Icerya purchasi Maskell (Hemiptera: Monophlebidae) Control Using Low Carbon Footprint Oligonucleotide Insecticides
Abstract
:1. Introduction
2. Results
2.1. Search for the oligoICER-11 Target mRNA
2.2. Mortality of I. purhcasi after Treatment with oligoICER-11 in the Natural Habitat
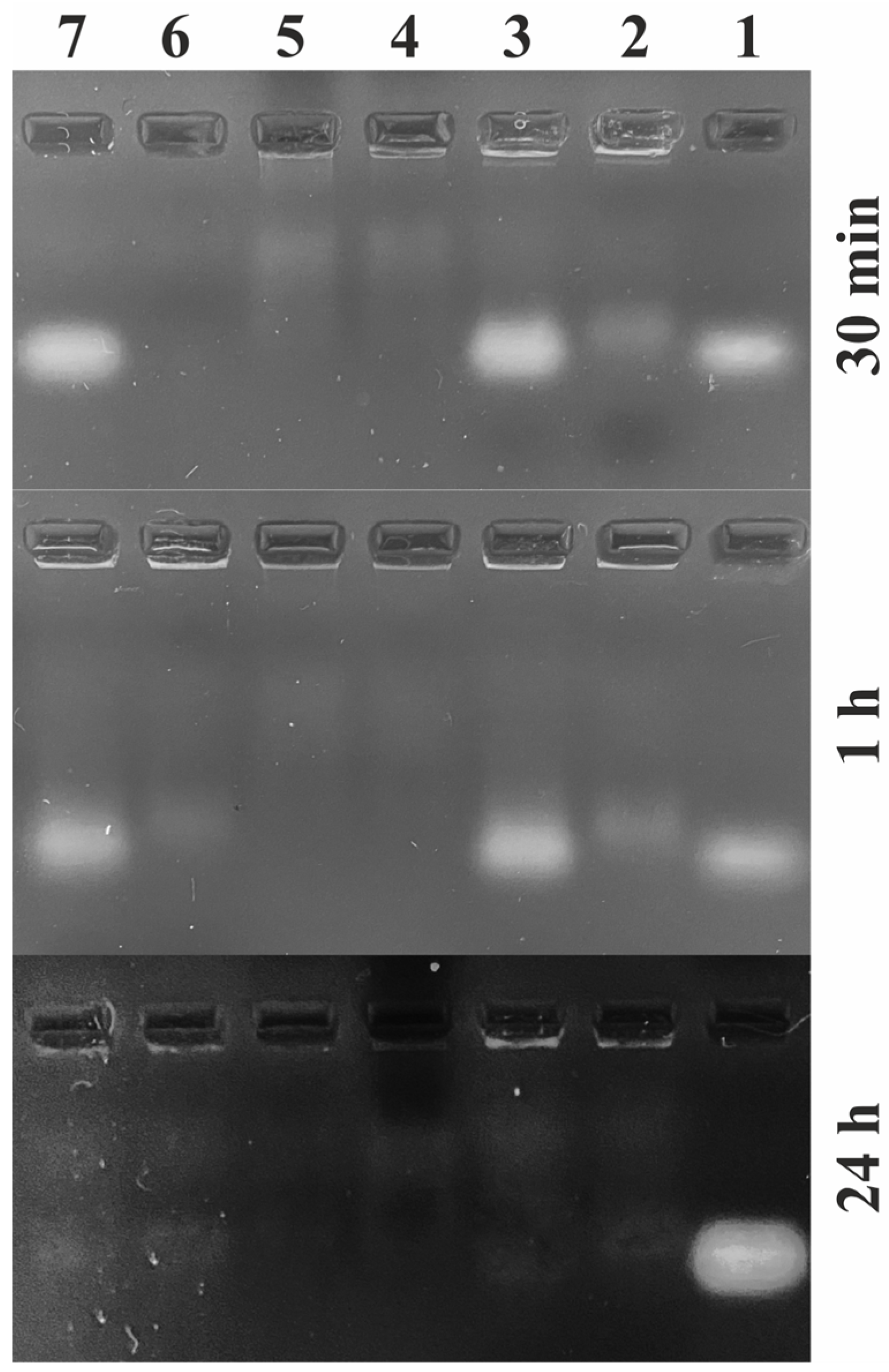
2.3. Biodegradability of Oligonucleotides with the Participation of Tissue Deoxyribonucleases
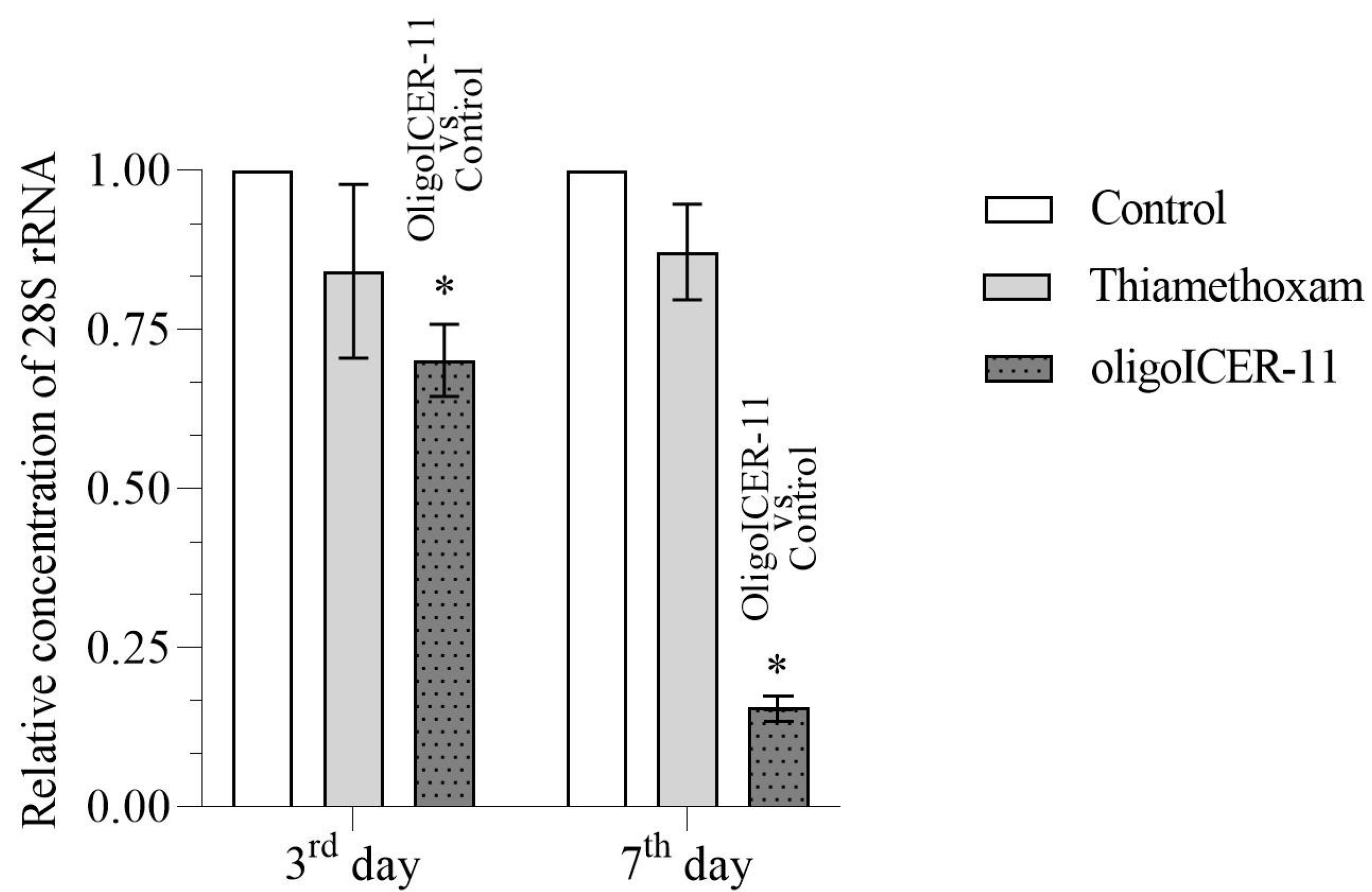
2.4. The oligoICER-11 Olinscide Significantly Decreases the Concentration of the 28S rRNA in I. purchasi Larvae
3. Materials and Methods
3.1. Origin of I. purchasi
3.2. Applied DNA Sequence (Olinscide oligoICER-11)
3.3. Neonicotinoid Insecticide Treatment
3.4. Assessment of the Quality of Oligonucleotide Synthesis by the MALDI-TOF Method
3.5. Search for I. purchasi 28S rRNA Fragment, Complementary of the oligoICER-11 Sequence
3.6. Quantification of I. purchasi 28S rRNA Gene Concentration
3.7. DNA Nuclease Activity Analyses
3.8. Statistical Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biber-Freudenberger, L.; Ziemacki, J.; Tonnang, H.E.; Borgemeister, C. Future Risks of Pest Species under Changing Climatic Conditions. PLoS ONE 2016, 11, e0153237. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. A comparison of absolute performance of different correlative and mechanistic species distribution models in an independent area. Ecol. Evol. 2016, 6, 5973–5986. [Google Scholar] [CrossRef] [Green Version]
- Pareek, A.; Meena, B.M.; Sharma, S.; Teterwal, M.L.; Kalyan, R.K.; Meena, B.L. Impact of climate change on insect pests and their management strategies. In Climate Change and Sustainable Agriculture; Kumar, P.S., Kanwat, M., Meena, P.D., Kumar, V., Alone, R.A., Eds.; New India Publishing Agency: New Delhi, India, 2017; pp. 253–286. [Google Scholar]
- Wells, C.N.; Tonkyn, D. Changes in the Geographic Distribution of the Diana Fritillary (Speyeria diana: Nymphalidae) under Forecasted Predictions of Climate Change. Insects 2018, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocmánková, E.; Trnka, M.; Juroch, J.; Dubrovský, M.; Semerádová, D.; Mozny, M.; Žalud, Z. Impact of climate change on the occurrence and activity of harmful organisms. Plant. Prot. Sci. 2010, 45, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Fand, B.B.; Kamble, A.L.; Kumar, M. Will climate change pose serious threat to crop pest management: A critical review? Int. J. Sci. Res. Publ. 2012, 2, 1–14. [Google Scholar]
- Winter, M.; Schweiger, O.; Klotz, S.; Nentwig, W.; Andriopoulos, P.; Arianoutsou, M.; Basnou, C.; Delipetrou, P.; Didžiulis, V.; Hejda, M.; et al. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl. Acad. Sci. USA 2009, 106, 21721–21725. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef] [Green Version]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
- Seebens, H.; Essl, F.; Dawson, W.; Fuentes, N.; Moser, D.; Pergl, J.; Pyšek, P.; Kleunen, M.; Weber, E.; Winter, M.; et al. Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Chang. Biol. 2015, 21, 4128–4140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.J.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef] [Green Version]
- Heimpel, G.E.; Yang, Y.; Hill, J.D.; Ragsdale, D.W. Environmental consequences of invasive species: Greenhouse gas emissions of insecticide use and the role of biological control in reducing emissions. PLoS ONE 2013, 8, e72293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaliappan, S.B.; Gunasekaran, Y.; Smyrna, R.; Meena, R.S. Soil and Environmental Management. In Sustainable Management of Soil and Environment; Meena, R., Kumar, S., Bohra, J., Jat, M., Eds.; Springer: Singapore, 2019; pp. 1–28. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–28. [Google Scholar] [CrossRef]
- Kollar, J.; Bakay, L.; Pastor, M. First record of cottony cushion scale Icerya purchasi (Hemiptera, Monophlebidae) in Slovakia. Plant. Prot. Sci. 2016, 52, 217–219. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shi, J. Predicting the Potential Global Geographical Distribution of Two Icerya Species under Climate Change. Forests 2020, 11, 684. [Google Scholar] [CrossRef]
- Rubiales, D.; Fernández-Aparicio, M. First report of cottony-cushion scale (Icerya purchasi) on red berried mistletoe (Viscum cruciatum). Entomol. Res. 2009, 39, 95–96. [Google Scholar] [CrossRef]
- García Morales, M.; Denno, B.D.; Miller, D.R.; Miller, G.L.; Ben-Dov, Y.; Hardy, N. ScaleNet: A literature-based model of scale insect biology and systematics. Database 2016, 2016, bav118. [Google Scholar] [CrossRef]
- Zhao, G.S.; Zhou, W.C. Exotic pests and diseases of plants in Taiwan. J. Taiwan Agric. Res. 1994, 2, 25–27. [Google Scholar]
- Tang, F.T.; Hao, J.J. The Margarodidae and Others of China; Chinese Agricultural Science and Technology: Beijing, China, 1995; p. 157. [Google Scholar]
- Calderón Alvarez, C.; Causton, C.E.; Hoddle, M.S.; Van Driesche, R.G.; Stanek, E.J. Monitoring the effects of Rodolia cardinalis on Icerya purchasi populations on the Galapagos Islands. BioControl 2012, 57, 167–179. [Google Scholar] [CrossRef]
- Roque-Albelo, L. Population decline of Galápagos endemic Lepidoptera on Volcán Alcedo (Isabela Island, Galápagos Islands, Ecuador): An effect of the introduction of the cottony cushion scale? Bull. Inst. R. Sci. Nat. Belg. Entomol. 2003, 73, 177–180. [Google Scholar]
- Hoddle, M.; Ramirez, C.; Hoddle, C.; Loayza, C.; Lincango, M.P.; Driesche, R.; Causton, C. Post release evaluation of Rodolia cardinalis (Coleoptera: Coccinellidae) for control of Icerya purchasi (Hemiptera: Monophlebidae) in the Galapagos Islands. Biol. Control. 2013, 67, 262–274. [Google Scholar] [CrossRef]
- Ak, K.; Akça, I.; Saruhan, I. Biological control of Icerya purchasi Maskell (Hemiptera: Margarodidae) with Rodolia cardinalis Mulsant (Coleoptera: Coccinellidae) in a Cherry Laurel Orchard. Int. J. Agric. Innov. Res. 2017, 5, 1077–1080. [Google Scholar]
- Tozlu, E.; Tekiner, N.; Tozlu, G.; Kotan, R.; Çalmaşur, Ö.; Gokturk, T.; Dadaşoğlu, F. The Investigation of the Biological Control of Icerya purchasi Maskell, 1878 (Hemiptera: Margarodidae) with Entomopathogenic Fungi and Bacteria. Alinteri J. Agric. Sci. 2020, 35, 50–56. [Google Scholar] [CrossRef]
- Urbaneja, A.; Grout, T.G.; Gravena, S.; Wu, F.; Cen, Y.; Stansly, P. Citrus pests in a global world. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 333–348. [Google Scholar] [CrossRef]
- Carruthers, R.I.; Hural, K. Fungi as naturally occurring entomopathogens. Symp. Mol. Cell. Biol. 1990, 112, 115–138. [Google Scholar]
- Inglis, G.D.; Goettel, M.S.; Butt, T.M.; Strasser, H. Use of hyphomycetous fungi for managing insect pests. In Fungi as Biocontrol Agents: Progress Problems and Potential; Butt, T.M., Jackson, C., Magan, N., Eds.; CABI Publishing: Wallingford, UK, 2001; pp. 23–69. [Google Scholar]
- Bhattarai, S.S.; Koirala Bishwokarma, S.; Gurung, S.; Dhami, P.; Bishwokarma, Y. Efficacy of entomopathogens for control of blue pumpkin beetle (Aulacophora nigripennis Motschulsky, 1857) in sponge gourd (Luffa cylindrica) under laboratory condition at Paklihawa, Nepal. Glob. J. Biol. Agric. Health Sci. 2016, 5, 102–105. [Google Scholar]
- Oberemok, V.V.; Laikova, K.V.; Repetskaya, A.I.; Kenyo, I.M.; Gorlov, M.V.; Kasich, I.N.; Krasnodubets, A.M.; Gal’chinsky, N.; Fomochkina, I.; Zaitsev, A.S.; et al. A Half-Century History of Applications of Antisense Oligonucleotides in Medicine, Agriculture and Forestry: We Should Continue the Journey. Molecules 2018, 23, 1302. [Google Scholar] [CrossRef] [Green Version]
- Oberemok, V.V.; Gal’chinsky, N.V.; Useinov, R.Z.; Novikov, I.A.; Puzanova, Y.V.; Filatov, R.I.; Kouakou, N.J.; Kouame, K.F.; Kra, K.D.; Laikova, K.V. Four Most Pathogenic Superfamilies of Insect Pests of Suborder Sternorrhyncha: Invisible Superplunderers of Plant Vitality. Insects 2023, 14, 462. [Google Scholar] [CrossRef]
- Seifer, G.; Rapold, T.; Gisin, V. Process for Preparing Thiamethoxam. CZ Patent 300093B6, 28 January 2009. [Google Scholar]
- Gal’chinsky, N.; Useinov, R.; Yatskova, E.; Laikova, K.; Novikov, I.; Gorlov, M.; Trikoz, N.; Sharmagiy, A.; Plugatar, Y.; Oberemok, V. A breakthrough in the efficiency of contact DNA insecticides: Rapid high mortality rates in the sap-sucking insects Dynaspidiotus britannicus Comstock and Unaspis euonymi Newstead. J. Plant. Prot. Res. 2020, 60, 220–223. [Google Scholar] [CrossRef]
- Useinov, R.; Gal’chinsky, N.; Yatskova, E.; Novikov, I.; Puzanova, Y.; Trikoz, N.; Sharmagiy, A.; Plugatar, Y.; Laikova, K.; Oberemok, V. To bee or not to bee: Creating DNA insecticides to replace non-selective organophosphate insecticides for use against the soft scale insect Ceroplastes japonicus Green. J. Plant. Prot. Res. 2020, 60, 406–409. [Google Scholar] [CrossRef]
- Oberemok, V.V.; Useinov, R.Z.; Skorokhod, O.A.; Gal’chinsky, N.V.; Novikov, I.A.; Makalish, T.P.; Yatskova, E.V.; Sharmagiy, A.K.; Golovkin, I.O.; Gninenko, Y.I.; et al. Oligonucleotide Insecticides for Green Agriculture: Regulatory Role of Contact DNA in Plant–Insect Interactions. Int. J. Mol. Sci. 2022, 23, 15681. [Google Scholar] [CrossRef] [PubMed]
- Cech, R.; Leisch, F.; Zaller, J.G. Pesticide Use and Associated Greenhouse Gas Emissions in Sugar Beet, Apples, and Viticulture in Austria from 2000 to 2019. Agriculture 2022, 12, 879. [Google Scholar] [CrossRef]
- Dias, N.; Stein, C.A. Antisense oligonucleotides: Basic concepts and mechanisms. Mol. Cancer Ther. 2002, 1, 347–355. [Google Scholar] [PubMed]
- Oberemok, V.V.; Laikova, K.V.; Zaitsev, A.S.; Temirova, Z.Z.; Gal’chinsky, N.V.; Nyadar, P.M.; Shumskykh, M.N.; Zubarev, I.V. The need for the application of modern chemical insecticides and environmental consequences of their use: A mini review. J. Plant. Prot. Res. 2017, 57, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Oberemok, V.V.; Laikova, K.; Shumskykh, M.; Kenyo, I.; Kasich, I.; Deri, K.; Seidosmanova, E.; Krasnodubets, K.; Bekirova, V.; Gal’chinsky, N. A primary attempt of Leptinotarsa decemlineata control using contact DNA insecticide based on short antisense oligonucleotide of its CYP6B gene. J. Plant. Prot. Res. 2018, 58, 106–108. [Google Scholar] [CrossRef]
- Oberemok, V.V.; Laikova, K.V.; Gal’chinsky, N.V.; Useinov, R.Z.; Novikov, I.A.; Temirova, Z.Z.; Shumskykh, M.N.; Krasnodubets, A.M.; Repetskaya, A.I.; Dyadichev, V.; et al. DNA insecticide developed from the Lymantria dispar 5.8S ribosomal RNA gene provides a novel biotechnology for plant protection. Sci. Rep. 2019, 9, 6197. [Google Scholar] [CrossRef] [Green Version]
- Paule, M.R.; White, R.J. Transcription by RNA polymerases I and III. Nucleic Acids Res. 2000, 28, 1283–1298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Zaitsev, A.S.; Omel’chenko, O.V.; Nyadar, P.M.; Oberemok, V.V. Influence of DNA oligonucleotides used as insecticides on biochemical parameters of Quercus robur and Malus domestica. Bull. Transylvania Univ. Bras. Ser. II For. Wood Ind. Agric. Food Eng. 2015, 8, 37–46. [Google Scholar]
- Oberemok, V.V.; Nyadar, P.; Zaytsev, O.; Levchenko, N.; Shiyntum, H.; Omelchenko, O. Pioneer evaluation of the possible side effects of the DNA insecticides on wheat (Triticum aestivum L.). Int. J. Biochem. Biophys. 2013, 1, 57–63. [Google Scholar] [CrossRef]
- Oberemok, V.V.; Laikova, V.K.; Zaitsev, S.A.; Nyadar, M.P.; Shumskykh, N.M.; Gninenko, I.Y. DNA insecticides based on iap3 gene fragments of cabbage looper and gypsy moth nuclear polyhedrosis viruses show selectivity for non-target insects. Arch. Biol. Sci. 2015, 67, 785–792. [Google Scholar] [CrossRef]
- Damha, M.J.; Giannaris, P.A.; Zabarylo, S.V. An improved procedure for derivatization of controlled-pore glass beads for solid-phase oligonucleotide synthesis. Nucleic Acids Res. 1990, 18, 3813–3821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Siwkowski, A.; Lima, W.F.; Olsen, P.; Ravikumar, V.T. Antisense oligonucleotides: Efficient synthesis of 2′-O-methoxyethyl phosphorothioate oligonucleotides using 4,5-dicyanoimidazole. Are these oligonucleotides comparable to those synthesized using 1H-tetrazole as coupling activator? Bioorg. Med. Chem. 2006, 14, 5049–5060. [Google Scholar] [CrossRef] [PubMed]
- Shakhgil’dyan, G.Y.; Piyanzina, K.I.; Stepko, A.A.; Natyrov, A.; Mikhailov, A.; Savinkov, V.I.; Sigaev, V. Nanoporous Glass with Controlled Pore Size for High-Efficiency Synthesis of Oligonucleotides. Glass Ceram. 2019, 75, 377–382. [Google Scholar] [CrossRef]
- Prakash, T.P.; Wan, W.B.; Low, A.; Yu, J.; Chappell, A.E.; Gaus, H.; Kinberger, G.A.; Østergaard, M.E.; Migawa, M.T.; Swayze, E.E.; et al. Solid-phase synthesis of 5′-triantennary N-acetylgalactosamine conjugated antisense oligonucleotides using phosphoramidite chemistry. Bioorg. Med. Chem. Lett. 2015, 25, 4127–4130. [Google Scholar] [CrossRef]
- Bonora, G.M.; Biancotto, G.; Maffini, M.; Scremin, C.L. Large scale, liquid phase synthesis of oligonucleotides by the phosphoramidite approach. Nucleic Acids Res. 1993, 21, 1213–1217. [Google Scholar] [CrossRef] [Green Version]
- Bonora, G.M.; Zaramella, S.; Veronese, F.M. Synthesis by high-efficiency liquid-phase (HELP) method of oligonucleotides conjugated with high-molecular weight polyethylene glycols (PEGs). Biol. Proced. Online 1998, 1, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Lönnberg, H. Synthesis of oligonucleotides on a soluble support. Beilstein J. Org. Chem. 2017, 3, 1368–1387. [Google Scholar] [CrossRef]
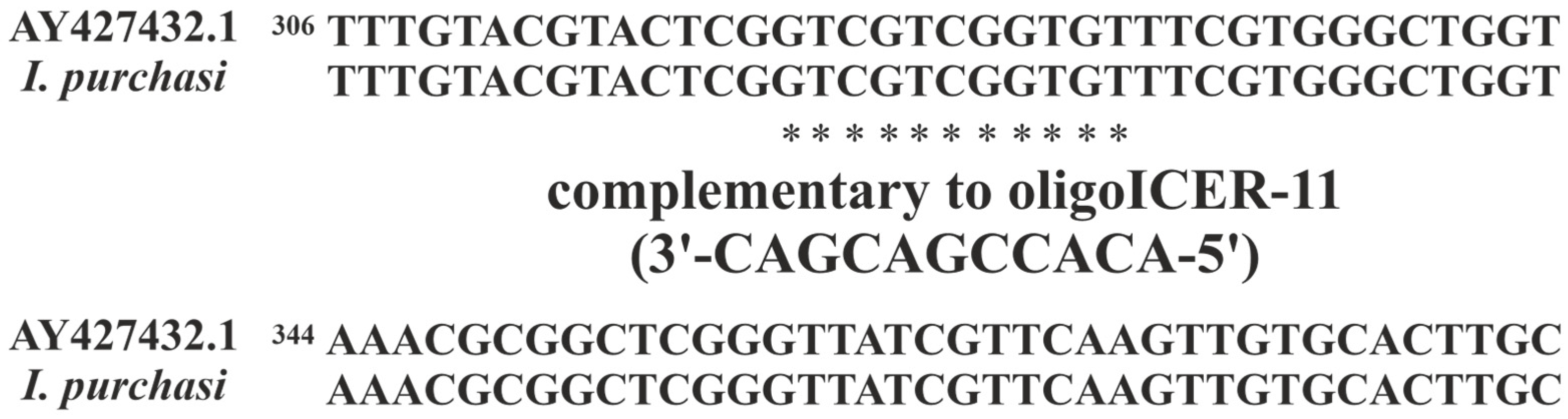
| Insecticides against I. purchasi | Corresponding Compound | Reagent and Solvents | t CO2/t Ratio | Reference |
|---|---|---|---|---|
| Synthesis of oligonucleotide insecticides (DNA insecticides, olinscides) on a solid-phase carrier on an automatic DNA synthesizer does not lead to greenhouse gas emissions | ||||
| Oligonucleotide insecticide (oligoICER-11) | Antisense oligonucleotide | Amidites, tetrazole, 1-methylimidazole, triethylamine, acetic or propionic anhydride, pyridine, iodine, acetic acid, trichloroacetic acid, dichloromethane, acetonitrile | ~0 | |
| Synthesis of frequently used chlorothiazole neonicotinoid insecticides results in greenhouse gas emissions | ||||
| Chlorothiazole (Thiamethoxam) | Thiamethoxam | S-methyl-N-nitroisothiourea, methylamine, N-methyl-N′-nitroguanidine, formaldehyde, formic acid, tetrahydro-1,3,5-oxadiazine, 2-chloro-5-chloromethylthiazole, dimethylformamide, potassium carbonate | 0.351 | [35] |
| Day | Control (Water) | Thiamethoxam | oligoICER-11 |
|---|---|---|---|
| 3rd | 14.02 ± 2.79 | 28.67 ± 3.51 * | 42.92 ± 2.25 * |
| 7th | 20.08 ± 6.25 | 33.67 ± 2.52 * | 45.05 ± 4.74 * |
| 10th | 23.7 ± 8.87 | 35.33 ± 2.08 | 70.55 ± 0.77 * |
| Day | Control (Water) | oligoICER-11 |
|---|---|---|
| 1st | 6.01 ± 0.06 | 6.02 ± 0.04 |
| 7th | 6.04 ± 0.04 | 6.05 ± 0.04 |
| Oligonucleotide | Resulting m/z | Theoretical m/z |
|---|---|---|
| oligoICER-11 | 3295.72 | 3294.61 |
| I. purchasi 28S rRNA gene forward primer | 6189.09 | 6181.08 |
| I. purchasi 28S rRNA gene reverse primer | 6142.76 | 6134.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gal’chinsky, N.V.; Yatskova, E.V.; Novikov, I.A.; Useinov, R.Z.; Kouakou, N.J.; Kouame, K.F.; Kra, K.D.; Sharmagiy, A.K.; Plugatar, Y.V.; Laikova, K.V.; et al. Icerya purchasi Maskell (Hemiptera: Monophlebidae) Control Using Low Carbon Footprint Oligonucleotide Insecticides. Int. J. Mol. Sci. 2023, 24, 11650. https://doi.org/10.3390/ijms241411650
Gal’chinsky NV, Yatskova EV, Novikov IA, Useinov RZ, Kouakou NJ, Kouame KF, Kra KD, Sharmagiy AK, Plugatar YV, Laikova KV, et al. Icerya purchasi Maskell (Hemiptera: Monophlebidae) Control Using Low Carbon Footprint Oligonucleotide Insecticides. International Journal of Molecular Sciences. 2023; 24(14):11650. https://doi.org/10.3390/ijms241411650
Chicago/Turabian StyleGal’chinsky, Nikita V., Ekaterina V. Yatskova, Ilya A. Novikov, Refat Z. Useinov, Nanan J. Kouakou, Kra F. Kouame, Kouadio D. Kra, Alexander K. Sharmagiy, Yuri V. Plugatar, Kateryna V. Laikova, and et al. 2023. "Icerya purchasi Maskell (Hemiptera: Monophlebidae) Control Using Low Carbon Footprint Oligonucleotide Insecticides" International Journal of Molecular Sciences 24, no. 14: 11650. https://doi.org/10.3390/ijms241411650







