoulos, I.N.; Yu, S.; et al. BRCA Locus-Specific Loss of Heterozygosity in Germline BRCA1 and BRCA2 Carriers. Nat. Commun. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yost, S.; Ruark, E.; Alexandrov, L.B.; Rahman, N. Insights into BRCA Cancer Predisposition from Integrated Germline and Somatic Analyses in 7632 Cancers. JNCI Cancer Spectr. 2019, 3, pkz028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsson, P.; Bandlamudi, C.; Cheng, M.L.; Srinivasan, P.; Chavan, S.S.; Friedman, N.D.; Rosen, E.Y.; Richards, A.L.; Bouvier, N.; Selcuklu, S.D.; et al. Tumour Lineage Shapes BRCA-Mediated Phenotypes. Nature 2019, 571, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib Following Platinum-Based Chemotherapy in Newly Diagnosed Patients (Pts) with Advanced Ovarian Cancer (OC) and a BRCA1/2 Mutation (BRCAm): Phase III SOLO1 Trial. Ann. Oncol. 2018, 29, viii727. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.M.; Matulonis, U.A.; Malander, S.; Provencher, D.; Mahner, S.; Follana, P.; Waters, J.; Berek, J.S.; Woie, K.; Oza, A.M.; et al. Niraparib Maintenance Therapy in Patients with Recurrent Ovarian Cancer After a Partial Response to the Last Platinum-Based Chemotherapy in the ENGOT-OV16/NOVA Trial. J. Clin. Oncol. 2019, 37, 2968–2973. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Casadei, S.; Nord, A.S.; et al. Germline and Somatic Mutations in Homologous Recombination Genes Predict Platinum Response and Survival in Ovarian, Fallopian Tube, and Peritoneal Carcinomas. Clin. Cancer Res. 2014, 20, 764–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernards, S.S.; Pennington, K.; Harrell, M.I.; Agnew, K.J.; Norquist, B.M.; Swisher, E.M. Overall Survival in BRCA1 or RAD51C Methylated vs Mutated Ovarian Carcinoma Following Primary Treatment with Platinum Chemotherapy. Gynecol. Oncol. 2017, 145, 24. [Google Scholar] [CrossRef]
- Kondrashova, O.; Topp, M.; Nesic, K.; Lieschke, E.; Ho, G.-Y.; Harrell, M.I.; Zapparoli, G.V.; Hadley, A.; Holian, R.; Boehm, E.; et al. Methylation of All BRCA1 Copies Predicts Response to the PARP Inhibitor Rucaparib in Ovarian Carcinoma. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondrashova, O.; Nguyen, M.; Shield-Artin, K.; Tinker, A.V.; Teng, N.N.H.; Harrell, M.I.; Kuiper, M.J.; Ho, G.-Y.; Barker, H.; Jasin, M.; et al. Secondary Somatic Mutations Restoring RAD51C and RAD51D Associated with Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 2017, 7, 984–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| n = 237 Patients | |
|---|---|
| Median age at diagnosis (IQR) * | 62.0 (56.0–68.0) |
| Histological type, n (%) | |
| - High-grade serous carcinoma | 205 (86.5) |
| - Low-grade serous carcinoma | 4 (1.7) |
| - Clear-cell carcinoma | 2 (0.8) |
| - Carcinosarcoma | 4 (1.7) |
| - High-grade endometrioid carcinoma | 15 (6.3) |
| - Undifferentiated carcinoma | 7 (3.0) |
| FIGO stage, n (%) | |
| - I | 8 (3.3) |
| - II | 10 (4.2) |
| - III | 126 (53.2) |
| - IV | 45 (19.0) |
| - NA | 48 (20.3) |
| Type of samples, n (%) | |
| - Biopsy | 93 (39.2) |
| - Surgical samples | 127 (53.6) |
| - NA | 17 (7.2) |
| Sample collection, n (%) | |
| - Primitive | 141 (59.4%) |
| - Primitive post-neoadjuvant treatment | 62 (26.2%) |
| - Relapse | 1 (0.4%) |
| - Relapse post chemotherapy | 27 (11.4%) |
| - NA | 6 (2.5%) |
| Gene | Variant | Protein | Functional Domain | Variant Type | Germline | Allelic Frequency | LOH | TP53 Associated Mutation | Allelic Frequency |
|---|---|---|---|---|---|---|---|---|---|
| BRCA1 | c.134+3A>C | - | - | Splicing | No | 0.43 | Yes | c.375+1G>T | 0.45 |
| BRCA1 | c.1121del | p.Thr374fs | - | Frameshift | Yes | 0.7 | Yes | c.394A>G; p.Lys132Glu | 0.63 |
| BRCA1 | c.212+3A>G | - | Ring finger | Splicing | Yes | 0.85 | Yes | c.673-1G>C | 0.55 |
| BRCA1 | c.1674del | p.Gly559Valfs*13 | - | Nonsense | Yes | 0.67 | Yes | c.742C>T; p.Arg248Trp | 0.57 |
| BRCA1 | c.68_69del | p.Glu23fs | Ring finger + NES1 | Frameshift | Yes | 0.46 | Inconclusive | No | WT |
| BRCA1 | c.190T>C | p.Cys64Arg | Ring finger | Missense | No | 0.19 | Yes | c.578A>T; p.His193Leu | 0.24 |
| BRCA1 | c.5266dup | p.Gln1756_Asp1757fs | Linker | Frameshift | Yes | 0.77 | Yes | c.351del; p.Gly117fs | 0.29 |
| BRCA1 | c.5468-2A>G | - | BRCT2/AD2 | Splicing | Yes | 0.50 | No | c.403T>C; p.Cys135Arg | 0.18 |
| BRCA1 | c.5266dup | p.Gln1756_Asp1757fs | Linker | Frameshift | Yes | 0.74 | Yes | c.840A>C; p.Arg280Ser | 0.62 |
| BRCA1 | c.514C>T | p.Gln172* | - | Nonsense | No | 0.04 | Yes | c.375+5del | 0.05 |
| BRCA1 | c.81-1G>C | - | Ring finger + NES1 | Splicing | No | 0.14 | Yes | c.518T>C; p.Val173Ala | 0.09 |
| BRCA2 | c.2612C>A | p.Ser871* | - | Nonsense | Yes | 0.89 | Yes | NR | NR |
| BRCA1 | c.815_824dup | p.Gly275_Thr276fs | - | Frameshift | No | 0.69 | Yes | c.743G>A; p.Arg248Gln | 0.61 |
| BRCA2 | c.6533_6542del | p.His2178Glnfs*10 | - | Deletion | No | 0.24 | Yes | c.1024C>T; p.Arg342Ter* | 0.29 |
| BRCA1 | c.4183C>T | p.Gln1395* | Coil coiled/AD1 | Nonsense | Yes | 0.62 | Yes | c.527G>T; p.Cys176Phe | 0.44 |
| BRCA1 | c.1789G>T | p.Glu597* | - | Nonsense | No | 0.16 | Yes | c.742C>T, p.Arg248Trp | 0.15 |
| BRCA1 | c.3001G>T | p.Glu1001* | - | Nonsense | Yes | 0.68 | Yes | c.395A>G; p.Lys132Arg | 0.27 |
| BRCA1 | c.5503C>T | p.Arg1835Stop | BRCT2/AD2 | Nonsense | Yes | 0.12 | Yes | c.395A>G; p.Lys132Arg | 0.20 |
| BRCA1 | c.523A>T | p.Lys175* | - | Nonsense | No | 0.39 | No | c.614A>G; p.Tyr205Cys | 0.54 |
| BRCA2 | c.7952del | p.Arg2651fs | Helical domain | Frameshift | No | 0.47 | Yes | c.681_682insT; p.Ser227_Asp228fs | 0.42 |
| BRCA1 | c.4065_4068del | p.Asn1355fs | AD1 | Frameshift | Yes | 0.81 | Yes | c.824G>A; p.Cys275Tyr | 0.62 |
| BRCA1 | c.2389G>T | p.Glu797* | - | Nonsense | No | 0.41 | Yes | c.586C>T; p.Arg196* | 0.26 |
| BRCA2 | c.6591_6592del | p.Thr2197fs | - | Frameshift | Yes | 0.87 | Yes | NR | NR |
| BRCA2 | c.2612C>A | p.Ser871* | - | Nonsense | No | 0.48 | No | c.394A>G; p.Lys132Glu | 0.15 |
| BRCA2 | c.8487+1G>A | - | - | Splicing | No | 1 | Yes | No | WT |
| BRCA2 | c.3785C>G | p.Ser1262* | - | Nonsense | Yes | 0.28 | Yes | c.1025G>A; p.Arg342Gln | 0.35 |
| BRCA2 | c.2612C>A | p.Ser871* | - | Nonsense | Yes | 0.69 | Yes | c.524G>A; p.Arg175His | 0.42 |
| BRCA1 | c.4658del | p.Leu1553fs | AD1 | Frameshift | Inconclusive | 0.87 | Yes | c.376-2A>G | 0.70 |
| BRCA1 | c.3257T>G | p.Leu1086* | - | Nonsense | Yes | 0.55 | No | c.824G>A; p.Cys275Tyr | 0.22 |
| BRCA1 | c.1063A>T | p.Lys355* | - | Nonsense | No | 0.61 | Yes | c.396G>T; p.Lys132Asn | 0.64 |
| BRCA1 | c.3008_3009del | p.Phe1003fs | - | Frameshift | Yes | 0.80 | Yes | c.1010G>T; p.Arg337Leu | 0.52 |
| BRCA1 | c.2679_2682del | p.Lys893fs | - | Frameshift | Yes | 0.63 | Yes | c.818 G>T; p.Arg273Leu | 0.48 |
| BRCA2 | c.2492del | p.Val831fs | - | Frameshift | No | 0.21 | Yes | c.818G>T; p.Arg273Leu | 0.23 |
| BRCA1 | c.4072G>T | p.Glu1358* | AD1 | Nonsense | No | 0.60 | Yes | c.403T>G; p.Cys135Gly | 0.62 |
| BRCA1 | c.3995del | p.Gly1332fs | AD1 | Frameshift | No | 0.28 | Yes | c.783-1_784delinsCTT; p.? | 0.20 |
| BRCA1 | c.4868C>G | p.Ala1623Gly | AD2 | Missense | No | 0.089 | Yes | c.644G>T, p.Ser215Ile | 0.0587 |
| BRCA1 | c.4658del | p.Leu1553fs | AD1 | Frameshift | Yes | 0.87 | Yes | c.376-2A>G | 0.70 |
| BRCA2 | c.5345_5346del | p.Gln1782fs | - | Frameshift | Yes | 0.66 | Yes | c.307_308insGAAAACCT; p.Tyr103_Gln104fs | 0.32 |
| BRCA2 | c.3233_3234insT | p.Val1078_Ser1079fs | - | Frameshift | Yes | 0.76 | Yes | c.262del; p.Ala88fs | 0.68 |
| BRCA2 | c.5682C>G | p.Tyr1894* | - | Nonsense | Yes | 0.91 | Yes | No | - |
| BRCA2 | c.1834G>T | p.Glu612* | - | Nonsense | No | 0.04 | Yes | c.388C>T; p.Leu130Phe | 0.0294 |
| BRCA2 | c.413_417del | - | Frameshift | No | 0.02 | Inconclusive | No | - | |
| BRCA1 | Deletion exon 21 to 24 | p.? | - | Frameshift | Yes | 0.8 * | Yes | c.151del; p.Glu51fs | 0.52 |
| TP53 Variant Type | Frequency |
|---|---|
| Missense | 102 (60%) |
| Frameshift | 34 (20%) |
| Nonsense | 20 (12%) |
| Splicing | 13 (8%) |
| Total | 169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borcoman, E.; Santana dos Santos, E.; Genestie, C.; Pautier, P.; Lacroix, L.; Caputo, S.M.; Cabaret, O.; Guillaud-Bataille, M.; Michels, J.; Auguste, A.; et al. Combined Tumor-Based BRCA1/2 and TP53 Mutation Testing in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 11570. https://doi.org/10.3390/ijms241411570
Borcoman E, Santana dos Santos E, Genestie C, Pautier P, Lacroix L, Caputo SM, Cabaret O, Guillaud-Bataille M, Michels J, Auguste A, et al. Combined Tumor-Based BRCA1/2 and TP53 Mutation Testing in Ovarian Cancer. International Journal of Molecular Sciences. 2023; 24(14):11570. https://doi.org/10.3390/ijms241411570
Chicago/Turabian StyleBorcoman, Edith, Elizabeth Santana dos Santos, Catherine Genestie, Patricia Pautier, Ludovic Lacroix, Sandrine M. Caputo, Odile Cabaret, Marine Guillaud-Bataille, Judith Michels, Aurelie Auguste, and et al. 2023. "Combined Tumor-Based BRCA1/2 and TP53 Mutation Testing in Ovarian Cancer" International Journal of Molecular Sciences 24, no. 14: 11570. https://doi.org/10.3390/ijms241411570
APA StyleBorcoman, E., Santana dos Santos, E., Genestie, C., Pautier, P., Lacroix, L., Caputo, S. M., Cabaret, O., Guillaud-Bataille, M., Michels, J., Auguste, A., Leary, A., & Rouleau, E. (2023). Combined Tumor-Based BRCA1/2 and TP53 Mutation Testing in Ovarian Cancer. International Journal of Molecular Sciences, 24(14), 11570. https://doi.org/10.3390/ijms241411570






