Transcriptome Dynamics during Spike Differentiation of Wheat Reveal Amazing Changes in Cell Wall Metabolic Regulators
Abstract
1. Introduction
2. Results
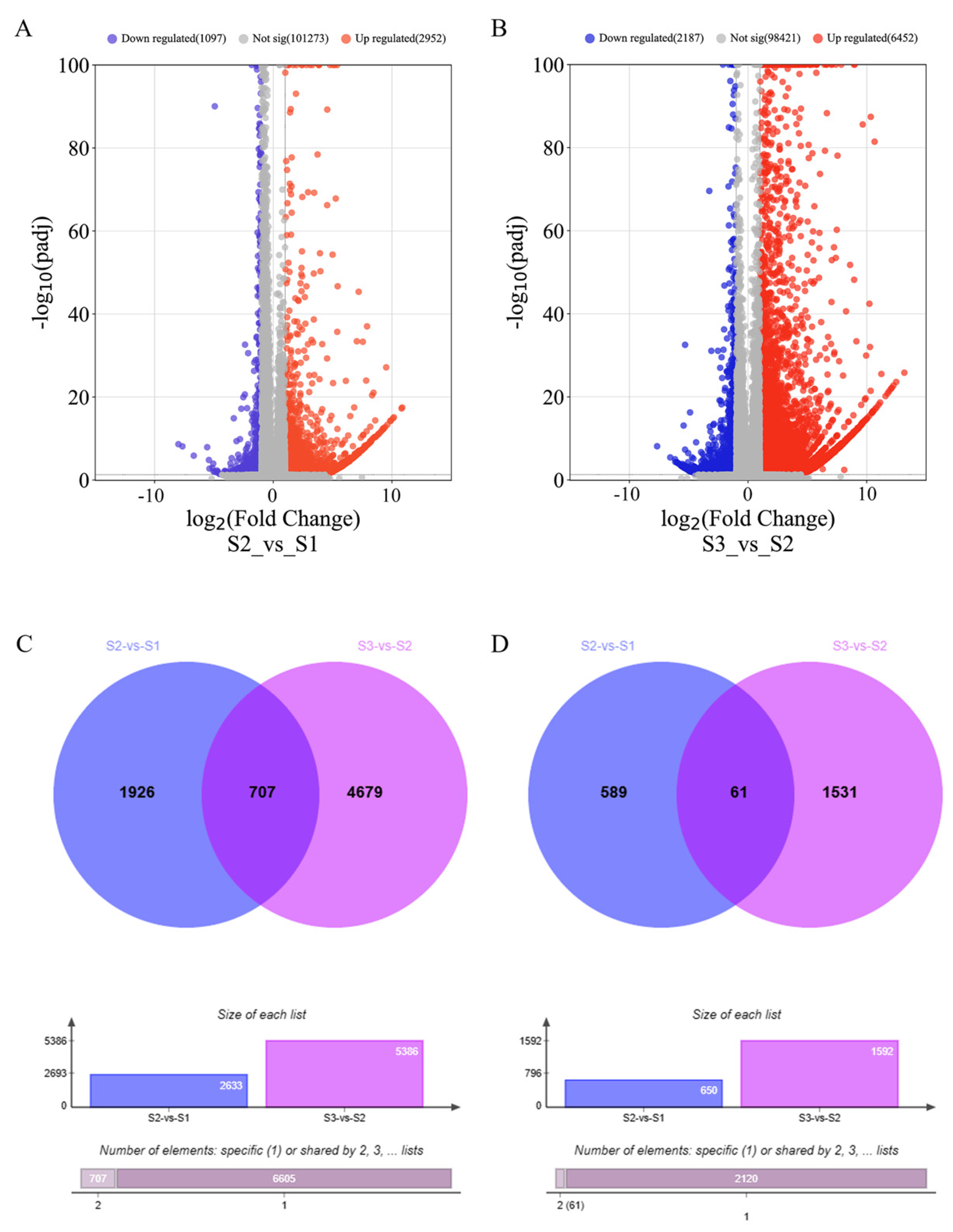
2.1. Analysis of Transcriptome Expression Profile and Differentially Expressed Genes during Wheat Spike Development
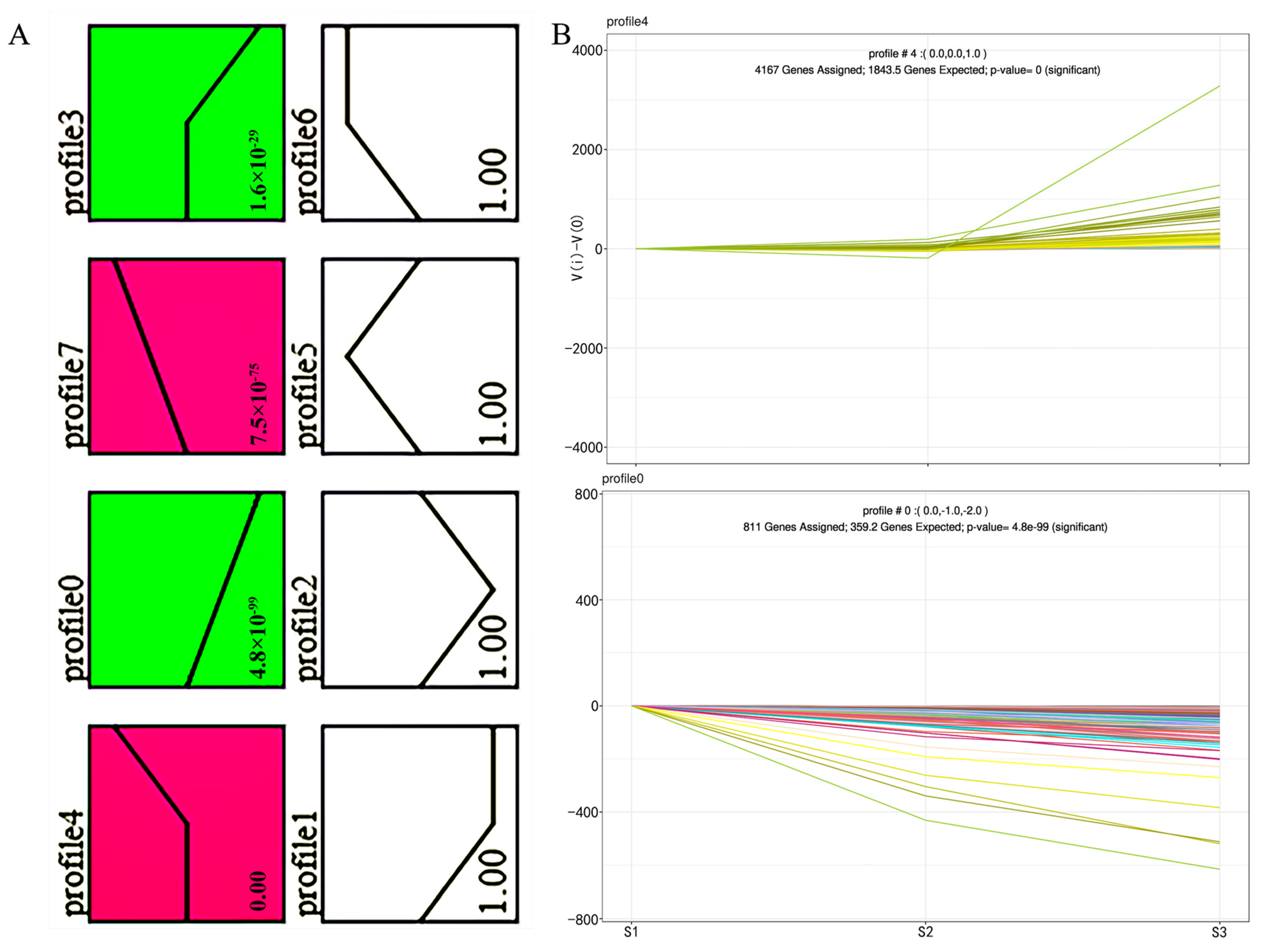
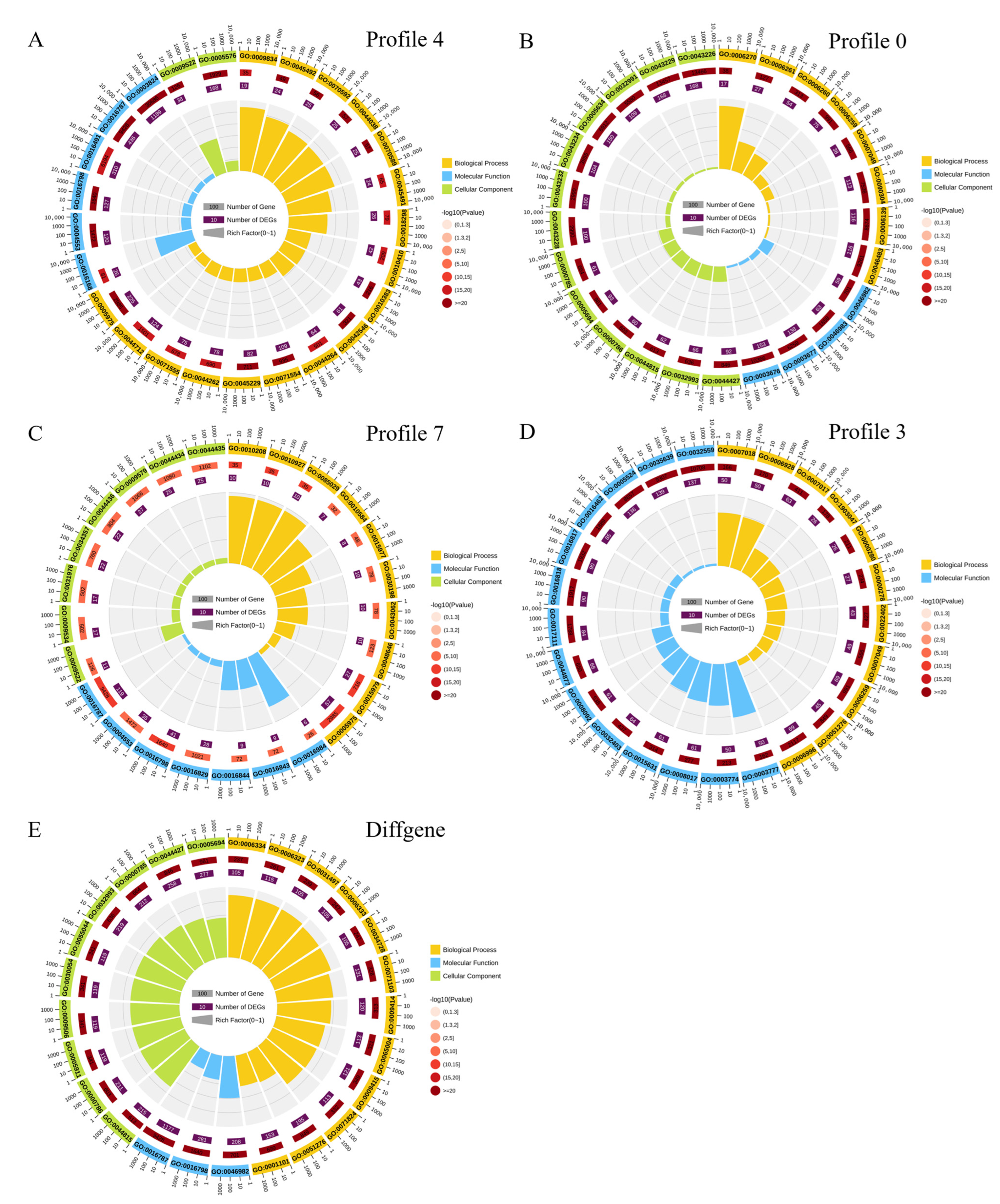
2.2. STC and GO Annotation Analysis of DEGs
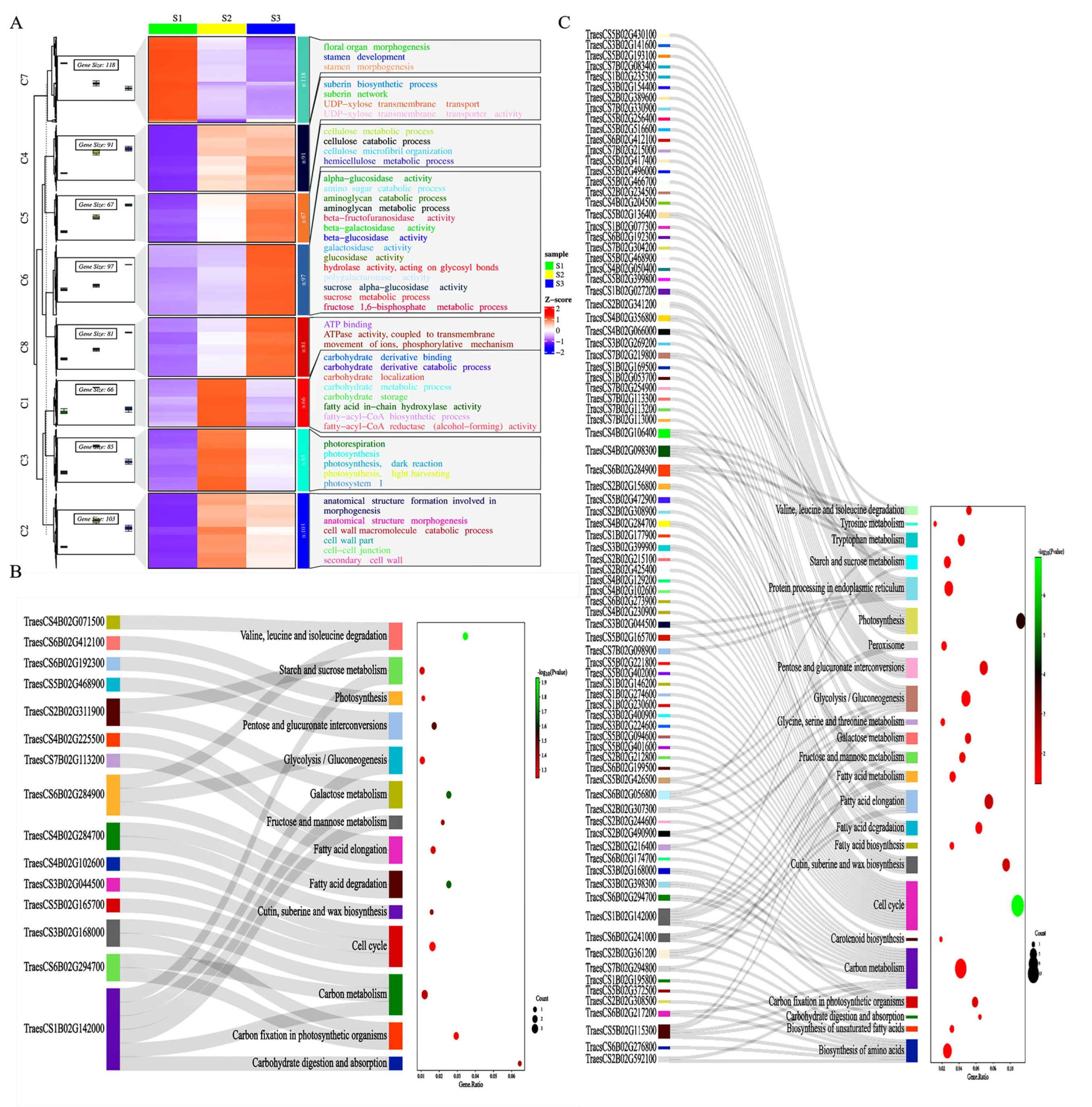
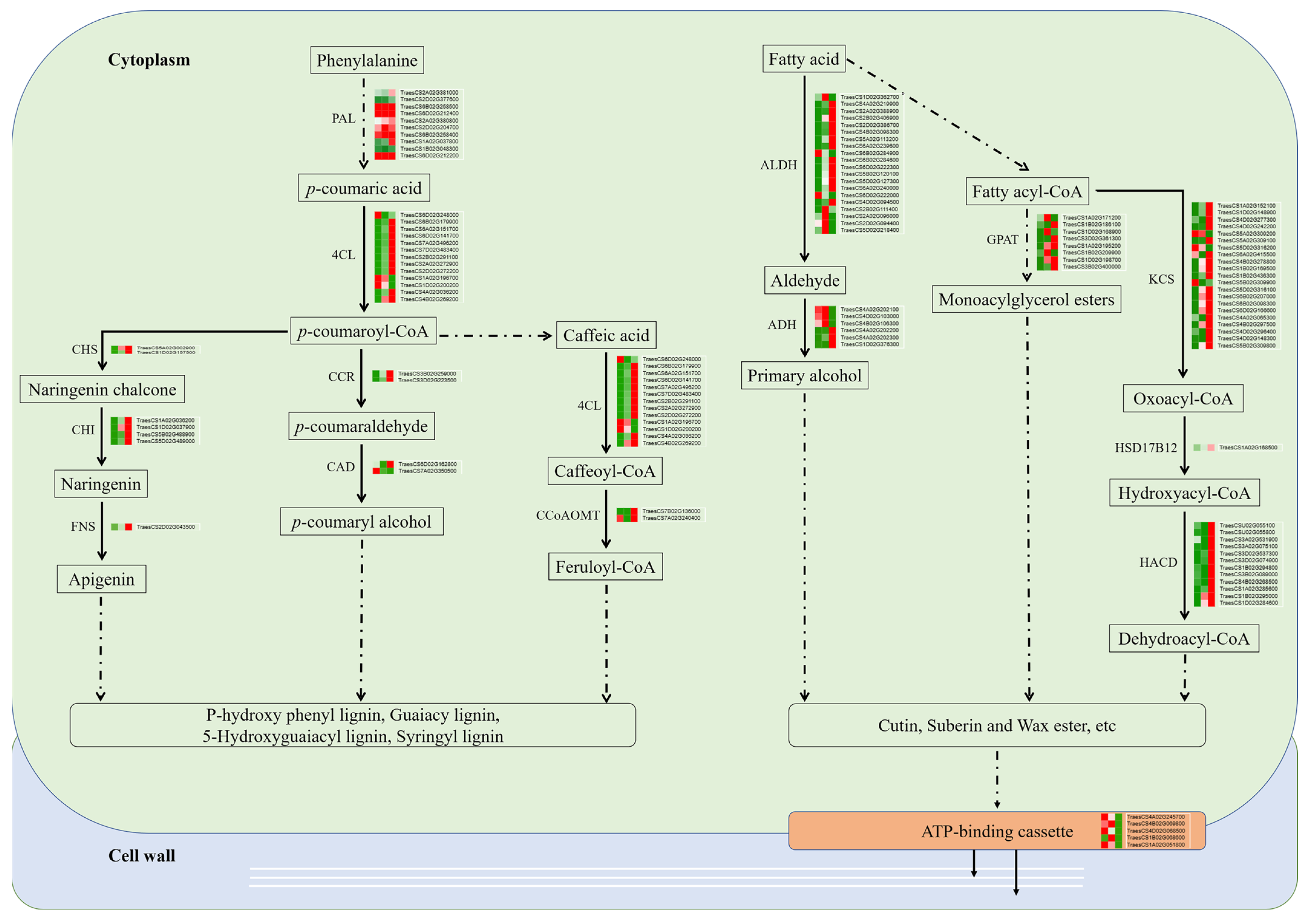
2.3. MapMan Analysis Uncovered the Involvement of Active Cell Walls and Carbohydrate Metabolism during Spike Development
2.4. Validation of Differentially Expressed Genes by qRT-PCR
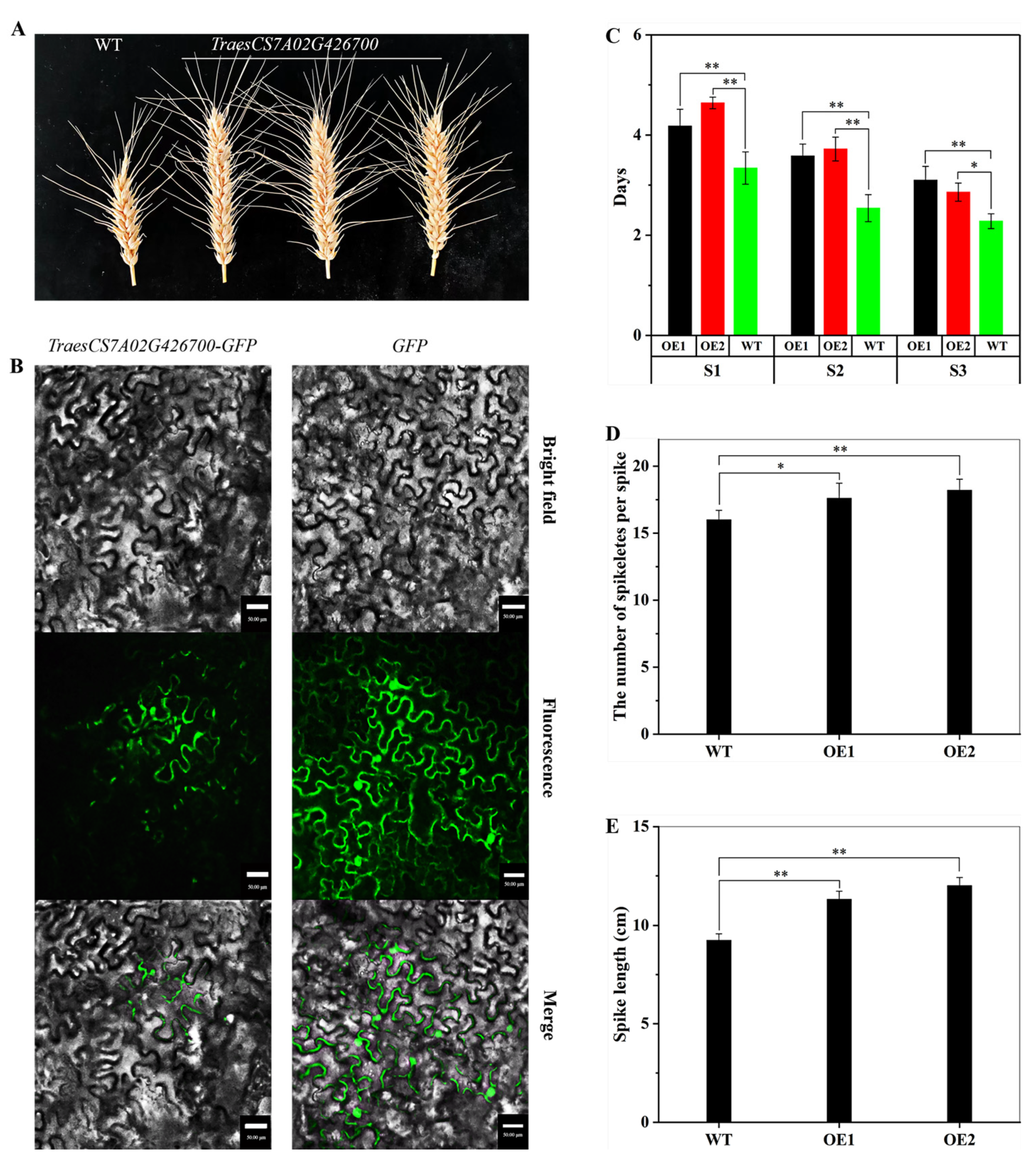
2.5. Experimental Verification of Candidate Spike Regulator Gene
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Gene Function Annotation and Enrichment Analysis
4.3. Series Test of Cluster (STC) Analysis of DEGs
4.4. MapMan Analysis
4.5. RNA Extraction and Real-Time Quantitative PCR
4.6. Construction of pCAMBIA1301-TraesCS7A02G426700 Vector for Agrobacterium-Mediated Wheat Transformation
4.7. Subcellular Localization of The TraesCS7A02G426700
4.8. Microscopic Observation of WT and Transgenic Plants
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yang, Y.; Lin, X.; Xiao, J. Deciphering spike architecture formation towards yield improvement in wheat. J. Genet. Genom. 2023. [Google Scholar] [CrossRef] [PubMed]
- Shitsukawa, N.; Kinjo, H.; Takumi, S.; Murai, K. Heterochronic development of the floret meristem determines grain number per spikelet in diploid, tetraploid and hexaploid wheats. Ann. Bot. 2009, 104, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-Q.; Wang, N.; Wang, X.-L.; Zhang, X.S. Architecture of Wheat Inflorescence: Insights from Rice. Trends Plant Sci. 2019, 24, 802–809. [Google Scholar] [CrossRef]
- Theißen, G.; Saedler, H. Floral quartets. Nature 2001, 409, 469–471. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, Z. Molecular Control of Grass Inflorescence Development. Annu. Rev. Plant Biol. 2014, 65, 553–578. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Schnurbusch, T. A genetic playground for enhancing grain number in cereals. Trends Plant Sci. 2012, 17, 91–101. [Google Scholar] [CrossRef]
- Tanaka, W.; Pautler, M.; Jackson, D.; Hirano, H.-Y. Grass Meristems II: Inflorescence Architecture, Flower Development and Meristem Fate. Plant Cell Physiol. 2013, 54, 313–324. [Google Scholar] [CrossRef]
- Liu, K.; Sun, X.; Ning, T.; Duan, X.; Wang, Q.; Liu, T.; An, Y.; Guan, X.; Tian, J.; Chen, J. Genetic dissection of wheat panicle traits using linkage analysis and a genome-wide association study. Theor. Appl. Genet. 2018, 131, 1073–1090. [Google Scholar] [CrossRef]
- Simons, K.J. Molecular Characterization of the Major Wheat Domestication Gene Q. Genetics 2005, 172, 547–555. [Google Scholar] [CrossRef]
- Miao, L.; Mao, X.; Wang, J.; Liu, Z.; Zhang, B.; Li, W.; Chang, X.; Reynolds, M.; Wang, Z.; Jing, R. Elite Haplotypes of a Protein Kinase Gene TaSnRK2.3 Associated with Important Agronomic Traits in Common Wheat. Front. Plant Sci. 2017, 08, 368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-G.; Lv, G.-D.; Li, B.; Wang, J.-J.; Zhao, Y.; Kong, F.-M.; Guo, Y.; Li, S.-S. Isolation and characterization of the TaSnRK2.10 gene and its association with agronomic traits in wheat (Triticum aestivum L.). PLoS ONE 2017, 12, e0174425. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Song, G.; Guan, J.; Chen, K.; Jia, M.; Huang, D.; Wu, J.; Zhang, L.; Kong, X.; Geng, S.; et al. Transcriptome Profiling of Wheat Inflorescence Development from Spikelet Initiation to Floral Patterning Identified Stage-Specific Regulatory Genes. Plant Physiol. 2017, 174, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.B.; Nalam, V.J.; Zemetra, R.S.; Riera-Lizarazu, O. Mapping the compactum locus in wheat (Triticum aestivum L.) and its relationship to other spike morphology genes of the Triticeae. Euphytica 2007, 163, 193–201. [Google Scholar] [CrossRef]
- Boden, S.A.; Cavanagh, C.; Cullis, B.R.; Ramm, K.; Greenwood, J.; Finnegan, E.J.; Trevaskis, B.; Swain, S.M. Ppd-1 is a key regulator of inflorescence architecture and paired spikelet development in wheat. Nat. Plants 2015, 1, 14016. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, X.; Zhao, M.; Zhang, W.; Li, B.; An, D.; Li, J.; Zhang, A.; Liu, R.; Liu, X. A Genome-wide View of Transcriptome Dynamics During Early Spike Development in Bread Wheat. Sci. Rep. 2018, 8, 15338. [Google Scholar] [CrossRef]
- The International Wheat Genome Sequencing Consortium (IWGSC); Appels, R.; Eversole, K.; Feuillet, C.; Keller, B.; Rogers, J.; Stein, N.; Pozniak, C.J.; Stein, N.; Choulet, F.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef]
- Shi, X.; Cui, F.; Han, X.; He, Y.; Zhao, L.; Zhang, N.; Zhang, H.; Zhu, H.; Liu, Z.; Ma, B.; et al. Comparative genomic and transcriptomic analyses uncover the molecular basis of high nitrogen-use efficiency in the wheat cultivar Kenong 9204. Mol. Plant 2022, 15, 1440–1456. [Google Scholar] [CrossRef]
- Reiter, W.-D. Biosynthesis and properties of the plant cell wall. Curr. Opin. Plant Biol. 2002, 5, 536–542. [Google Scholar] [CrossRef]
- Meents, M.; Watanabe, Y.; Samuels, A.L. The cell biology of secondary cell wall biosynthesis. Ann. Bot. 2018, 121, 1107–1125. [Google Scholar] [CrossRef]
- Perrot, T.; Pauly, M.; Ramírez, V. Emerging roles of β-glucanases in plant development and adaptative responses. Plants 2022, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Lv, D.; Yan, X.; Subburaj, S.; Ge, P.; Li, X.; Hu, Y.; Yan, Y. Proteome characterization of developing grains in bread wheat cultivars (Triticum aestivum L.). BMC Plant Biol. 2012, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Eimert, K.; Villand, P.; Kilian, A.; Kleczkowski, L.A. Cloning and characterization of several cDNAs for UDP-glucose pyrophosphorylase from barley (Hordeum vulgare) tissues. Gene 1996, 170, 227–232. [Google Scholar] [CrossRef]
- Ap Rees, T.; Leja, M.; Macdonald, F.; Green, J. Nucleotide sugars and starch synthesis in spadix of Arum maculatum and suspension cultures of Glycine max. Phytochemistry 1984, 23, 2463–2468. [Google Scholar] [CrossRef]
- Hara, Y.; Yokoyama, R.; Osakabe, K.; Toki, S.; Nishitani, K. Function of xyloglucan endotransglucosylase/hydrolases in rice. Ann. Bot. 2013, 114, 1309–1318. [Google Scholar] [CrossRef]
- Hazen, S.P.; Scott-Craig, J.S.; Walton, J.D. Cellulose Synthase-Like Genes of Rice. Plant Physiol. 2002, 128, 336–340. [Google Scholar] [CrossRef]
- Oraby, H.; Venkatesh, B.; Dale, B.; Ahmad, R.; Ransom, C.; Oehmke, J.; Sticklen, M. Enhanced conversion of plant biomass into glucose using transgenic rice-produced endoglucanase for cellulosic ethanol. Transgenic Res. 2007, 16, 739–749. [Google Scholar] [CrossRef]
- Stahl, U. Cloning and Functional Characterization of a Phospholipid: Diacylglycerol Acyltransferase from Arabidopsis. Plant Physiol. 2004, 135, 1324–1335. [Google Scholar] [CrossRef]
- Joo, J.-Y.; Kim, M.-S.; Sung, J. Transcriptional Changes of Cell Wall Organization Genes and Soluble Carbohydrate Alteration during Leaf Blade Development of Rice Seedlings. Plants 2021, 10, 823. [Google Scholar] [CrossRef]
- Hu, R.; Xu, Y.; Yu, C.; He, K.; Tang, Q.; Jia, C.; He, G.; Wang, X.; Kong, Y.; Zhou, G. Transcriptome analysis of genes involved in secondary cell wall biosynthesis in developing internodes of Miscanthus lutarioriparius. Sci. Rep. 2017, 7, 9034. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Kadoya, K.; Iiyama, K. Bonding of hydroxycinnamic acids to lignin: Ferulic and p-coumaric acids are predominantly linked at the benzyl position of lignin, not the β-position, in grass cell walls. Phytochemistry 2001, 57, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.C.; Willis, S.C.; Paterson, A.E.J. Intraprotoplasmic and wall-localised formation of arabinoxylan-bound diferulates and larger ferulate coupling-products in maize cell-suspension cultures. Planta 2000, 211, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; Nishitani, K. A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of Arabidopsis. Plant Cell Physiol. 2001, 42, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Eveland, A.L.; Goldshmidt, A.; Pautler, M.; Morohashi, K.; Liseron-Monfils, C.; Lewis, M.W.; Kumari, S.; Hiraga, S.; Yang, F.; Unger-Wallace, E.; et al. Regulatory modules controlling maize inflorescence architecture. Genome Res. 2013, 24, 431–443. [Google Scholar] [CrossRef]
- Furutani, I.; Sukegawa, S.; Kyozuka, J. Genome-wide analysis of spatial and temporal gene expression in rice panicle development. Plant J. 2006, 46, 503–511. [Google Scholar] [CrossRef]
- Digel, B.; Pankin, A.; von Korff, M. Global Transcriptome Profiling of Developing Leaf and Shoot Apices Reveals Distinct Genetic and Environmental Control of Floral Transition and Inflorescence Development in Barley. Plant Cell 2015, 27, 2318–2334. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Tian, C.; Sajjad, M.; Gao, C.; Tong, Y.; Wang, X.; Jiao, Y. Transcriptome Association Identifies Regulators of Wheat Spike Architecture. Plant Physiol. 2017, 175, 746–757. [Google Scholar] [CrossRef]
- Dobrovolskaya, O.; Pont, C.; Sibout, R.; Martinek, P.; Badaeva, E.; Murat, F.; Chosson, A.; Watanabe, N.; Prat, E.; Gautier, N.; et al. FRIZZY PANICLE Drives Supernumerary Spikelets in Bread Wheat. Plant Physiol. 2014, 167, 189–199. [Google Scholar] [CrossRef]
- Hagemann, W. Comparative morphology of acrogenous branch systems and phylogenetic considerations. II. Angiosperms. Acta Biotheor. 1990, 38, 207–242. [Google Scholar] [CrossRef]
- Guo, Z.; Schnurbusch, T. Variation of floret fertility in hexaploid wheat revealed by tiller removal. J. Exp. Bot. 2015, 66, 5945–5958. [Google Scholar] [CrossRef] [PubMed]
- Mollet, J.C.; Leroux, C.; Dardelle, F.; Lehner, A. Cell wall composition, biosynthesis and remodeling during pollen tube growth. Plants 2013, 2, 107–147. [Google Scholar] [CrossRef] [PubMed]
- Verhertbruggen, Y.; Marcus, S.E.; Chen, J.; Knox, J.P. Cell Wall Pectic Arabinans Influence the Mechanical Properties of Arabidopsis thaliana Inflorescence Stems and Their Response to Mechanical Stress. Plant Cell Physiol. 2013, 54, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Gookin, T.E.; Hunter, D.A.; Reid, M.S. Temporal analysis of alpha and beta-expansin expression during floral opening and se-nescence. Plant Sci. 2003, 164, 769–781. [Google Scholar] [CrossRef]
- Takahashi, R.; Fujitani, C.; Yamaki, S.; Yamada, K. Analysis of the cell wall loosening proteins during rose flower opening. In Proceedings of the International Conference on Quality Management in Supply Chains of Ornamentals 755, Bangkok, Thailand, 3–6 December 2007; pp. 483–488. [Google Scholar]
- Yamada, K.; Takahashi, R.; Fujitani, C.; Mishima, K.; Yoshida, M.; Joyce, D.C.; Yamaki, S. Cell wall extensibility and effect of cell-wall-loosening proteins during rose flower opening. J. Jpn. Soc. Hortic. Sci. 2009, 78, 242–251. [Google Scholar] [CrossRef]
- O’Donoghue, E.M.; Somerfield, S.D.; Heyes, J.A. Organization of cell walls in Sandersonia aurantiaca floral tissue. J. Exp. Bot. 2002, 53, 513–523. [Google Scholar] [CrossRef]
- Watanabe, Y.; Niki, T.; Norikoshi, R.; Nakano, M.; Ichimura, K. Soluble carbohydrate concentration and expression of expansin and xyloglucan en-dotransglucosylase/hydrolase genes in epidermal and parenchyma cells during lily flower opening. J. Plant Physiol. 2022, 270, 153615. [Google Scholar] [CrossRef]
- Azeez, A.; Sane, A.P.; Tripathi, S.K.; Bhatnagar, D.; Nath, P. The gladiolus GgEXPA1 is a GA-responsive alpha-expansin gene expressed ubiquitously during expansion of all floral tissues and leaves but repressed during organ senescence. Postharvest Biol. Technol. 2010, 58, 48–56. [Google Scholar] [CrossRef]
- Franková, L.; Fry, S.C. Biochemistry and physiological roles of enzymes that ‘cut and paste’ plant cell-wall polysaccharides. J. Exp. Bot. 2013, 64, 3519–3550. [Google Scholar] [CrossRef]
- Van Sandt, V.S.T.; Suslov, D.; Verbelen, J.-P.; Vissenberg, K. Xyloglucan Endotransglucosylase Activity Loosens a Plant Cell Wall. Ann. Bot. 2007, 100, 1467–1473. [Google Scholar] [CrossRef]
- Shi, J.; Cui, M.; Yang, L.; Kim, Y.-J.; Zhang, D. Genetic and Biochemical Mechanisms of Pollen Wall Development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bonawitz, N.D.; Weng, J.-K.; Chapple, C. The Growth Reduction Associated with Repressed Lignin Biosynthesis in Arabidopsis thaliana Is Independent of Flavonoids. Plant Cell 2010, 22, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Han, Y.; Li, D.; Lin, Y.; Cai, Y. Systematic Analysis of the 4-Coumarate:Coenzyme A Ligase (4CL) Related Genes and Expression Profiling during Fruit Development in the Chinese Pear. Genes 2016, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Schmid, K.M.; Ohlrogge, J.B. Lipid metabolism in plants. Biochem. Lipids Lipoproteins Membr. 2008, 97–130. [Google Scholar] [CrossRef]
- Llewellyn, D.; Finnegan, E.; Ellis, J.; Dennis, E.; Peacock, W. Structure and expression of an alcohol dehydrogenase 1 gene from Pisum sativum (cv. “Greenfeast”). J. Mol. Biol. 1987, 195, 115–123. [Google Scholar] [CrossRef]
- Millar, A.A.; Kunst, L. Very-long-chain fatty acid biosynthesis is controlled through the expression and specificity of the condensing enzyme. Plant J. 1997, 12, 121–131. [Google Scholar] [CrossRef]
- Chen, G.; Komatsuda, T.; Ma, J.F.; Nawrath, C.; Pourkheirandish, M.; Tagiri, A.; Hu, Y.-G.; Sameri, M.; Li, X.; Zhao, X.; et al. An ATP-binding cassette subfamily G full transporter is essential for the retention of leaf water in both wild barley and rice. Proc. Natl. Acad. Sci. USA 2011, 108, 12354–12359. [Google Scholar] [CrossRef]
- Hyodo, H.; Yamakawa, S.; Takeda, Y.; Tsuduki, M.; Yokota, A.; Nishitani, K.; Kohchi, T. Active gene expression of a xyloglucan endotransglucosylase/hydrolase gene, XTH9, in inflorescence apices is related to cell elongation in Arabidopsis thaliana. Plant Mol. Biol. 2003, 52, 473–482. [Google Scholar] [CrossRef]
- Miedes, E.; Lorences, E.P. Xyloglucan endotransglucosylase/hydrolases (XTHs) during tomato fruit growth and ripening. J. Plant Physiol. 2009, 166, 489–498. [Google Scholar] [CrossRef]
- Genovesi, V.; Fornalé, S.; Fry, S.C.; Ruel, K.; Ferrer, P.; Encina, A.; Sonbol, F.-M.; Bosch, J.; Puigdomènech, P.; Rigau, J.; et al. ZmXTH1, a new xyloglucan endotransglucosylase/hydrolase in maize, affects cell wall structure and composition in Arabidopsis thaliana*. J. Exp. Bot. 2008, 59, 875–889. [Google Scholar] [CrossRef]
- Bonnett, O.T. The development of the wheat spike. J. Agric. Res. 1936, 53, 445–451. [Google Scholar]
- Fisher, J.E. Developmental morphology of the inflorescence in hexaploid wheat cultivars with and without the cultivar Norin 10 in their ancestry. Can. J. Plant Sci. 1973, 53, 7–15. [Google Scholar] [CrossRef]
- Wright, G.W.; Simon, R.M. A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics 2003, 19, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Ramoni, M.F.; Sebastiani, P.; Kohane, I.S. Cluster analysis of gene expression dynamics. Proc. Natl. Acad. Sci. USA 2002, 99, 9121–9126. [Google Scholar] [CrossRef]
- Thimm, O.; Bläsing, O.; Gibon, Y.; Nagel, A.; Meyer, S.; Krüger, P.; Selbig, J.; Müller, L.A.; Rhee, S.Y.; Stitt, M. Mapman: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004, 37, 914–939. [Google Scholar] [CrossRef]
- Yang, Y.; Li, R.; Qi, M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J. 2000, 22, 543–551. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Liu, Y.; Shen, Y.; Zhang, D.; Li, W. Transcriptome Dynamics during Spike Differentiation of Wheat Reveal Amazing Changes in Cell Wall Metabolic Regulators. Int. J. Mol. Sci. 2023, 24, 11666. https://doi.org/10.3390/ijms241411666
Han J, Liu Y, Shen Y, Zhang D, Li W. Transcriptome Dynamics during Spike Differentiation of Wheat Reveal Amazing Changes in Cell Wall Metabolic Regulators. International Journal of Molecular Sciences. 2023; 24(14):11666. https://doi.org/10.3390/ijms241411666
Chicago/Turabian StyleHan, Junjie, Yichen Liu, Yiting Shen, Donghai Zhang, and Weihua Li. 2023. "Transcriptome Dynamics during Spike Differentiation of Wheat Reveal Amazing Changes in Cell Wall Metabolic Regulators" International Journal of Molecular Sciences 24, no. 14: 11666. https://doi.org/10.3390/ijms241411666
APA StyleHan, J., Liu, Y., Shen, Y., Zhang, D., & Li, W. (2023). Transcriptome Dynamics during Spike Differentiation of Wheat Reveal Amazing Changes in Cell Wall Metabolic Regulators. International Journal of Molecular Sciences, 24(14), 11666. https://doi.org/10.3390/ijms241411666





