Berberine Reduces Lipid Accumulation in Obesity via Mediating Transcriptional Function of PPARδ
Abstract
:1. Introduction
2. Results
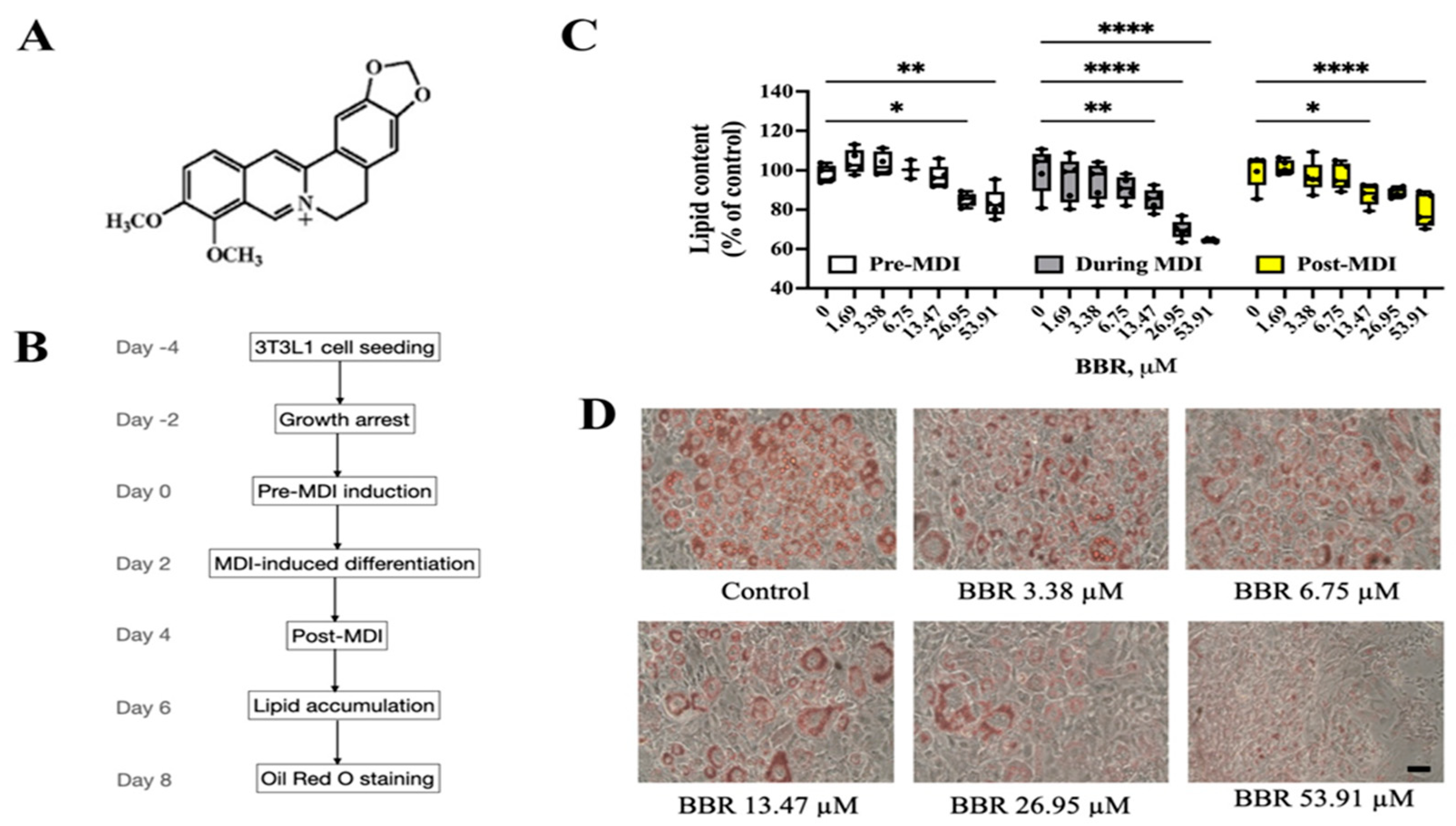
2.1. BBR Reduced Lipid Accumulation in 3T3L1 Cells
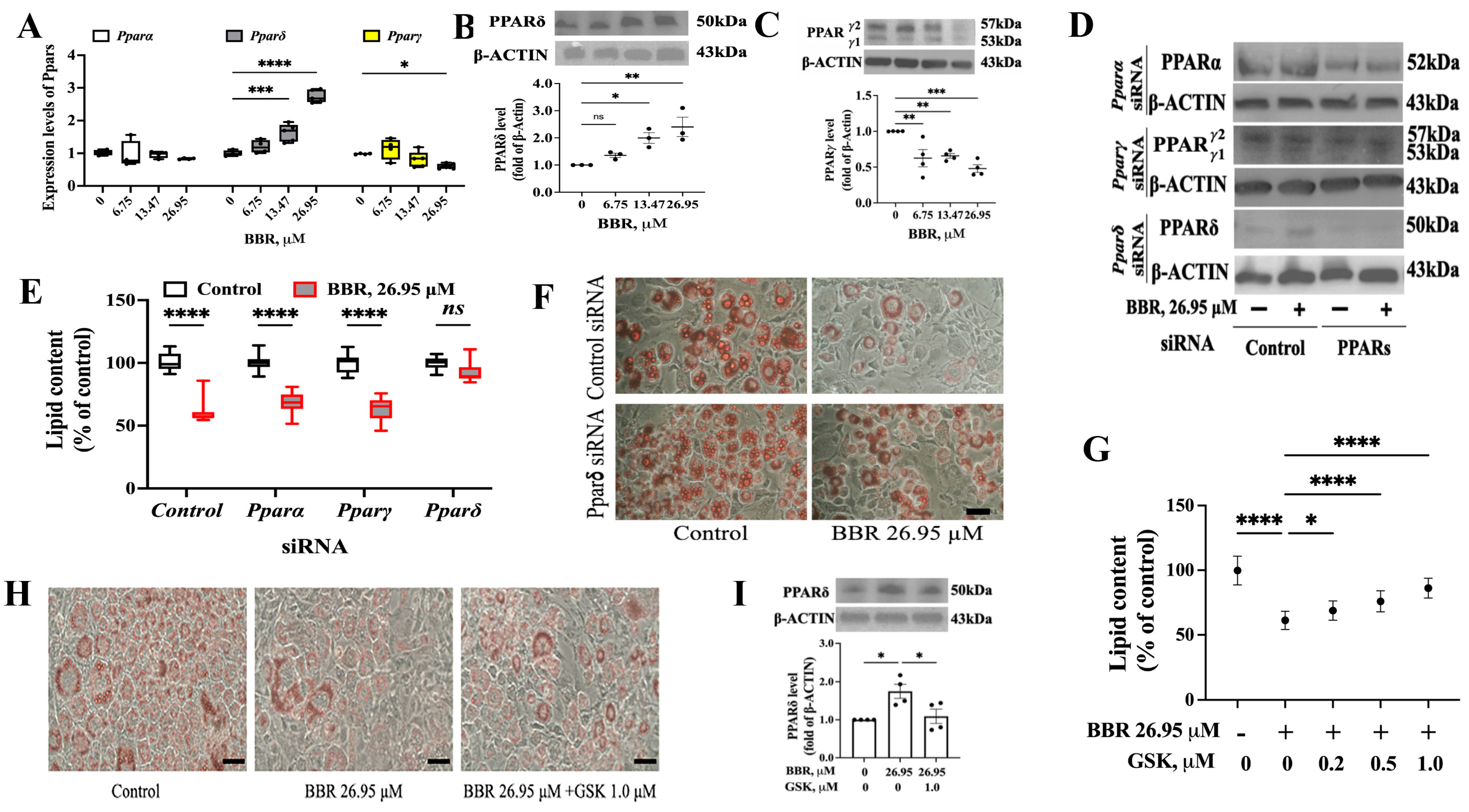
2.2. PPARδ Was Involved in the Effect of BBR on 3T3L1 Cells
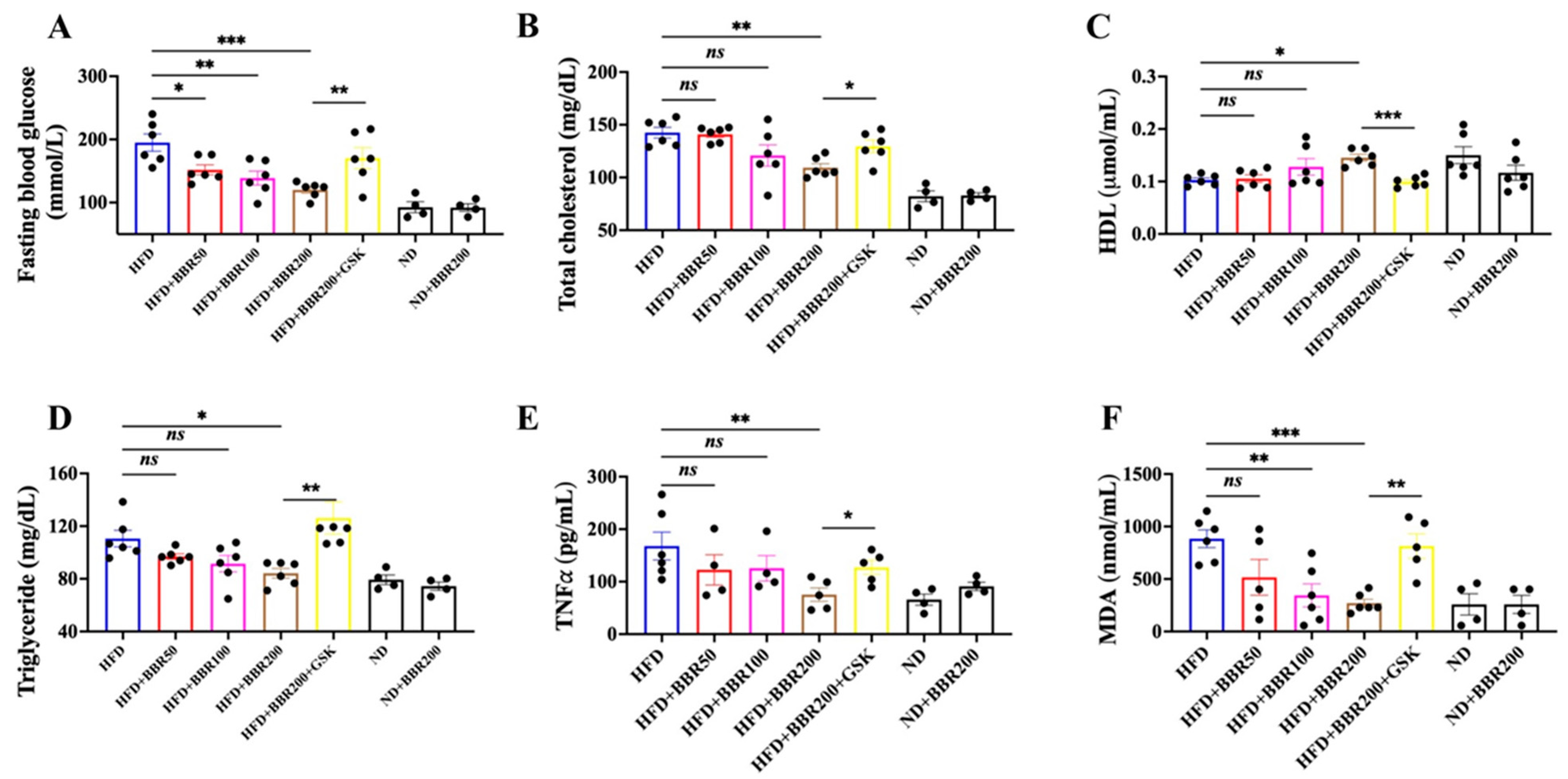
2.3. In Vivo Anti-Obesity Effects of BBR Depended on PPARδ
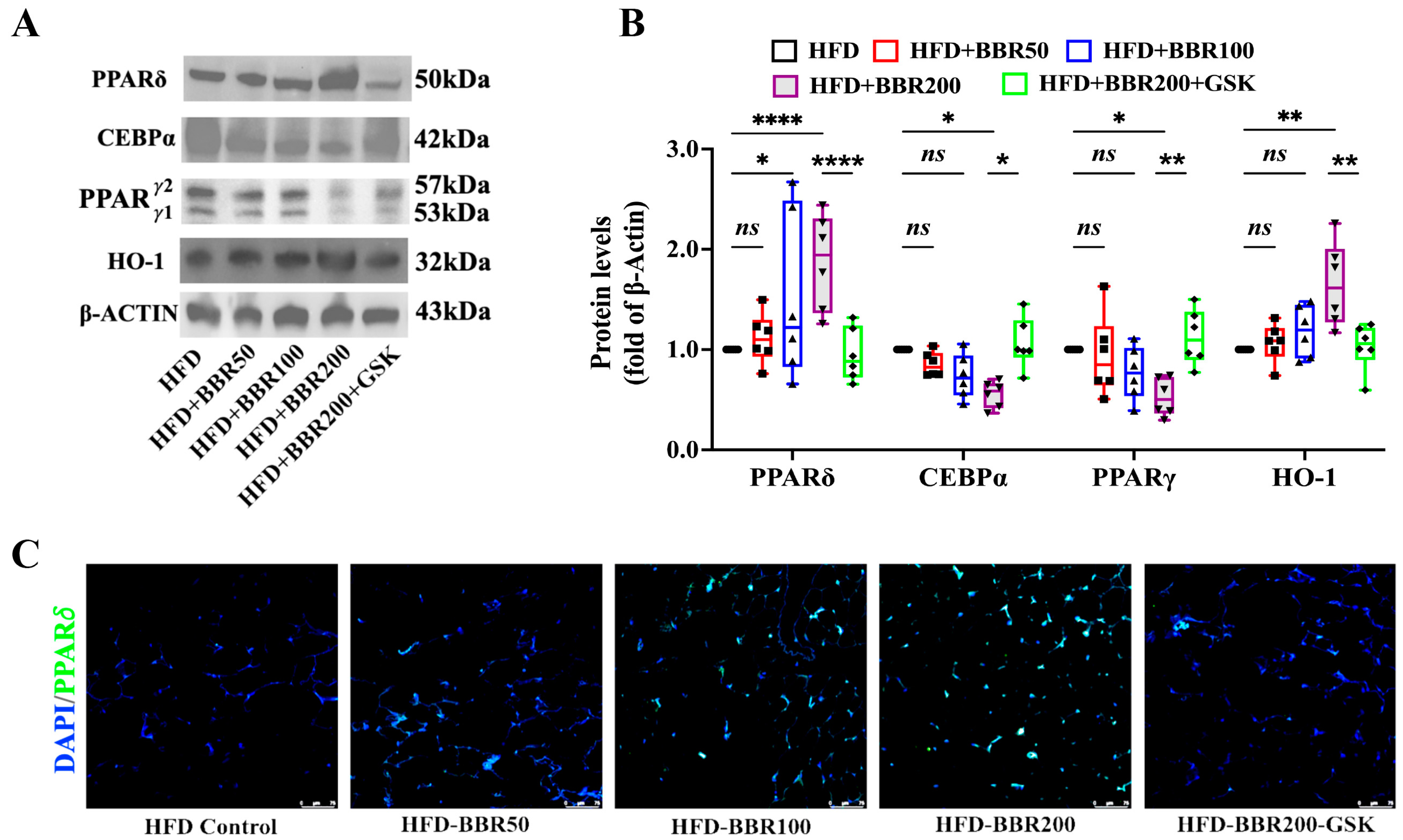
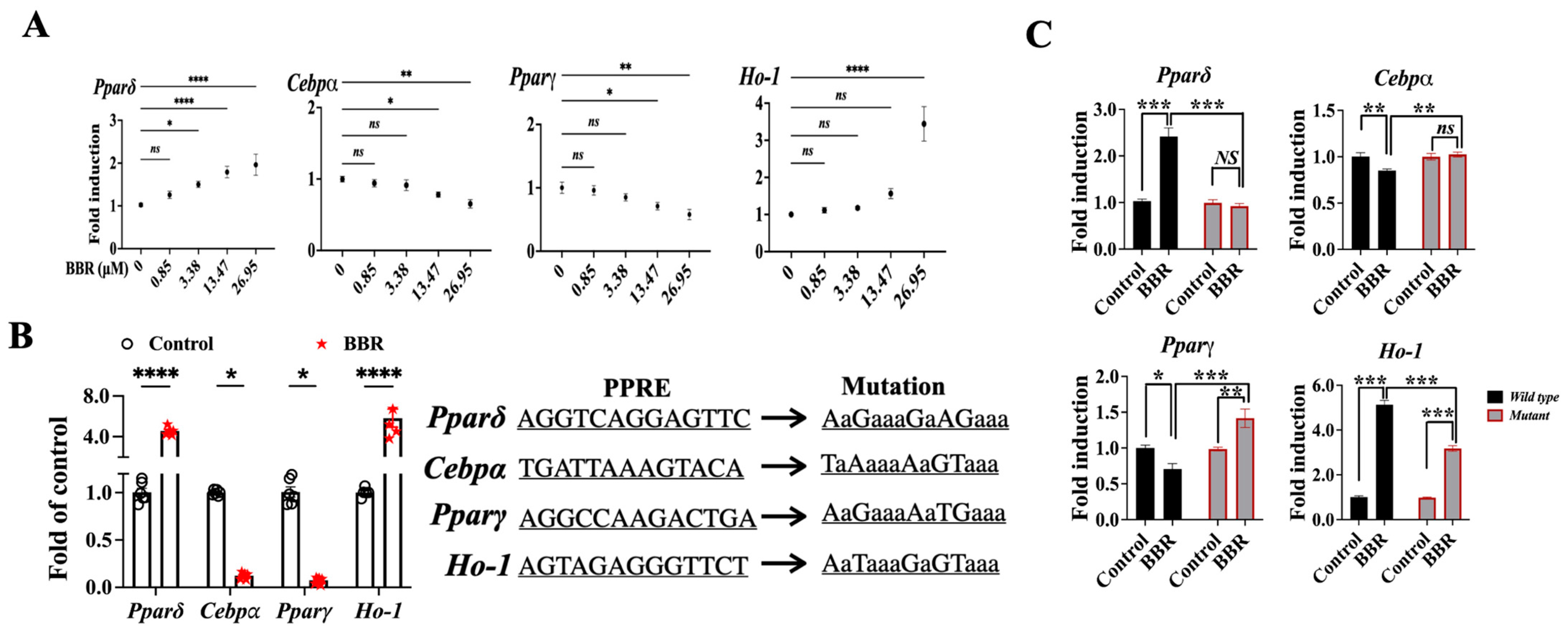
2.4. BBR Activated PPARδ to Regulate Downstream Genes
2.5. BBR Regulated Cebpα, Pparγ, and Ho-1 through Mediating the Transcriptional Function PPARδ
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Treatment
4.3. Mice Experiment
4.4. Histological Examination and Immunostaining
4.5. qPCR and WB Analysis
4.6. Luciferase Reporter Assay and Chromatin Immunoprecipitation Coupled PCR/qPCR Assay
4.7. Statistical Analysis
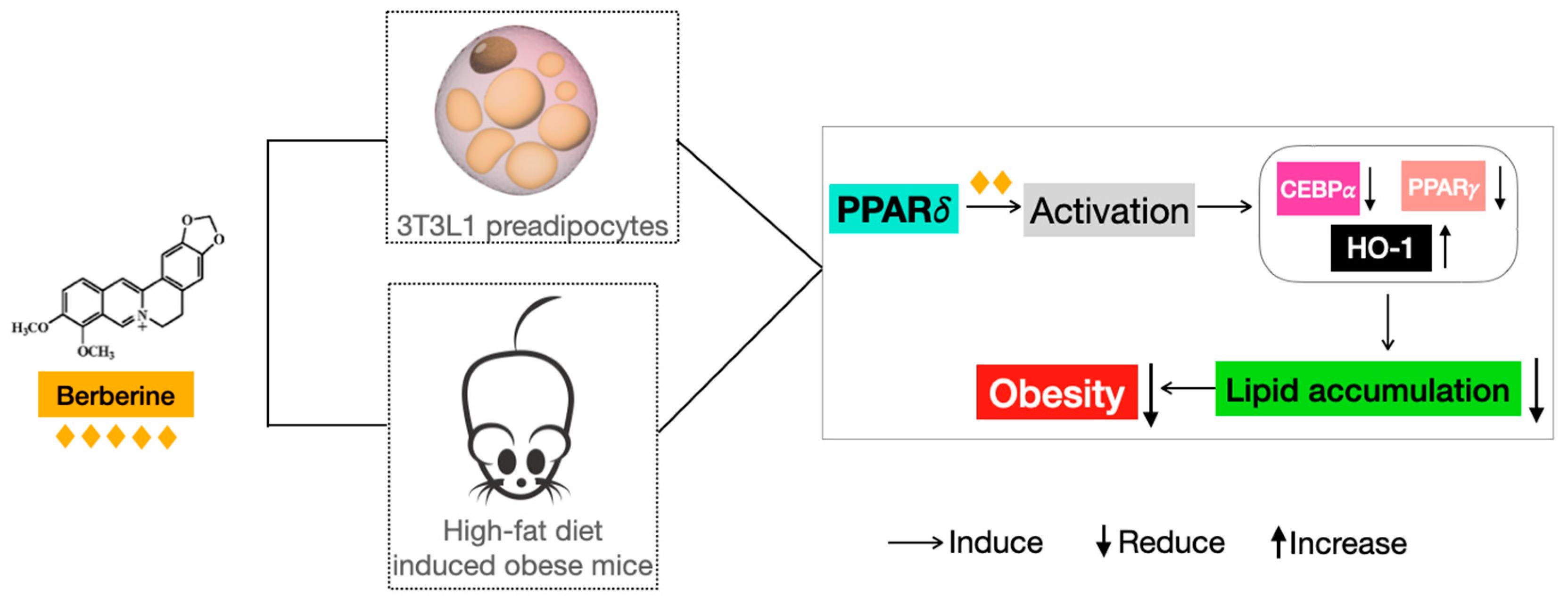
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobstein, T.; Brinsden, H.; Neveux, M. World Obesity Atlas 2022; World Obesity Federation: London, UK, 2022. [Google Scholar]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, C.; Petersen, M.B.; Sorensen, T.I.A. Will the COVID-19 pandemic worsen the obesity epidemic? Nat. Rev. Endocrinol. 2020, 16, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Du, S.F.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254266. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Tronieri, J.S.; Butryn, M.L. Lifestyle modification approaches for the treatment of obesity in adults. Am. Psychol. 2020, 75, 235–251. [Google Scholar] [CrossRef]
- Wadden, T.A.; Webb, V.L.; Moran, C.H.; Bailer, B.A. Lifestyle modification for obesity: New developments in diet, physical activity, and behavior therapy. Circulation 2012, 125, 1157–1170. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- Pilitsi, E.; Farr, O.M.; Polyzos, S.A.; Perakakis, N.; Nolen-Doerr, E.; Papathanasiou, A.-E.; Mantzoros, C.S. Pharmacotherapy of obesity: Available medications and drugs under investigation. Metabolism 2019, 92, 170–192. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef]
- Gagnon, C.; Schafer, A.L. Bone Health After Bariatric Surgery. JBMR Plus 2018, 2, 121–133. [Google Scholar] [CrossRef] [PubMed]
- van Beek, A.P.; Emous, M.; Laville, M.; Tack, J. Dumping syndrome after esophageal, gastric or bariatric surgery: Pathophysiology, diagnosis, and management. Obes. Rev. 2017, 18, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Duval, C.; Muller, M.; Kersten, S. PPARs, Obesity, and Inflammation. PPAR Res. 2007, 2007, 95974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, J.P.; Akiyama, T.E.; Meinke, P.T. PPARs: Therapeutic targets for metabolic disease. Trends Pharmacol. Sci. 2005, 26, 244–251. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as Nuclear Receptors for Nutrient and Energy Metabolism. Molecules 2019, 24, 2545. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-H.; Sheng, J.-Q.; Xie, W.-H.; Luo, X.-Q.; Xue, Y.-N.; Xu, G.-L.; Chen, C. Mechanism and Basis of Traditional Chinese Medicine Against Obesity: Prevention and Treatment Strategies. Front. Pharmacol. 2021, 12, 615895. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Lou, G.; Zeng, H.-R.; Hu, J.; Huang, Q.; Peng, W.; Yang, X.-B. Coptidis Rhizoma: A comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm. Biol. 2019, 57, 193–225. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Luo, K.; Zhang, J.M.; Ni, P.; Xiong, W.; Luo, X.; Xu, G.; Liu, H.; Zeng, Z. Additional file 1 of Obese rats intervened with Rhizoma coptidis revealed differential gene expression and microbiota by serum metabolomics. BMC Complement. Med. 2021, 21, 208. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Wang, S.; Cai, F.; Zheng, G.; Lu, A.; Yu, X.; Jiang, M. Treatment Principles of Obesity with Chinese Herbal Medicine: Literature Analysis by Text Mining. Engineering 2013, 5, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Gu, D.; Li, J.; Cui, K.; Zhang, Y. Effects and Action Mechanisms of Berberine and Rhizoma coptidis on Gut Microbes and Obesity in High-Fat Diet-Fed C57BL/6J Mice. PLoS ONE 2011, 6, e24520. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-J.; Jung, E.; Shim, I. Berberine for Appetite Suppressant and Prevention of Obesity. BioMed Res. Int. 2020, 2020, 3891806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Li, B.; Meng, X.; Wang, J.; Zhang, Y.; Yao, S.; Ma, Q.; Jin, L.; Yang, J.; et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014, 5, 5493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Davies, G.E. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia 2010, 81, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, C.; Yang, J.; Zhang, T.; Zhou, Q. Berberine is a potent agonist of peroxisome proliferator activated receptor alpha. Front. Biosci. 2016, 21, 1052–1060. [Google Scholar]
- Shou, J.W.; Li, X.X.; Tang, Y.S.; Lim-Ho Kong, B.; Wu, H.Y.; Xiao, M.J.; Cheung, C.K.; Shaw, P.C. Novel mechanistic insight on the neuroprotective effect of berberine: The role of PPARdelta for antioxidant action. Free Radic. Biol. Med. 2022, 181, 62–71. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, T.; Ma, G.; Zheng, L.; Jiang, X.; Yang, F.; Wang, Z.; Li, N.; He, Z.; Song, X.; et al. Berberine modulates deacetylation of PPARgamma to promote adipose tissue remodeling and thermogenesis via AMPK/SIRT1 pathway. Int. J. Biol. Sci. 2021, 17, 3173–3187. [Google Scholar] [CrossRef]
- Hu, Y.; Davies, G. Berberine increases expression of GATA-2 and GATA-3 during inhibition of adipocyte differentiation. Phytomedicine 2009, 16, 864–873. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Ma, S.-R.; Zuo, Z.-Y.; Wu, Y.-B.; Kong, W.-J.; Wang, A.-P.; Jiang, J.-D. Berberine inhibits adipocyte differentiation, proliferation and adiposity through down-regulating galectin-3. Sci. Rep. 2019, 9, 13415. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Tang, K.; Chen, R.; Nie, H.; Liang, S.; Zhang, J.; Zhang, Y.; Yang, Q. Berberine attenuates hepatic oxidative stress in rats with non-alcoholic fatty liver disease via the Nrf2/ARE signalling pathway. Exp. Ther. Med. 2019, 17, 2091–2098. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Zhang, Y.; Gong, Z.; Sheng, X.; Li, Z.; Zhang, W.; Qin, Y. Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARgamma pathway. Biochem. Biophys. Res. Commun. 2006, 348, 571–578. [Google Scholar] [CrossRef]
- Sun, S.; Yang, Y.; Xiong, R.; Ni, Y.; Ma, X.; Hou, M.; Chen, L.; Xu, Z.; Chen, L.; Ji, M. Oral berberine ameliorates high-fat diet-induced obesity by activating TAS2Rs in tuft and endocrine cells in the gut. Life Sci. 2022, 311 Pt A, 121141. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Xu, J.; Xue, Z.; Zhang, M.; Pang, X.; Zhang, X.; Zhao, L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci. Rep. 2015, 5, 14405. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.H.; Liu, X.Z.; Pan, W.; Zou, D.J. Berberine protects against diet-induced obesity through regulating metabolic endotoxemia and gut hormone levels. Mol. Med. Rep. 2017, 15, 2765–2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, R.M.; Barish, G.D.; Wang, Y.-X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Zhou, S.W.; Bin Zhang, K.; Tang, J.L.; Guang, L.X.; Ying, Y.; Xu, Y.; Zhang, L.; Li, D.D. Chronic Effects of Berberine on Blood, Liver Glucolipid Metabolism and Liver PPARs Expression in Diabetic Hyperlipidemic Rats. Biol. Pharm. Bull. 2008, 31, 1169–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhou, S. Berberine regulates peroxisome proliferator-activated receptors and positive transcription elongation factor b expression in diabetic adipocytes. Eur. J. Pharmacol. 2010, 649, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.W.; Shaw, P.C. Berberine activates PPARdelta and promotes gut microbiota-derived butyric acid to suppress hepatocellular carcinoma. Phytomedicine 2023, 115, 154842. [Google Scholar] [CrossRef] [PubMed]
- Dickey, A.S.; Sanchez, D.N.; Arreola, M.; Sampat, K.R.; Fan, W.; Arbez, N.; Akimov, S.; Van Kanegan, M.J.; Ohnishi, K.; Gilmore-Hall, S.K.; et al. PPARdelta activation by bexarotene promotes neuroprotection by restoring bioenergetic and quality control homeostasis. Sci. Transl. Med. 2017, 9, eaal2332. [Google Scholar] [CrossRef] [Green Version]
- Adhikary, T.; Wortmann, A.; Schumann, T.; Finkernagel, F.; Lieber, S.; Roth, K.; Toth, P.M.; Diederich, W.E.; Nist, A.; Stiewe, T.; et al. The transcriptional PPARbeta/delta network in human macrophages defines a unique agonist-induced activation state. Nucleic Acids Res. 2015, 43, 5033–5051. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Tang, H.; Deng, R.; Wang, N.; Zhang, Y.; Wang, Y.; Liu, Y.; Li, F.; Wang, X.; Zhou, L. Berberine Suppresses Adipocyte Differentiation via Decreasing CREB Transcriptional Activity. PLoS ONE 2015, 10, e0125667. [Google Scholar] [CrossRef]
- Jang, J.; Jung, Y.; Seo, S.J.; Kim, S.-M.; Shim, Y.J.; Cho, S.H.; Chung, S.-I.; Yoon, Y. Berberine activates AMPK to suppress proteolytic processing, nuclear translocation and target DNA binding of SREBP-1c in 3T3-L1 adipocytes. Mol. Med. Rep. 2017, 15, 4139–4147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Wang, X.; Yang, Y.; Wu, L.; Li, F.; Zhang, R.; Yuan, G.; Wang, N.; Chen, M.; Ning, G. Berberine attenuates cAMP-induced lipolysis via reducing the inhibition of phosphodiesterase in 3T3-L1 adipocytes. Biochim. Et Biophys. Acta 2011, 1812, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhao, Y.; Zhu, H.; Liang, N.; Ma, K.Y.; Lei, L.; He, W.-S.; et al. Beneficial effects of tea water extracts on the body weight and gut microbiota in C57BL/6J mice fed with a high-fat diet. Food Funct. 2019, 10, 2847–2860. [Google Scholar] [CrossRef]
- Shou, J.-W.; Cheung, C.-K.; Gao, J.; Shi, W.-W.; Shaw, P.-C. Berberine Protects C17.2 Neural Stem Cells From Oxidative Damage Followed by Inducing Neuronal Differentiation. Front. Cell. Neurosci. 2019, 13, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Groups | Treatment Information |
|---|---|
| ND | Normal C57BL/6J mice + normal diet+ vehicle |
| ND + BBR200 | Normal C57BL/6J mice + BBR 200 mg/kg/day, i.g. |
| HFD | Obese C57BL/6J mice + HFD + vehicle |
| HFD + BBR50 | Obese C57BL/6J mice + HFD + BBR 50 mg/kg/day, i.g. |
| HFD + BBR100 | Obese C57BL/6J mice + HFD + BBR 100 mg/kg/day, i.g. |
| HFD + BBR200 | Obese C57BL/6J mice + HFD + BBR 200 mg/kg/day, i.g. |
| HFD + BBR20 + GSK | Obese C57BL/6J mice + HFD + BBR 200 mg/kg/day, i.g. + GSK 1 mg/kg/day, i.p. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shou, J.-W.; Shaw, P.-C. Berberine Reduces Lipid Accumulation in Obesity via Mediating Transcriptional Function of PPARδ. Int. J. Mol. Sci. 2023, 24, 11600. https://doi.org/10.3390/ijms241411600
Shou J-W, Shaw P-C. Berberine Reduces Lipid Accumulation in Obesity via Mediating Transcriptional Function of PPARδ. International Journal of Molecular Sciences. 2023; 24(14):11600. https://doi.org/10.3390/ijms241411600
Chicago/Turabian StyleShou, Jia-Wen, and Pang-Chui Shaw. 2023. "Berberine Reduces Lipid Accumulation in Obesity via Mediating Transcriptional Function of PPARδ" International Journal of Molecular Sciences 24, no. 14: 11600. https://doi.org/10.3390/ijms241411600






