Genome-Wide Datasets of Chicories (Cichorium intybus L.) for Marker-Assisted Crop Breeding Applications: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Literature Research
2.2. Data Collection and Maps Drawing
3. Results and Discussion
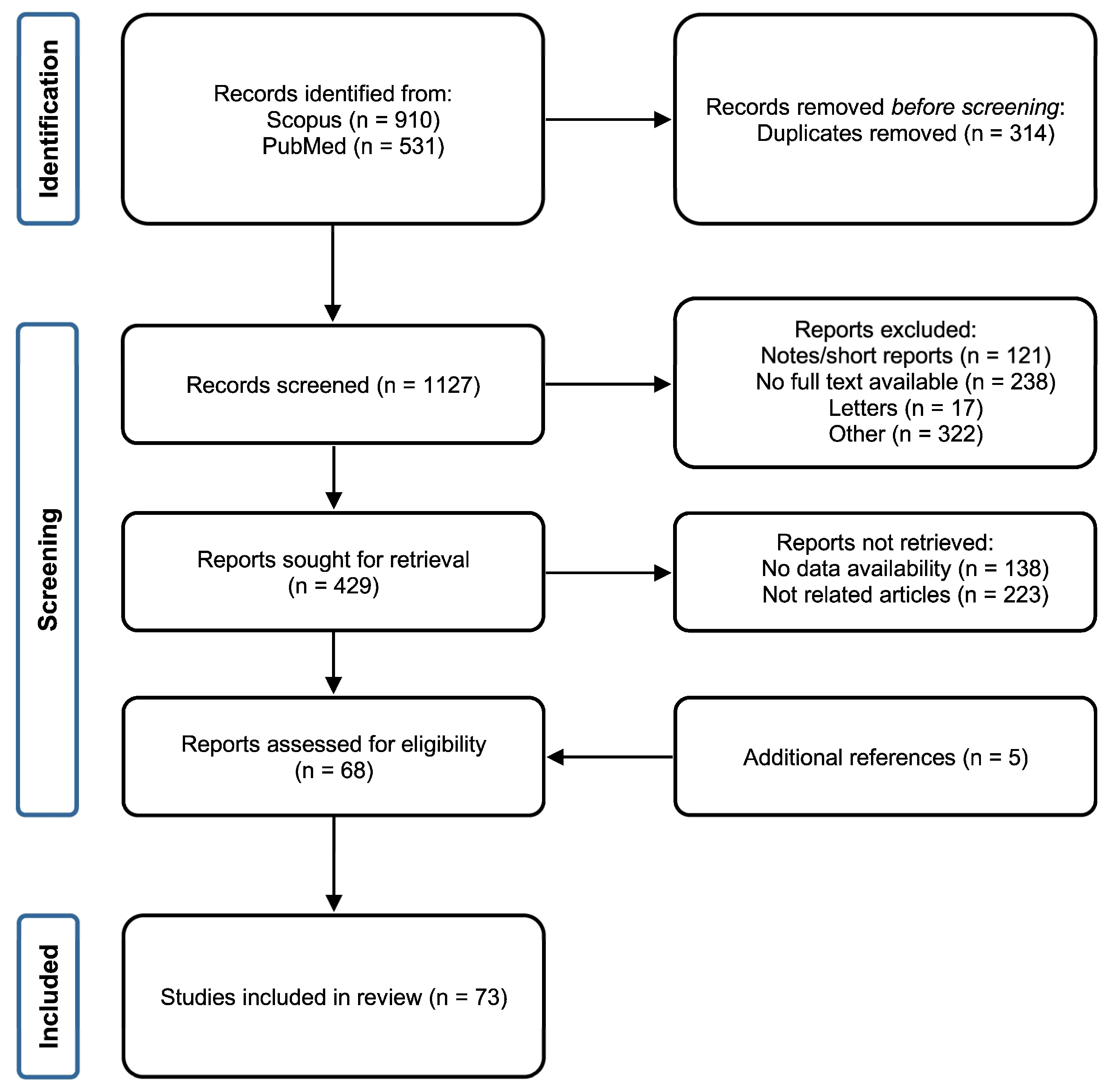
3.1. Screening Literature Results
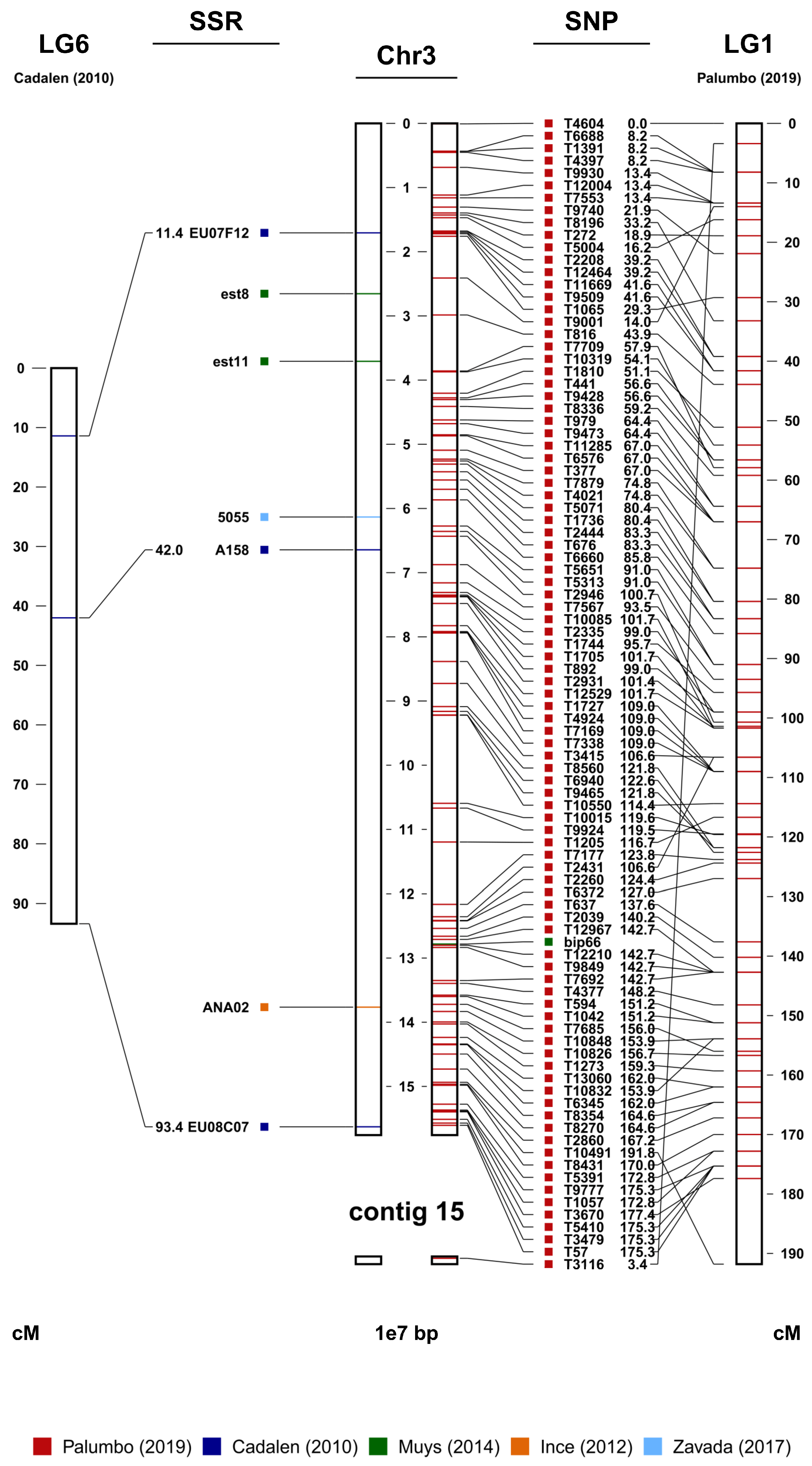
3.2. Toward a Genetic Genomic Map of Chicory Useful for Marker-Assisted Breeding
3.3. A Comprehensive Map for Marker-Assisted Selection Purposes
3.3.1. Reproductive Barriers
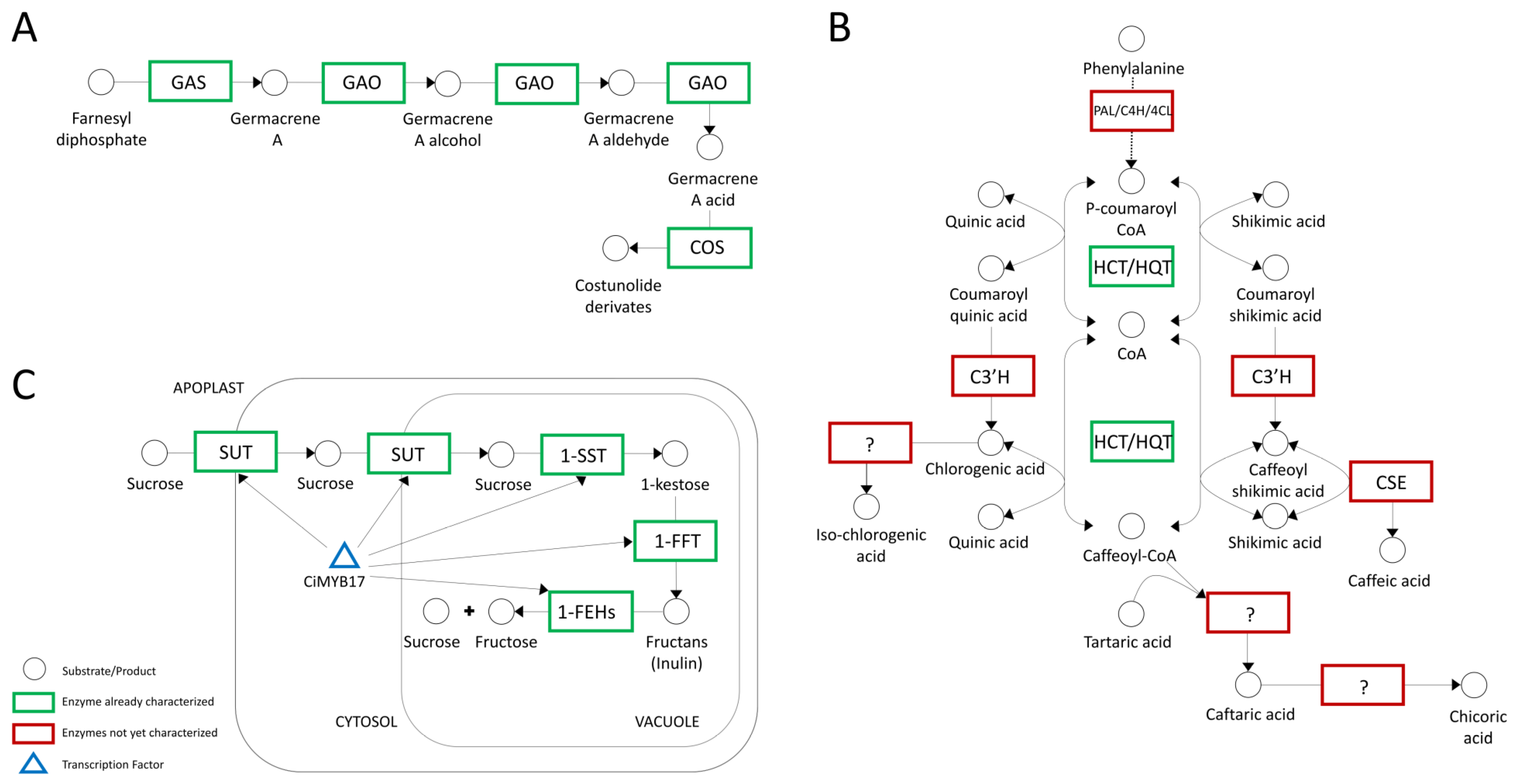
3.3.2. Chicory, the Special Bitter-Taste Vegetable—STL Biosynthesis
3.3.3. Hydroxycinnamate Biosynthesis (HCA)
3.3.4. Inulin Metabolism
3.3.5. Biotic and Abiotic Stresses
3.3.6. Lilac-Blue Color
3.3.7. Flowering Time
3.3.8. Somatic Embryogenesis (SE)
3.3.9. Red Discoloration
3.3.10. Gene Normalization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raulier, P.; Maudoux, O.; Notté, C.; Draye, X.; Bertin, P. Exploration of Genetic Diversity within Cichorium endivia and Cichorium intybus with Focus on the Gene Pool of Industrial Chicory. Genet. Resour. Crop Evol. 2015, 63, 243–259. [Google Scholar] [CrossRef]
- Stephens, J.M. Chicory-Cichorium intybus L.; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 1994. [Google Scholar]
- Lucchin, M.; Varotto, S.; Barcaccia, G.; Parrini, P. Chicory and Endive. In Vegetables I: Asteraceae, Brassicaceae, Chenopodicaceae, and Cucurbitaceae; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; pp. 3–48. ISBN 978-0-387-30443-4. [Google Scholar]
- Barcaccia, G.; Ghedina, A.; Lucchin, M. Current Advances in Genomics and Breeding of Leaf Chicory (Cichorium intybus L.). Agriculture 2016, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Galla, G.; Ghedina, A.; Tiozzo, S.C.; Barcaccia, G. Toward a First High-Quality Genome Draft for Marker-Assisted Breeding in Leaf Chicory, Radicchio (Cichorium intybus L.). In Plant Genomics; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Bahmani, M. Overview of Medicinal Plants Used for Cardiovascular System Disorders and Diseases in Ethnobotany of Different Areas in Iran. J. Herbmed. Pharmacol. 2016, 5, 39–44. [Google Scholar]
- Al-Snafi, A.E. Medical Importance of Cichorium intybus. IOSR J. Pharm. 2016, 6, 41–56. [Google Scholar]
- Perović, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a Food Ingredient—Nutritional Composition, Bioactivity, Safety, and Health Claims: A Review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Testone, G.; Sobolev, A.P.; Mele, G.; Nicolodi, C.; Gonnella, M.; Arnesi, G.; Biancari, T.; Giannino, D. Leaf Nutrient Content and Transcriptomic Analyses of Endive (Cichorium endivia) Stressed by Downpour-Induced Waterlog Reveal a Gene Network Regulating Kestose and Inulin Contents. Hortic. Res. 2021, 8, 92. [Google Scholar] [CrossRef]
- Wei, H.; Bausewein, A.; Steininger, H.; Su, T.; Zhao, H.; Harms, K.; Greine, S.; Rausch, T. Linking Expression of Fructan Active Enzymes, Cell Wall Invertases and Sucrose Transporters with Fructan Profiles in Growing Taproot of Chicory (Cichorium intybus): Impact of Hormonal and Environmental Cues. Front. Plant Sci. 2016, 7, 1806. [Google Scholar] [CrossRef] [Green Version]
- Barcaccia, G.; Pal Lo Ttin, I.L.; Soattin, M.; Lazzarin, R.; Parrini, P.; Lucchin, M.; Weber, W.E. Genomic DNA Fingerprints as a Tool for Identifying Cultivated Types of Radicchio (Cichorium intybus L.) from Veneto, Italy. Plant Breed. 2002, 122, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Gonthier, L.; Blassiau, C.; Mörchen, M.; Cadalen, T.; Poiret, M.; Hendriks, T.; Quillet, M.C. High-Density Genetic Maps for Loci Involved in Nuclear Male Sterility (NMS1) and Sporophytic Self-Incompatibility (S-Locus) in Chicory (Cichorium intybus L., Asteraceae). Theor. Appl. Genet. 2013, 126, 2103–2121. [Google Scholar] [CrossRef]
- Patella, A.; Scariolo, F.; Palumbo, F.; Barcaccia, G. Genetic Structure of Cultivated Varieties of Radicchio (Cichorium intybus L.): A Comparison between F1 Hybrids and Synthetics. Plants 2019, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Scariolo, F.; Palumbo, F.; Barcaccia, G. Molecular Characterization and Genetic Structure Evaluation of Breeding Populations of Fennel (Foeniculum vulgare Mill.). Agronomy 2022, 12, 542. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Das Kumar, B. Molecular Markers: It’s Application in Crop Improvement. J. Crop Sci. Biotechnol. 2009, 12, 169–181. [Google Scholar] [CrossRef]
- Sabev, P.; Valkova, N.; Todorovska, E.G. Molecular Markers and Their Application in Cotton Breeding: Progress and Future Perspectives. Bulg. J. Agric. Sci. 2020, 26, 816–828. [Google Scholar]
- Semagn, K.; Bjørnstad, Å.; Ndjiondjop, M.N. Progress and Prospects of Marker Assisted Backcrossing as a Tool in Crop Breeding Programs. Afr. J. Biotechnol. 2006, 5, 2588–2603. [Google Scholar]
- Palumbo, F.; Galla, G.; Vitulo, N.; Barcaccia, G. First Draft Genome Sequencing of Fennel (Foeniculum vulgare Mill.): Identification of Simple Sequence Repeats and Their Application in Marker-Assisted Breeding. Mol. Breed. 2018, 38, 122. [Google Scholar] [CrossRef]
- Barcaccia, G.; Palumbo, F.; Scariolo, F.; Vannozzi, A.; Borin, M.; Bona, S. Potentials and Challenges of Genomics for Breeding Cannabis Cultivars. Front. Plant Sci. 2020, 11, 573299. [Google Scholar] [CrossRef]
- Fan, W.; Wang, S.; Wang, H.; Wang, A.; Jiang, F.; Liu, H.; Zhao, H.; Xu, D.; Zhang, Y. The Genomes of Chicory, Endive, Great Burdock and Yacon Provide Insights into Asteraceae Palaeo-Polyploidization History and Plant Inulin Production. Mol. Ecol. Resour. 2022, 22, 3124–3140. [Google Scholar] [CrossRef]
- De Simone, M.; Morgante, M.; Lucchin, M.; Parrini, P.; Marocco, A. A First Linkage Map of Cichorium intybus L. Using a One-Way Pseudo-Testcross and PCR-Derived Markers. Mol. Breed. 1997, 3, 415–425. [Google Scholar] [CrossRef]
- Van Stallen, N.; Vandenbussche, B.; Verdoodt, V.; De Proft, M. Construction of a Genetic Linkage Map for Witloof (Cichorium L. var foliosum Hegi). Plant Breed. 2003, 122, 521–525. [Google Scholar] [CrossRef]
- Van Stallen, N.; Vandenbussche, B.; Londers, E.; Noten, V.; De Proft, M. QTL Analysis of Important Pith Characteristics in a Cross between Two Inbred lines of Chicory (Cichorium intybus var foliosum): A Preliminary Study. Plant Breed. 2005, 124, 54–58. [Google Scholar]
- Cassan, L.; Moreau, L.; Segouin, S.; Bellamy, A.; Falque, M.; Limami, A.M. Genetic Map Construction and Quantitative Trait Loci (QTL) Mapping for Nitrogen Use Efficiency and Its Relationship with Productivity and Quality of the Biennial Crop Belgian Endive (Cichorium intybus L.). J. Plant Physiol. 2010, 167, 1253–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadalen, T.; Mörchen, M.; Blassiau, C.; Clabaut, A.; Scheer, I.; Hilbert, J.L.; Hendriks, T.; Quillet, M.C. Development of SSR Markers and Construction of a Consensus Genetic Map for Chicory (Cichorium intybus L.). Mol. Breed. 2010, 25, 699–722. [Google Scholar] [CrossRef]
- Ghedina, A.; Galla, G.; Cadalen, T.; Hilbert, J.L.; Caenazzo, S.T.; Barcaccia, G. A Method for Genotyping Elite Breeding Stocks of Leaf Chicory (Cichorium intybus L.) by Assaying Mapped Microsatellite Marker Loci Genetics. BMC Res. Notes 2015, 8, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muys, C.; Thienpont, C.N.; Dauchot, N.; Maudoux, O.; Draye, X.; Cutsem, P. Van Integration of AFLPs, SSRs and SNPs Markers into a New Genetic Map of Industrial Chicory (Cichorium intybus L. var Sativum). Plant Breed. 2014, 133, 130–137. [Google Scholar] [CrossRef]
- Palumbo, F.; Qi, P.; Pinto, V.B.; Devos, K.M.; Barcaccia, G. Construction of the First Snp-Based Linkage Map Using Genotyping-by-Sequencing and Mapping of the Male-Sterility Gene in Leaf Chicory. Front. Plant Sci. 2019, 10, 276. [Google Scholar] [CrossRef]
- Ince, G.A. A Contig-Based Microsatellite Marker Approach and Its Application in Cichorium ESTs. Rom. Biotechnol. Lett. 2012, 17, 7033. [Google Scholar]
- Závada, T.; Malik, R.J.; Kesseli, R.V. Population Structure in Chicory (Cichorium intybus): A Successful U.S. Weed since the American Revolutionary War. Ecol. Evol. 2017, 7, 4209–4219. [Google Scholar] [CrossRef] [Green Version]
- Závada, T.; Malik, R.J.; Mazumder, L.; Kesseli, R.V. Radical Shift in the Genetic Composition of New England Chicory Populations. J. Ecol. 2023, 111, 391–399. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Boudiar, R.; Casas, A.M.; Igartua, E.; Contreras-Moreira, B. BARLEYMAP: Physical and Genetic Mapping of Nucleotide Sequences and Annotation of Surrounding Loci in Barley. Mol. Breed. 2015, 35, 13. [Google Scholar] [CrossRef]
- Rana, M.; Sood, A.; Hussain, W.; Kaldate, R.; Sharma, T.R.; Gill, R.K.; Kumar, S.; Singh, S. Gene Pyramiding and Multiple Character Breeding. In Lentils; Singh, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 83–124. ISBN 978-0-12-813522-8. [Google Scholar]
- Kushanov, F.N.; Turaev, O.S.; Ernazarova, D.K.; Gapparov, B.M.; Oripova, B.B.; Kudratova, M.K.; Rafieva, F.U.; Khalikov, K.K.; Erjigitov, D.S.; Khidirov, M.T.; et al. Genetic Diversity, QTL Mapping, and Marker-Assisted Selection Technology in Cotton (Gossypium spp.). Front. Plant Sci. 2021, 12, 779386. [Google Scholar] [CrossRef]
- Barcaccia, G.; Tiozzo Caenazzo, S. New Male Sterile Mutant of Leaf Chicory, Including Radicchio, Used to Produce Chicory Plants and Seeds with Traits Such as Male Sterility Exhibiting Cytological Phenotype with Shapeless, Small and Shrunken Microspores in Dehiscent Anthers. European Patent EP2713705A1, 5 January 2014. Available online: https://patents.google.com/patent/EP2713705B1 (accessed on 12 January 2023).
- Palumbo, F.; Draga, S.; Magon, G.; Gabelli, G.; Vannozzi, A.; Farinati, S.; Scariolo, F.; Lucchin, M.; Barcaccia, G. MIK2 Is a Candidate Gene of the S-Locus for Sporophytic Self-Incompatibility (SSI) in Chicory (Cichorium intybus, Asteraceae). Front. Plant Sci. 2023, 14, 1204538. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Kodde, J.; Verstappen, F.W.A.; Altug, I.G.; De Kraker, J.W.; Wallaart, T.E. Isolation and Characterization of Two Germacrene A Synthase CDNA Clones from Chicory. Plant Physiol. 2002, 129, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.T.; Göpfert, J.C.; Ikezawa, N.; MacNevin, G.; Kathiresan, M.; Conrad, J.; Spring, O.; Ro, D.K. Biochemical Conservation and Evolution of Germacrene A Oxidase in Asteraceae. J. Biol. Chem. 2010, 285, 16588–16598. [Google Scholar] [CrossRef] [Green Version]
- Cankar, K.; Van Houwelingen, A.; Bosch, D.; Sonke, T.; Bouwmeester, H.; Beekwilder, J. A Chicory Cytochrome P450 Mono-Oxygenase CYP71AV8 for the Oxidation of (+)-Valencene. FEBS Lett. 2011, 585, 178–182. [Google Scholar] [CrossRef]
- Testone, G.; Mele, G.; Di Giacomo, E.; Gonnella, M.; Renna, M.; Tenore, G.C.; Nicolodi, C.; Frugis, G.; Iannelli, M.A.; Arnesi, G.; et al. Insights into the Sesquiterpenoid Pathway by Metabolic Profiling and de Novo Transcriptome Assembly of Stem-Chicory (Cichorium intybus Cultigroup “Catalogna”). Front. Plant Sci. 2016, 7, 1676. [Google Scholar] [CrossRef] [Green Version]
- Legrand, G.; Delporte, M.; Khelifi, C.; Harant, A.; Vuylsteker, C.; Mörchen, M.; Hance, P.; Hilbert, J.L.; Gagneul, D. Identification and Characterization of Five BAHD Acyltransferases Involved in Hydroxycinnamoyl Ester Metabolism in Chicory. Front. Plant Sci. 2016, 7, 741. [Google Scholar] [CrossRef]
- Van Den Ende, W.; Lammens, W.; Van Laere, A.; Schroeven, L.; Le Roy, K. Donor and Acceptor Substrate Selectivity among Plant Glycoside Hydrolase Family 32 Enzymes. FEBS J. 2009, 276, 5788–5798. [Google Scholar] [CrossRef]
- Van Den Ende, W.; Michiels, A.; Wonterghem, V.; Clerens, S.P.; Laere, A.J. Van Defoliation Induces Fructan 1-Exohydrolase II in Witloof Chicory Roots. Cloning and Purification of Two Isoforms, Fructan 1-Exohydrolase IIa and Fructan 1-Exohydrolase IIb. Mass Fingerprint of the Fructan 1-Exohydrolase II Enzymes 1. Plant Physiol. 2001, 126, 1186–1195. [Google Scholar] [CrossRef] [Green Version]
- Michiels, A.; Van Laere, A.; Van Den Ende, W.; Tucker, M. Expression Analysis of a Chicory Fructan 1-Exohydrolase Gene Reveals Complex Regulation by Cold. J. Exp. Bot. 2004, 55, 1325–1333. [Google Scholar] [CrossRef]
- Wei, H.; Bausewein, A.; Greiner, S.; Dauchot, N.; Harms, K.; Rausch, T. CiMYB17, a Stress-Induced Chicory R2R3-MYB Transcription Factor, Activates Promoters of Genes Involved in Fructan Synthesis and Degradation. New Phytol. 2017, 215, 281–298. [Google Scholar] [CrossRef] [Green Version]
- Palms, B.; Goupil, P.; De Almeida Engler, J.; Van Der Straeten, D.; Van Montagu, M.; Rambour, S. Evidence for the Nitrate-Dependent Spatial Regulation of the Nitrate Reductase Gene in Chicory Roots. Planta 1996, 200, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Adomat, C.; Böger, P. Cloning, Sequence, Expression, and Characterization of Protoporphyrinogen IX Oxidase from Chicory. Pestic. Biochem. Physiol. 2000, 66, 49–62. [Google Scholar] [CrossRef]
- Mingeot, D.; Dauchot, N.; Van Cutsem, P.; Watillon, B. Characterisation of Two Cold Induced Dehydrin Genes from Cichorium intybus L. Mol. Biol. Rep. 2009, 36, 1995–2001. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Lin, M.; Lin, Z.; Zhao, L.; Zhao, G.; Li, Q.; Yin, X. Identification, Functional Characterization, and Expression Pattern of a NaCl-Inducible Vacuolar Na+/H+ Antiporter in Chicory (Cichorium intybus L.). Plant Growth Regul. 2015, 75, 605–614. [Google Scholar] [CrossRef]
- Liang, M.; Chen, D.; Lin, M.; Zheng, Q.; Huang, Z.; Lin, Z.; Zhao, G. Isolation and Characterization of Two DREB1 Genes Encoding Dehydration-Responsive Element Binding Proteins in Chicory (Cichorium intybus). Plant Growth Regul. 2014, 73, 45–55. [Google Scholar] [CrossRef]
- Seitz, C.; Ameres, S.; Schlangen, K.; Forkmann, G.; Halbwirth, H. Multiple Evolution of Flavonoid 3′,5′-Hydroxylase. Planta 2015, 242, 561–573. [Google Scholar] [CrossRef]
- Périlleux, C.; Pieltain, A.; Jacquemin, G.; Bouché, F.; Detry, N.; D’Aloia, M.; Thiry, L.; Aljochim, P.; Delansnay, M.; Mathieu, A.S.; et al. A Root Chicory MADS Box Sequence and the Arabidopsis Flowering Repressor FLC Share Common Features That Suggest Conserved Function in Vernalization and De-Vernalization Responses. Plant J. 2013, 75, 390–402. [Google Scholar] [CrossRef]
- Hendriks, T.; Scheer, I.; Quillet, M.-C.; Randoux, B.; Delbreil, B.; Vasseur, J.; Hilbert, J.-L. A Nonsymbiotic Hemoglobin Gene Is Expressed during Somatic Embryogenesis in Cichorium. Biochim. Biophys. Acta BBA 1998, 1443, 193–197. [Google Scholar] [CrossRef]
- Galland, R.; Randoux, B.; Vasseur, J.; Hilbert, J.-L. A Glutathione S-Transferase CDNA Identified by MRNA Differential Display Is Upregulated during Somatic Embryogenesis in Cichorium. Biochim. Biophys. Acta BBA Gene Struct. Expr. 2001, 1522, 212–216. [Google Scholar] [CrossRef]
- Helleboid, S.; Chapman, A.; Hendriks, T.; Inzé, D.; Vasseur, J.; Hilbert, J.-L. Cloning of β-1,3-Glucanases Expressed during Cichorium Somatic Embryogenesis. Plant Mol. Biol. 2000, 42, 377–386. [Google Scholar] [CrossRef]
- Randoux, B.; Quillet, M.-C.; Rambaud, C.; Vasseur, J.; Hilbert, J.-L. Characterisation of CDNAs Homologous to Rab5-GTP Binding Protein Expressed during Early Somatic Embryogenesis in Chicory. Plant Sci. 2002, 162, 413–422. [Google Scholar] [CrossRef]
- Salman, A.; Goupil, P.; Filgueiras, H.; Charles, F.; Ledoigt, G.; Sallanon, H. Controlled Atmosphere and Heat Shock Affect PAL1 and HSP90 MRNA Accumulation in Fresh-Cut Endive (Cichorium intybus L.). Eur. Food Res. Technol. 2008, 227, 721–726. [Google Scholar] [CrossRef]
- Delporte, M.; Legrand, G.; Hilbert, J.L.; Gagneul, D. Selection and Validation of Reference Genes for Quantitative Real-Time PCR Analysis of Gene Expression in Cichorium intybus. Front. Plant Sci. 2015, 6, 651. [Google Scholar] [CrossRef] [Green Version]
- Maroufi, A.; Van Bockstaele, E.; De Loose, M. Validation of Reference Genes for Gene Expression Analysis in Chicory (Cichorium intybus) Using Quantitative Real-Time PCR. BMC Mol. Biol. 2010, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Eenink, A.H. Compatibility and Incompatibility in Witloof-Chicory (Cichorium intybus L.). 2. The Incompatibility System. Euphytica 1981, 30, 77–85. [Google Scholar] [CrossRef]
- Peña-Espinoza, M.; Valente, A.H.; Thamsborg, S.M.; Simonsen, H.T.; Boas, U.; Enemark, H.L.; López-Muñoz, R.; Williams, A.R. Antiparasitic Activity of Chicory (Cichorium intybus) and Its Natural Bioactive Compounds in Livestock: A Review; BioMed Central Ltd.: London, UK, 2018; Volume 11. [Google Scholar]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids Lactones: Benefits to Plants and People. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [Green Version]
- Channotiya, J.; Tiwari, A.; Taj, G.; Verma, A.K.; Dubey, A. In-Silico and Molecular Docking Studies on Germacrene A Synthase Enzyme and Sesuiterpene Lactone (Lactucin) Involved in Antimalarial Activity of Cichorium intybus. Netw. Model. Anal. Health Inform. Bioinform. 2021, 10, 24. [Google Scholar] [CrossRef]
- De Kraker, J.-W.; Franssen, M.C.R.; Dalm, M.C.F.; De Groot, A.; Bouwmeester, H.J. Biosynthesis of Germacrene A Carboxylic Acid in Chicory Roots. Demonstration of a Cytochrome P450 (+)-Germacrene A Hydroxylase and NADP-Dependent Sesquiterpenoid Dehydrogenase(s) Involved in Sesquiterpene Lactone Biosynthesis. Plant Physiol. 2001, 125, 1930–1940. [Google Scholar] [CrossRef] [Green Version]
- Bogdanović, M.; Cankar, K.; Todorović, S.; Dragicević, M.; Simonović, A.; van Houwelingen, A.; Schijlen, E.; Schipper, B.; Gagneul, D.; Hendriks, T.; et al. Tissue Specific Expression and Genomic Organization of Bitter Sesquiterpene Lactone Biosynthesis in Cichorium intybus L. (Asteraceae). Ind. Crops Prod. 2019, 129, 253–260. [Google Scholar] [CrossRef]
- Van Laere, A.; Van Den Ende, W. Inulin Metabolism in Dicots: Chicory as a Model System. Plant Cell Environ. 2002, 25, 803–813. [Google Scholar] [CrossRef]
- Shoorideh, H.; Peighambari, S.A.; Omidi, M.; Naghavi, M.R.; Maroufi, A. Spatial Expression of Genes in Inulin Biosynthesis Pathway in Wild and Root Type Chicory. J. Agric. Sci. Technol. 2018, 20, 1049–1058. [Google Scholar]
- Ikezawa, N.; Göpfert, J.C.; Nguyen, D.T.; Kim, S.U.; O’Maille, P.E.; Spring, O.; Ro, D.K. Lettuce Costunolide Synthase (CYP71BL2) and Its Homolog (CYP71BL1) from Sunflower Catalyze Distinct Regio- and Stereoselective Hydroxylations in Sesquiterpene Lactone Metabolism. J. Biol. Chem. 2011, 286, 21601–21611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Majdi, M.; Cankar, K.; Goedbloed, M.; Charnikhova, T.; Verstappen, F.W.A.; de Vos, R.C.H.; Beekwilder, J.; van der Krol, S.; Bouwmeester, H.J. Reconstitution of the Costunolide Biosynthetic Pathway in Yeast and Nicotiana Benthamiana. PLoS ONE 2011, 6, e23255. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.M.; Saillard, N.; Yang, T.; Franssen, M.C.R.; Bouwmeester, H.J.; Jongsma, M.A. Biosynthesis of Sesquiterpene Lactones in Pyrethrum (Tanacetum cinerariifolium). PLoS ONE 2013, 8, e65030. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Han, X.; Liu, X.; Wang, Q.; Zhang, J.; Zhao, H.; Tang, J.; Luo, K.; Zhai, Z.; et al. The Chromosome-Scale Assembly of Endive (Cichorium endivia) Genome Provides Insights into the Sesquiterpenoid Biosynthesis. Genomics 2022, 114, 110400. [Google Scholar] [CrossRef]
- Rees, S.; Harborne, J. The Role of Sesquiterpene Lactones and Phenolics in the Chemical Defence of the Chicory Plant. Phytochemistry 1985, 24, 2225–2231. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Content of Insoluble Bound Phenolics in Millets and Their Contribution to Antioxidant Capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Siquet, C.; Orrù, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A.; et al. Lipophilic Phenolic Antioxidants: Correlation between Antioxidant Profile, Partition Coefficients and Redox Properties. Bioorg. Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef]
- Lallemand, L.A.; Zubieta, C.; Lee, S.G.; Wang, Y.; Acajjaoui, S.; Timmins, J.; McSweeney, S.; Jez, J.M.; McCarthy, J.G.; McCarthy, A.A. A Structural Basis for the Biosynthesis of the Major Chlorogenic Acids Found in Coffee. Plant Physiol. 2012, 160, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Bahri, M.; Hance, P.; Grec, S.; Quillet, M.C.; Trotin, F.; Hilbert, J.L.; Hendriks, T. A “Novel” Protocol for the Analysis of Hydroxycinnamic Acids in Leaf Tissue of Chicory (Cichorium intybus L., Asteraceae). Sci. World J. 2012, 2012, 142983. [Google Scholar] [CrossRef] [Green Version]
- Kandeler, R.; Ullrich, W.R. Symbolism of Plants: Examples from European-Mediterranean Culture Presented with Biology and History of Art. J. Exp. Bot. 2009, 60, 3297–3299. [Google Scholar] [CrossRef]
- Sonnante, G.; D’Amore, R.; Blanco, E.; Pierri, C.L.; de Palma, M.; Luo, J.; Tucci, M.; Martin, C. Novel Hydroxycinnamoyl-Coenzyme a Quinate Transferase Genes from Artichoke Are Involved in the Synthesis of Chlorogenic Acid. Plant Physiol. 2010, 153, 1224–1238. [Google Scholar] [CrossRef] [Green Version]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, Health Benefits and Food Applications; Elsevier: Amsterdam, The Netherlands, 2016; Volume 147. [Google Scholar]
- Raninen, K.; Lappi, J.; Mykkänen, H.; Poutanen, K. Dietary Fiber Type Reflects Physiological Functionality: Comparison of Grain Fiber, Inulin, and Polydextrose. Nutr. Rev. 2011, 69, 9–21. [Google Scholar] [CrossRef]
- Stoyanova, S.; Geuns, J.; Hideg, É.; Van Den Ende, W. The Food Additives Inulin and Stevioside Counteract Oxidative Stress. Int. J. Food Sci. Nutr. 2011, 62, 207–214. [Google Scholar] [CrossRef]
- Kusch, U.; Greiner, S.; Steininger, H.; Meyer, A.D.; Corbière-Divialle, H.; Harms, K.; Rausch, T. Dissecting the Regulation of Fructan Metabolism in Chicory (Cichorium intybus) Hairy Roots. New Phytol. 2009, 184, 127–140. [Google Scholar] [CrossRef]
- van Arkel, J.; Vergauwen, R.; Sévenier, R.; Hakkert, J.C.; van Laere, A.; Bouwmeester, H.J.; Koops, A.J.; van der Meer, I.M. Sink Filling, Inulin Metabolizing Enzymes and Carbohydrate Status in Field Grown Chicory (Cichorium intybus L.). J. Plant Physiol. 2012, 169, 1520–1529. [Google Scholar] [CrossRef]
- Jian, H.; Lu, K.; Yang, B.; Wang, T.; Zhang, L.; Zhang, A.; Wang, J.; Liu, L.; Qu, C.; Li, J. Genome-Wide Analysis and Expression Profiling of the SUC and SWEET Gene Families of Sucrose Transporters in Oilseed Rape (Brassica napus L.). Front. Plant Sci. 2016, 7, 1464. [Google Scholar] [CrossRef] [Green Version]
- Péron, T.; Candat, A.; Montiel, G.; Veronesi, C.; Macherel, D.; Delavault, P.; Simier, P. New Insights into Phloem Unloading and Expression of Sucrose Transporters in Vegetative Sinks of the Parasitic Plant Phelipanche ramosa L. (Pomel). Front. Plant Sci. 2017, 7, 2048. [Google Scholar] [CrossRef] [Green Version]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-Sink Transport of Sugar and Regulation by Environmental Factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [Green Version]
- Alves, H.L.S.; Matiolli, C.C.; Soares, R.C.; Almadanim, M.C.; Oliveira, M.M.; Abreu, I.A. Carbon/Nitrogen Metabolism and Stress Response Networks—Calcium-Dependent Protein Kinases as the Missing Link? J. Exp. Bot. 2021, 72, 4190–4201. [Google Scholar] [CrossRef]
- Di Martino, C.; Torino, V.; Minotti, P.; Pietrantonio, L.; Del Grosso, C.; Palmieri, D.; Palumbo, G.; Crawford, T.W.; Carfagna, S. Mycorrhized Wheat Plants and Nitrogen Assimilation in Coexistence and Antagonism with Spontaneous Colonization of Pathogenic and Saprophytic Fungi in a Soil of Low Fertility. Plants 2022, 11, 924. [Google Scholar] [CrossRef]
- Kelly, S. The Amount of Nitrogen Used for Photosynthesis Modulates Molecular Evolution in Plants. Mol. Biol. Evol. 2018, 35, 1616–1625. [Google Scholar] [CrossRef] [Green Version]
- Hoff, J.E.; Wilcox, G.E.; Jones, C.M. The Effect of Nitrate and Ammonium Nitrogen on the Free Amino Acid Composition of Tomato Plants and Tomato Fruit. J. Am. Soc. Hortic. Sci. 1994, 99, 27–30. [Google Scholar] [CrossRef]
- Hirel, B.; Bertin, P.; Quilleré, I.; Bourdoncle, W.; Attagnant, C.; Dellay, C.; Gouy, A.; Cadiou, S.; Retailliau, C.; Falque, M.; et al. Towards a Better Understanding of the Genetic and Physiological Basis for Nitrogen Use Efficiency in Maize. Plant Physiol. 2001, 125, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Koch, M.; Breithaupt, C.; Kiefersauer, R.; Freigang, J.; Huber, R.; Messerschmidt, A. Crystal Structure of Protoporphyrinogen IX Oxidase: A Key Enzyme in Haem and Chlorophyll Biosynthesis. EMBO J. 2004, 23, 1720–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayan, F.E.; Barker, A.; Tranel, P.J. Origins and Structure of Chloroplastic and Mitochondrial Plant Protoporphyrinogen Oxidases: Implications for the Evolution of Herbicide Resistance. Pest. Manag. Sci. 2018, 74, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ahn, Y.O.; Nam, J.-W.; Hong, M.-K.; Song, N.; Kim, T.; Yu, G.-H.; Sung, S.-K. Biochemical and Physiological Mode of Action of Tiafenacil, a New Protoporphyrinogen IX Oxidase-Inhibiting Herbicide. Pestic. Biochem. Physiol. 2018, 152, 38–44. [Google Scholar] [CrossRef]
- Park, M.; Randel, W.J.; Kinnison, D.E.; Bourassa, A.E.; Degenstein, D.A.; Roth, C.Z.; McLinden, C.A.; Sioris, C.E.; Livesey, N.J.; Santee, M.L. Variability of Stratospheric Reactive Nitrogen and Ozone Related to the QBO. J. Geophys. Res. Atmos. 2017, 122, 10103–10118. [Google Scholar] [CrossRef]
- Duke, S.O.; Rebeiz, C.A. Porphyrin Biosynthesis as a Tool in Pest Management, an Overview. In Porphyric Pesticides; ACS Symposium Series; ACS: Washington, DC, USA, 1994. [Google Scholar]
- Hallahan, B.J.; Camilleri, P.; Smith, A.; Bowyer, J.R. Mode of Action Studies on a Chiral Diphenyl Ether Peroxidizing Herbicide: Correlation between Differential Inhibition of Protoporphyrinogen IX Oxidase Activity and Induction of Tetrapyrrole Accumulation by the Enantiomers. Plant Physiol. 1992, 100, 1211–1216. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: A Commonalty in the Response of Plants to Dehydration and Low Temperature. Physiol. Plant 1997, 100, 291–296. [Google Scholar] [CrossRef]
- Puhakainen, T.; Hess, M.W.; Mäkelä, P.; Svensson, J.; Heino, P.; Palva, E.T. Overexpression of Multiple Dehydrin Genes Enhances Tolerance to Freezing Stress in Arabidopsis. Plant Mol. Biol. 2004, 54, 743–753. [Google Scholar] [CrossRef]
- Hundertmark, M.; Hincha, D.K. LEA (Late Embryogenesis Abundant) Proteins and Their Encoding Genes in Arabidopsis Thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, P.; Chakrabarty, D. Dehydrin in the Past Four Decades: From Chaperones to Transcription Co-Regulators in Regulating Abiotic Stress Response. Curr. Res. Biotechnol. 2021, 3, 249–259. [Google Scholar] [CrossRef]
- Abdul Aziz, M.; Sabeem, M.; Mullath, S.K.; Brini, F.; Masmoudi, K. Plant Group II LEA Proteins: Intrinsically Disordered Structure for Multiple Functions in Response to Environmental Stresses. Biomolecules 2021, 11, 1662. [Google Scholar] [CrossRef]
- Kohan-Baghkheirati, E.; Bagherieh-Najjar, M.; Abdolzadeh, A.; Geisler-Lee, J. Altered DREB1A Gene Expression in Arabidopsis Thaliana Leads to Change in Root Growth, Antioxidant Enzymes Activity, and Response to Salinity but Not to Cold. J. Genet. Resour. 2018, 4, 90–104. [Google Scholar] [CrossRef]
- Abedi, S.; Iranbakhsh, A.; Ardebili, Z.O.; Ebadi, M. Nitric Oxide and Selenium Nanoparticles Confer Changes in Growth, Metabolism, Antioxidant Machinery, Gene Expression, and Flowering in Chicory (Cichorium intybus L.): Potential Benefits and Risk Assessment. Environ. Sci. Pollut. Res. 2021, 28, 3136–3148. [Google Scholar] [CrossRef]
- Donde, R.; Gupta, M.K.; Gouda, G.; Kumar, J.; Vadde, R.; Sahoo, K.K.; Dash, S.K.; Behera, L. Computational Characterization of Structural and Functional Roles of DREB1A, DREB1B and DREB1C in Enhancing Cold Tolerance in Rice Plant. Amino Acids 2019, 51, 839–853. [Google Scholar] [CrossRef]
- Kidokoro, S.; Watanabe, K.; Ohori, T.; Moriwaki, T.; Maruyama, K.; Mizoi, J.; Myint Phyu Sin Htwe, N.; Fujita, Y.; Sekita, S.; Shinozaki, K.; et al. Soybean DREB1/CBF-Type Transcription Factors Function in Heat and Drought as Well as Cold Stress-Responsive Gene Expression. Plant J. 2015, 81, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Katan, M.B. Absorption, Metabolism and Health Effects of Dietary Flavonoids in Man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic Compounds and Their Role in Oxidative Processes in Fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Verpoorte, R.; van der Heijden, R.; Memelink, J. Engineering the Plant Cell Factory for Secondary Metabolite Production. Transgenic Res. 2000, 9, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed. Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Amalesh, S.; Gouranga, D.; Sanjoy Kumar, D. Roles of Flavonoids in Plants. Int. J. Pharm. Sci. Technol. 2011, 6, 12–35. [Google Scholar]
- Karami, O.; Aghavaisi, B.; Mahmoudi Pour, A. Molecular Aspects of Somatic-to-Embryogenic Transition in Plants. J. Chem. Biol. 2009, 2, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, J.L. Somatic Embryogenesis: A Model for Early Development in Higher Plants. Plant Cell 1993, 5, 1411–1423. [Google Scholar] [CrossRef] [Green Version]
- Legrand, S.; Hendriks, T.; Hubert, J.L.; Quillet, M.C. Characterization of Expressed Sequence Tags Obtained by SSH during Somatic Embryogenesis in Cichorium intybus L. BMC Plant Biol. 2007, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Lucau-Danila, A.; Laborde, L.; Legrand, S.; Huot, L.; Hot, D.; Lemoine, Y.; Hilbert, J.L.; Hawkins, S.; Quillet, M.C.; Hendriks, T.; et al. Identification of Novel Genes Potentially Involved in Somatic Embryogenesis in Chicory (Cichorium intybus L.). BMC Plant Biol. 2010, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Grimault, V.; Helleboid, S.; Vasseur, J.; Hilbert, J.-L. Co-Localization of ß-1,3-Glucanases and Callose during Somatic Embryogenesis in Cichorium. Plant Signal. Behav. 2007, 2, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.; Rahman, I.A.; Islam, T.; Ghosh, A. Genome-Wide Identification and Expression Analysis of Glutathione S-Transferase Gene Family in Tomato: Gaining an Insight to Their Physiological and Stress-Specific Roles. PLoS ONE 2017, 12, e0187504. [Google Scholar] [CrossRef] [Green Version]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-Transferase Enzymes in Plant-Pathogen Interactions. Front. Plant Sci. 2018, 871, 1836. [Google Scholar] [CrossRef] [Green Version]
- Kays, S.J. Preharvest Factors Affecting Appearance. Postharvest Biol. Technol. 1999, 15, 233–247. [Google Scholar] [CrossRef]
- Hilton, H.W.; Clifford, S.C.; Wurr, D.C.E.; Burton, K.S. The Influence of Agronomic Factors on the Visual Quality of Field-Grown, Minimally-Processed Lettuce. J. Hortic. Sci. Biotechnol. 2009, 84, 193–198. [Google Scholar] [CrossRef]
- Hunter, P.J.; Chadwick, M.; Graceson, A.; Hambidge, A.; Hand, P.; Heath, J.; Lignou, S.; Oruna-Concha, M.J.; Pink, D.; Rada, B.; et al. Elucidation of the Biochemical Pathways Involved in Two Distinct Cut-Surface Discolouration Phenotypes of Lettuce. Postharvest Biol. Technol. 2022, 183, 111753. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Tsironi, T.N. Application of Processing and Packaging Hurdles for Fresh-Cut Fruits and Vegetables Preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef]
- Belen Martin-Diana, A.; Rico, D.; Barry-Ryan, C.; Mulcahy, J.; Frias, J.; Henehan, G.T.M. Effect of Heat Shock on Browning-Related Enzymes in Minimally Processed Iceberg Lettuce and Crude Extracts. Biosci. Biotechnol. Biochem. 2005, 69, 1677–1685. [Google Scholar] [CrossRef] [Green Version]
- Gimeno, J.; Eattock, N.; Van Deynze, A.; Blumwald, E. Selection and Validation of Reference Genes for Gene Expression Analysis in Switchgrass (Panicum virgatum) Using Quantitative Real-Time RT-PCR. PLoS ONE 2014, 9, e91474. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Yan, H.; Jiang, X.; Zhang, X.; Zhang, Y.; Huang, X.; Zhang, Y.; Miao, J.; Xu, B.; Frazier, T.; et al. Evaluation of Candidate Reference Genes for Normalization of Quantitative RT-PCR in Switchgrass Under Various Abiotic Stress Conditions. Bioenergy Res. 2014, 7, 1201–1211. [Google Scholar] [CrossRef]

| Purpose | Topic | Keywords Terms Searched |
|---|---|---|
| Development of a consensus map for marker-assisted breeding | Identification of SSR sequences | “SSR” OR “microsatellite” |
| Identification of SNP sequences | “SNP” OR “SNV” | |
| Development of a comprehensive map for marker-assisted selection | Reproductive barriers | “male-sterility” OR “self-incompatibility” OR “CMS” OR “NMS” OR “SSI” OR “S-locus” |
| Sesquiterpene lactone biosynthesis | “sesquiterpene lactones” OR “STL” OR “lactucin” | |
| Hydroxycinnamates | “hydroxycinnamates” OR “HCA” OR “chlorogenic acid” | |
| Inulin metabolism | “inulin” OR “fructan” | |
| Stress response | “stressors” OR “stress” OR “biotic stress” OR “abiotic stress” OR “stress response” OR “stress tolerance” | |
| Blue-lilac color | “flavonoids” OR “anthocyanin” OR “flower color” | |
| Flowering time | “flowering” OR “flowering time” OR “flowering response” | |
| Somatic embryogenesis | “somatic embryogenesis” OR “SE” | |
| Red discoloration | “discoloration” OR “cuttings response” | |
| Gene normalization | “gene normalization” OR “reference genes” OR “data normalization” |
| References | De Simone et al. [21] | Van Stallen et al. [22] | Van Stallen et al. [23] | Cassan et al. [24] | Cadalen et al. [25] | Gonthier et al. [12] | Muys et al. [27] | Palumbo et al. [28] | Consensus Map (from Fan et al. [20]) |
|---|---|---|---|---|---|---|---|---|---|
| LG ⟷ Chr | N.a. | N.a. | N.a. | N.a. | LG8 | LG8 | LG5 | LG2 | Chr1 |
| LG3 | LG3 | LG6 | LG3 | Chr2 | |||||
| LG6 | LG6 | LG8 | LG1 | Chr3 | |||||
| LG1 | LG1 | LG3 | LG6 | Chr4 | |||||
| LG2 | LG2 | LG9 | LG5 | Chr5 | |||||
| LG9 | LG9 | LG4 | LG8 | Chr6 | |||||
| LG5 | LG5 | LG1 | LG7 | Chr7 | |||||
| LG7 | LG7 | LG2 | LG4 | Chr8 | |||||
| LG4 | LG4 | LG7 | LG9 | Chr9 | |||||
| Markers | 16 RAPDs; 72 SAMPLs; 283 AFLPs | 129 RAPDs | RAPDs | 73 RAPDs; 9 SSRs | 472 SSRs | SSRs; AFLPs | 170 AFLPs; 28 SSRs; 27 EST-SNPs; 12 EST-SSRs | 727 SNPs | 579 SNPs; 60 SSRs |
| Length | 1201 cM | 609.6 cM | N.a. | 987 cM | 878 cM | N.a. | 1208 cM | 1413 cM | 1280 Mb |
| Source | C. intybus (radicchio) × C. endivia (escarole) | C. intybus (witloof) | C. intybus (witloof) | C. intybus (witloof) | C. intybus (industrial); C. intybus (witloof) | C. intybus (industrial) | C. intybus (industrial); | C. intybus (radicchio) | C. intybus (Grassland Puna) |
| Traits/Topic | Gene or Marker Locus | Methods | Citation |
|---|---|---|---|
| Reproductive barriers 1 | NMS-related (NMS1, NMS2) | Genetic mapping (AFLP-based SCARs) | [12] |
| ms1 (MYB103-like) | Genetic mapping (SSR; SNP) | [25,28,35] | |
| S-related (S1-S4), MIK2 | Genetic mapping (AFLP-based SCARs) | [12,36] | |
| Sesquiterpene lactone biosynthesis (STL) 2 | GASlo, GASsh | cDNA library construction; expression analysis in E. coli | [37] |
| GAO | Analyses of the metabolites from transgenic yeast; immunoblot analysis; in vitro enzyme assay; GC—MS enzyme assay | [38] | |
| CYP71AV8 | Isolation; cloning; coexpression in yeasts; GC—MS analysis | [39] | |
| GASsh2, GAS1, GAO, COS | RNA sequencing, transcriptome assembly, functional annotation, gene expression analyses, qPCR | [40] | |
| Hydroxycinnamates (HCAs) 3 | HCT1, HCT2, HQT1, HQT2, HQT3 | In vitro assays of recombinant proteins in E. coli, transient expression in N. benthamiana, SDS—PAGE and immunoblot analysis | [41] |
| Inulin metabolism 4 | 1-FEH I | Cloning, MALDI-TOF and Q-TOF analyses, and expression in transgenic potato tubers | [42] |
| 1-FEH IIa, 1-FEH IIb | Sequencing, Q-TOF analyses, RNA isolation, RT—PCR, and subcloning | [43] | |
| 1-FEH IIa2 | Northern blot hybridization, Transient expression analysis, Promoter analysis | [44] | |
| 1- FEH IIb2, CiMYB17, SUT1, SUT2, SUT3,1-SST, 1-FFT | RNAseq, yeast one-hybrid assay, transfection experiments, transient expression in grapevine (Vitis vinifera), and qPCR | [10,45] | |
| Stress response 5 | nia gene | Northern blot analysis, ln-situ hybridization, cloning, and sequencing | [46] |
| PPX1 | Ion exchange chromatography, construction of a cDNA library, cloning, Expression analysis in E. coli, and ProTox assay | [47] | |
| CAld5H (bip41) | Genetic mapping (SNP) | [27] | |
| DHN1, DHN2 | Southern blot analysis, Northern blot analysis, cloning, and transcription promoter analysis | [48] | |
| CiNHX1 | Cloning of CiDREB1, transformation in E. coli, qPCR, and subcellular localization | [49] | |
| DREB1A, DREB1B | Subcloning, sequencing, qPCR, protein localization, and functional expression in a yeast mutant | [50] | |
| Blue-lilac color 6 | F3’H, F3’5’H | Extraction of anthocyanins, cloning of F3’H and F3 5 H cDNAs, and expression analysis in yeast | [51] |
| Flowering time 7 | FL1 gene | Cloning, qPCR, construction of transgenics, and transformation in Arabidopsis via floral dip method | [52] |
| Somatic embryogenesis 8 | nsHb | Construction of cDNA library in a phage lambda and integration in E. coli, differential screening of the cDNA library, and Northern blot analysis | [53] |
| chi-GST1 | RT—PCR, Northern blot analysis, and expression of the protein in E. coli, | [54] | |
| CG1 | qPCR, RACE PCR, in vivo expression of cDNAs in Escherichia coli, Southern blot analysis, and Northern blot analysis | [55] | |
| GTP1/2 | Cloning, library construction and screening, Northern blot analysis, (RACE) PCR, RT—PCR, and expression analysis in BL21 bacterial cells | [56] | |
| Red discoloration 9 | PAL1, PAL2 | Color analysis, RNA extraction, cDNA synthesis, and qPCR | [57] |
| Gene normalization 10 | βTUB, UBQ10, SAND, Clath, TIP41, PP2AA3, CYP5, ACT2, PROF, ACT7 | Determination of reference gene expression stability using geNorm, NormFinder and BestKeeper | [58] |
| ACT, EF-1αM, NADHD, His-H3, rRNA, TUB | Determination of reference gene expression stability using geNorm, NormFinder and BestKeeper | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Draga, S.; Gabelli, G.; Palumbo, F.; Barcaccia, G. Genome-Wide Datasets of Chicories (Cichorium intybus L.) for Marker-Assisted Crop Breeding Applications: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 11663. https://doi.org/10.3390/ijms241411663
Draga S, Gabelli G, Palumbo F, Barcaccia G. Genome-Wide Datasets of Chicories (Cichorium intybus L.) for Marker-Assisted Crop Breeding Applications: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2023; 24(14):11663. https://doi.org/10.3390/ijms241411663
Chicago/Turabian StyleDraga, Samela, Giovanni Gabelli, Fabio Palumbo, and Gianni Barcaccia. 2023. "Genome-Wide Datasets of Chicories (Cichorium intybus L.) for Marker-Assisted Crop Breeding Applications: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 24, no. 14: 11663. https://doi.org/10.3390/ijms241411663
APA StyleDraga, S., Gabelli, G., Palumbo, F., & Barcaccia, G. (2023). Genome-Wide Datasets of Chicories (Cichorium intybus L.) for Marker-Assisted Crop Breeding Applications: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 24(14), 11663. https://doi.org/10.3390/ijms241411663







