Implications of the Cultivation of Rosemary and Thyme (Lamiaceae) in Plant Communities for the Development of Antioxidant Therapies
Abstract
1. Introduction
2. Results
2.1. Determination of Polyphenolic Compounds Using Spectrophotometric Methods
2.2. Identification and Quantification of Polyphenolic Compounds Using Ultra-High Performance Liquid Chromatography–MS (UHPLC–MS)
2.3. Identification of Polyphenolic Compounds Using FT–ICR MS
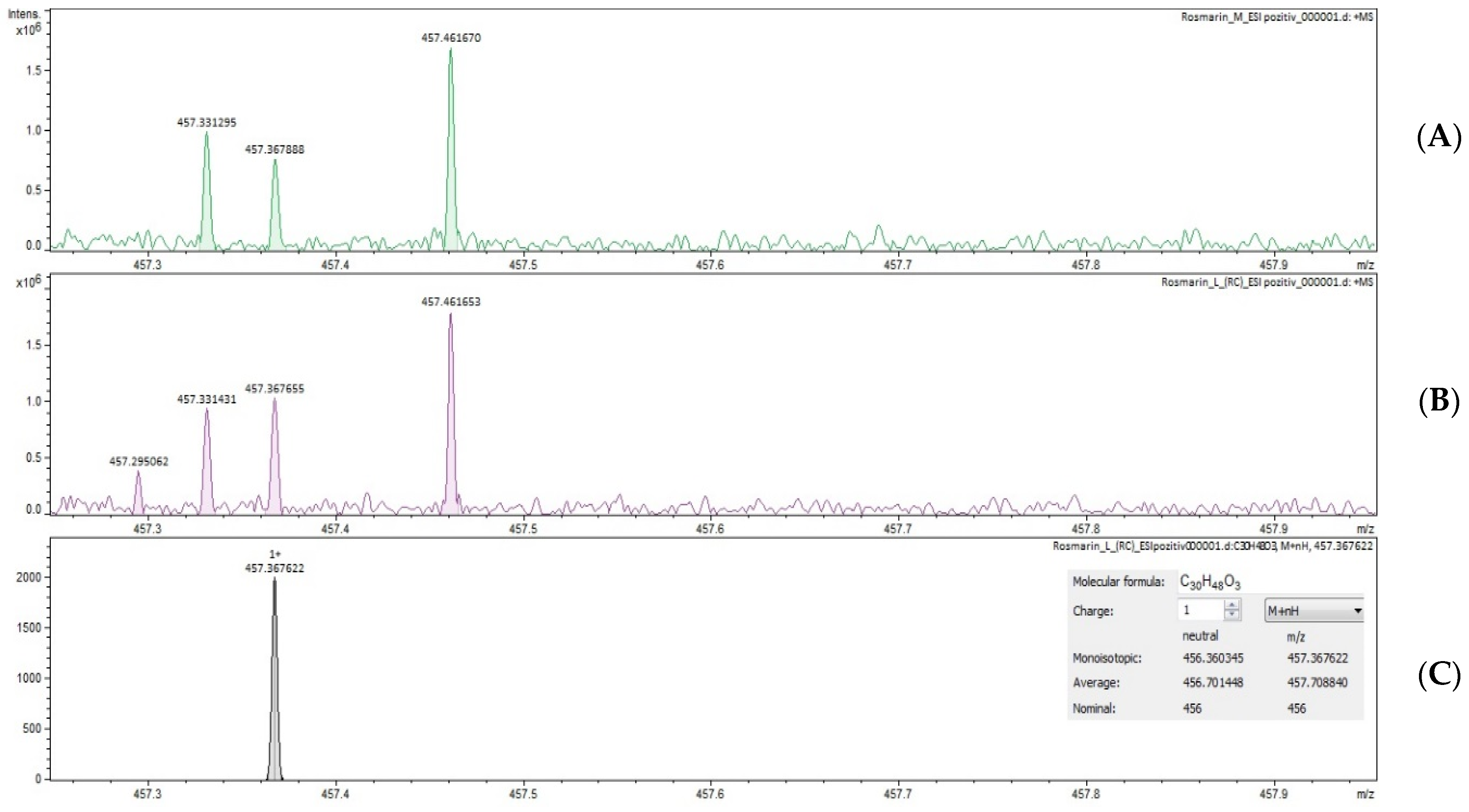
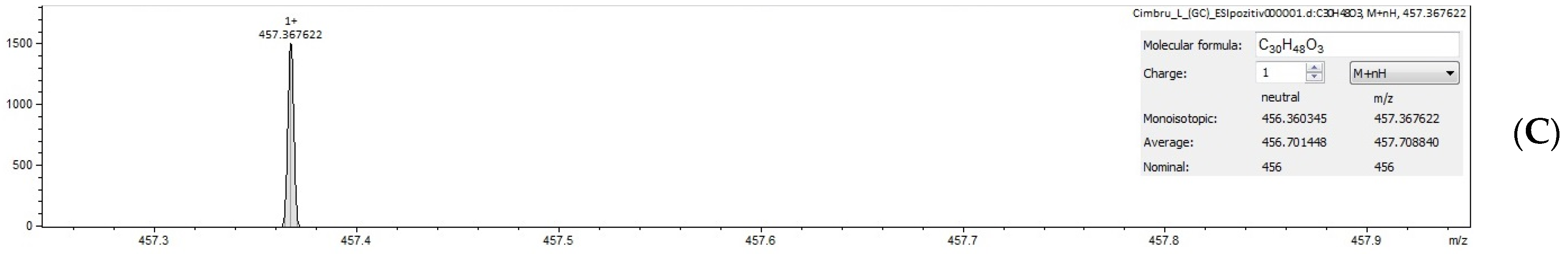
2.4. Identification of Triterpenic Acids by UHPLC–MS and FT–ICR MS (ESI+ and ESI−)
2.5. In Vitro Evaluation of Antioxidant Activity
2.5.1. DPPH Free Radical-Scavenging Activity
2.5.2. ABTS Method
2.5.3. FRAP Assay
2.6. Statistical Analysis
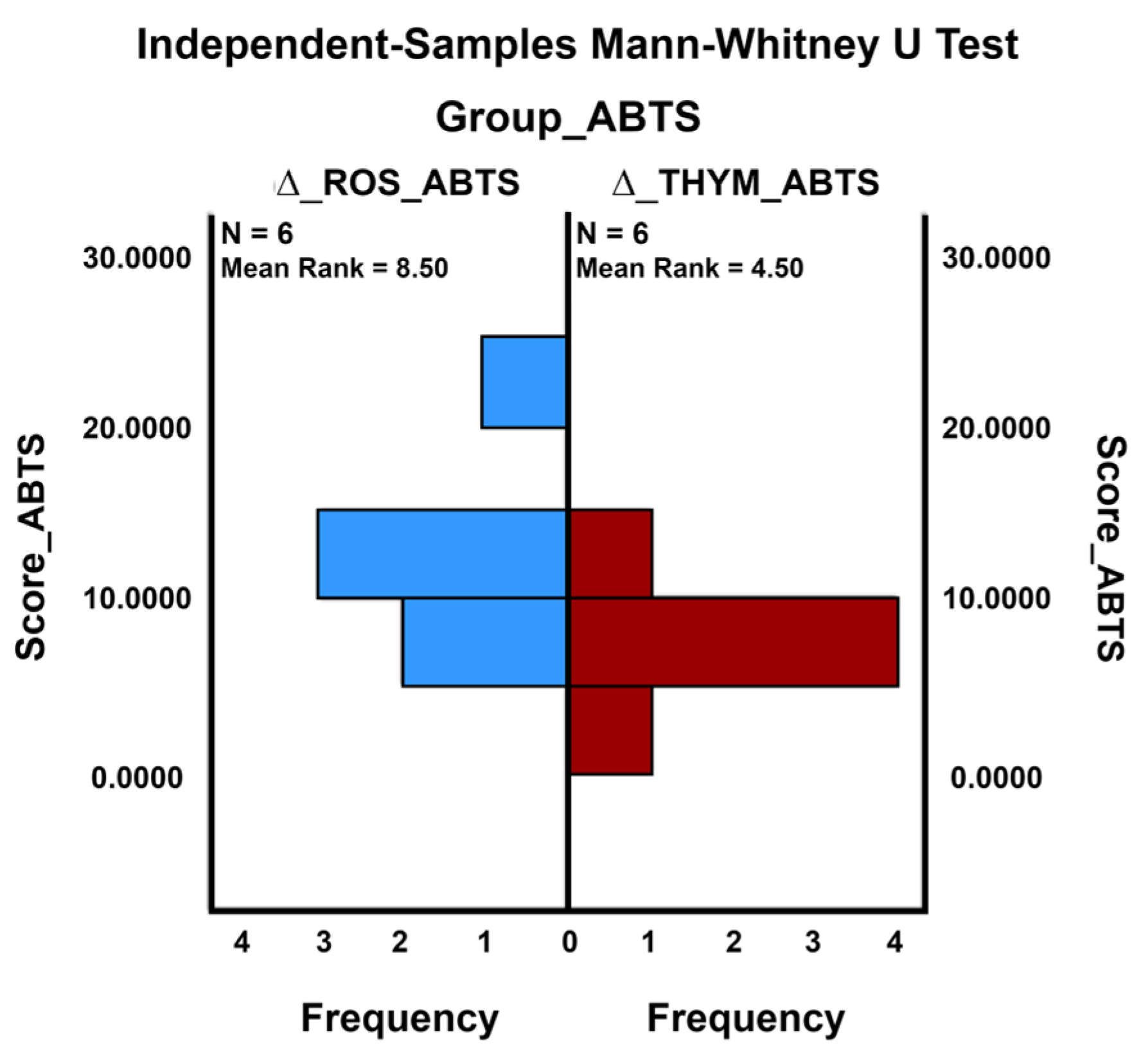
2.6.1. Descriptive Analysis and Comparison of Antioxidant Activity between Common and Control Crops
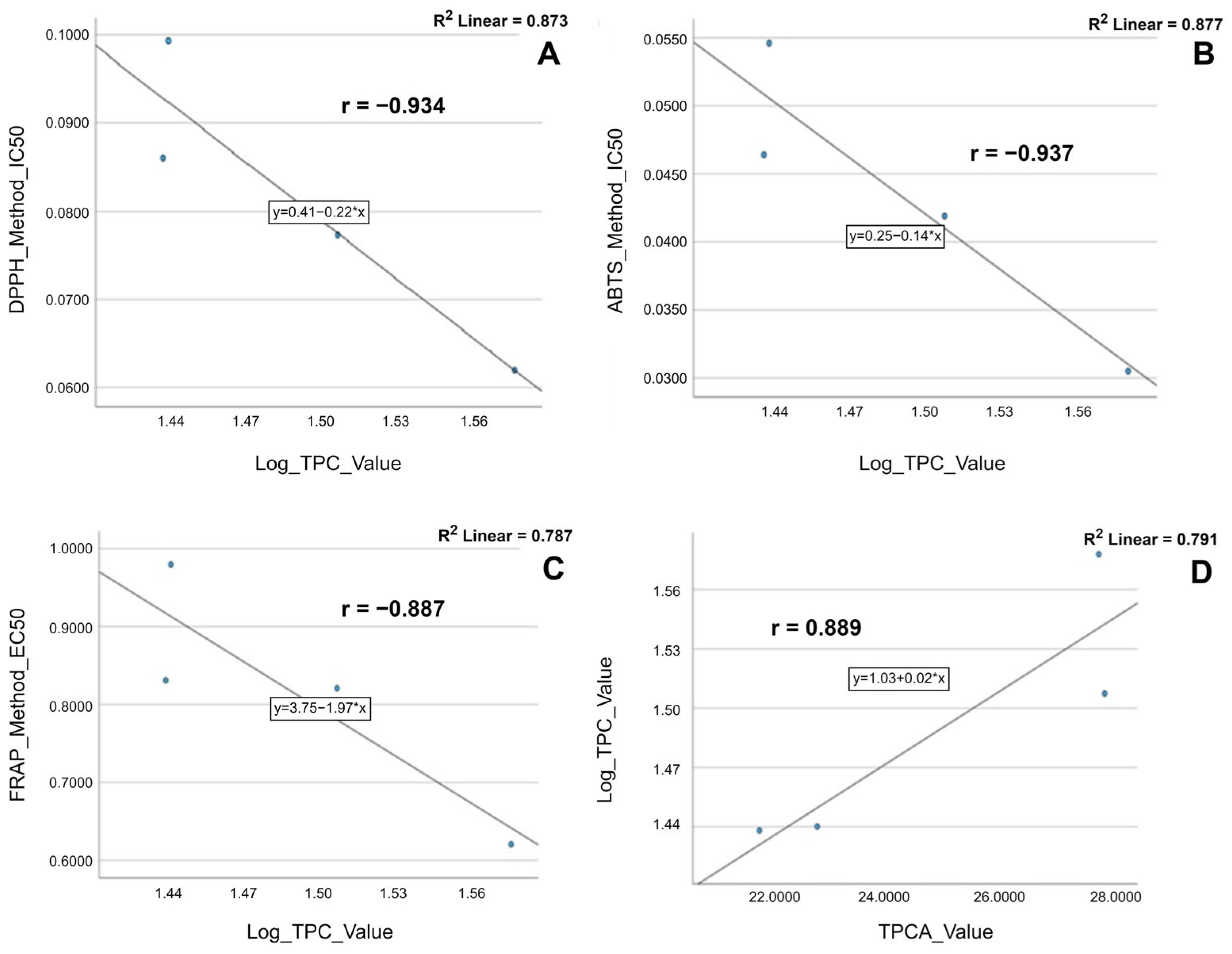
2.6.2. Pearson Correlation between Phenolic Constituents’ Content (TPCAs and TPC) and Antioxidant Activities (IC50 and EC50)
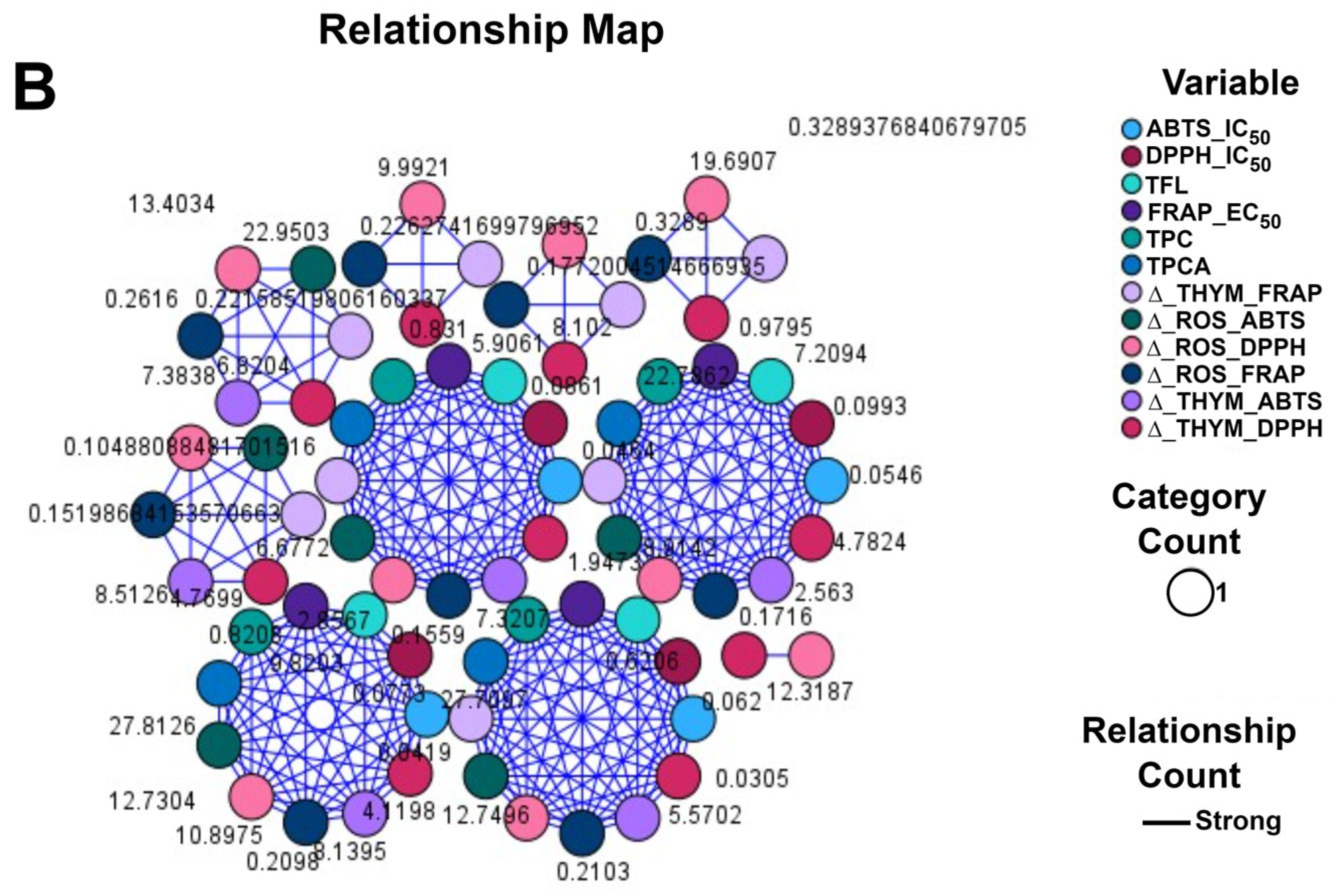
2.6.3. Correlations between Antioxidant Variables Expressed by Inhibition and Optical Density
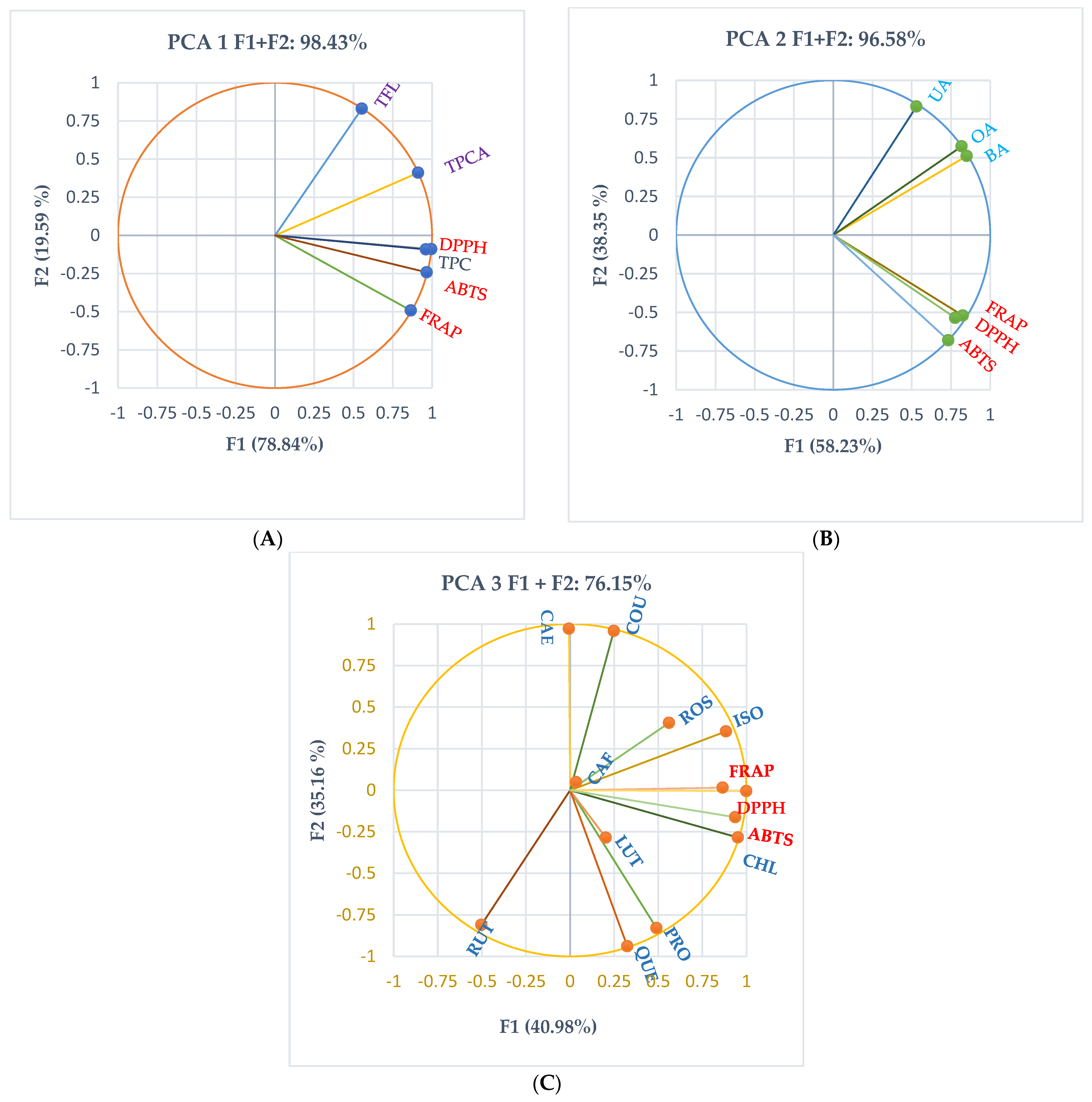
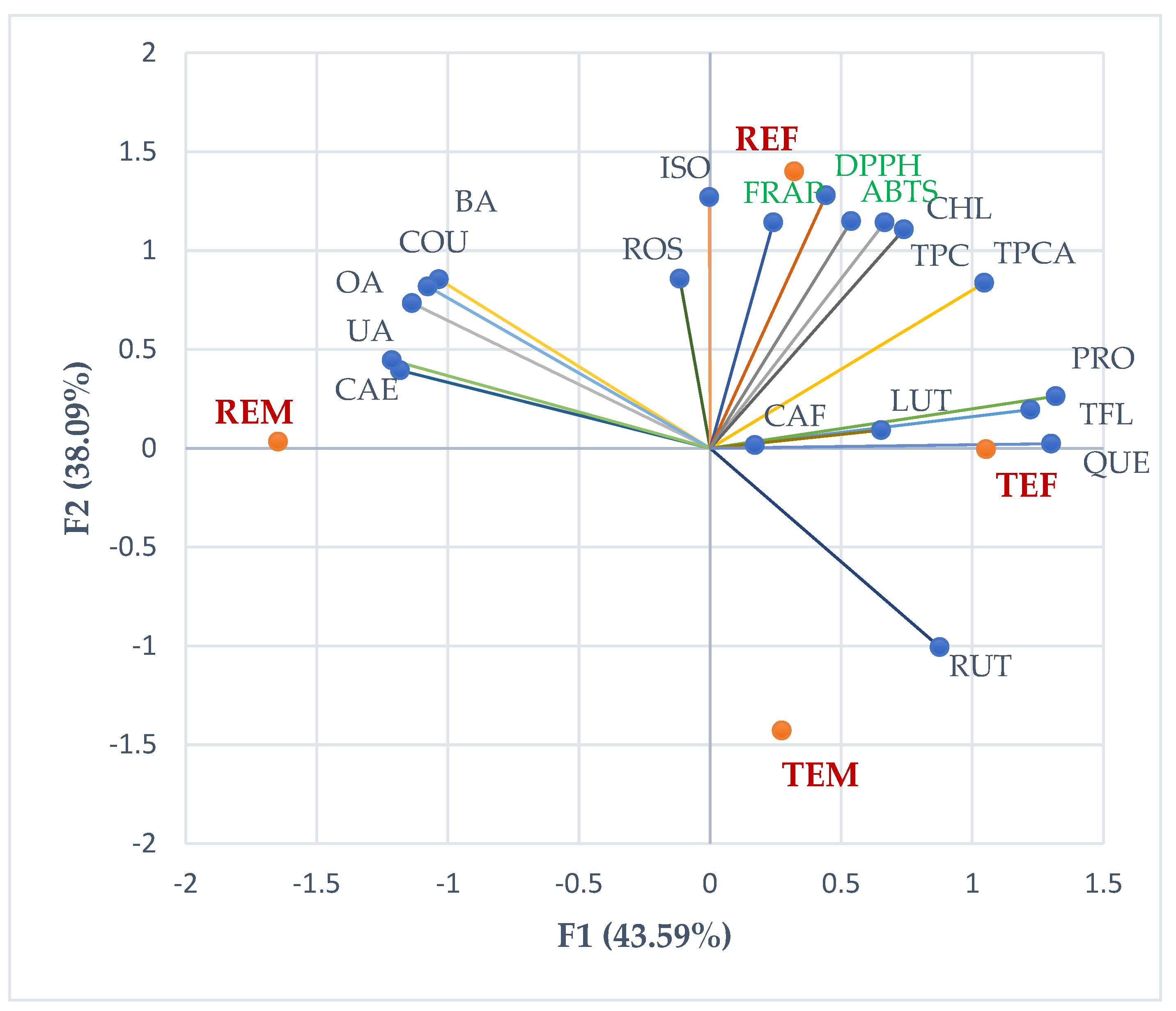
2.6.4. Principal Component Analysis
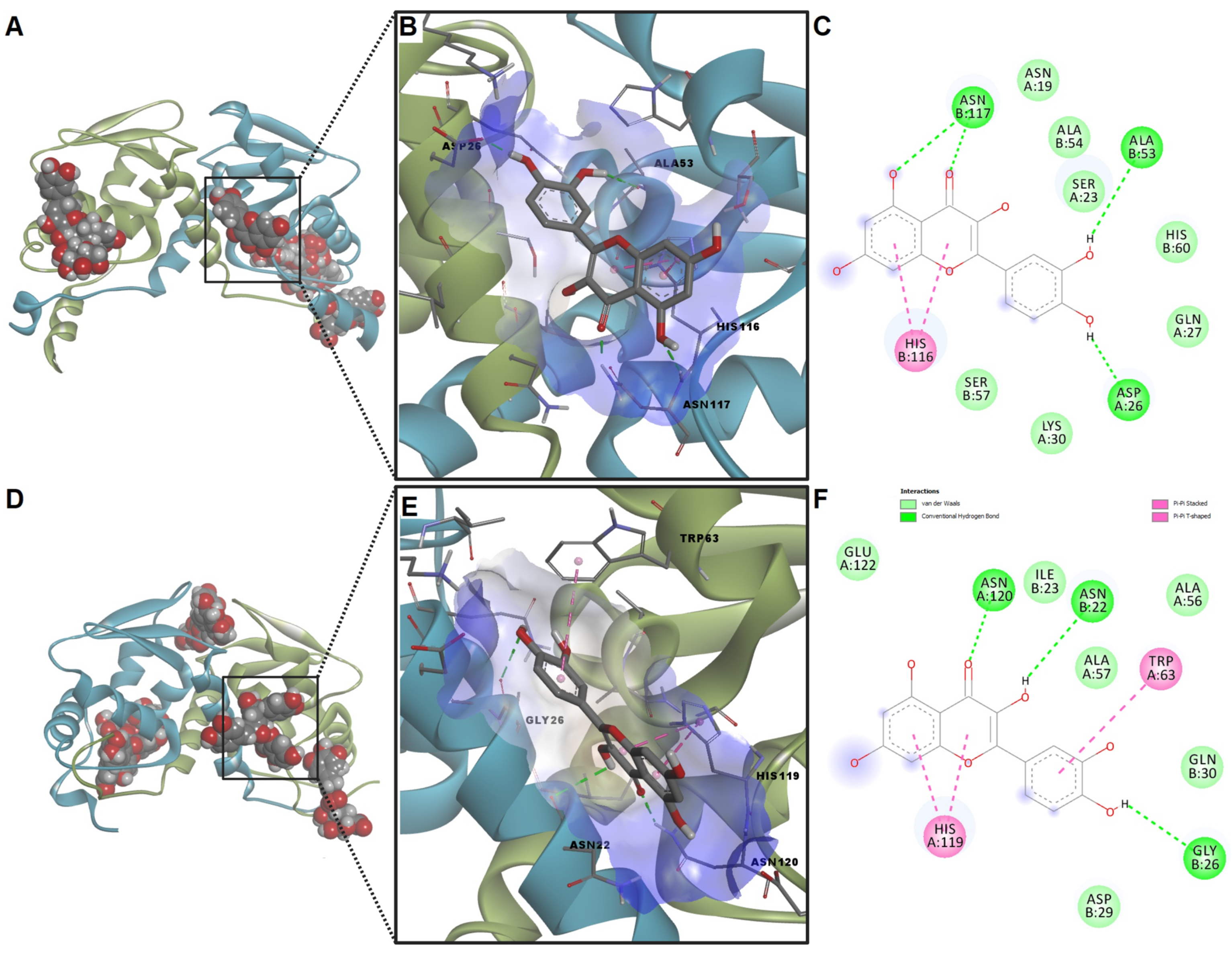
2.7. Molecular Docking Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Plant Materials
4.1.2. Plant Extracts
4.2. Phenolic Constituent Analysis
4.2.1. Determination of Total Flavonoid Content
4.2.2. Determination of Total Phenolic Acid Content
4.2.3. Determination of Total Phenolic Content
4.2.4. Identification and Quantification of Polyphenolic Compounds by Ultra-High Performance Liquid Chromatography–MS (UHPLC–MS)
4.2.5. Identification of Polyphenolic Compounds by High-Resolution Electrospray Ionization Mass Spectrometry (HR ESI–MS)
4.3. In Vitro Antioxidant Activity
4.3.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free Radical-Scavenging Method
4.3.2. ABTS Method
4.3.3. Ferric-Reducing Antioxidant Power Assay (FRAP Assay)
4.4. Molecular Docking
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Packard, V.L. The International Encyclopedia of Geography: People, the Earth, Environment and Technology. Ref. Rev. 2018, 32, 31–32. [Google Scholar] [CrossRef]
- Selim, A.; Bari, E.; Rahaman, M.H.; Rahman, M.M. Phytosociology and Biodiversity of Roadside Herbs in a Salinity-Affected Coastal Area of Bangladesh. Heliyon 2021, 7, 07813. [Google Scholar] [CrossRef]
- Pott, R. Phytosociology: A Modern Geobotanical Method. Plant Biosyst. 2011, 145, 9–18. [Google Scholar] [CrossRef]
- Dengler, J.; Chytrý, M.; Ewald, J. Phytosociology. Encycl. Ecol. 2008, 2767–2779. [Google Scholar] [CrossRef]
- Werle, I.S.; Zanon, A.J.; Streck, N.A.; Schaedler, C.E.; Porta, F.S.D.; Barbieri, G.F.; Ulguim, A.D.R.; Tseng, T.M. Technology Levels in Cassava Cultivation Alter Phytosociology of Weeds. HortScience 2021, 56, 787. [Google Scholar] [CrossRef]
- Luță, E.A.; Ghica, M.; Costea, T.; Gîrd, C.E. Phytosociological Study and Its Influence on the Biosynthesis of Active Compounds of Two Medicinal Plants Mentha piperita L. and Melissa officinalis L. Farmacia 2020, 68, 919–924. [Google Scholar] [CrossRef]
- Luță, E.A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Ghica, M.; Mihai, D.P.; Olaru, O.T.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; et al. The Influence of Phytosociological Cultivation and Fertilization on Polyphenolic Content of Menthae and Melissae Folium and Evaluation of Antioxidant Properties through In Vitro and In Silico Methods. Plants 2022, 11, 2398. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Duffy, S.; So, A.; Murphy, T.H. Activation of Endogenous Antioxidant Defenses in Neuronal Cells Prevents Free Radical-Mediated Damage. J. Neurochem. 1998, 71, 69–77. [Google Scholar] [CrossRef]
- Kwong-Han, K.; Zunaina, E.; Hanizasurana, H.; Che-Badariah, A.A.; Che-Maraina, C.H. Comparison of Catalase, Glutathione Peroxidase and Malondialdehyde Levels in Tears among Diabetic Patients with and without Diabetic Retinopathy. J. Diabetes Metab. Disord. 2022, 21, 681–688. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal Prospects of Antioxidants: A Review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar]
- Ahuja, M.; Kaidery, N.A.; Dutta, D.; Attucks, O.C.; Kazakov, E.H.; Gazaryan, I.; Matsumoto, M.; Igarashi, K.; Sharma, S.M.; Thomas, B. Harnessing the Therapeutic Potential of the Nrf2/Bach1 Signaling Pathway in Parkinson’s Disease. Antioxidants 2022, 11, 1780. [Google Scholar] [CrossRef]
- Tsukumo, S.; Unno, M.; Muto, A.; Takeuchi, A.; Kometani, K.; Kurosaki, T.; Igarashi, K.; Saito, T. Bach2 Maintains T Cells in a Naive State by Suppressing Effector Memory-Related Genes. Proc. Natl. Acad. Sci. USA 2013, 110, 10735–10740. [Google Scholar] [CrossRef]
- Qi, Y.; Huang, Y.; Dong, Y.; Zhang, W.; Xia, F.; Bai, H. Effective Improvement of the Oxidative Stability of Acer Truncatum Bunge Seed Oil, a New Woody Oil Food Resource, by Rosemary Extract. Antioxidants 2023, 12, 889. [Google Scholar] [CrossRef]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus Officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef]
- Satoh, T.; Trudler, D.; Oh, C.K.; Lipton, S.A. Potential Therapeutic Use of the Rosemary Diterpene Carnosic Acid for Alzheimer’s Disease, Parkinson’s Disease, and Long-COVID through NRF2 Activation to Counteract the NLRP3 Inflammasome. Antioxidants 2022, 11, 124. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Murcia, M.A.; Jordán, M.J.; Bañón, S. Assessment of Rosemary (Rosmarinus officinalis L.) Extract as Antioxidant in Jelly Candies Made with Fructan Fibres and Stevia. Antioxidants 2020, 9, 1289. [Google Scholar] [CrossRef]
- Sánchez-Marzo, N.; Pérez-Sánchez, A.; Barrajón-Catalán, E.; Castillo, J.; Herranz-López, M.; Micol, V. Rosemary Diterpenes and Flavanone Aglycones Provide Improved Genoprotection against UV-Induced DNA Damage in a Human Skin Cell Model. Antioxidants 2020, 9, 255. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Nicolescu, A.; Dias, M.I.; Pinela, J.; Barros, L.; Añibarro-Ortega, M.; Stojković, D.; Carević, T.; Mocan, A.; et al. Thymus Species from Romanian Spontaneous Flora as Promising Source of Phenolic Secondary Metabolites with Health-Related Benefits. Antioxidants 2023, 12, 390. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Aazza, S.; Dandlen, S.A.; Majdoub, N.; Lyoussi, B.; Raposo, S.; Antunes, M.D.; Gomes, V.; Miguel, M.G. Antioxidant Activity of Thyme Waste Extract in O/W Emulsions. Antioxidants 2019, 8, 243. [Google Scholar] [CrossRef]
- Pandur, E.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Antioxidant and Anti-Inflammatory Effects of Thyme (Thymus vulgaris L.) Essential Oils Prepared at Different Plant Phenophases on Pseudomonas Aeruginosa LPS-Activated THP-1 Macrophages. Antioxidants 2022, 11, 1330. [Google Scholar] [CrossRef]
- Dauqan, E.M.; Abdullah, A. Medicinal and Functional Values of Thyme (Thymus vulgaris L.) Herb. J. Appl. Biol. Biotechnol. 2017, 5, 017–022. [Google Scholar] [CrossRef]
- Abolghasemi, R.; Haghighi, M.; Solgi, M.; Mobinikhaledi, A. Rapid Synthesis of ZnO Nanoparticles by Waste Thyme (Thymus vulgaris L.). Int. J. Environ. Sci. Technol. 2019, 16, 6985–6990. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, N.; Fu, Y.J.; Wang, W.; Luo, M.; Zhao, C.J.; Zu, Y.G.; Liu, X.L. Chemical Composition and Antimicrobial Activity of the Essential Oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Nartop, P.; Gurel, A.; Bedir, E.; Vardar-Sukan, F. Determination of Phenolic Content and Antioxidant Activity of Extracts Obtained from Rosmarinus Officinalis’ Calli. J. Plant Physiol. 2007, 164, 1536–1542. [Google Scholar] [CrossRef]
- Begum, A.; Sandhya, S.; Ali, S.S.; Vinod, K.R.; Reddy, S.; Banji, D. An In-Depth Review on the Medicinal Flora Rosmarinus Officinalis (Lamiaceae). Acta Sci. Pol. Technol. Aliment. 2013, 12, 61–73. Available online: https://pubmed.ncbi.nlm.nih.gov/24584866/ (accessed on 14 March 2023).
- Rahbardar, M.G.; Hosseinzadeh, H. Therapeutic Effects of Rosemary (Rosmarinus officinalis L.) and Its Active Constituents on Nervous System Disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100–1112. [Google Scholar] [CrossRef]
- Cheung, S.; Tai, J. Anti-Proliferative and Antioxidant Properties of Rosemary Rosmarinus Officinalis. Oncol. Rep. 2007, 17, 1525–1531. Available online: https://pubmed.ncbi.nlm.nih.gov/17487414/ (accessed on 14 March 2023).
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus Officinalis L.: An Update Review of Its Phytochemistry and Biological Activity. Futur. Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef]
- AL-Megrin, W.A.; AlSadhan, N.A.; Metwally, D.M.; Al-Talhi, R.A.; El-Khadragy, M.F.; Abdel-Hafez, L.J.M. Potential Antiviral Agents of Rosmarinus Officinalis Extract against Herpes Viruses 1 and 2. Biosci. Rep. 2020, 40, 20200992. [Google Scholar] [CrossRef]
- Murata, K.; Noguchi, K.; Kondo, M.; Onishi, M.; Watanabe, N.; Okamura, K.; Matsuda, H. Promotion of Hair Growth by Rosmarinus Officinalis Leaf Extract. Phyther. Res. 2013, 27, 212–217. [Google Scholar] [CrossRef]
- Jugl-Chizzola, M.; Spergser, J.; Schilcher, F.; Novak, J.; Bucher, A.; Gabler, C.; Hagmüller, W.; Zitterl-Eglseer, K. Effects of Thymus vulgaris L. as Feed Additive in Piglets and against Haemolytic E. Coli in Vitro. Berl. Munch Tierarztl. Wochenschr. 2005, 118, 495–501. Available online: https://pubmed.ncbi.nlm.nih.gov/16318274/ (accessed on 14 March 2023).
- Pezzei, C.K.; Schönbichler, S.A.; Hussain, S.; Kirchler, C.G.; Huck-Pezzei, V.A.; Popp, M.; Krolitzek, J.; Bonn, G.K.; Huck, C.W. Near-Infrared and Mid-Infrared Spectroscopic Techniques for a Fast and Nondestructive Quality Control of Thymi Herba. Planta Med. 2018, 84, 420–427. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int. J. Clin. Med. 2015, 06, 635–642. [Google Scholar] [CrossRef]
- Basch, E.; Ulbricht, C.; Hammerness, P.; Bevins, A.; Sollars, D. Thyme (Thymus vulgaris L.), Thymol. J. Herb. Pharmacother. 2004, 4, 49–67. [Google Scholar] [CrossRef]
- Ghica, A.; Drumea, V.; Moroșan, A.; Mihaiescu, D.E.; Costea, L.; Luță, E.A.; Mihai, D.P.; Balaci, D.T.; Fița, A.C.; Olaru, O.T.; et al. Phytochemical Screening and Antioxidant Potential of Selected Extracts from Betula alba var. pendula Roth., Glycyrrhiza glabra L., and Avena sativa L. Plants 2023, 12, 2510. [Google Scholar] [CrossRef]
- Sewale, B.; Mammo, S. Analysis of Floristic Composition and Plant Community Types in Kenech Natural Forest, Kaffa Zone, Ethiopia. Trees For. People 2022, 7, 100170. [Google Scholar] [CrossRef]
- CBD Strategy and Action Plan—Romanian (English Version). National Strategy and Action Plan for Biodiversity Conservation 2014–2020. Chapter 4. National Strategy for Biodiversity Conservation. 2017, pp. 36–52. Available online: https://www.cbd.int/doc/world/ro/ro-nbsap-v3-en.pdf (accessed on 6 March 2023).
- Luță, E.A.; Ghica, M.; Gîrd, C.E. The Initiation of a Phytosociological Study on Certain Types of Medicinal Plants. Agriculture 2022, 12, 283. [Google Scholar] [CrossRef]
- Chapter 2.8.14. Tannins in Herbal Drugs (01/2008:20814). In European Pharmacopoeia—11th Ed. European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2023; pp. 1415–1416+1496–1498.
- Costea, L.; Chițescu, C.L.; Boscencu, R.; Ghica, M.; Lupuliasa, D.; Mihai, D.P.; Deculescu-Ioniță, T.; Dutu, L.E.; Popescu, M.L.; Luță, E.A.; et al. The Polyphenolic Profile and Antioxidant Activity of Five Vegetal Extracts with Hepatoprotective Potential. Plants 2022, 11, 1680. [Google Scholar] [CrossRef]
- Koga, K.; Shibata, H.; Yoshino, K.; Nomoto, K. Effects of 50% Ethanol Extract from Rosemary (Rosmarinus officinalis) on α-Glucosidase Inhibitory Activity and the Elevation of Plasma Glucose Level in Rats, and Its Active Compound. J. Food Sci. 2006, 71, S507–S512. [Google Scholar] [CrossRef]
- Costea, L.; Ghica, M.; Costea, T.; Gîrd, C.E. Spectrophotometric Evaluation of Flavonoids, Phenolcarboxylic Acids and Total Phenolic Contents of Several Indigenous Herbal Products With Potential Hepatoprotective Effect. Farmacia 2021, 69, 1176–1181. [Google Scholar] [CrossRef]
- Adesanwo, J.K.; Makinde, O.O.; Obafemi, C.A. Phytochemical Analysis and Antioxidant Activity of Methanol Extract and Betulinic Acid Isolated from the Roots of Tetracera Potatoria. J. Pharm. Res. 2013, 6, 903–907. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Activity of Caffeic Acid (3,4-Dihydroxycinnamic Acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Jayanthi, R.; Subash, P. Antioxidant Effect of Caffeic Acid on Oxytetracycline Induced Lipid Peroxidation in Albino Rats. Indian J. Clin. Biochem. 2010, 25, 371–375. [Google Scholar] [CrossRef]
- Iwahashi, H.; Ishii, T.; Sugata, R.; Kido, R. The Effects of Caffeic Acid and Its Related Catechols on Hydroxyl Radical Formation by 3-Hydroxyanthranilic Acid, Ferric Chloride, and Hydrogen Peroxide. Arch. Biochem. Biophys. 1990, 276, 242–247. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 634. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C. Lipid Peroxidation and Antioxidants as Biomarkers of Tissue Damage. Clin. Chem. 1995, 41, 1819–1828. Available online: https://pubmed.ncbi.nlm.nih.gov/7497639/ (accessed on 14 March 2023).
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Romanova, D.; Vachálkova, A.; Čipák, L.; Ovesná, Z.; Rauko, P. Study of Antioxidant Effect of Apigenin, Luteolin and Quercetin by DNA Protective Method. Neoplasma 2001, 48, 104–107. Available online: https://pubmed.ncbi.nlm.nih.gov/11478688/ (accessed on 14 March 2023).
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin Induces Apoptotic Cell Death via Antioxidant Activity in Human Colon Cancer Cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant Activity, Total Phenolic and Total Flavonoid Contents of Whole Plant Extracts Torilis Leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Duan, S.C.; Kwon, S.J.; Eom, S.H. Effect of Thermal Processing on Color, Phenolic Compounds, and Antioxidant Activity of Faba Bean (Vicia faba L.) Leaves and Seeds. Antioxidants 2021, 10, 1207. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Manap, M.Y.A.; Khatib, A.; Razis, A.F.A. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between Phenolic Content and Antioxidant Properties in Twenty-Four Plant Species of Traditional Ethnoveterinary Use in the Mediterranean Area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative Analysis of Bioactive Phenolic Compounds Composition from 26 Medicinal Plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef]
- Leonov, A.; Arlia-Ciommo, A.; Piano, A.; Svistkova, V.; Lutchman, V.; Medkour, Y.; Titorenko, V.I. Longevity Extension by Phytochemicals. Molecules 2015, 20, 6544–6572. [Google Scholar] [CrossRef]
- Konan, M.K.; Koffi, E.N.D.; Cisse, I.; Adima, A.A.; Bekro, Y.A. Phytochemical, Nutritional and Antioxidant Capacity of Five Ivorian Edible Leaves Aqueous Extracts. J. Appl. Pharm. Sci. 2016, 6, 082–086. [Google Scholar] [CrossRef]
- Bonesi, M.; Loizzo, M.R.; Acquaviva, R.; Malfa, G.A.; Aiello, F.; Tundis, R. Anti-Inflammatory and Antioxidant Agents from Salvia Genus (Lamiaceae): An Assessment of the Current State of Knowledge. Antiinflamm. Antiallergy. Agents Med. Chem. 2017, 16, 70–86. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, K.; Ban, L.; Mao, Y.; Hou, C.; Li, J. Silage Fermentation: A Potential Biological Approach for the Long-Term Preservation and Recycling of Polyphenols and Terpenes in Globe Artichoke (Cynara scolymus L.) by-Products. Molecules 2020, 25, 3302. [Google Scholar] [CrossRef]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol Induces Antioxidant Pathways in Keratinocytes by Targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef]
- Attucks, O.C.; Jasmer, K.J.; Hannink, M.; Kassis, J.; Zhong, Z.; Gupta, S.; Victory, S.F.; Guzel, M.; Polisetti, D.R.; Andrews, R.; et al. Induction of Heme Oxygenase I (HMOX1) by HPP-4382: A Novel Modulator of Bach1 Activity. PLoS ONE 2014, 9, e101044. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, S.; Zhao, C.; Zhou, Y.; Zhang, L.; Liu, H.; Zhou, P.; Li, S.; Fu, L.; Zheng, Z.; et al. Genome-Scale CRISPR Knockout Screening Identifies BACH1 as a Key Regulator of Aflatoxin B1-Induced Oxidative Damage. Antioxidants 2022, 11, 1787. [Google Scholar] [CrossRef]
- Rosemary Leaf (01/2013:1560). In European Pharmacopoeia—11th Ed. European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2023; pp. 1699–1700.
- Thymi Herba (07/2014:0865). In European Pharmacopoeia—11th Ed. European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2023; pp. 1752–1754.
- Munekata, P.E.S.; Alcántara, C.; Žugčić, T.; Abdelkebir, R.; Collado, M.C.; García-Pérez, J.V.; Jambrak, A.R.; Gavahian, M.; Barba, F.J.; Lorenzo, J.M. Impact of Ultrasound-Assisted Extraction and Solvent Composition on Bioactive Compounds and in Vitro Biological Activities of Thyme and Rosemary. Food Res. Int. 2020, 134, 109242. [Google Scholar] [CrossRef]
- Gîrd, C.E.; Duțu, L.E.; Popescu, M.L.; Nencu, I.; Costea, T. Farmacognozie Practică (Laboratory Guide—“Carol Davila” University); Editura Universitară “Carol Davila”: Bucharest, Romania, 2020; Volume 1, pp. 30–42. [Google Scholar]
- Lamuela-Raventós, R.M. Folin-Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. Meas. Antioxid. Act. Capacit. Recent Trends Appl. 2017, 6, 107–115. [Google Scholar] [CrossRef]
- Liu, L.; Song, C.; Tian, S.; Zhang, Q.; Cai, X.; Liu, Y.; Liu, Z.; Wang, W. Structural Characterization of Sulfur-Containing Aromatic Compounds in Heavy Oils by FT-ICR Mass Spectrometry with a Narrow Isolation Window. Fuel 2019, 240, 40–48. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, Y.G.; Lee, Y.K.; Pack, S.P.; Jung, J.Y.; Jang, K.S. Chemical Characterization of Dissolved Organic Matter in Moist Acidic Tussock Tundra Soil Using Ultra-High Resolution 15T FT-ICR Mass Spectrometry. Biotechnol. Bioprocess Eng. 2017, 22, 637–646. [Google Scholar] [CrossRef]
- He, C.; Zhang, Y.; Li, Y.; Zhuo, X.; Li, Y.; Zhang, C.; Shi, Q. In-House Standard Method for Molecular Characterization of Dissolved Organic Matter by FT-ICR Mass Spectrometry. ACS Omega 2020, 5, 11730–11736. [Google Scholar] [CrossRef]
- Hur, M.; Oh, H.; Kim, S. Optimized Automatic Noise Level Calculations for Broadband FT-ICR Mass Spectra of Petroleum Give More Reliable and Faster Peak Picking Results. Bull. Korean Chem. Soc. 2009, 30, 2665–2668. [Google Scholar] [CrossRef]
- Al Hamwi, M.; Ela, M.A.; El-Lakany, A.; Bakkour, Y.; Mahmoud, Z. FTICR/MS Analysis of Micromeria Fruticosa and Teucrium Polium Growing in Lebanon. Pharmacogn. J. 2022, 14, 112–127. [Google Scholar] [CrossRef]
- Ohta, D.; Kanaya, S.; Suzuki, H. Application of Fourier-Transform Ion Cyclotron Resonance Mass Spectrometry to Metabolic Profiling and Metabolite Identification. Curr. Opin. Biotechnol. 2010, 21, 35–44. [Google Scholar] [CrossRef]
- Maia, M.; Ferreira, A.E.N.; Laureano, G.; Marques, A.P.; Torres, V.M.; Silva, A.B.; Matos, A.R.; Cordeiro, C.; Figueiredo, A.; Silva, M.S. Vitis Vinifera “Pinot Noir” Leaves as a Source of Bioactive Nutraceutical Compounds. Food Funct. 2019, 10, 3822–3827. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. J. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Ebskamp, M.J.M.; Paul, M.J.; Jeuken, M.J.W.; Weisbeek, P.J.; Smeekens, S.C.M. Improved Performance of Transgenic Fructan-Accumulating Tobacco under Drought Stress. Plant Physiol. 1995, 107, 125–130. [Google Scholar] [CrossRef]
- Ito, N.; Watanabe-Matsui, M.; Igarashi, K.; Murayama, K. Crystal Structure of the Bach1 BTB Domain and Its Regulation of Homodimerization. Genes Cells 2009, 14, 167–178. [Google Scholar] [CrossRef]
- Rosbrook, G.O.; Stead, M.A.; Carr, S.B.; Wright, S.C. The Structure of the Bach2 POZ-Domain Dimer Reveals an Intersubunit Disulfide Bond. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 26–34. [Google Scholar] [CrossRef]
- Land, H.; Humble, M.S. YASARA: A Tool to Obtain Structural Guidance in Biocatalytic Investigations. In Protein Engineering; Humana Press: New York, NY, USA, 2018; Volume 1685, pp. 43–67. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program for Chemistry Aware Data Visualization and Analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Vochita, G.; Gherghel, D.; Mihai, C.T.; Rambu, D.; Calcan, S.I.; Costache, T.; Cucolea, I.E.; Matei, E.; et al. In Vitro Anticancer Activity and Oxidative Stress Biomarkers Status Determined by Usnea barbata (L.) f.h. Wigg. Dry Extracts. Antioxidants 2021, 10, 1141. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Aschie, M.; Bucur, L.; Rambu, D.; Costache, T.; Cucolea, I.E.; Vochita, G.; Gherghel, D.; et al. Usnic Acid and Usnea barbata (L.) F.H. Wigg. Dry Extracts Promote Apoptosis and DNA Damage in Human Blood Cells through Enhancing ROS Levels. Antioxidants 2021, 10, 1171. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Musuc, A.M.; Mitu, M.A.; Atkinson, I.; et al. In Vitro Anticancer Activity of Mucoadhesive Oral Films Loaded with Usnea barbata (L.) F. H. Wigg Dry Acetone Extract, with Potential Applications in Oral Squamous Cell Carcinoma Complementary Therapy. Antioxidants 2022, 11, 1934. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Karampelas, O.; Musuc, A.M.; Atkinson, I.; et al. Evaluation of Usnea barbata (L.) Weber ex F.H. Wigg Extract in Canola Oil Loaded in Bioadhesive Oral Films for Potential Applications in Oral Cavity Infections and Malignancy. Antioxidants 2022, 11, 1601. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Mitu, M.A.; Musuc, A.M.; Petrescu, S.; et al. Design, Characterization, and Anticancer and Antimicrobial Activities of Mucoadhesive Oral Patches Loaded with Usnea barbata (L.) F. H. Wigg Ethanol Extract F-UBE-HPMC. Antioxidants 2022, 11, 1801. [Google Scholar] [CrossRef]


| Plant Extract | TFL (mg/g Eq Expressed in Rutin) | TPCAs (mg/g Eq Expressed in Chlorogenic Acid) | TPC (mg/g Eq Expressed in Tannic Acid) |
|---|---|---|---|
| REM | 59.061 ± 4.01 | 217.756 ± 43.13 | 236.058 ± 17.88 |
| REF | 78.323 ± 12.47 | 277.097 ± 18.60 | 378.336 ± 38.70 |
| TEM | 72.094 ± 18.04 | 227.862 ± 36.85 | 275.507 ± 22.57 |
| TEF | 98.203 ± 13.65 | 278.126 ± 45.05 | 321.678 ± 40.01 |
| Sample Name | REM | REF | TEM | TEF |
|---|---|---|---|---|
| Protocatechuic acid | 16.820 ± 1.25 | 39.442 ± 1.03 | 32.742 ± 1.09 | 47.581 ± 1.50 |
| Rutin | ND | ND | 12.734 ± 1.22 | 11.776 ± 0.93 |
| Caffeic acid | 524.433 ± 1.22 | 357.742 ± 1.87 | 356.001 ± 1.73 | 684.231 ± 1.56 |
| Chlorogenic acid | 21.507 ± 1.15 | 68.022 ± 1.53 | 23.873 ± 0.60 | 46.341 ± 1.27 |
| Luteolin | 87.172 ± 1.09 | 53.355 ± 0.81 | 29.278 ±0.92 | 341.923 ± 1.25 |
| Kaempferol | 4.934 ± 0.75 | 2.485 ± 0.78 | 1.492 ± 0.46 | 2.312 ± 0.61 |
| Rosmarinic acid | 34.681 ± 1.41 | 33.204 ± 1.46 | 27.485 ± 1.80 | 35.947 ± 1.58 |
| Quercetin | ND | 19.793 ± 1.12 | 18.933 ± 1.36 | 20.057 ± 1.90 |
| Isoquercitrin | 143.862 ± 1.55 | 167.290 ± 1.91 | 85.778 ± 0.75 | 151.538 ± 0.88 |
| Ferulic acid | ND | ND | ND | ND |
| p-Coumaric acid | 61.683 ± 1.13 | 39.832 ± 1.85 | 5.174 ± 1.61 | 8.021 ± 1.18 |
| Sample Name | m/z | REM | REF | TEM | TEF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ESI+ | ESI− | ESI+ | ESI− | ESI+ | ESI− | ESI+ | ESI− | ESI+ | ESI− | |
| Protocatechuic acid | 155.033 | 153.019 | + | − | + | − | + | − | + | − |
| Rutin | 611.160 | 609.146 | + | − | + | − | + | + | + | + |
| Caffeic acid | 181.049 | 179.034 | + | + | + | + | + | + | + | + |
| Chlorogenic acid | 355.102 | 353.087 | + | + | + | + | + | − | + | − |
| Luteolin | 287.055 | 285.040 | + | + | + | + | + | + | + | + |
| Kaempferol | 287.055 | 285.040 | + | + | + | + | + | + | + | + |
| Rosmarinic acid | 361.091 | 359.077 | + | + | + | + | + | + | + | + |
| Quercetin | 303.049 | 301.035 | − | + | + | + | + | + | + | + |
| Isoquercitrin | 465.102 | 463.088 | + | + | + | + | + | + | + | + |
| Ferulic acid | 195.065 | 193.050 | + | + | + | + | − | + | − | + |
| p-Coumaric acid | 165.054 | 163.040 | − | + | − | + | + | + | + | + |
| Plant Extract | REM | REF | TEM | TEF | |
|---|---|---|---|---|---|
| Triterpenic acids (μg compound/g extract) | BA | 10.972 ± 1.13 | 8.754 ± 0.72 | ND | ND |
| OA | 11.446 ± 0.93 | 8.511 ± 0.63 | 1.302 ± 0.21 | 1.952 ± 0.14 | |
| UA | 11.672 ± 0.81 | 6.224 ± 0.72 | 3.503 ± 0.43 | 4.912 ± 0.24 | |
| DPPH_REM | DPPH_TEM | DPPH_REF | DPPH_TEF | ∆_ROS_DPPH | ∆_THYM_DPPH |
|---|---|---|---|---|---|
| 26.8293 | 28.2696 | 29.6860 | 30.2169 | 2.8567 | 1.9473 |
| 32.2557 | 29.9825 | 37.8352 | 34.7649 | 5.5795 | 4.7824 |
| 36.3526 | 32.1799 | 47.1130 | 37.7501 | 10.7604 | 5.5702 |
| 38.1699 | 35.5120 | 49.0674 | 39.6318 | 10.8975 | 4.1198 |
| 42.0245 | 38.5409 | 51.9401 | 43.3108 | 9.9156 | 4.7699 |
| 46.8293 | 44.2517 | 60.2327 | 51.0721 | 13.4034 | 6.8204 |
| 53.4162 | 46.8773 | 63.4083 | 58.4584 | 9.9921 | 11.5811 |
| 54.4747 | 50.8127 | 70.5213 | 58.9147 | 16.0466 | 8.1020 |
| 60.1467 | 54.7481 | 79.8374 | 66.5479 | 19.6907 | 11.7998 |
| 64.2053 | 56.3985 | 88.4043 | 68.7172 | 24.1990 | 12.3187 |
| ABTS_REM | ABTS_TEM | ABTS_REF | ABTS_TEF | ∆_ROS_ABTS | ∆_THYM_ABTS |
|---|---|---|---|---|---|
| 23.3391 | 21.1628 | 30.0163 | 28.4835 | 6.6772 | 7.3207 |
| 31.1780 | 31.3178 | 40.0922 | 33.8808 | 8.9142 | 2.5630 |
| 37.0152 | 34.0213 | 49.7648 | 44.6948 | 12.7496 | 10.6735 |
| 47.3598 | 40.4797 | 60.0902 | 48.6192 | 12.7304 | 8.1395 |
| 54.1619 | 47.9070 | 64.9578 | 56.4196 | 10.7959 | 8.5126 |
| 57.7477 | 52.8924 | 80.6980 | 60.2762 | 22.9503 | 7.3838 |
| FRAP_REM | FRAP_TEM | FRAP_REF | FRAP_TEF | ∆_ROS_FRAP | ∆_THYM_FRAP |
|---|---|---|---|---|---|
| 0.2772 | 0.2749 | 0.4331 | 0.2859 | 0.1559 | 0.0110 |
| 0.3248 | 0.3139 | 0.4964 | 0.3265 | 0.1716 | 0.0126 |
| 0.3821 | 0.3734 | 0.5924 | 0.3748 | 0.2103 | 0.0014 |
| 0.4347 | 0.4147 | 0.6445 | 0.4134 | 0.2098 | −0.0013 |
| 0.4662 | 0.4737 | 0.7071 | 0.4968 | 0.2409 | 0.0231 |
| 0.5324 | 0.5048 | 0.7940 | 0.5539 | 0.2616 | 0.0491 |
| 0.6161 | 0.5460 | 0.8952 | 0.5972 | 0.2791 | 0.0512 |
| 0.6751 | 0.6537 | 0.9582 | 0.6851 | 0.2831 | 0.0314 |
| 0.7753 | 0.6759 | 1.1042 | 0.7841 | 0.3289 | 0.1082 |
| Levene’s Test | t-Test for Equality of Means | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Significance | Mean Diff. | Std. Error Diff. | 95% CI of the Difference | ||||
| One-Sided p | Two-Sided p | Lower Bound | Upper Bound | ||||||||
| Score | Equal variances assumed | 1.807 | 0.196 | 2.231 | 18 | 0.019 | 0.039 * | 5.1530 | 2.3097 | 0.3004 | 10.0055 |
| Standardizer a | Point Estimate | 95% Confidence Interval | |||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Score | Cohen’s d | 5.1646986 | 0.998 | 0.051 | 1.920 |
| Hedges’ correction | 5.3931164 | 0.9555 * | 0.049 | 1.839 | |
| Glass’s delta | 3.6361205 | 1.417 | 0.304 | 2.480 | |
| Mann–Whitney U | 6.000 |
| Wilcoxon W | 27.000 |
| Test Statistic | 6.000 |
| Standard Error | 6.245 |
| Standardized Test Statistic (z) | −1.922 |
| Asymptotic Sig. (2-sided test)—p-value * | 0.055 |
| Standardizer a | Point Estimate | 90% Confidence Interval | |||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Score | Cohen’s d | 0.0738839 | 0.918 | 0.059 | 1.749 |
| Hedges’ correction | 0.0778539 | 0.871 * | 0.056 | 1.660 | |
| Glass’s delta | 0.0899359 | 0.754 | −0.133 | 1.593 | |
| Levene’s Test | t-Test for Equality of Means | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Significance | Mean Diff. | Std. Error Diff. | 95% CI of the Difference | ||||
| One-Sided p | Two-Sided p | Lower Bound | Upper Bound | ||||||||
| Score | Equal variances assumed | 0.896 | 0.358 | −2.578 | 16 | 0.010 | 0.020 * | −0.2379 | 0.0923 | −0.4336 | −0.0423 |
| Vegetal Extract | TPC (mg/g Eq Expressed in Tannic Acid) | TPCAs (mg/g Eq Expressed in Chlorogenic Acid) | DPPH IC50 (mg/mL) | ABTS IC50 (mg/mL) | FRAP EC50 (mg/mL) |
|---|---|---|---|---|---|
| REM | 236.058 ± 17.88 | 217.756 ± 43.13 | 0.0861 ± 0.0003 | 0.0464 ± 0.0002 | 0.8310 ± 0.0002 |
| TEM | 275.507 ± 22.57 | 227.862 ± 36.85 | 0.0993 ± 0.0005 | 0.0546 ± 0.0004 | 0.9795 ± 0.0001 |
| REF | 378.336 ± 38.70 | 277.097 ± 18.60 | 0.0620 ± 0.0004 | 0.0305 ± 0.0002 | 0.6206 ± 0.0001 |
| TEF | 321.678 ± 40.01 | 278.126 ± 45.05 | 0.0773 ± 0.0004 | 0.0419 ± 0.0009 | 0.8208 ± 0.0000 |
| DPPH_IC50 | ABTS_IC50 | FRAP_EC50 | Log_TPC_Value | TPCAs_Value | ||
|---|---|---|---|---|---|---|
| DPPH_ Method_IC50 | Pearson Correlation | 1 | 0.997 *** | 0.978 ** | −0.934 * | −0.793 |
| Sig. (2-tailed) | 0.003 | 0.022 | 0.066 | 0.207 | ||
| N | 4 | 4 | 4 | 4 | 4 | |
| ABTS_ Method_IC50 | Pearson Correlation | 0.997 *** | 1 | 0.988 ** | −0.937 * | −0.765 |
| Sig. (2-tailed) | 0.003 | 0.012 | 0.063 | 0.235 | ||
| N | 4 | 4 | 4 | 4 | 4 | |
| FRAP_ Method_EC50 | Pearson Correlation | 0.978 ** | 0.988 ** | 1 | −0.887 | −0.657 |
| Sig. (2-tailed) | 0.022 | 0.012 | 0.113 | 0.343 | ||
| N | 4 | 4 | 4 | 4 | 4 | |
| BACH1 | BACH2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | ΔG (kcal/mol) | LE | Hydrogen Bonds | Pi Interactions | ΔG (kcal/mol) | LE | Hydrogen Bonds | Pi Interactions |
| Protocatechuic acid | −5.46 | 0.497 | Lys127 | Lys100, Val103, Lys127 | −5.37 | 0.488 | Thr70, Asn72 | − |
| Rutin | −7.22 | 0.168 | Ser4, Ile97, Ser99, Asn102 | Lys82, Ile97 | −7.45 | 0.173 | Tyr14, Asn22, Ala57, Arg117, His119 | − |
| Caffeic acid | −5.91 | 0.455 | Pro86, Thr93, Lys95 | Pro86, Ile97 | −6.03 | 0.464 | Ala113 | Phe128 |
| Chlorogenic acid | −6.66 | 0.267 | Gln43, Ile97, Ser99, Asn102 | Val81 | −7.03 | 0.281 | Ala113, Ser124, Ser127 | Phe128 |
| Luteolin | −7.01 | 0.334 | Pro86, Lys95, Ser99 | Lyes82, Pro86, Ile97 | −7.72 | 0.368 | Cys37 | Arg52(A), Arg52(B) |
| Kaempferol | −7.31 | 0.348 | − | Pro86, Ile97 | −7.06 | 0.336 | His119 | Cys58, Phe93, Met118, Leu121 |
| Rosmarinic acid | −7.05 | 0.271 | Pro86, Thr93 | Val8, Ile97 | −7.12 | 0.274 | Asn22, Asp29, Lys32 | Leu25, Trp63 |
| Quercetin | −6.59 | 0.300 | Asp26, Ala53, Asn117 | His116 | −7.47 | 0.340 | Asn22, Gly26, Asn120 | Trp63, His119 |
| Isoquercitrin | −6.45 | 0.195 | Ser7, Ile97, Glu101 | Lys82, Glu85, Pro86 | −7.97 | 0.242 | Arg52, Val67, Asn72 | Asp38, Arg52 |
| Ferulic acid | −5.88 | 0.420 | Phe123 | Lys100, Lys127 | −6.38 | 0.456 | Leu36, Arg52 | − |
| p-Coumaric acid | −6.17 | 0.514 | Val8 | Leu126, Lys127 | −6.21 | 0.518 | Tyr14, Cys125 | Phe93, Met118, Leu121 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luță, E.-A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Mihai, D.P.; Popescu, L.; Bejenaru, L.E.; Bejenaru, C.; Popovici, V.; Olaru, O.T.; et al. Implications of the Cultivation of Rosemary and Thyme (Lamiaceae) in Plant Communities for the Development of Antioxidant Therapies. Int. J. Mol. Sci. 2023, 24, 11670. https://doi.org/10.3390/ijms241411670
Luță E-A, Biță A, Moroșan A, Mihaiescu DE, Mihai DP, Popescu L, Bejenaru LE, Bejenaru C, Popovici V, Olaru OT, et al. Implications of the Cultivation of Rosemary and Thyme (Lamiaceae) in Plant Communities for the Development of Antioxidant Therapies. International Journal of Molecular Sciences. 2023; 24(14):11670. https://doi.org/10.3390/ijms241411670
Chicago/Turabian StyleLuță, Emanuela-Alice, Andrei Biță, Alina Moroșan, Dan Eduard Mihaiescu, Dragoș Paul Mihai, Liliana Popescu, Ludovic Everard Bejenaru, Cornelia Bejenaru, Violeta Popovici, Octavian Tudorel Olaru, and et al. 2023. "Implications of the Cultivation of Rosemary and Thyme (Lamiaceae) in Plant Communities for the Development of Antioxidant Therapies" International Journal of Molecular Sciences 24, no. 14: 11670. https://doi.org/10.3390/ijms241411670
APA StyleLuță, E.-A., Biță, A., Moroșan, A., Mihaiescu, D. E., Mihai, D. P., Popescu, L., Bejenaru, L. E., Bejenaru, C., Popovici, V., Olaru, O. T., & Gîrd, C. E. (2023). Implications of the Cultivation of Rosemary and Thyme (Lamiaceae) in Plant Communities for the Development of Antioxidant Therapies. International Journal of Molecular Sciences, 24(14), 11670. https://doi.org/10.3390/ijms241411670









