GSK3β Inhibition Ameliorates Atherosclerotic Calcification
Abstract
:1. Introduction
2. Results
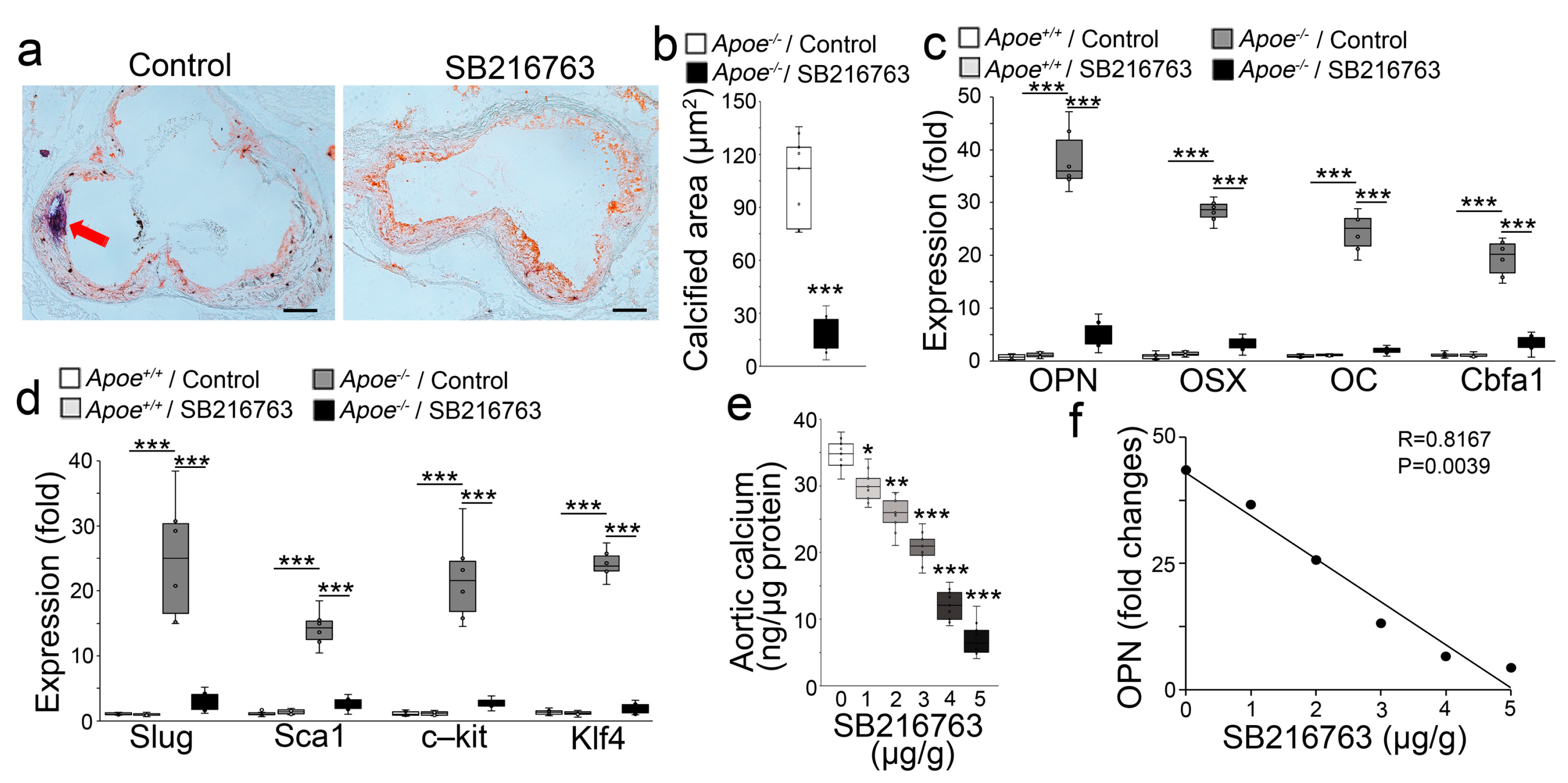
2.1. GSK3β Inhibition Reduced the Calcification of Atherosclerotic Lesions
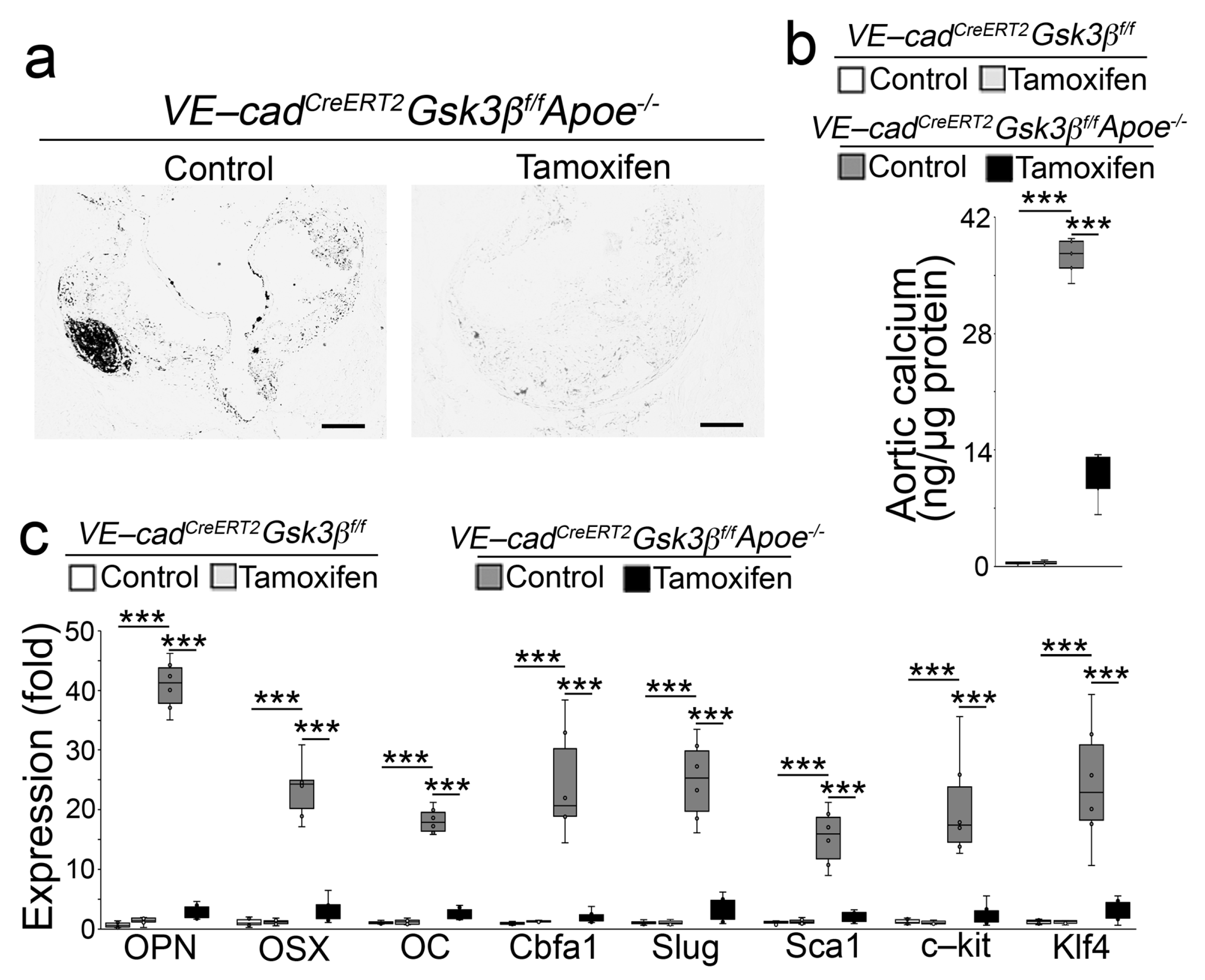
2.2. Endothelial Specifc GSK3β Deletion Limited the Calcification of Atherosclerotic Lesions
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. RNA Analysis
4.3. Immunoblotting
4.4. Quantification of Aortic Calcium
4.5. Lesion Quantification
4.6. Oil Red O Staining
4.7. Von Kossa Staining
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sage, A.P.; Tintut, Y.; Demer, L.L. Regulatory mechanisms in vascular calcification. Nat. Rev. Cardiol. 2010, 7, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Shaw, L.J.; Liu, S.T.; Weinstein, S.R.; Mosler, T.P.; Tseng, P.H.; Flores, F.R.; Callister, T.Q.; Raggi, P.; Berman, D.S. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J. Am. Coll. Cardiol. 2007, 49, 1860–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Li, K.; Wei, W.; Ding, S. Chemical approaches to stem cell biology and therapeutics. Cell Stem Cell 2013, 13, 270–283. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Jumabay, M.; Ly, A.; Radparvar, M.; Cubberly, M.R.; Bostrom, K.I. A role for the endothelium in vascular calcification. Circ. Res. 2013, 113, 495–504. [Google Scholar] [CrossRef]
- Medici, D.; Shore, E.M.; Lounev, V.Y.; Kaplan, F.S.; Kalluri, R.; Olsen, B.R. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 2010, 16, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, K.I.; Yao, J.; Guihard, P.J.; Blazquez-Medela, A.M.; Yao, Y. Endothelial-mesenchymal transition in atherosclerotic lesion calcification. Atherosclerosis 2016, 253, 124–127. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Bennett, B.J.; Wang, X.; Rosenfeld, M.E.; Giachelli, C.; Lusis, A.J.; Bostrom, K.I. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ. Res. 2010, 107, 485–494. [Google Scholar] [CrossRef]
- Han, J.K.; Chang, S.H.; Cho, H.J.; Choi, S.B.; Ahn, H.S.; Lee, J.; Jeong, H.; Youn, S.W.; Lee, H.J.; Kwon, Y.W.; et al. Direct conversion of adult skin fibroblasts to endothelial cells by defined factors. Circulation 2014, 130, 1168–1178. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Wu, X.; Qiao, X.; Zhang, D.; Zhang, L.; Ma, J.A.; Cai, X.; Bostrom, K.I.; Yao, Y. Shifting osteogenesis in vascular calcification. JCI Insight 2021, 6, e143023. [Google Scholar] [CrossRef]
- Coghlan, M.P.; Culbert, A.A.; Cross, D.A.; Corcoran, S.L.; Yates, J.W.; Pearce, N.J.; Rausch, O.L.; Murphy, G.J.; Carter, P.S.; Roxbee Cox, L.; et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 2000, 7, 793–803. [Google Scholar] [CrossRef] [Green Version]
- Bostrom, K.I.; Qiao, X.; Zhao, Y.; Wu, X.; Zhang, L.; Ma, J.A.; Ji, J.; Cai, X.; Yao, Y. GSK3beta Inhibition Reduced Vascular Calcification in Ins2(Akita/+) Mice. Int. J. Mol. Sci. 2023, 24, 5971. [Google Scholar] [CrossRef]
- Yao, J.; Guihard, P.J.; Blazquez-Medela, A.M.; Guo, Y.; Moon, J.H.; Jumabay, M.; Bostrom, K.I.; Yao, Y. Serine Protease Activation Essential for Endothelial-Mesenchymal Transition in Vascular Calcification. Circ. Res. 2015, 117, 758–769. [Google Scholar] [CrossRef]
- Rayasam, G.V.; Tulasi, V.K.; Sodhi, R.; Davis, J.A.; Ray, A. Glycogen synthase kinase 3: More than a namesake. Br. J. Pharmacol. 2009, 156, 885–898. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.E.; Ko, R.; Jung, H.J.; Lee, S.Y. Glycogen synthase kinase 3beta promotes osteogenic differentiation of murine adipose-derived stromal cells. PLoS ONE 2013, 8, e54551. [Google Scholar] [CrossRef]
- Kitajima, K.; Nakajima, M.; Kanokoda, M.; Kyba, M.; Dandapat, A.; Tolar, J.; Saito, M.K.; Toyoda, M.; Umezawa, A.; Hara, T. GSK3beta inhibition activates the CDX/HOX pathway and promotes hemogenic endothelial progenitor differentiation from human pluripotent stem cells. Exp. Hematol. 2016, 44, 68–74.e10. [Google Scholar] [CrossRef] [Green Version]
- Yamamizu, K.; Matsunaga, T.; Uosaki, H.; Fukushima, H.; Katayama, S.; Hiraoka-Kanie, M.; Mitani, K.; Yamashita, J.K. Convergence of Notch and beta-catenin signaling induces arterial fate in vascular progenitors. J. Cell Biol. 2010, 189, 325–338. [Google Scholar] [CrossRef]
- Szabo-Rogers, H.; Yakob, W.; Liu, K.J. Frontal Bone Insufficiency in Gsk3beta Mutant Mice. PLoS ONE 2016, 11, e0149604. [Google Scholar] [CrossRef] [Green Version]
- Wrana, J.L. Crossing Smads. Sci. STKE 2000, 2000, re1. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef]
- Ju, W.; Hoffmann, A.; Verschueren, K.; Tylzanowski, P.; Kaps, C.; Gross, G.; Huylebroeck, D. The bone morphogenetic protein 2 signaling mediator Smad1 participates predominantly in osteogenic and not in chondrogenic differentiation in mesenchymal progenitors C3H10T1/2. J. Bone Miner. Res. 2000, 15, 1889–1899. [Google Scholar] [CrossRef]
- Luzi, E.; Marini, F.; Sala, S.C.; Tognarini, I.; Galli, G.; Brandi, M.L. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J. Bone Miner. Res. 2008, 23, 287–295. [Google Scholar] [CrossRef]
- Saijilafu; Hur, E.M.; Liu, C.M.; Jiao, Z.; Xu, W.L.; Zhou, F.Q. PI3K-GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nat. Commun. 2013, 4, 2690. [Google Scholar] [CrossRef] [Green Version]
- Corada, M.; Nyqvist, D.; Orsenigo, F.; Caprini, A.; Giampietro, C.; Taketo, M.M.; Iruela-Arispe, M.L.; Adams, R.H.; Dejana, E. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev. Cell 2010, 18, 938–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Pan, W. GSK3: A multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 2010, 35, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Alva, J.A.; Zovein, A.C.; Monvoisin, A.; Murphy, T.; Salazar, A.; Harvey, N.L.; Carmeliet, P.; Iruela-Arispe, M.L. VE-Cadherin-Cre-recombinase transgenic mouse: A tool for lineage analysis and gene deletion in endothelial cells. Dev. Dyn. 2006, 235, 759–767. [Google Scholar] [CrossRef]
- Stoller, J.K.; Aboussouan, L.S. Alpha1-antitrypsin deficiency. Lancet 2005, 365, 2225–2236. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, X.; Zhao, Y.; Yang, Y.; Wu, X.; Zhang, L.; Ma, J.A.; Ji, J.; Boström, K.I.; Yao, Y. GSK3β Inhibition Ameliorates Atherosclerotic Calcification. Int. J. Mol. Sci. 2023, 24, 11638. https://doi.org/10.3390/ijms241411638
Cai X, Zhao Y, Yang Y, Wu X, Zhang L, Ma JA, Ji J, Boström KI, Yao Y. GSK3β Inhibition Ameliorates Atherosclerotic Calcification. International Journal of Molecular Sciences. 2023; 24(14):11638. https://doi.org/10.3390/ijms241411638
Chicago/Turabian StyleCai, Xinjiang, Yan Zhao, Yang Yang, Xiuju Wu, Li Zhang, Jocelyn A. Ma, Jaden Ji, Kristina I. Boström, and Yucheng Yao. 2023. "GSK3β Inhibition Ameliorates Atherosclerotic Calcification" International Journal of Molecular Sciences 24, no. 14: 11638. https://doi.org/10.3390/ijms241411638




