Graphene Nanocomposites as Innovative Materials for Energy Storage and Conversion—Design and Headways
Abstract
:1. Introduction
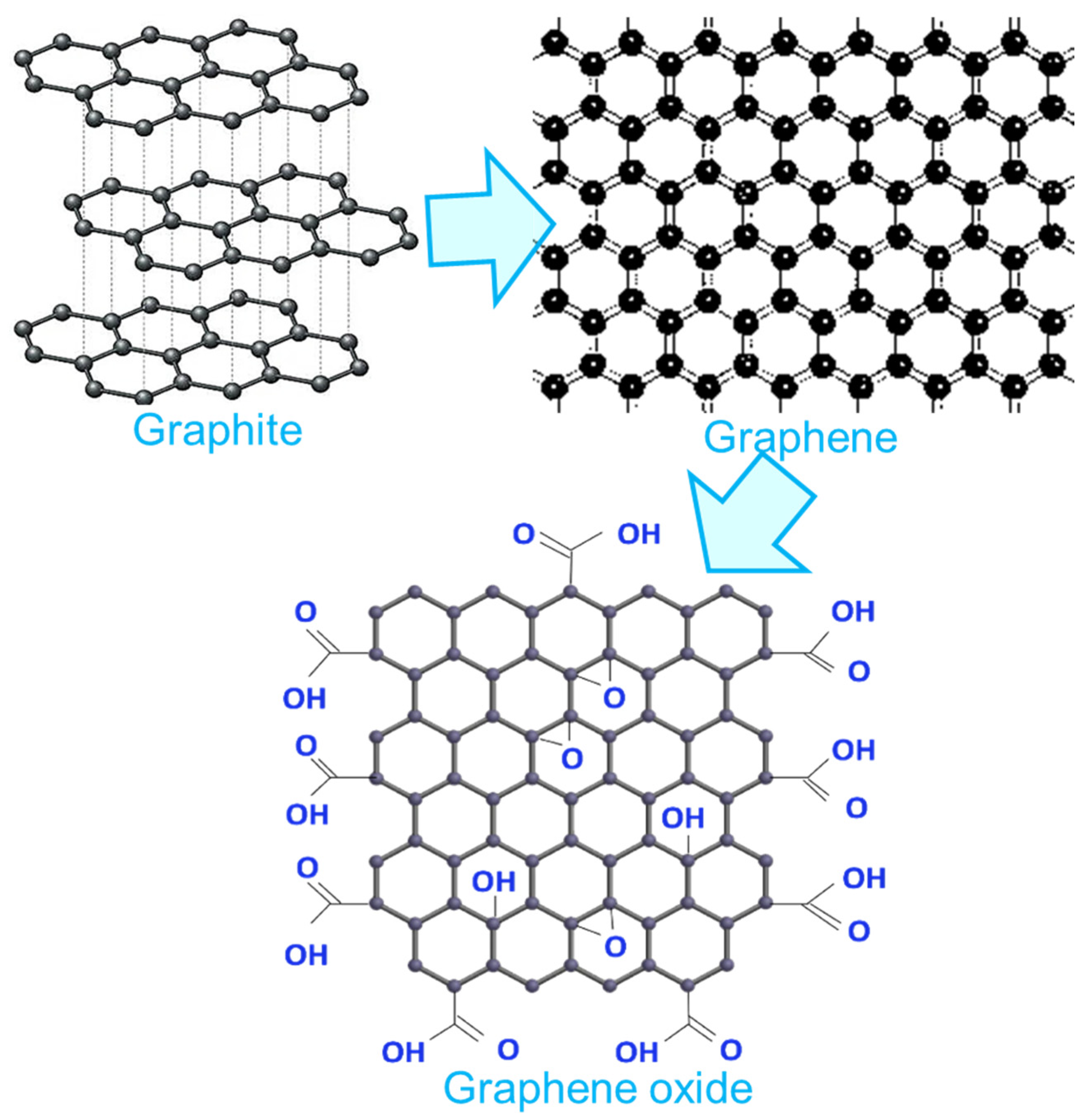
2. Graphene: An Exclusive Nanocarbon
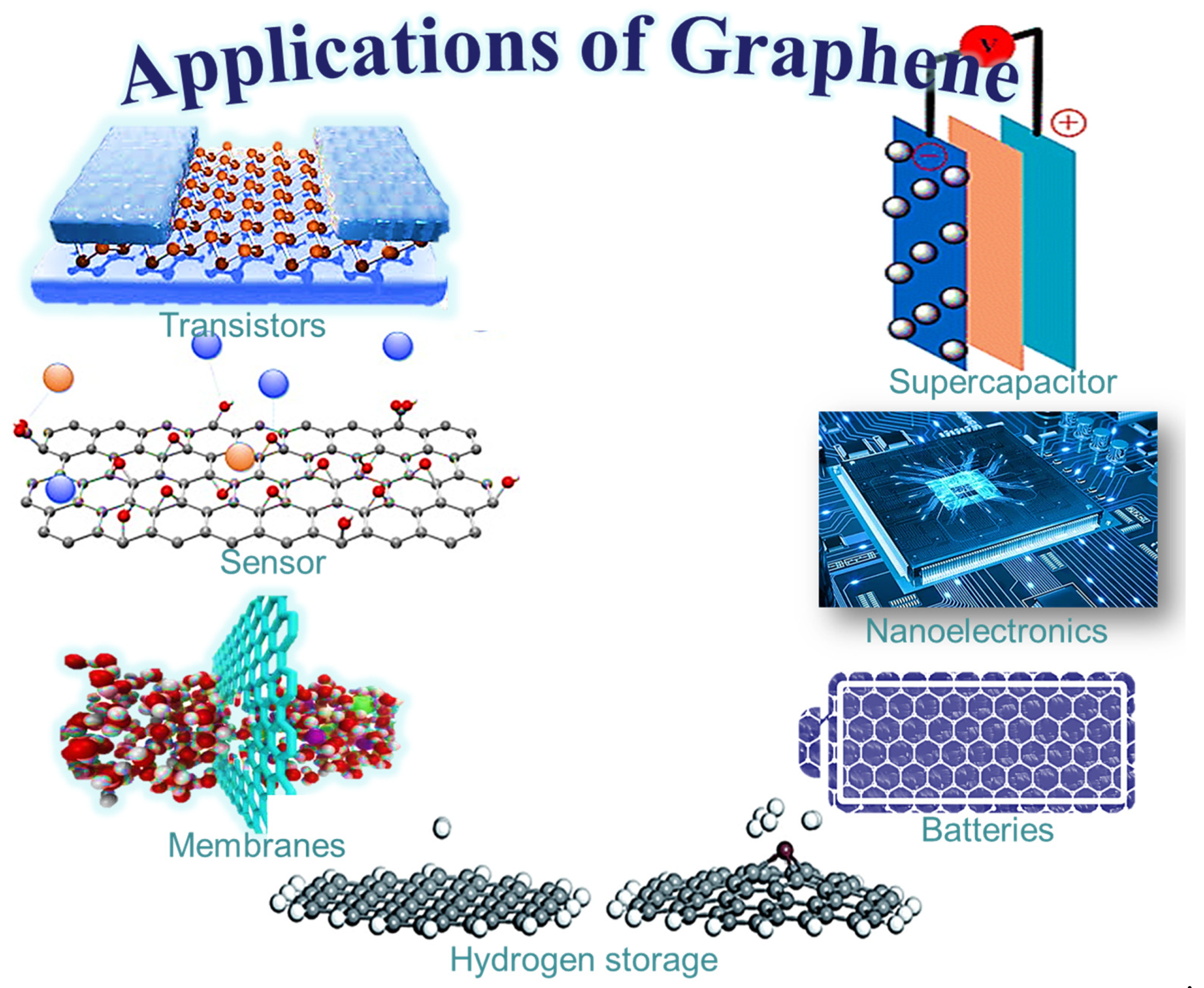
3. Graphene-Derived Nanocomposites: Innovative Materials
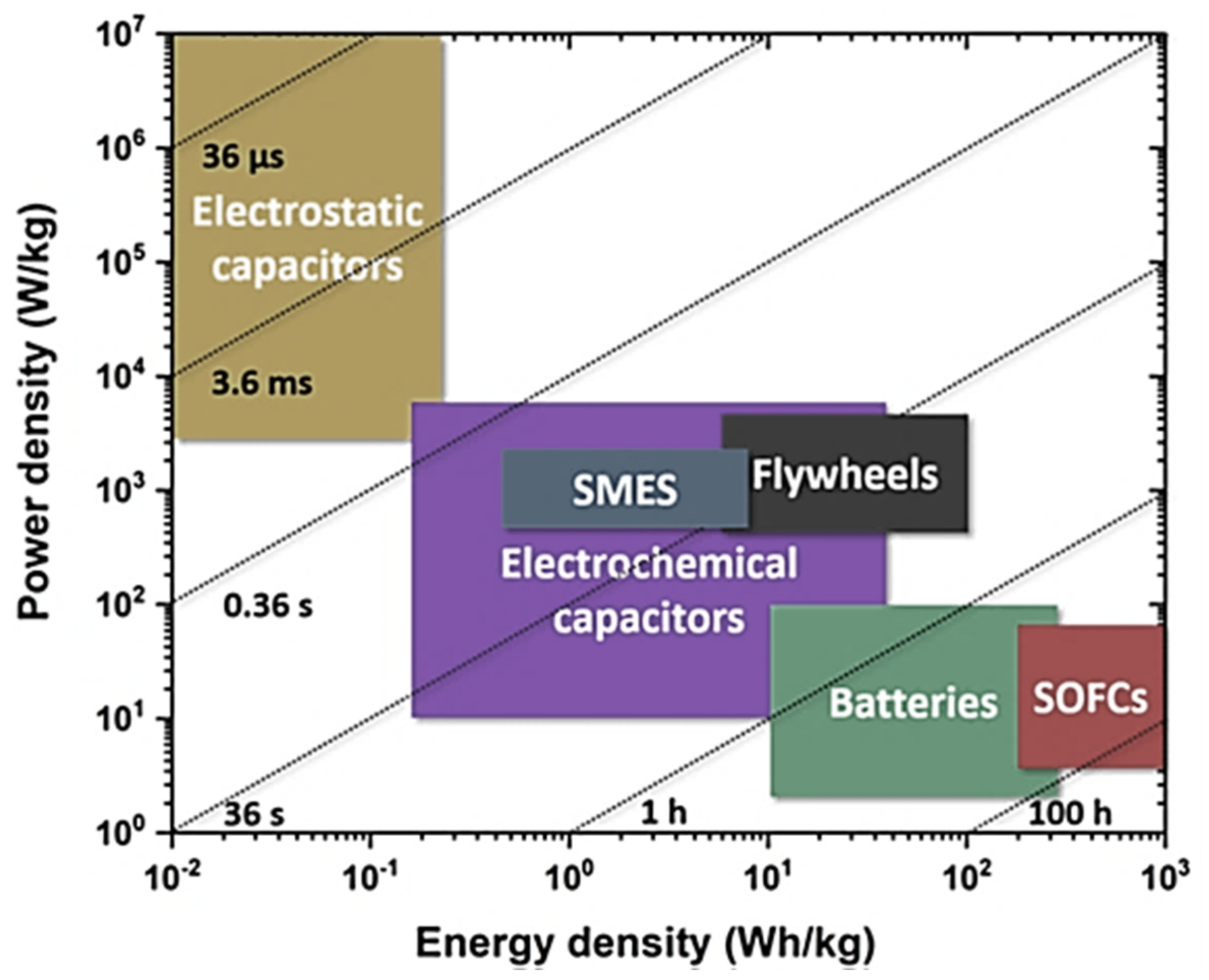
4. Graphene Consequent Nanocomposites in Supercapacitors
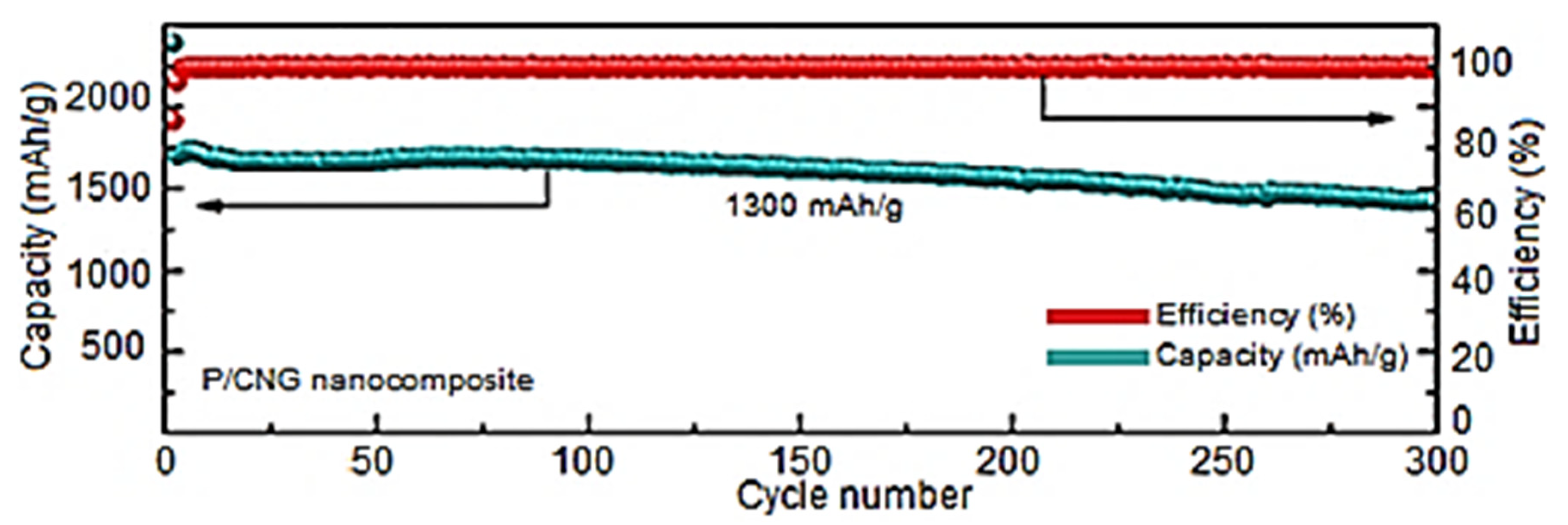
5. Graphene Nanocomposites towards Li-ion Batteries
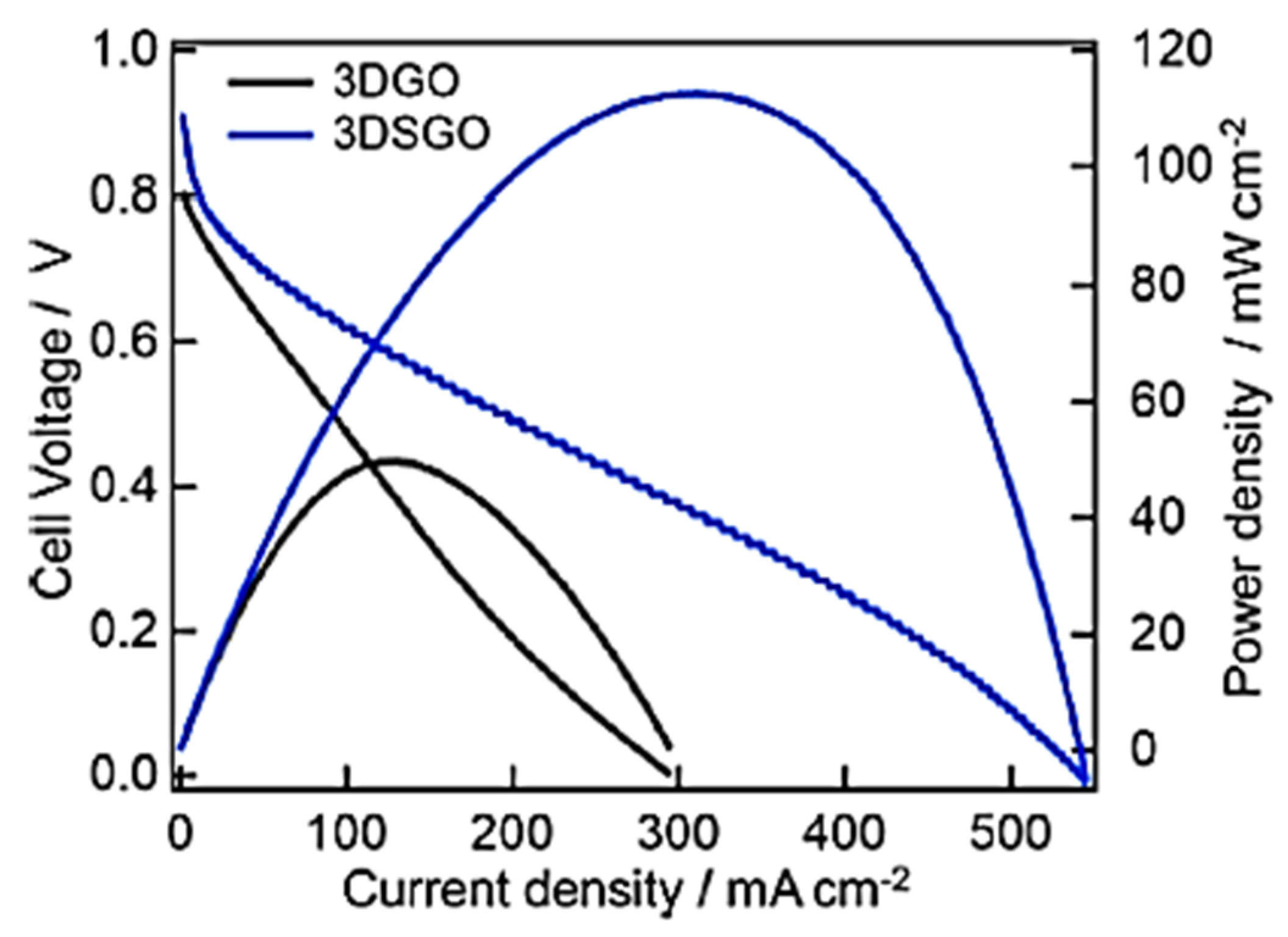
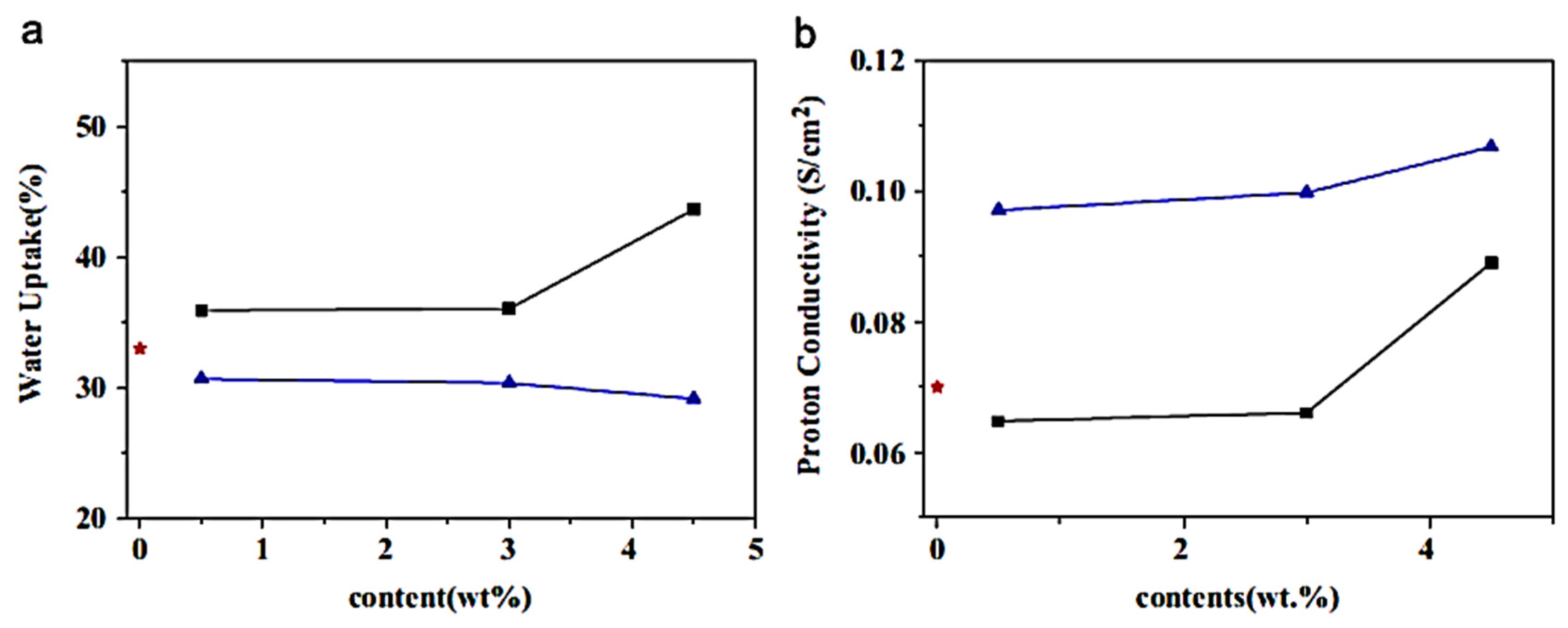
6. Graphene Nanocomposites for Fuel Cells
7. Challenges and Future
8. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fraenza, C.C.; Greembaum, S.; Suarez, S.N. Nuclear Magnetic Resonance Relaxation Pathways in Electrolytes for Energy Storage. Int. J. Mol. Sci. 2023, 24, 10373. [Google Scholar] [CrossRef]
- Tero, R.; Hagiwara, Y.; Saito, S. Domain Localization by Graphene Oxide in Supported Lipid Bilayers. Int. J. Mol. Sci. 2023, 24, 7999. [Google Scholar] [CrossRef]
- Park, L.; Kim, H.-S.; Jang, W.; Ji, M.-K.; Ryu, J.-H.; Cho, H.; Lim, H.-P. Antibacterial Evaluation of Zirconia Coated with Plasma-Based Graphene Oxide with Photothermal Properties. Int. J. Mol. Sci. 2023, 24, 8888. [Google Scholar] [CrossRef] [PubMed]
- Polyakova, P.V.; Baimova, J.A. Mechanical Properties of Graphene Networks under Compression: A Molecular Dynamics Simulation. Int. J. Mol. Sci. 2023, 24, 6691. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A.; Ahmad, I.; Eisa, M.; Maaza, M.; Khan, H. Manufacturing Strategies for Graphene Derivative Nanocomposites—Current Status and Fruitions. Nanomanufacturing 2023, 3, 1–19. [Google Scholar] [CrossRef]
- Adnan, M. The Future of Energy Storage: Advancements and Roadmaps for Lithium-Ion Batteries. Int. J. Mol. Sci. 2023, 24, 7457. [Google Scholar]
- Wang, F.; Han, Y.; Feng, X.; Xu, R.; Li, A.; Wang, T.; Deng, M.; Tong, C.; Li, J.; Wei, Z. Mesoporous Carbon-Based Materials for Enhancing the Performance of Lithium-Sulfur Batteries. Int. J. Mol. Sci. 2023, 24, 7291. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Z. Supercapacitor and supercapattery as emerging electrochemical energy stores. Int. Mater. Rev. 2017, 62, 173–202. [Google Scholar] [CrossRef] [Green Version]
- Smaisim, G.F.; Abed, A.M.; Al-Madhhachi, H.; Hadrawi, S.K.; Al-Khateeb, H.M.M.; Kianfar, E. Graphene-based important carbon structures and nanomaterials for energy storage applications as chemical capacitors and supercapacitor electrodes: A review. BioNanoScience 2023, 13, 219–248. [Google Scholar] [CrossRef]
- Fallahifar, R.; Kalantar, M. Optimal planning of lithium ion battery energy storage for microgrid applications: Considering capacity degradation. J. Energy Storage 2023, 57, 106103. [Google Scholar] [CrossRef]
- Sengodu, P.; Deshmukh, A.D. Conducting polymers and their inorganic composites for advanced Li-ion batteries: A review. Rsc Adv. 2015, 5, 42109–42130. [Google Scholar] [CrossRef]
- Wang, X.; Duan, L.; Zheng, N. Thermodynamic analysis of a novel tri-generation system integrated with a solar energy storage and solid oxide fuel cell–Gas turbine. Appl. Therm. Eng. 2023, 219, 119648. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Ahmad, S. Surface-active antimicrobial and anticorrosive Oleo-Polyurethane/graphene oxide nanocomposite coatings: Synergistic effects of in-situ polymerization and π-π interaction. Prog. Org. Coat. 2019, 127, 168–180. [Google Scholar] [CrossRef]
- Mohan, T.; Kanny, K. Green Nanofillers for Polymeric Materials. In Green Nanomaterials; Springer: Singapore, 2020; pp. 99–138. [Google Scholar]
- Siwal, S.S.; Zhang, Q.; Devi, N.; Thakur, V.K. Carbon-based polymer nanocomposite for high-performance energy storage applications. Polymers 2020, 12, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooney, M.; Nyayachavadi, A.; Rondeau-Gagné, S. Eco-friendly semiconducting polymers: From greener synthesis to greener processability. J. Mater. Chem. C 2020, 8, 14645–14664. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, Z.; Yang, Z.; Ke, L.; Kitipornchai, S.; Yang, J. Functionally graded graphene reinforced composite structures: A review. Eng. Struct. 2020, 210, 110339. [Google Scholar] [CrossRef]
- Ghabussi, A.; Ashrafi, N.; Shavalipour, A.; Hosseinpour, A.; Habibi, M.; Moayedi, H.; Babaei, B.; Safarpour, H. Free vibration analysis of an electro-elastic GPLRC cylindrical shell surrounded by viscoelastic foundation using modified length-couple stress parameter. Mech. Based Des. Struct. Mach. 2021, 49, 738–762. [Google Scholar] [CrossRef]
- Huo, J.; Zhang, G.; Ghabussi, A.; Habibi, M. Bending analysis of FG-GPLRC axisymmetric circular/annular sector plates by considering elastic foundation and horizontal friction force using 3D-poroelasticity theory. Compos. Struct. 2021, 276, 114438. [Google Scholar] [CrossRef]
- Ghabussi, A.; Habibi, M.; NoormohammadiArani, O.; Shavalipour, A.; Moayedi, H.; Safarpour, H. Frequency characteristics of a viscoelastic graphene nanoplatelet–reinforced composite circular microplate. J. Vib. Control 2021, 27, 101–118. [Google Scholar] [CrossRef]
- Yang, H. A review of supercapacitor-based energy storage systems for microgrid applications. In Proceedings of 2018 IEEE Power & Energy Society General Meeting (PESGM), Portland, OR, USA, 5–10 August 2018; pp. 1–5. [Google Scholar]
- Novoselov, K.S.; Fal, V.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Usachov, D.; Adamchuk, V.; Haberer, D.; Grüneis, A.; Sachdev, H.; Preobrajenski, A.; Laubschat, C.; Vyalikh, D. Quasifreestanding single-layer hexagonal boron nitride as a substrate for graphene synthesis. Phys. Rev. B 2010, 82, 075415. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhang, Y.; Chen, P.; Li, Y.; Liu, M.; Gao, T.; Ma, D.; Chen, Y.; Cheng, Z.; Qiu, X. Toward single-layer uniform hexagonal boron nitride–graphene patchworks with zigzag linking edges. Nano Lett. 2013, 13, 3439–3443. [Google Scholar] [CrossRef] [PubMed]
- Girit, Ç.Ö.; Meyer, J.C.; Erni, R.; Rossell, M.D.; Kisielowski, C.; Yang, L.; Park, C.-H.; Crommie, M.; Cohen, M.L.; Louie, S.G. Graphene at the edge: Stability and dynamics. Science 2009, 323, 1705–1708. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Navarro, C.; Meyer, J.C.; Sundaram, R.S.; Chuvilin, A.; Kurasch, S.; Burghard, M.; Kern, K.; Kaiser, U. Atomic structure of reduced graphene oxide. Nano Lett. 2010, 10, 1144–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.Y.; Ruiz-Vargas, C.S.; Van Der Zande, A.M.; Whitney, W.S.; Levendorf, M.P.; Kevek, J.W.; Garg, S.; Alden, J.S.; Hustedt, C.J.; Zhu, Y. Grains and grain boundaries in single-layer graphene atomic patchwork quilts. Nature 2011, 469, 389–392. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Geim, A.K. Nobel Lecture: Random walk to graphene. Rev. Mod. Phys. 2011, 83, 851. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Tour, J.M. Top-down versus bottom-up fabrication of graphene-based electronics. Chem. Mater. 2014, 26, 163–171. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Bao, Q.; Duh, J.-G.; Chang, C.-T. Top-down dispersion meets bottom-up synthesis: Merging ultranano silicon and graphene nanosheets for superior hybrid anodes for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 9986–9997. [Google Scholar] [CrossRef]
- Zhang, Z.; Fraser, A.; Ye, S.; Merle, G.; Barralet, J. Top-down bottom-up graphene synthesis. Nano Futures 2019, 3, 042003. [Google Scholar] [CrossRef]
- Wei, C.; Negishi, R.; Ogawa, Y.; Akabori, M.; Taniyasu, Y.; Kobayashi, Y. Turbostratic multilayer graphene synthesis on CVD graphene template toward improving electrical performance. Jpn. J. Appl. Phys. 2019, 58, SIIB04. [Google Scholar] [CrossRef]
- Cabrero-Vilatela, A.; Weatherup, R.S.; Braeuninger-Weimer, P.; Caneva, S.; Hofmann, S. Towards a general growth model for graphene CVD on transition metal catalysts. Nanoscale 2016, 8, 2149–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Wang, M.; Jang, S.K.; Jang, W.J.; Kim, M.; Park, S.Y.; Kim, S.W.; Kahng, S.J.; Choi, J.Y.; Ruoff, R.S.; Song, Y.J. A Platform for Large-Scale Graphene Electronics–CVD Growth of Single-Layer Graphene on CVD-Grown Hexagonal Boron Nitride. Adv. Mater. 2013, 25, 2746–2752. [Google Scholar] [CrossRef]
- Liang, J.; Li, L.; Tong, K.; Ren, Z.; Hu, W.; Niu, X.; Chen, Y.; Pei, Q. Silver nanowire percolation network soldered with graphene oxide at room temperature and its application for fully stretchable polymer light-emitting diodes. ACS Nano 2014, 8, 1590–1600. [Google Scholar] [CrossRef]
- Narayanam, P.K.; Botcha, V.D.; Ghosh, M.; Major, S.S. Growth and Photocatalytic Behaviour of Transparent Reduced GO-ZnO Nanocomposite Sheets. Nanotechnology 2019, 30, 485601. [Google Scholar] [CrossRef]
- Kausar, A. Potential of polymer/graphene nanocomposite in electronics. Am. J. Nanosci. Nanotechnol. Res. 2018, 6, 55–63. [Google Scholar]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Zandiatashbar, A.; Lee, G.-H.; An, S.J.; Lee, S.; Mathew, N.; Terrones, M.; Hayashi, T.; Picu, C.R.; Hone, J.; Koratkar, N. Effect of defects on the intrinsic strength and stiffness of graphene. Nat. Commun. 2014, 5, 3186. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-T.; Chang, Y.; Wang, H.; Liu, G.; Chen, S.; Wang, Y.; Liu, Y.; Cao, A. Folding/aggregation of graphene oxide and its application in Cu2+ removal. J. Colloid Interface Sci. 2010, 351, 122–127. [Google Scholar] [CrossRef]
- Wang, W.-N.; Jiang, Y.; Biswas, P. Evaporation-induced crumpling of graphene oxide nanosheets in aerosolized droplets: Confinement force relationship. J. Phys. Chem. Lett. 2012, 3, 3228–3233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xia, G.; Du, M.; Lu, Y.; Xu, H. Scotch-tape-like exfoliation effect of graphene quantum dots for efficient preparation of graphene nanosheets in water. Appl. Surf. Sci. 2019, 483, 52–59. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.-t.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.S. Interlayer Distance Controlled Graphene, Supercapacitor and Method of Producing the Same. U.S. Patent US20150103469A1, 26 February 2019. [Google Scholar]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.J.; Zeng, X.L.; Dang, C.Y. Graphene Composites. Handb. Graphene 2019, 1, 1–25. [Google Scholar]
- Han, J.T.; Jang, J.I.; Cho, J.Y.; Hwang, J.Y.; Woo, J.S.; Jeong, H.J.; Jeong, S.Y.; Seo, S.H.; Lee, G.-W. Synthesis of nanobelt-like 1-dimensional silver/nanocarbon hybrid materials for flexible and wearable electronics. Sci. Rep. 2017, 7, 4931. [Google Scholar] [CrossRef] [Green Version]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for biology and medicine: Sensing, imaging, and drug delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, A.; D’Agostino, M.; Pavoni, E.; Ardiccioni, C.; Motta, S.; Crippa, P.; Biagetti, G.; Notarstefano, V.; Rexha, J.; Perta, N. SARS-CoV-2 multi-variant rapid detector based on graphene transistor functionalized with an engineered dimeric ACE2 receptor. Nano Today 2023, 48, 101729. [Google Scholar] [CrossRef]
- Bi, J.; Du, Z.; Sun, J.; Liu, Y.; Wang, K.; Du, H.; Ai, W.; Huang, W. On the Road to the Frontiers of Lithium-ion Batteries: A Review and Outlook of Graphene Anodes. Adv. Mater. 2023, 2210734. [Google Scholar] [CrossRef]
- Macili, A.; Vlamidis, Y.; Pfusterschmied, G.; Leitgeb, M.; Schmid, U.; Heun, S.; Veronesi, S. Study of hydrogen absorption in a novel three-dimensional graphene structure: Towards hydrogen storage applications. Appl. Surf. Sci. 2023, 156375. [Google Scholar] [CrossRef]
- Tang, C.; Titirici, M.-M.; Zhang, Q. A review of nanocarbons in energy electrocatalysis: Multifunctional substrates and highly active sites. J. Energy Chem. 2017, 26, 1077–1093. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, V.; Jayaraman, A. Theory and simulation studies of effective interactions, phase behavior and morphology in polymer nanocomposites. Soft Matter 2014, 10, 13–38. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Bose, S.; Hong, C.E.; Uddin, M.E.; Khanra, P.; Kim, N.H.; Lee, J.H. Preparation of functionalized graphene/linear low density polyethylene composites by a solution mixing method. Carbon 2011, 49, 1033–1037. [Google Scholar] [CrossRef]
- Vadukumpully, S.; Paul, J.; Mahanta, N.; Valiyaveettil, S. Flexible conductive graphene/poly (vinyl chloride) composite thin films with high mechanical strength and thermal stability. Carbon 2011, 49, 198–205. [Google Scholar] [CrossRef]
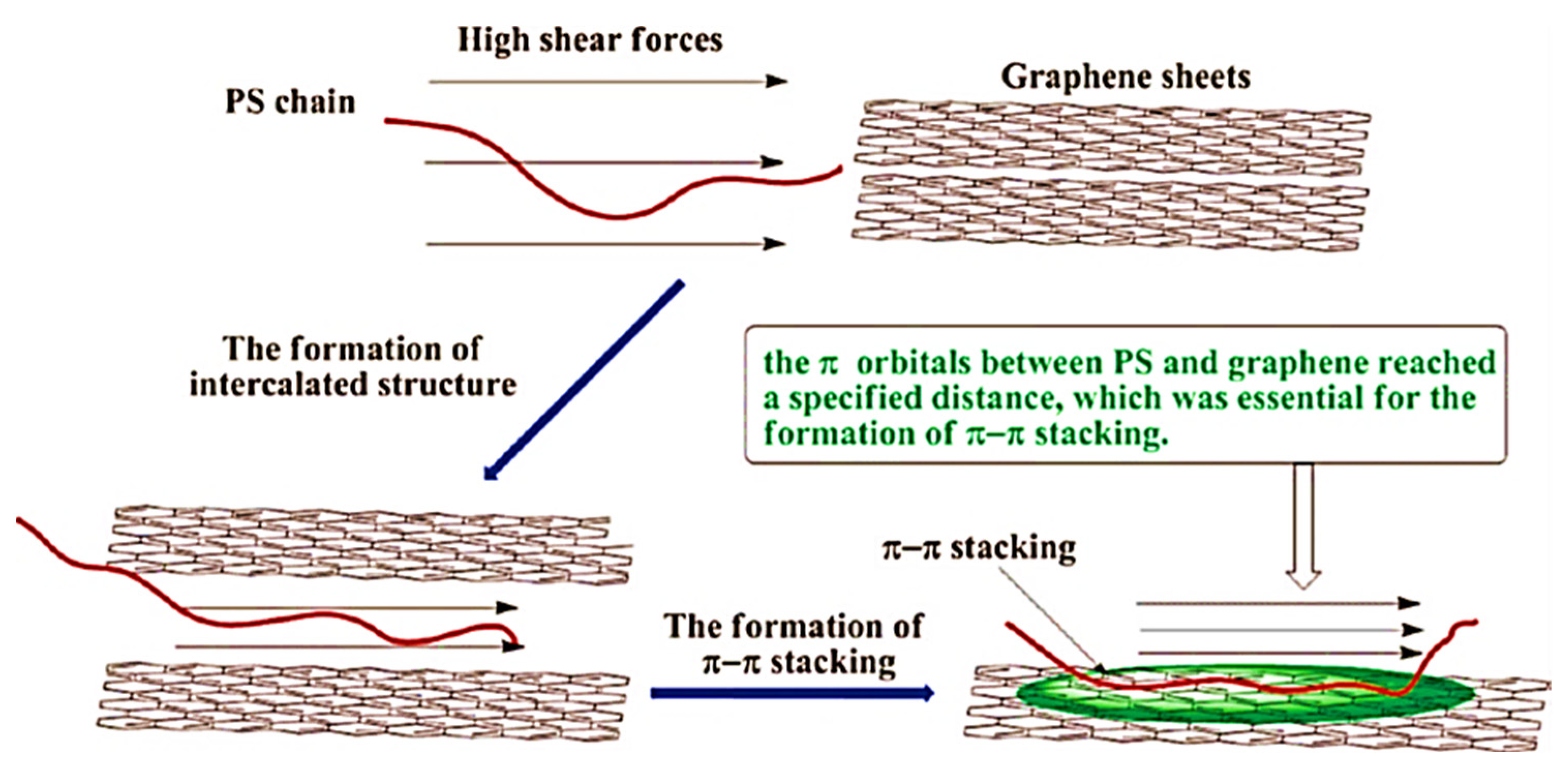
- Shen, B.; Zhai, W.; Chen, C.; Lu, D.; Wang, J.; Zheng, W. Melt blending in situ enhances the interaction between polystyrene and graphene through π–π stacking. ACS Appl. Mater. Interfaces 2011, 3, 3103–3109. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, G.; Zhao, S.; Cui, J.; Gao, A.; Yan, Y. Fabrication of pristine graphene-based conductive polystyrene composites towards high performance and light-weight. Compos. Sci. Technol. 2018, 159, 232–239. [Google Scholar] [CrossRef]
- He, F.; Lam, K.-H.; Fan, J.; Chan, L.H. Improved dielectric properties for chemically functionalized exfoliated graphite nanoplates/syndiotactic polystyrene composites prepared by a solution-blending method. Carbon 2014, 80, 496–503. [Google Scholar] [CrossRef]
- Mohammadsalih, Z.G.; Inkson, B.J.; Chen, B. The effect of dispersion condition on the structure and properties of polystyrene/graphene oxide nanocomposites. Polym. Compos. 2021, 42, 320–328. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, J.; Yuan, W. Preparation of a poly (methyl methacrylate)-reduced graphene oxide composite with enhanced properties by a solution blending method. Eur. Polym. J. 2012, 48, 1674–1682. [Google Scholar] [CrossRef]
- Balasubramaniyan, R.; Pham, V.H.; Jang, J.; Hur, S.H.; Chung, J.S. A one pot solution blending method for highly conductive poly (methyl methacrylate)-highly reduced graphene nanocomposites. Electron. Mater. Lett. 2013, 9, 837–839. [Google Scholar] [CrossRef]
- Ma, N.; Yang, D.; Riaz, S.; Wang, L.; Wang, K. Aging Mechanism and Models of Supercapacitors: A Review. Technologies 2023, 11, 38. [Google Scholar] [CrossRef]
- Liu, S.; Yu, T.; Wu, Y.; Li, W.; Li, B. Evolution of cellulose into flexible conductive green electronics: A smart strategy to fabricate sustainable electrodes for supercapacitors. RSC Adv. 2014, 4, 34134–34143. [Google Scholar] [CrossRef]
- Alsaad, A.M.; Aljarrah, I.A.; Ahmad, A.; Al-Bataineh, Q.M.; Shariah, A.; Al-Akhras, M.A.; Telfah, A.D. The structural, optical, thermal, and electrical properties of synthesized PEO/GO thin films. Appl. Phys. A 2022, 128, 676. [Google Scholar] [CrossRef]
- Singh, J.; Dhaliwal, A.; Sharma, K.; Sehgal, R.; Kumar, V. Conductive polymer-based composite photocatalysts for environment and energy applications. In Conjugated Polymers for Next-Generation Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 505–538. [Google Scholar]
- Albarqouni, Y.M.; Lee, S.P.; Ali, G.A.; Ethiraj, A.S.; Algarni, H.; Chong, K.F. Facile synthesis of reduced graphene oxide aerogel in soft drink as supercapacitor electrode. J. Nanostruct. Chem. 2022, 12, 417–427. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Iro, Z.S.; Subramani, C.; Dash, S. A brief review on electrode materials for supercapacitor. Int. J. Electrochem. Sci 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Liu, X.; Li, K. Energy storage devices in electrified railway systems: A review. Transp. Saf. Environ. 2020, 2, 183–201. [Google Scholar] [CrossRef]
- Yao, K.; Chen, S.; Rahimabady, M.; Mirshekarloo, M.S.; Yu, S.; Tay, F.E.H.; Sritharan, T.; Lu, L. Nonlinear dielectric thin films for high-power electric storage with energy density comparable with electrochemical supercapacitors. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 1968–1974. [Google Scholar]
- Yang, L.; Kong, X.; Li, F.; Hao, H.; Cheng, Z.; Liu, H.; Li, J.-F.; Zhang, S. Perovskite lead-free dielectrics for energy storage applications. Prog. Mater. Sci. 2019, 102, 72–108. [Google Scholar] [CrossRef]
- Kausar, A. N-Doped Graphene and Polymer Sequent Nanocomposite—Nitty-Gritties and Scoping Insights. Polym. Plast. Technol. Mater. 2023, 62, 1347–1363. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Zhao, T.; Aldaghri, O.; Eisa, M. Graphene in Polymeric Nanocomposite Membranes—Current State and Progress. Processes 2023, 11, 927. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Zhao, T.; Aldaghri, O.; Eisa, M. Polymer/Graphene Nanocomposites via 3D and 4D Printing—Design and Technical Potential. Processes 2023, 11, 868. [Google Scholar] [CrossRef]
- Xu, F.; Gao, M.; Wang, H.; Liu, H.; Yan, F.; Zhao, H.; Yao, Q. Polymer-based graphene composite molding: A review. RSC Adv. 2023, 13, 2538–2551. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, S.; Xu, K.; Zhang, Y.; Zhang, L.; Lou, G.; Wu, Y.; Zhu, E.; Chen, H.; Shen, Z. Sustainable activated carbons from dead ginkgo leaves for supercapacitor electrode active materials. Chem. Eng. Sci. 2018, 181, 36–45. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X. Conducting polymers directly coated on reduced graphene oxide sheets as high-performance supercapacitor electrodes. J. Phys. Chem. C 2012, 116, 5420–5426. [Google Scholar] [CrossRef]
- Chandra, S.; Patel, M.D.; Lang, H.; Bahadur, D. Dendrimer-functionalized magnetic nanoparticles: A new electrode material for electrochemical energy storage devices. J. Power Sources 2015, 280, 217–226. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Selvaganesh, S.V.; Mathiyarasu, J.; Phani, K.; Yegnaraman, V. Chemical synthesis of PEDOT–Au nanocomposite. Nanoscale Res. Lett. 2007, 2, 546. [Google Scholar] [CrossRef] [Green Version]
- Ezeigwe, E.R.; Tan, M.T.; Khiew, P.S.; Siong, C.W. One-step green synthesis of graphene/ZnO nanocomposites for electrochemical capacitors. Ceram. Int. 2015, 41, 715–724. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, A.K.; Pradhan, D. High performance solid-state asymmetric supercapacitor using green synthesized graphene–WO3 nanowires nanocomposite. Acs Sustain. Chem. Eng. 2017, 5, 10128–10138. [Google Scholar] [CrossRef]
- Çıplak, Z.; Yıldız, A.; Yıldız, N. Green preparation of ternary reduced graphene oxide-au@ polyaniline nanocomposite for supercapacitor application. J. Energy Storage 2020, 32, 101846. [Google Scholar] [CrossRef]
- Arthisree, D.; Madhuri, W. Optically active polymer nanocomposite composed of polyaniline, polyacrylonitrile and green-synthesized graphene quantum dot for supercapacitor application. Int. J. Hydrog. Energy 2020, 45, 9317–9327. [Google Scholar] [CrossRef]
- Sumboja, A.; Wang, X.; Yan, J.; Lee, P.S. Nanoarchitectured current collector for high rate capability of polyaniline based supercapacitor electrode. Electrochim. Acta 2012, 65, 190–195. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mary, N. Biocompatible supercapacitor electrodes using green synthesised ZnO/Polymer nanocomposites for efficient energy storage applications. J. Energy Storage 2020, 28, 101275. [Google Scholar] [CrossRef]
- Burkhardt, S.E.; Bois, J.; Tarascon, J.-M.; Hennig, R.G.; Abruña, H.D. Li-carboxylate anode structure-property relationships from molecular modeling. Chem. Mater. 2013, 25, 132–141. [Google Scholar] [CrossRef]
- Xu, W.; Read, A.; Koech, P.K.; Hu, D.; Wang, C.; Xiao, J.; Padmaperuma, A.B.; Graff, G.L.; Liu, J.; Zhang, J.-G. Factors affecting the battery performance of anthraquinone-based organic cathode materials. J. Mater. Chem. 2012, 22, 4032–4039. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, L.; Hu, X.; Wang, Z.; Wik, T.; Pecht, M. A review of fractional-order techniques applied to lithium-ion batteries, lead-acid batteries, and supercapacitors. J. Power Sources 2018, 390, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Guo, R.; Li, T.; Li, F.; Liu, Z.; Zheng, M.; Wang, B.; Yang, Z.; Luo, H.; Wan, Y. Application of Polyaniline for Li-Ion Batteries, Lithium–Sulfur Batteries, and Supercapacitors. ChemSusChem 2019, 12, 1591–1611. [Google Scholar] [CrossRef]
- Wu, B.; Ren, Y.; Li, N. LiFePO4 Cathode Material. In Electric Vehicles; Soylu, S., Ed.; Intech: London, UK, 2011; pp. 199–216. [Google Scholar]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation—approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Jiao, L.; Chen, J. Tin nanodots encapsulated in porous nitrogen-doped carbon nanofibers as a free-standing anode for advanced sodium-ion batteries. Adv. Mater. 2015, 27, 6702–6707. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Luo, Y.; Lv, W.; Yu, W.; Wu, S.; Hou, P.; Yang, Q.; Meng, Q.; Liu, C.; Cheng, H.M. Vertically Aligned Carbon Nanotubes Grown on Graphene Paper as Electrodes in Lithium-Ion Batteries and Dye-Sensitized Solar Cells. Adv. Energy Mater. 2011, 1, 486–490. [Google Scholar] [CrossRef]
- Chang, C.-H.; Manthiram, A. Covalently grafted polysulfur–graphene nanocomposites for ultrahigh sulfur-loading lithium–polysulfur batteries. ACS Energy Lett. 2017, 3, 72–77. [Google Scholar] [CrossRef]
- Jiao, X.; Liu, Y.; Li, T.; Zhang, C.; Xu, X.; Kapitanova, O.O.; He, C.; Li, B.; Xiong, S.; Song, J. Crumpled nitrogen-doped graphene-wrapped phosphorus composite as a promising anode for lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 30858–30864. [Google Scholar] [CrossRef]
- Guo, C.X.; Wang, M.; Chen, T.; Lou, X.W.; Li, C.M. A Hierarchically Nanostructured Composite of MnO2/Conjugated Polymer/Graphene for High-Performance Lithium Ion Batteries. Adv. Energy Mater. 2011, 1, 736–741. [Google Scholar] [CrossRef]
- Li, Z.-F.; Zhang, H.; Liu, Q.; Liu, Y.; Stanciu, L.; Xie, J. Novel pyrolyzed polyaniline-grafted silicon nanoparticles encapsulated in graphene sheets as Li-ion battery anodes. ACS Appl. Mater. Interfaces 2014, 6, 5996–6002. [Google Scholar] [CrossRef]
- Guo, W.; Su, J.; Li, Y.-H.; Wan, L.-J.; Guo, Y.-G. Nitroxide radical polymer/graphene nanocomposite as an improved cathode material for rechargeable lithium batteries. Electrochim. Acta 2012, 72, 81–86. [Google Scholar] [CrossRef]
- Chae, C.; Kim, K.W.; Yun, Y.J.; Lee, D.; Moon, J.; Choi, Y.; Lee, S.S.; Choi, S.; Jeong, S. Polyethylenimine-mediated electrostatic assembly of MnO2 nanorods on graphene oxides for use as anodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 11499–11506. [Google Scholar] [CrossRef]
- Song, Z.; Xu, T.; Gordin, M.L.; Jiang, Y.-B.; Bae, I.-T.; Xiao, Q.; Zhan, H.; Liu, J.; Wang, D. Polymer–graphene nanocomposites as ultrafast-charge and-discharge cathodes for rechargeable lithium batteries. Nano Lett. 2012, 12, 2205–2211. [Google Scholar] [CrossRef]
- Beladi-Mousavi, S.M.; Sadaf, S.; Mahmood, A.M.; Walder, L. High Performance Poly (viologen)–Graphene Nanocomposite Battery Materials with Puff Paste Architecture. ACS Nano 2017, 11, 8730–8740. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, Y. Three-dimensional porous graphene networks and hybrids for lithium-ion batteries and supercapacitors. Chem 2017, 2, 171–200. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Wang, Y. Graphene-based nanocomposite anodes for lithium-ion batteries. Nanoscale 2014, 6, 11528–11552. [Google Scholar] [CrossRef]
- Riyanto, E.; Kristiantoro, T.; Martides, E.; Prawara, B.; Mulyadi, D. Lithium-ion battery performance improvement using two-dimensional materials. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Pourzare, K.; Mansourpanah, Y.; Farhadi, S. Advanced nanocomposite membranes for fuel cell applications: A comprehensive review. Biofuel Res. J. 2016, 3, 496–513. [Google Scholar] [CrossRef] [Green Version]
- Ozoemena, K.I. Nanostructured platinum-free electrocatalysts in alkaline direct alcohol fuel cells: Catalyst design, principles and applications. RSC Adv. 2016, 6, 89523–89550. [Google Scholar] [CrossRef] [Green Version]
- Rahimnejad, M.; Ghoreyshi, A.A.; Najafpour, G.; Jafary, T. Power generation from organic substrate in batch and continuous flow microbial fuel cell operations. Appl. Energy 2011, 88, 3999–4004. [Google Scholar] [CrossRef]
- Ding, H.; Wang, S.; Long, Y.; Chan, S.H. Non-aqueous solution synthesis of Pt-based nanostructures for fuel cell catalysts. Mater. Today Energy 2020, 100616. [Google Scholar] [CrossRef]
- Tan, Q.; Qu, T.; Shu, C.-Y.; Liu, Y.; He, Y.; Zhai, W.; Guo, S.-W.; Liu, L.; Liu, Y.-N. High-Performance Polymer Fiber Membrane Based Direct Methanol Fuel Cell System with Non-Platinum Catalysts. ACS Sustain. Chem. Eng. 2019, 7, 17145–17153. [Google Scholar] [CrossRef]
- Jung, H.-Y.; Roh, S.-H. Carbon nanofiber/polypyrrole nanocomposite as anode material in microbial fuel cells. J. Nanosci. Nanotechnol. 2017, 17, 5830–5833. [Google Scholar] [CrossRef]
- Chi, M.; He, H.; Wang, H.; Zhou, M.; Gu, T. Graphite felt anode modified by electropolymerization of nano-polypyrrole to improve microbial fuel cell (MFC) production of bioelectricity. J. Microb. Biochem. Technol. S 2013, 12. [Google Scholar] [CrossRef]
- Lv, Z.; Chen, Y.; Wei, H.; Li, F.; Hu, Y.; Wei, C.; Feng, C. One-step electrosynthesis of polypyrrole/graphene oxide composites for microbial fuel cell application. Electrochim. Acta 2013, 111, 366–373. [Google Scholar] [CrossRef]
- Yuan, H.; Deng, L.; Chen, Y.; Yuan, Y. MnO2/Polypyrrole/MnO2 multi-walled-nanotube-modified anode for high-performance microbial fuel cells. Electrochim. Acta 2016, 196, 280–285. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, G.-Q.; Yuan, G.-E.; Liu, H.-Y.; Liu, J.-D.; Yang, F.-L. Anti-fouling performance and mechanism of anthraquinone/polypyrrole composite modified membrane cathode in a novel MFC–aerobic MBR coupled system. RSC Adv. 2015, 5, 22533–22543. [Google Scholar] [CrossRef]
- Rahman, M.A.; Yagyu, J.; Islam, M.S.; Fukuda, M.; Wakamatsu, S.; Tagawa, R.; Feng, Z.; Sekine, Y.; Ohyama, J.; Hayami, S. Three-Dimensional Sulfonated Graphene Oxide Proton Exchange Membranes for Fuel Cells. ACS Appl. Nano Mater. 2023, 6, 1707–1713. [Google Scholar] [CrossRef]
- Lee, D.; Yang, H.; Park, S.; Kim, W. Nafion/graphene oxide composite membranes for low humidifying polymer electrolyte membrane fuel cell. J. Membr. Sci. 2014, 452, 20–28. [Google Scholar] [CrossRef]
- Yuan, X.-H.; Yan, G.-D.; Li, H.-T.; Liu, X.; Su, C.-Q.; Wang, Y.-P. Research on energy management strategy of fuel cell–battery–supercapacitor passenger vehicle. Energy Rep. 2022, 8, 1339–1349. [Google Scholar] [CrossRef]
- Xue, Y.; Sun, S.; Wang, Q.; Dong, Z.; Liu, Z. Transition metal oxide-based oxygen reduction reaction electrocatalysts for energy conversion systems with aqueous electrolytes. J. Mater. Chem. A 2018, 6, 10595–10626. [Google Scholar] [CrossRef]
- Ni, J.; Li, Y. Carbon nanomaterials in different dimensions for electrochemical energy storage. Adv. Energy Mater. 2016, 6, 1600278. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Wan, C.; Jiao, Y.; Liang, D.; Wu, Y.; Li, J. A geologic architecture system-inspired micro-/nano-heterostructure design for high-performance energy storage. Adv. Energy Mater. 2018, 8, 1802388. [Google Scholar] [CrossRef]
- Ouyang, W.; Sun, J.; Memon, J.; Wang, C.; Geng, J.; Huang, Y. Scalable preparation of three-dimensional porous structures of reduced graphene oxide/cellulose composites and their application in supercapacitors. Carbon 2013, 62, 501–509. [Google Scholar] [CrossRef]
- Yang, X.; Fei, B.; Ma, J.; Liu, X.; Yang, S.; Tian, G.; Jiang, Z. Porous nanoplatelets wrapped carbon aerogels by pyrolysis of regenerated bamboo cellulose aerogels as supercapacitor electrodes. Carbohydr. Polym. 2018, 180, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Mensah-Darkwa, K.; Zequine, C.; Kahol, P.K.; Gupta, R.K. Supercapacitor energy storage device using biowastes: A sustainable approach to green energy. Sustainability 2019, 11, 414. [Google Scholar] [CrossRef] [Green Version]
- Holze, R. Composites of Intrinsically Conducting Polymers with Carbonaceous Materials for Supercapacitors—An Update. Univers. J. Electrochem. 2023, 1, 3. [Google Scholar]
- Akhter, J.S.; Ahmad, A.; Sharma, R.K.; Singh, R.; Mohd, A. Polymers/graphene derivative–based nanocomposites as electrode materials for supercapacitors. In Advances in Electronic Materials for Clean Energy Conversion and Storage Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 451–474. [Google Scholar]
- Wang, Z.; Liu, L.; Zhang, Y.; Huang, Y.; Liu, J.; Zhang, X.; Liu, X.; Teng, H.; Zhang, X.; Zhang, J. A Review of Graphene-Based Materials/Polymer Composite Aerogels. Polymers 2023, 15, 1888. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Xu, H.; Song, Y.; He, X. Polyimides as Promising Materials for Lithium-Ion Batteries: A Review. Nano-Micro Lett. 2023, 15, 135. [Google Scholar] [CrossRef]
- Lv, Z.-C.; Wang, P.-F.; Wang, J.-C.; Tian, S.-H.; Yi, T.-F. Key Challenges, Recent Advances and Future Perspectives of Rechargeable Lithium-Sulfur Batteries. J. Ind. Eng. Chem. 2023, 63, 68–88. [Google Scholar] [CrossRef]
- Moyseowicz, A.; Minta, D.; Gryglewicz, G. Conductive Polymer/Graphene-based Composites for Next Generation Energy Storage and Sensing Applications. ChemElectroChem 2023, 10, e202201145. [Google Scholar] [CrossRef]
- De Bortoli, B.F.; Camargo, M.C.R.; de Oliveira Polkowski, R.D.; de Albuquerque, R.F.C. Graphene: An overview of technology in the electric vehicles of the future. SAE Tech. Pap. 2023. [Google Scholar] [CrossRef]
- Starowicz, A.; Zieliński, M.; Rusanowska, P.; Dębowski, M. Microbial Fuel Cell Performance Boost through the Use of Graphene and Its Modifications. Energies 2023, 16, 576. [Google Scholar] [CrossRef]
- Fernández-Sotillo, A.; Ferreira-Aparicio, P. Durable corrosion-resistant coating based in graphene oxide for cost-effective fuel cells components. Iscience 2023, 26, 106569. [Google Scholar] [CrossRef]
- Barik, B.; Yun, Y.; Kumar, A.; Bae, H.; Namgung, Y.; Park, J.-Y.; Song, S.-J. Highly enhanced proton conductivity of single-step-functionalized graphene oxide/nafion electrolyte membrane towards improved hydrogen fuel cell performance. Int. J. Hydrog. Energy 2023, 48, 11029–11044. [Google Scholar] [CrossRef]
- Kausar, A. Advances in polymer-anchored carbon nanotube foam: A review. Polym. Plast. Technol. Mater. 2019, 58, 1965–1978. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Shi, K.; Yang, X.; Cranston, E.D.; Zhitomirsky, I. Efficient lightweight supercapacitor with compression stability. Adv. Funct. Mater. 2016, 26, 6437–6445. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Zhang, T.; Liu, Z. Nitrogen-doped graphene for supercapacitor with long-term electrochemical stability. Energy 2014, 70, 612–617. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Cullen, J.M. Toward a sustainable materials system. Science 2018, 360, 1396–1398. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
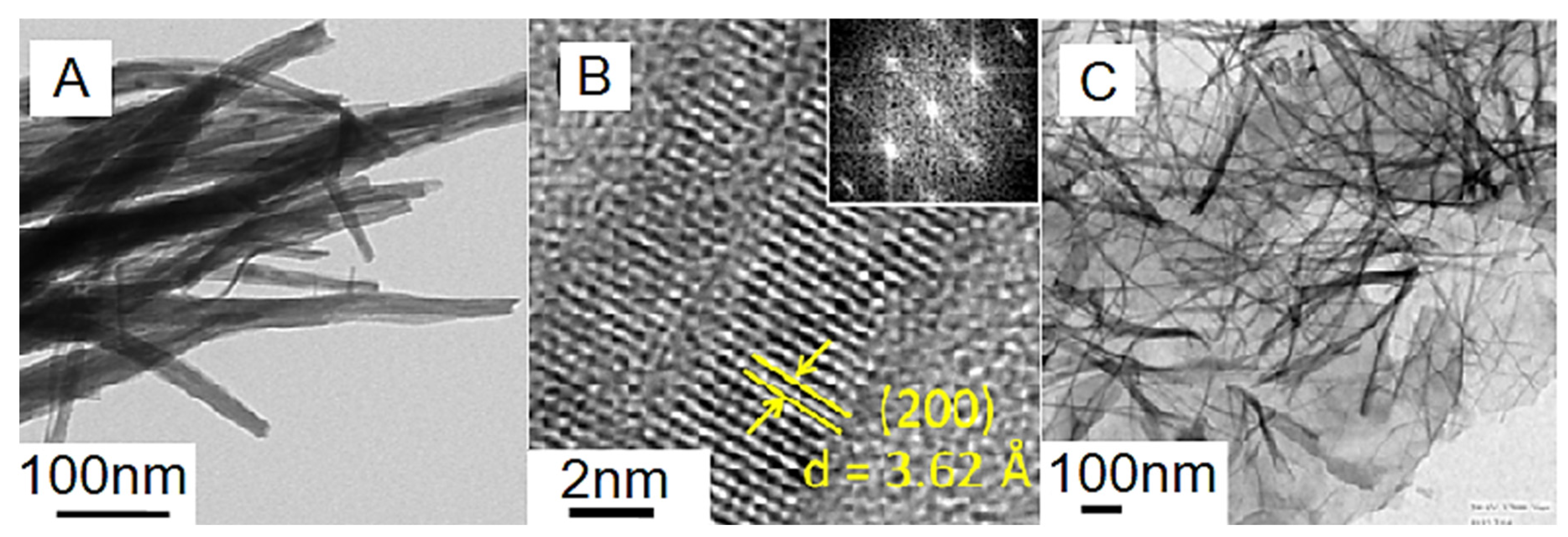
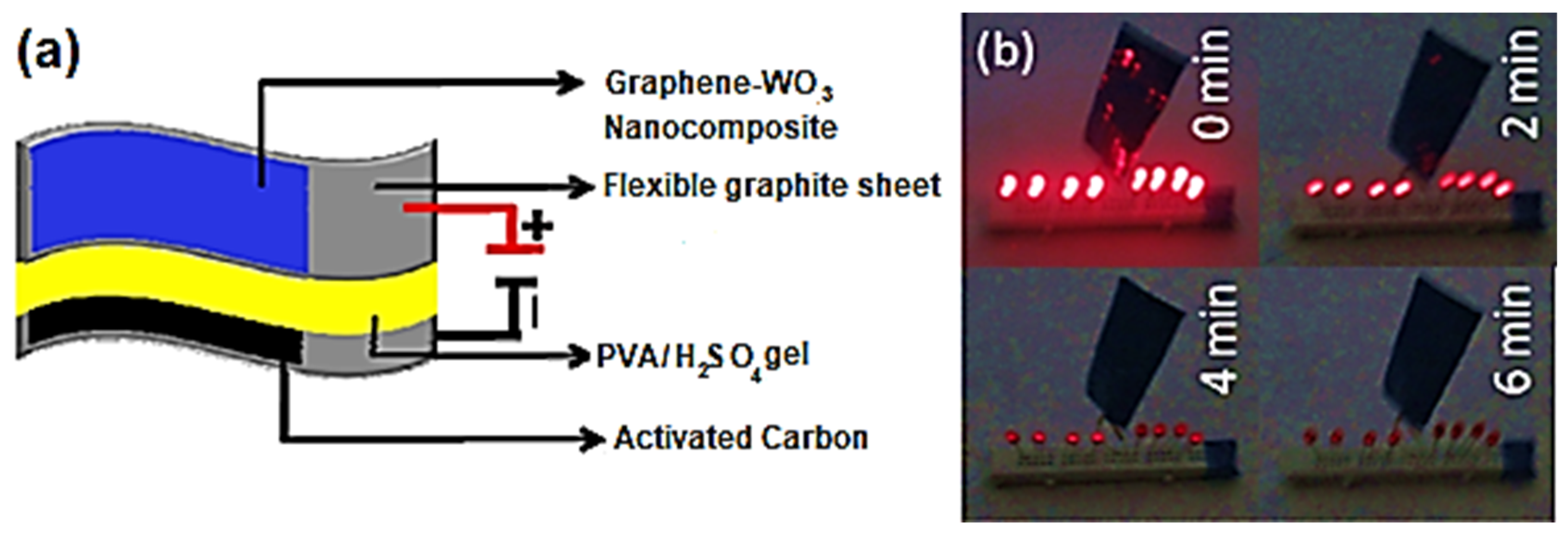
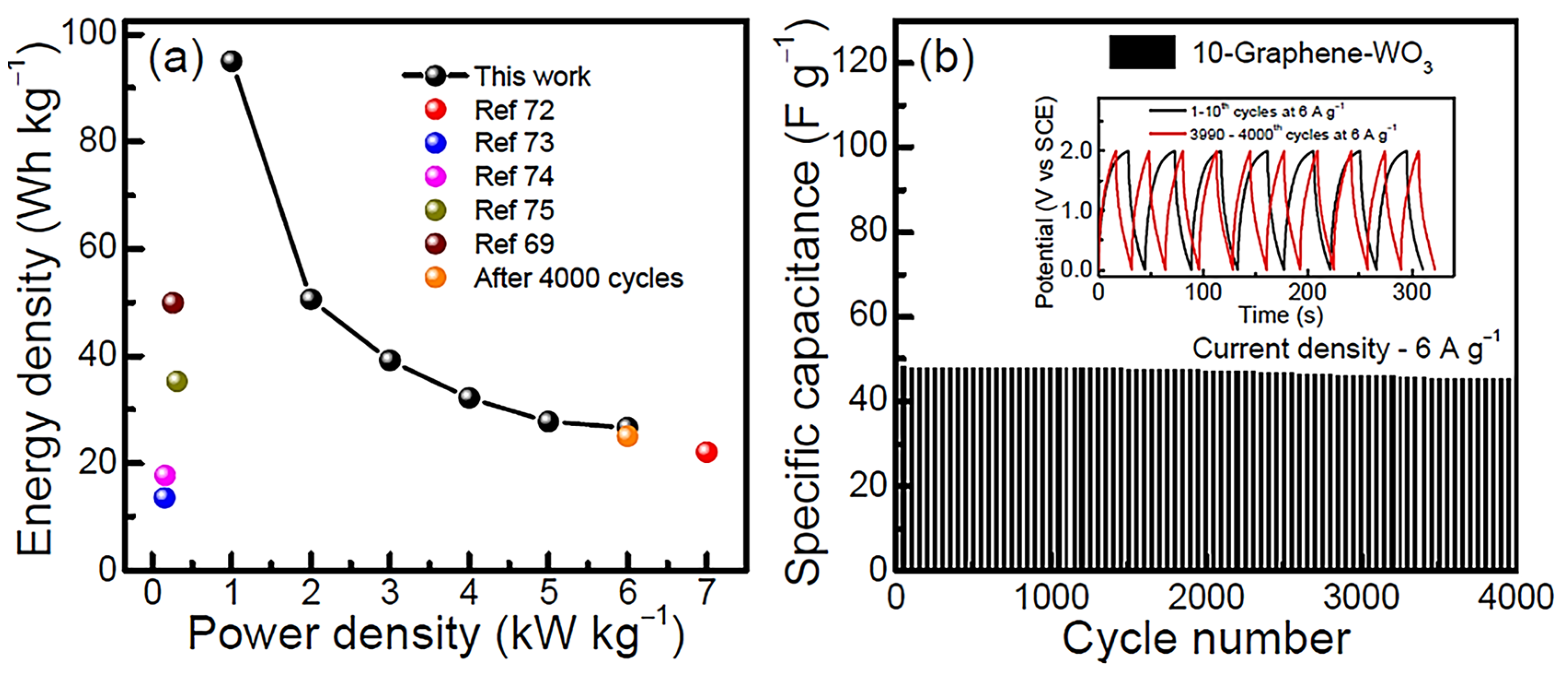
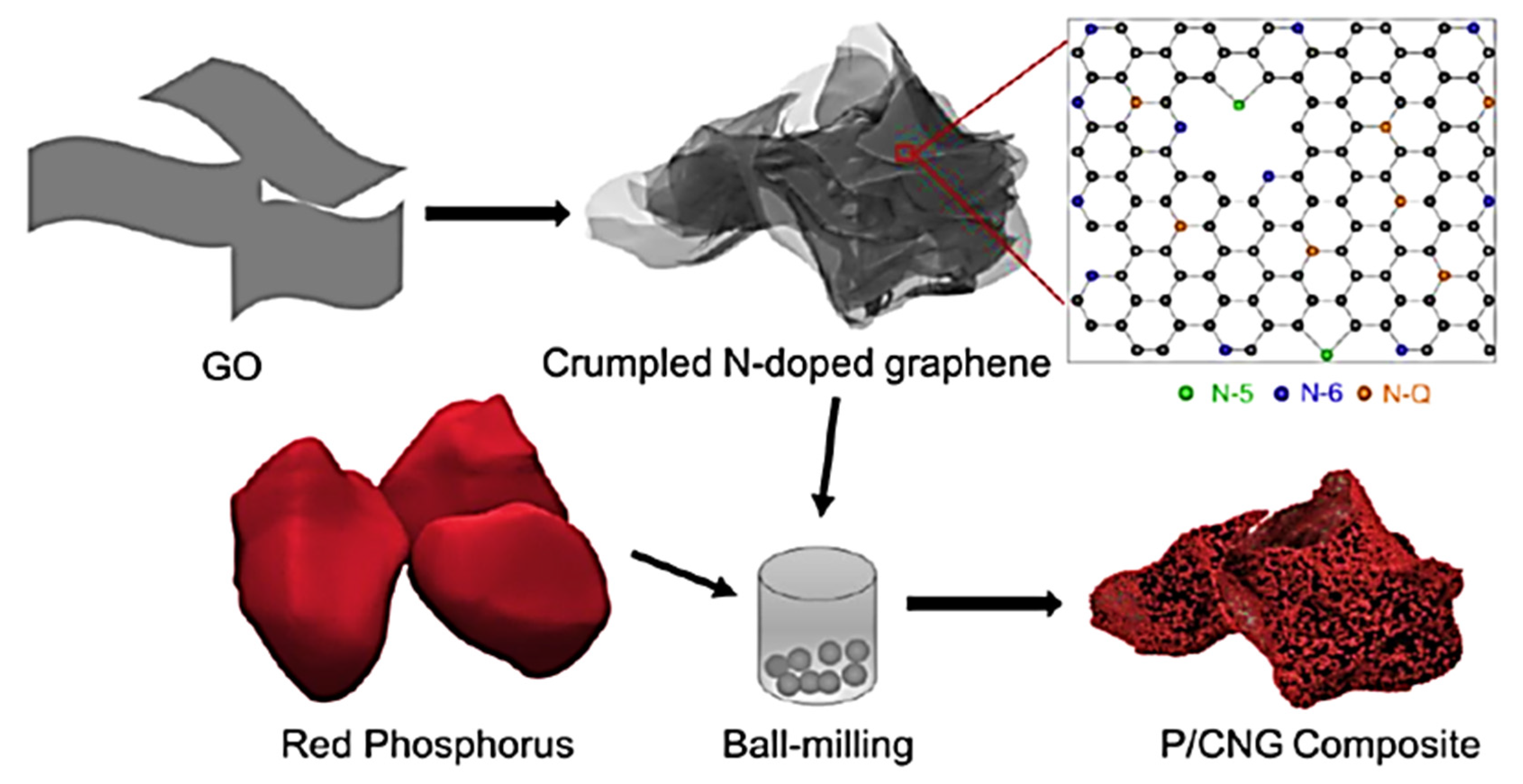


| Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| Nafion | 9.41 | 88.30 |
| Nafion/GO 0.5 | 65.16 | 31.85 |
| Nafion/GO 3.0 | 74.69 | 22.68 |
| Nafion/GO 4.5 | 79.47 | 19.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, A.; Ahmad, I.; Zhao, T.; Aldaghri, O.; Ibnaouf, K.H.; Eisa, M.H. Graphene Nanocomposites as Innovative Materials for Energy Storage and Conversion—Design and Headways. Int. J. Mol. Sci. 2023, 24, 11593. https://doi.org/10.3390/ijms241411593
Kausar A, Ahmad I, Zhao T, Aldaghri O, Ibnaouf KH, Eisa MH. Graphene Nanocomposites as Innovative Materials for Energy Storage and Conversion—Design and Headways. International Journal of Molecular Sciences. 2023; 24(14):11593. https://doi.org/10.3390/ijms241411593
Chicago/Turabian StyleKausar, Ayesha, Ishaq Ahmad, Tingkai Zhao, Osamah Aldaghri, Khalid H. Ibnaouf, and M. H. Eisa. 2023. "Graphene Nanocomposites as Innovative Materials for Energy Storage and Conversion—Design and Headways" International Journal of Molecular Sciences 24, no. 14: 11593. https://doi.org/10.3390/ijms241411593
APA StyleKausar, A., Ahmad, I., Zhao, T., Aldaghri, O., Ibnaouf, K. H., & Eisa, M. H. (2023). Graphene Nanocomposites as Innovative Materials for Energy Storage and Conversion—Design and Headways. International Journal of Molecular Sciences, 24(14), 11593. https://doi.org/10.3390/ijms241411593









