Involvement of Both Extrinsic and Intrinsic Apoptotic Pathways in Tridecylpyrrolidine-Diol Derivative-Induced Apoptosis In Vitro
Abstract
1. Introduction
2. Results
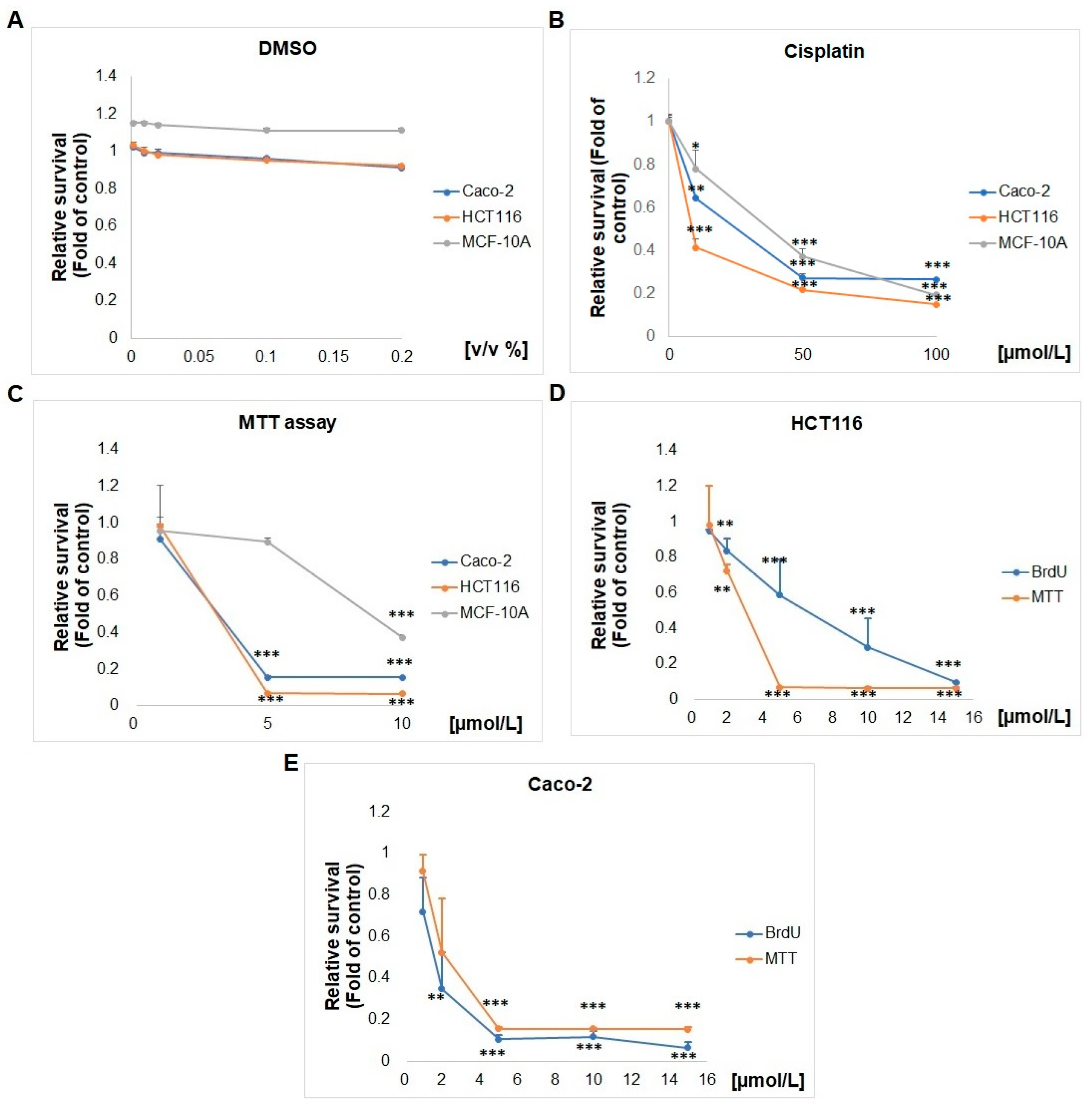
2.1. Effect of SS13 on Cell Viability and Proliferation
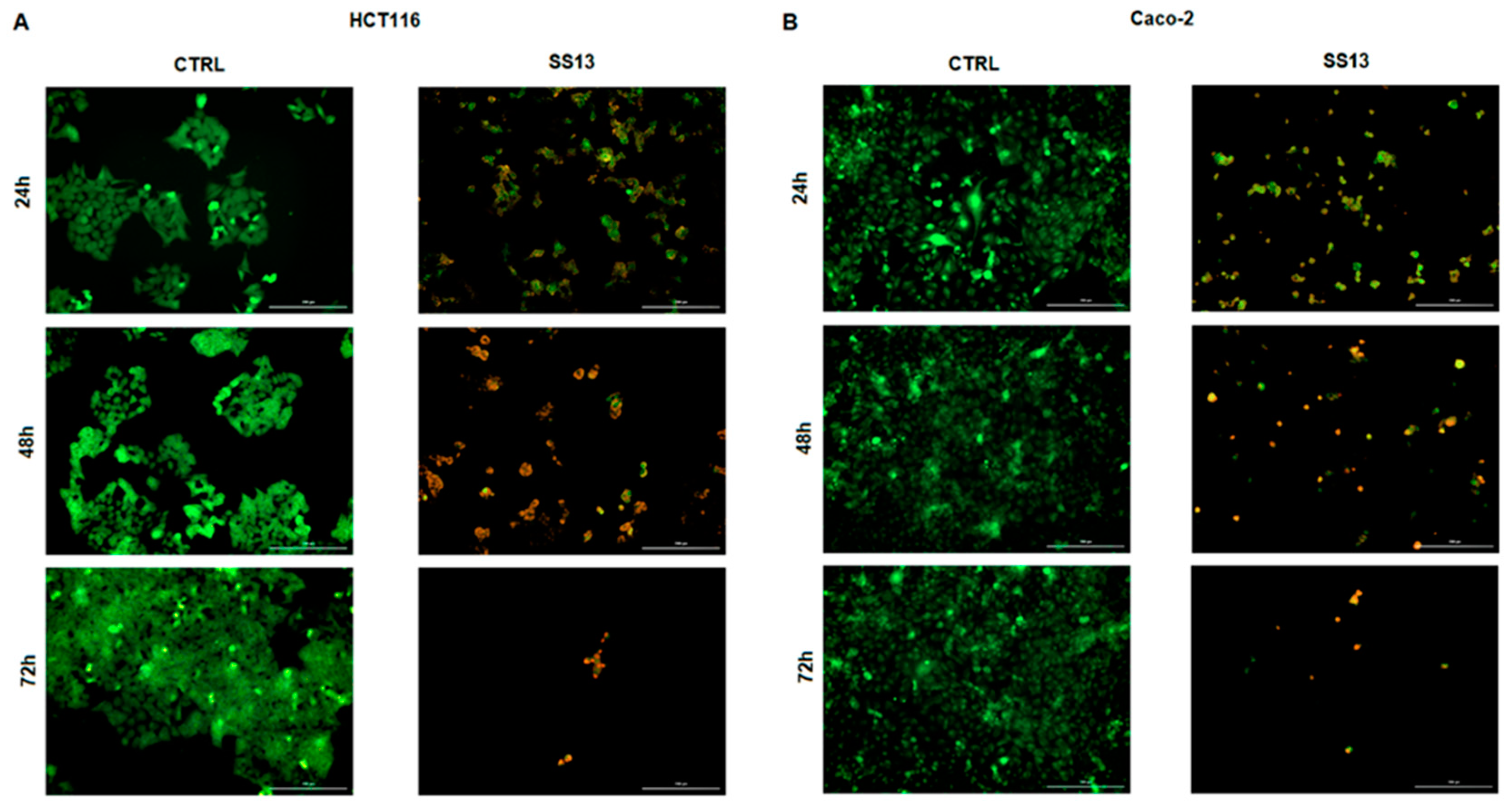
2.2. AO/PI Fluorescent Staining
2.3. Scratch Assay
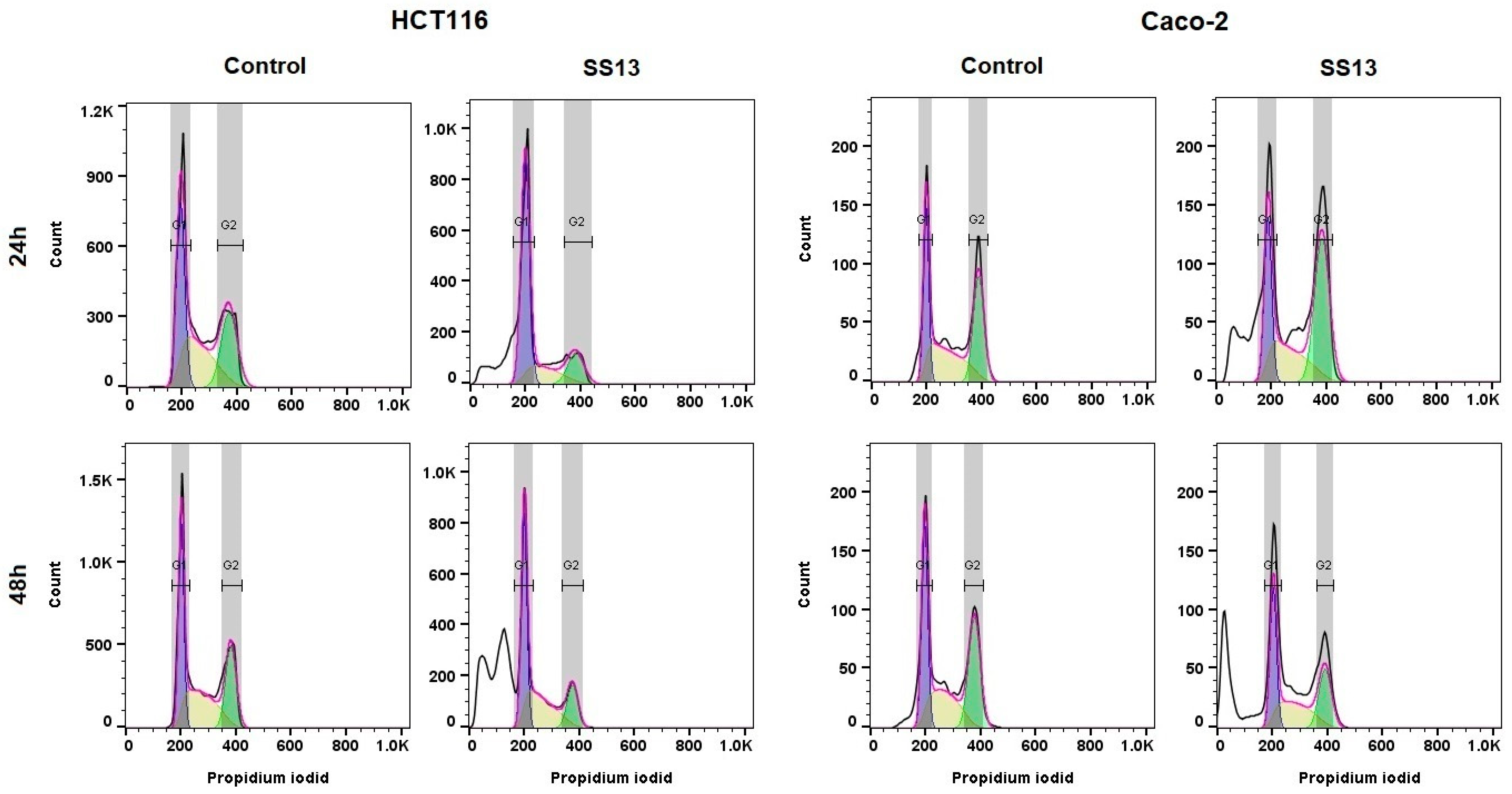
2.4. Cell Cycle Analysis
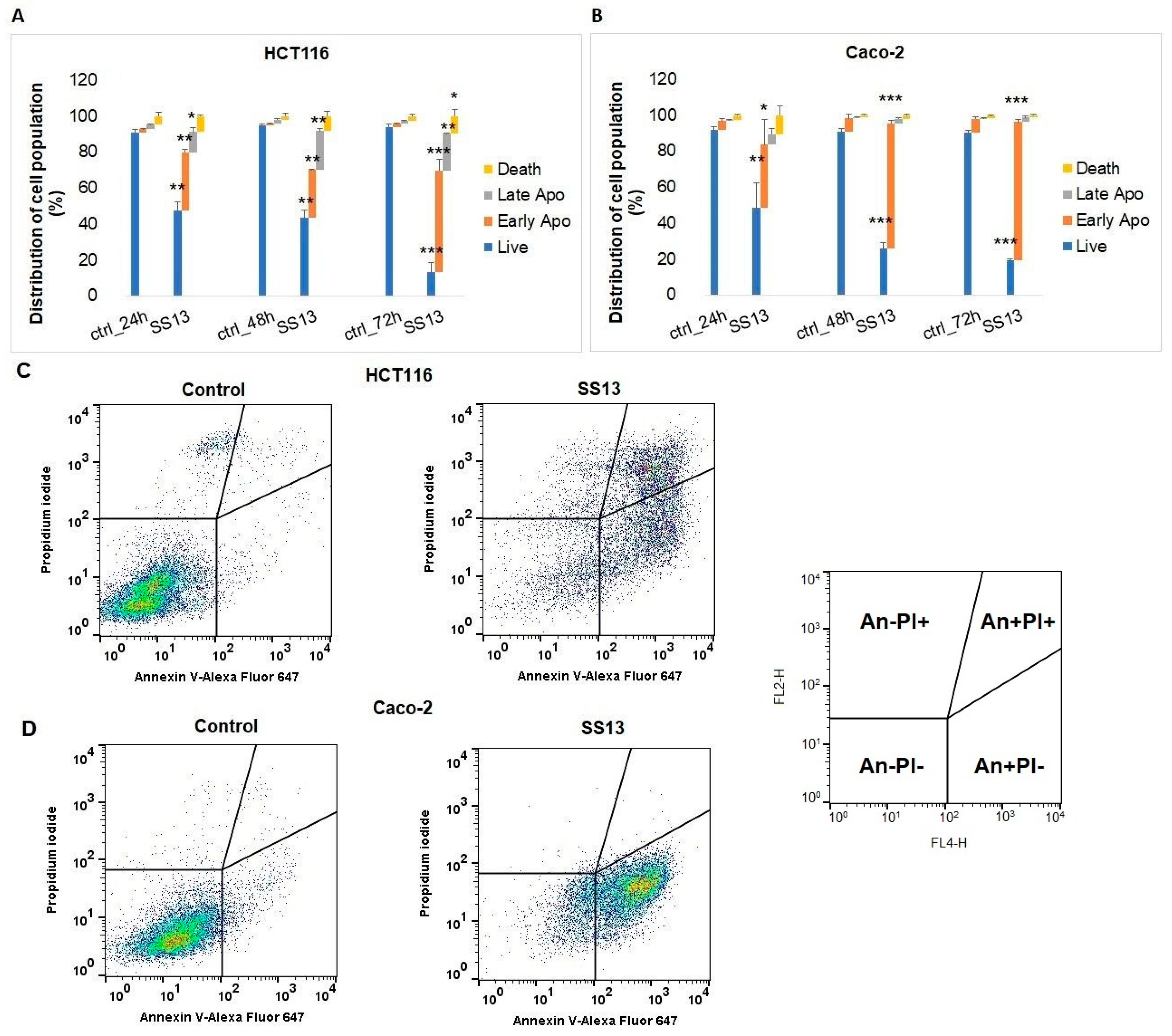
2.5. Annexin V-FITC/PI Staining
2.6. Effect of SS13 on Intrinsic and Extrinsic Apoptotic Pathways
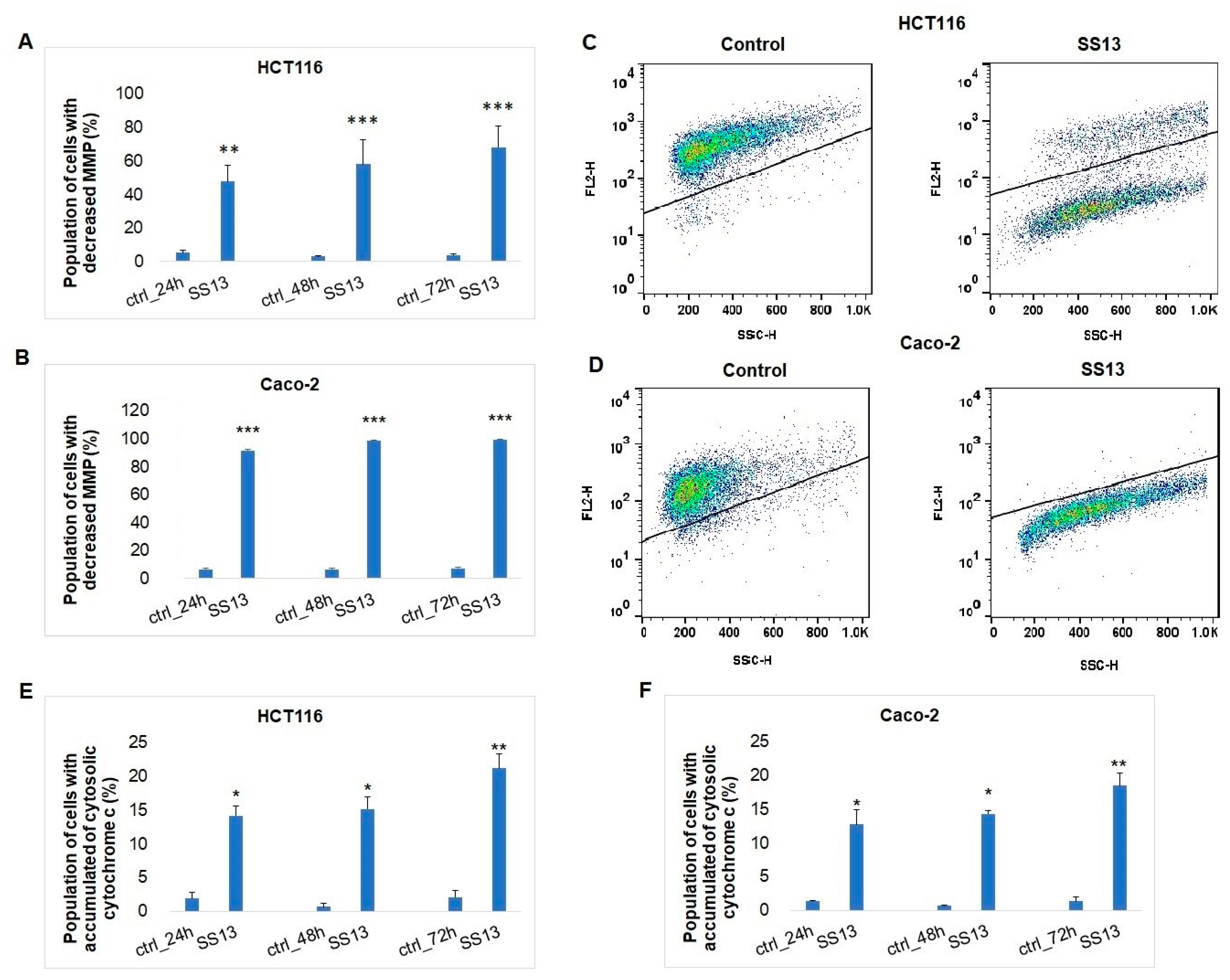
2.6.1. Activation of Intrinsic Apoptotic Pathway
Determination of Mitochondrial Membrane Potential, Cytochrome c Release and Apaf-1
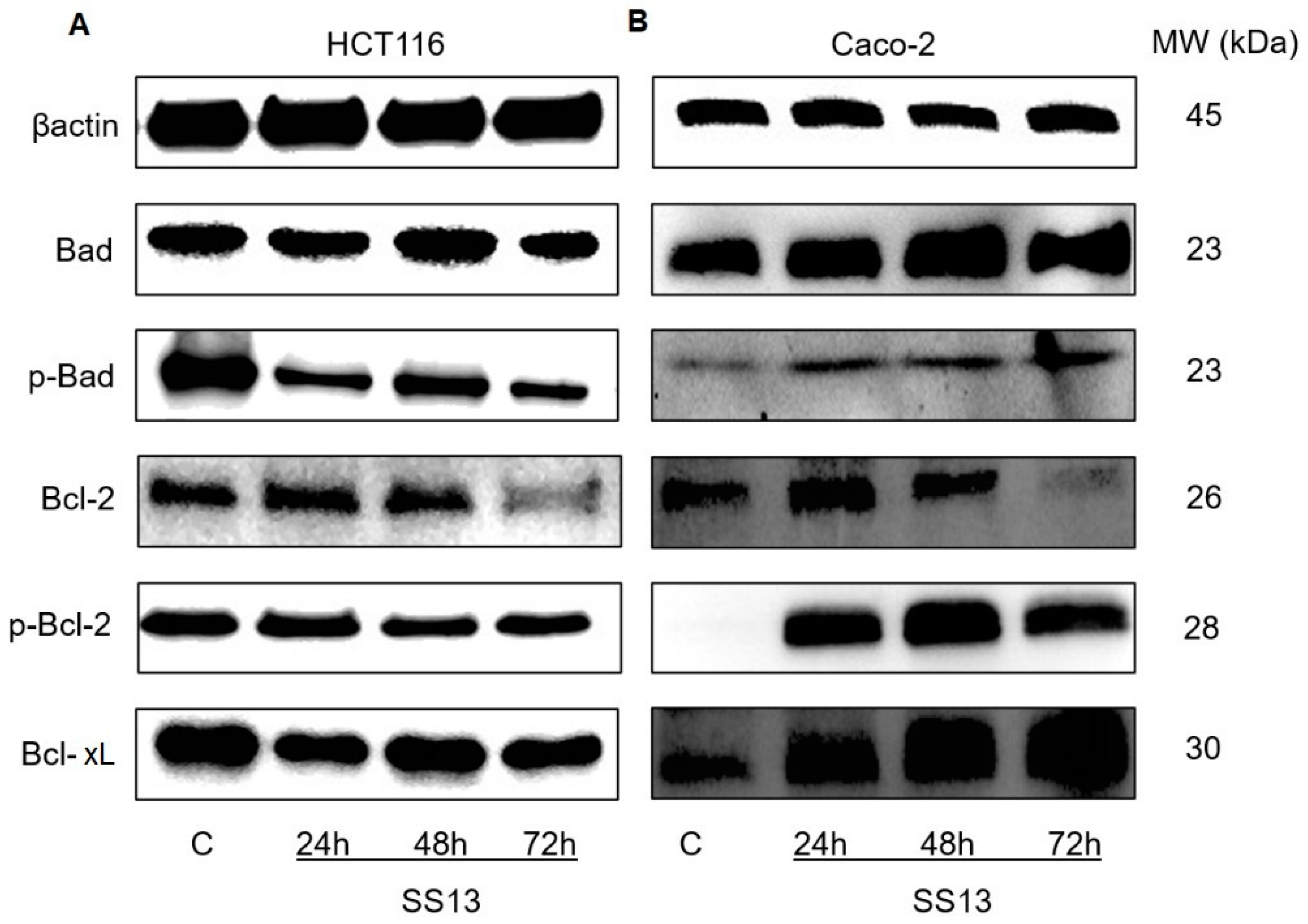
Changes in Bcl Family
Activity of Caspase 3/7 and PARP Cleavage
Inhibitor of Apoptosis Protein (IAP) Family
2.6.2. Activation of Extrinsic Apoptotic Pathway
3. Discussion
4. Materials and Methods
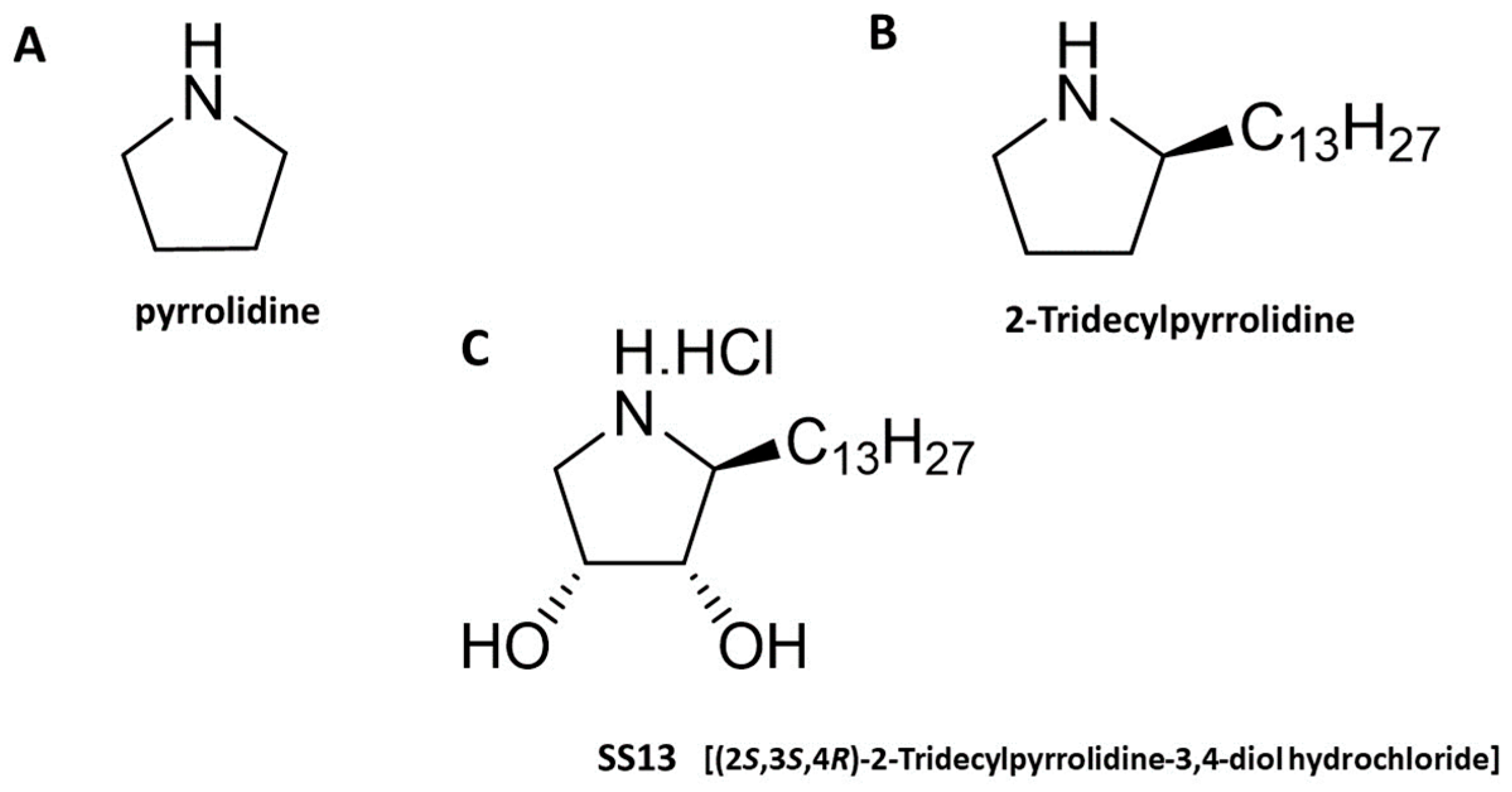
4.1. Tested Compound
4.2. Cell Cultures
4.3. MTT Assay
4.4. BrdU Proliferation Assay
4.5. AO/PI Staining
4.6. Scratch Assay
4.7. Flow Cytometric Analysis
4.7.1. Annexin V/PI Staining
4.7.2. Cell Cycle Analysis
4.7.3. Detection of Mitochondrial Membrane Potential Changes
4.7.4. Detection of Caspase 3/7 Activity
4.7.5. Analysis of Cytochrome c Release
4.7.6. Western Blot Analysis
4.7.7. RNA Isolation and Real-Time Polymerase Chain Reaction (RT-PCR)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Nordlinger, B.; Cervantes, A.; Group, E.G.W. Advanced colorectal cancer: Esmo clinical practice guidelines for treatment. Ann. Oncol. 2010, 21 (Suppl. S5), v93–v97. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D.; Group, E.G.W. Metastatic colorectal cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. S3), iii1–iii9. [Google Scholar] [CrossRef] [PubMed]
- Marmol, I.; Sanchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef]
- Wei, K.; Li, W.; Koike, K.; Pei, Y.; Chen, Y.; Nikaido, T. New amide alkaloids from the roots of Piper nigrum. J. Nat. Prod. 2004, 67, 1005–1009. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in Cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Takayama, H.; Ichikawa, T.; Kitajima, M.; Nonato, M.G.; Aimi, N. Isolation and structure elucidation of two new alkaloids, pandamarilactonine-c and -d, from Pandanus amaryllifolius and revision of relative stereochemistry of pandamarilactonine-a and -b by total synthesis. Chem. Pharm. Bull. 2002, 50, 1303–1304. [Google Scholar] [CrossRef]
- National Library of Medicine. Pyrrolidine; National Center for Biotechnology Information: Bethesda, MD, USA, 2004.
- Islam, M.T.; Mubarak, M.S. Pyrrolidine alkaloids and their promises in pharmacotherapy. Adv. Tradit. Med. 2020, 20, 13–22. [Google Scholar] [CrossRef]
- Meiyazhagan, G.; Raju, R.; Winfred, S.B.; Mannivanan, B.; Bhoopalan, H.; Shankar, V.; Sekar, S.; Venkatachalam, D.P.; Pitani, R.; Nagendrababu, V.; et al. Bioactivity studies of beta-lactam derived polycyclic fused pyrroli-dine/pyrrolizidine derivatives in dentistry: In vitro, in vivo and in silico studies. PLoS ONE 2015, 10, e0131433. [Google Scholar] [CrossRef]
- Katavic, P.L.; Venables, D.A.; Rali, T.; Carroll, A.R. Habbemines a and b, pyrrolidine alkaloids with human delta-opioid receptor binding affinity from the leaves of elaeocarpus habbemensis. J. Nat. Prod. 2007, 70, 866–868. [Google Scholar] [CrossRef]
- Girgis, A.S.; Panda, S.S.; Farag, I.S.; El-Shabiny, A.M.; Moustafa, A.M.; Ismail, N.S.; Pillai, G.G.; Panda, C.S.; Hall, C.D.; Katritzky, A.R. Synthesis, and qsar analysis of anti-oncological active spiro-alkaloids. Org. Biomol. Chem. 2015, 13, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.S.; Kiyatkin, E.A. Fluctuations in central and peripheral temperatures induced by intravenous nicotine: Central and peripheral contributions. Brain Res. 2011, 1383, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Mohamed, G.A.; Moharram, A.M.; Youssef, D.T. Aegyptolidines a and b: New pyrrolidine alkaloids from the fungus Aspergillus aegyptiacus. Phytochem. Lett. 2015, 12, 90–93. [Google Scholar] [CrossRef]
- Al-Massarani, S.M.; El-Gamal, A.A.; Al-Said, M.S.; Abdel-Kader, M.S.; Ashour, A.E.; Kumar, A.; Abdel-Mageed, W.M.; Al-Rehaily, A.J.; Ghabbour, H.A.; Fun, H.K. Studies on the red sea sponge Haliclona sp. For its chemical and cytotoxic properties. Pharmacogn. Mag. 2016, 12, 114–119. [Google Scholar] [CrossRef]
- Ji, J.; Sajjad, F.; You, Q.; Xing, D.; Fan, H.; Reddy, A.G.K.; Hu, W.; Dong, S. Synthesis and biological evaluation of substituted pyrrolidines and pyrroles as potential anticancer agents. Arch. Pharm. 2020, 353, e2000136. [Google Scholar] [CrossRef]
- Omar, H.A.; Zaher, D.M.; Srinivasulu, V.; Hersi, F.; Tarazi, H.; Al-Tel, T.H. Design, synthesis and biological evaluation of new pyrrolidine carboxamide analogues as potential chemotherapeutic agents for hepatocellular carcinoma. Eur. J. Med. Chem. 2017, 139, 804–814. [Google Scholar] [CrossRef]
- Chen, S.H.; Lin, J.K.; Liang, Y.C.; Pan, M.H.; Liu, S.H.; Lin-Shiau, S.Y. Involvement of activating transcription factors jnk, nf-kappab, and ap-1 in apoptosis induced by pyrrolidine dithiocarbamate/cu complex. Eur. J. Pharmacol. 2008, 594, 9–17. [Google Scholar] [CrossRef]
- Boga, S.B.; Alhassan, A.B.; Cooper, A.B.; Doll, R.; Shih, N.Y.; Shipps, G.; Deng, Y.; Zhu, H.; Nan, Y.; Sun, R.; et al. Discovery of 3(s)-thiomethyl pyrrolidine erk inhibitors for oncology. Bioorg. Med. Chem. Lett. 2018, 28, 2029–2034. [Google Scholar] [CrossRef]
- Al-Aamri, H.M.; Irving, H.R.; Bradley, C.; Meehan-Andrews, T. Intrinsic and extrinsic apoptosis responses in leukaemia cells following daunorubicin treatment. BMC Cancer 2021, 21, 438. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Rehmat, J.; Gul-e-Saba, C. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar]
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of action of bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008714. [Google Scholar] [CrossRef] [PubMed]
- Elena-Real, C.A.; Diaz-Quintana, A.; Gonzalez-Arzola, K.; Velazquez-Campoy, A.; Orzaez, M.; Lopez-Rivas, A.; Gil-Caballero, S.; De la Rosa, M.A.; Diaz-Moreno, I. Cytochrome c speeds up caspase cascade activation by blocking 14-3-3epsilon-dependent apaf-1 inhibition. Cell Death Dis. 2018, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhao, W.; Tong, P.; Li, P.; Zhao, Y.; Li, H.; Liang, J. Comprehensive molecular characterization of inhibitors of apoptosis proteins (iaps) for therapeutic targeting in cancer. BMC Med. Genom. 2020, 13, 7. [Google Scholar] [CrossRef]
- Della Ragione, F.; Cucciolla, V.; Borriello, A.; Della Pietra, V.; Manna, C.; Galletti, P.; Zappia, V. Pyrrolidine dithiocarbamate induces apoptosis by a cytochrome c-dependent mechanism. Biochem. Biophys. Res. Commun. 2000, 268, 942–946. [Google Scholar] [CrossRef]
- Shakeri, R.; Kheirollahi, A.; Davoodi, J. Apaf-1: Regulation and function in cell death. Biochimie 2017, 135, 111–125. [Google Scholar] [CrossRef]
- Blankenberg, F.; Narula, J.; Strauss, H.W. In vivo detection of apoptotic cell death: A necessary measurement for evaluating therapy for myocarditis, ischemia, and heart failure. J. Nucl. Cardiol. 1999, 6, 531–539. [Google Scholar] [CrossRef]
- Szántó, M.; Brunyánszki, A.; Kiss, B.; Nagy, L.; Gergely, P.; Virág, L.; Bai, P. Poly (adp-ribose) polymerase-2: Emerging transcriptional roles of a DNA-repair protein. Cell. Mol. Life Sci. 2012, 69, 4079–4092. [Google Scholar] [CrossRef]
- Ashkenazi, A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat. Rev. Drug Discov. 2008, 7, 1001–1012. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Haroon, M.; Baig, M.W.; Tariq, M.; Ahmad, Z.; Tahir, M.N.; Akhtar, T. Synthesis, crystal structure, anti-cancer, anti-inflammatory anti-oxidant and quantum chemical studies of 4-(pyrrolidine-2, 5-dione-1-yl) phenol. J. Mol. Struct. 2021, 1224, 129267. [Google Scholar] [CrossRef]
- Shyamsivappan, S.; Vivek, R.; Saravanan, A.; Arasakumar, T.; Subashini, G.; Suresh, T.; Shankar, R.; Mohan, P.S. Synthesis and x-ray study of dispiro 8-nitroquinolone analogues and their cytotoxic properties against human cervical cancer hela cells. Medchemcomm 2019, 10, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M. Ways of dying: Multiple pathways to apoptosis. Genes Dev. 2003, 17, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Mohammad, F.; Kotresha, D.; Menendez, J.C. Spirooxindole-pyrrolidine heterocyclic hybrids promotes apoptosis through activation of caspase-3. Bioorg. Med. Chem. 2019, 27, 2487–2498. [Google Scholar] [CrossRef]
- Yuan, S.; Akey, C.W. Apoptosome structure, assembly, and procaspase activation. Structure 2013, 21, 501–515. [Google Scholar] [CrossRef]
- Mullen, P. Parp cleavage as a means of assessing apoptosis. Methods Mol. Med. 2004, 88, 171–181. [Google Scholar]
- Van Delft, M.F.; Huang, D. How the bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006, 16, 203–213. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct activation of bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef]
- Stickles, X.B.; Marchion, D.C.; Bicaku, E.; Al Sawah, E.; Abbasi, F.; Xiong, Y.; Bou Zgheib, N.; Boac, B.M.; Orr, B.C.; Judson, P.L.; et al. Bad-mediated apoptotic pathway is associated with human cancer development. Int. J. Mol. Med. 2015, 35, 1081–1087. [Google Scholar] [CrossRef]
- Morais, C.; Pat, B.; Gobe, G.; Johnson, D.W.; Healy, H. Pyrrolidine dithiocarbamate exerts anti-proliferative and pro-apoptotic effects in renal cell carcinoma cell lines. Nephrol. Dial. Transpl. 2006, 21, 3377–3388. [Google Scholar] [CrossRef] [PubMed]
- Shitashige, M.; Toi, M.; Yano, T.; Shibata, M.; Matsuo, Y.; Shibasaki, F. Dissociation of bax from a bcl-2/bax heterodimer triggered by phosphorylation of serine 70 of bcl-2. J. Biochem. 2001, 130, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Kelkel, M.; Cerella, C.; Mack, F.; Schneider, T.; Jacob, C.; Schumacher, M.; Dicato, M.; Diederich, M. Ros-independent jnk activation and multisite phosphorylation of bcl-2 link diallyl tetrasulfide-induced mitotic arrest to apoptosis. Carcinogenesis 2012, 33, 2162–2171. [Google Scholar] [CrossRef]
- Li, Z.; Mbah, N.E.; Overmeyer, J.H.; Sarver, J.G.; George, S.; Trabbic, C.J.; Erhardt, P.W.; Maltese, W.A. The jnk signaling pathway plays a key role in methuosis (non-apoptotic cell death) induced by momipp in glioblastoma. BMC Cancer 2019, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Lu, M.; Petersen, S.; Ashkenazi, A. Apoptosis initiation through the cell-extrinsic pathway. Methods Enzymol. 2014, 544, 99–128. [Google Scholar] [PubMed]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef]
- Samraj, A.K.; Keil, E.; Ueffing, N.; Schulze-Osthoff, K.; Schmitz, I. Loss of caspase-9 provides genetic evidence for the type i/ii concept of cd95-mediated apoptosis. J. Biol. Chem. 2006, 281, 29652–29659. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Jia, Y.; Fan, Y.; Du, M.; Liu, A. Pdtc antagonized polysaccharide-induced apoptosis in mcf-7 cells through a caspase-8 mediated fas pathway. J. Funct. Foods 2013, 5, 1270–1278. [Google Scholar] [CrossRef]
- Fulda, S.; Vucic, D. Targeting iap proteins for therapeutic intervention in cancer. Nat. Rev. Drug Discov. 2012, 11, 109–124. [Google Scholar] [CrossRef]
- Ye, Q.; Cai, W.; Zheng, Y.; Evers, B.M.; She, Q.B. Erk and akt signaling cooperate to translationally regulate survivin expression for metastatic progression of colorectal cancer. Oncogene 2014, 33, 1828–1839. [Google Scholar] [CrossRef]
- Fábianová, D.; Pončáková, T.; Martinkova, M.; Fabian, M.; Fabišíková, M.; Pilatova, M.B.; Macejova, A.; Kuchar, J.; Jager, D. A straightforward approach toward cytotoxic pyrrolidine alkaloids: Novel analogues of natural broussonetines. Tetrahedron 2021, 96, 132380. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lefever, S.; Vandesompele, J.; Speleman, F.; Pattyn, F. Rtprimerdb: The portal for real-time pcr primers and probes. Nucleic Acids Res. 2009, 37, D942–D945. [Google Scholar] [CrossRef] [PubMed]



| Colon Cancer Cell Lines | Normal Epithelial Cell Line | ||
|---|---|---|---|
| HCT116 | Caco-2 | MCF-10A | |
| SS13 | 3.2 ± 0.1 | 2.2 ± 1.5 | 8.8 ± 0.1 |
| Cis-Pt | 9.0 ± 0.4 | 30.7 ± 0.8 | 25.9 ± 0.3 |
| Selectivity index for SS13 | 2.75 | 4 | 1 |
| Selectivity index for Cis-Pt | 2.88 | 0.84 | 1 |
| HCT116 | Time | Sub-G0/G1 | G1 | S | G2/M |
|---|---|---|---|---|---|
| ctrl | 24 h | 0.5 ± 0.4 | 35.1 ± 2.2 | 34.2 ± 5.0 | 30.3 ± 3.1 |
| SS13 | 14.1 ± 4.0 * | 46.1 ± 4.6 * | 21.9 ± 4.7 * | 18.0 ± 4.0 * | |
| ctrl | 48 h | 1.2 ± 0.5 | 35.2 ± 1.2 | 39.7 ± 3.7 | 24.0 ± 3.5 |
| SS13 | 41.4 ± 3.6 ** | 24.4 ± 4.2 * | 21.1 ± 1.6 * | 13.1 ± 2.8 * | |
| ctrl | 72 h | 0.9 ± 0.4 | 57.6 ± 3.2 | 27.4 ± 3.3 | 14.1 ± 3.5 |
| SS13 | 19.3 ± 3.1 * | 40.6 ± 2.8 * | 24.6 ± 3.4 | 15.5 ± 2.3 | |
| Caco-2 | |||||
| ctrl | 24 h | 1.3 ± 0.4 | 33.2 ± 2.7 | 31.0 ± 2.1 | 34.4 ± 3.2 |
| SS13 | 21.7 ± 8.6 * | 28.3 ± 5.2 * | 19.1 ± 1.9 * | 30.9 ± 3.5 | |
| ctrl | 48 h | 2.4 ± 1.4 | 33.4 ± 1.8 | 28.0 ± 2.3 | 36.2 ± 2.0 |
| SS13 | 32.3 ± 5.3 ** | 28.0 ± 1.4 * | 22.0 ± 3.3 * | 17.7 ± 4.2 | |
| ctrl | 72 h | 0.7 ± 0.5 | 35.5 ± 1.8 | 28.9 ± 1.7 | 34.9 ± 1.9 |
| SS13 | 24.2 ± 7.1 ** | 29.2 ± 2.2 * | 22.5 ± 1.1 * | 24.1 ± 3.9 * |
| Pro-Apoptotic | Anti-Apoptotic | ||||||
|---|---|---|---|---|---|---|---|
| Caco-2 | |||||||
| Gene | 24 h | 48 h | 72 h | Gene | 24 h | 48 h | 72 h |
| BAX | 2.514 ± 0.3 ** | 2.100 ± 0.2 ** | 5.236 ± 0.3 *** | BCL2 | 0.891 ± 0.1 | 2.234 ± 0.2 ** | 6.251 ± 0.6 *** |
| BIK | 2.863 ± 0.1 ** | 2.207 ± 0.2 ** | 0.393 ± 0.1 *** | BCL2L1 | 2.107 ± 0.1 ** | 3.775 ± 0.6 *** | 1.240 ± 0.7 |
| APAF1 | 0.565 ± 0.3 * | 3.668 ± 0.6 *** | 1.535 ± 0.4 * | XIAP | 1.260 ± 0.4 | 2.281 ± 0.2 ** | 0.402 ± 0.1 *** |
| CASP8 | 1.408 ± 0.01 * | 0.778 ± 0.1 | 0.441 ± 0.1 ** | BIRC5 | 1.905 ± 0.3 * | 1.849 ± 0.4 * | 1.049 ± 0.2 |
| TNF | 1.383 ± 0.3 | 2.136 ± 0.1 ** | 3.566 ± 0.4 *** | ||||
| FASLG | 1.265 ± 0.2 | 7.075 ± 0.5 *** | 0.704 ± 0.1* | ||||
| HCT116 | |||||||
| BAX | 1.831 ± 0.3 * | 6.548 ± 1.4 *** | 1.968 ± 0.2 * | BCL2 | 0.353 ± 0.2 *** | 0.281 ± 0.1 *** | 0.179 ± 0.4 *** |
| BIK | 0.512 ± 0.1 ** | 0.329 ± 0.1 *** | 3.241 ± 0.2 *** | BCL2L1 | 0.078 ± 0.3 *** | 0.117 ± 0.2 *** | 0.556 ± 0.2 ** |
| APAF1 | 0.742 ± 0.2* | 0.603 ± 0.1 ** | 3.564 ± 0.4 *** | XIAP | 0.313 ± 0.2 *** | 0.395 ± 0.2 *** | 0.409 ± 0.1 *** |
| CASP8 | 2.312 ± 0.3 ** | 1.496 ± 0.1 * | 1.021 ± 0.7 | BIRC5 | 2.301 ± 0.3 ** | 4.637 ± 0.4 *** | 10.460 ± 1.6 *** |
| TNF | 1.800 ± 0.2 * | 1.027 ± 0.4 | 0.646 ± 0.1 ** | ||||
| FASLG | 1.419 ± 0.1 * | 1.636 ± 0.1 * | 1.724 ± 0.3 * | ||||
| Primary Antibody | Mr (kDa) | Origin | Dilution | Catalogue No. | Manufacturer |
|---|---|---|---|---|---|
| Bad (D24A9) Rabbit mAb | 23 | Rabbit | 1:1000 | #9239 | Cell Signaling Technology®, Danvers, MA, USA |
| Phospho-Bad (Ser136) Antibody | 23 | Rabbit | 1:1000 | #9295 | |
| Phospho-Bcl-2 (Ser70) (5H2) Rabbit mAb | 28 | Rabbit | 1:1000 | #2827 | |
| Bcl-xL (54H6) Rabbit mAb | 30 | Rabbit | 1:1000 | #2764 | |
| Cleaved Caspase 8 (Asp374) (18C8) Rabbit mAb | 43/41/18 | Rabbit | 1:1000 | #9496 | |
| PARP (46D11) Rabbit mAb | 116/89 | Rabbit | 1:1000 | #9532 | |
| β-Actin (8H10D10) Mouse mAb | 45 | Mouse | 1:1000 | #3700 | |
| Anti-Bcl2 Antibody [100/D5] | 26 | Mouse | 1:500 | ab692 | Abcam |
| Secondary Antibody | Mr (kDa) | Origin | Dilution | Catalogue No. | Manufacturer |
| Anti-Rabbit IgG HRP-Linked Antibody | - | Goat | 1:1000 | #7074 | Cell Signaling Technology, Danvers, MA, USA |
| Anti-Mouse IgG HRP-Linked Antibody | - | Goat | 1:1000 | #7076 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | 5′-TGCACCACCAACTGCTTAGC-3′ | 5′-GGCATGGACTGTGGTCATGAG-3′ |
| BAX | 5′-CCCGAGAGGTCTTTTTCCGAG-3′ | 5′-CCAGCCCATGATGGTTCTGAT-3′ |
| BCL2 | 5′-TCCATGTCTTTGGACAACCA-3′ | 5′-CCAGCCCATGATGGTTCTGAT-3′ |
| BCL2L1 | 5′-TTACCTGAATGACCACCTA-3′ | 5′-ATTTCCGACTGAAGAGTGA-3′ |
| CASP8 | 5′-AGAGTCTGTGCCCAAATCAAC-3′ | 5′-GCTGCTTCTCTCTTTGCTGAA-3′ |
| FASLG | 5′-CACTTTGGGATTCTTTCCAT-3′ | 5′-GTGAGTTGAGGAGCTACAGA-3′ |
| TNF | 5′-GGCGTGGAGCTGAGAGATAAC-3′ | 5′-GGTGTGGGTGAGGAGCACAT-3′ |
| BIK | 5′-TCTGCAATTGTCACCGGTTA-3′ | 5′-TTGAGCACACCTGCTCCTC-3′ |
| XIAP | 5′-AGTGGTAGTCCTGTTTCAGCATCA-3′ | 5′-CCGCACGGTATCTCCTTCA-3′ |
| BIRC5 (survivin) | 5′-TGCCTGGCAGCCCTTTC-3′ | 5′-CCTCCAAGAAGGGCCAGTTC-3′ |
| APAF1 | 5′-TCCATGTATGGTGACCCATCC-3′ | 5′-AAGGTGGAGTACCACAGAGG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosalova, N.; Keselakova, A.; Kello, M.; Martinkova, M.; Fabianova, D.; Pilatova, M.B. Involvement of Both Extrinsic and Intrinsic Apoptotic Pathways in Tridecylpyrrolidine-Diol Derivative-Induced Apoptosis In Vitro. Int. J. Mol. Sci. 2023, 24, 11696. https://doi.org/10.3390/ijms241411696
Nosalova N, Keselakova A, Kello M, Martinkova M, Fabianova D, Pilatova MB. Involvement of Both Extrinsic and Intrinsic Apoptotic Pathways in Tridecylpyrrolidine-Diol Derivative-Induced Apoptosis In Vitro. International Journal of Molecular Sciences. 2023; 24(14):11696. https://doi.org/10.3390/ijms241411696
Chicago/Turabian StyleNosalova, Natalia, Alexandra Keselakova, Martin Kello, Miroslava Martinkova, Dominika Fabianova, and Martina Bago Pilatova. 2023. "Involvement of Both Extrinsic and Intrinsic Apoptotic Pathways in Tridecylpyrrolidine-Diol Derivative-Induced Apoptosis In Vitro" International Journal of Molecular Sciences 24, no. 14: 11696. https://doi.org/10.3390/ijms241411696
APA StyleNosalova, N., Keselakova, A., Kello, M., Martinkova, M., Fabianova, D., & Pilatova, M. B. (2023). Involvement of Both Extrinsic and Intrinsic Apoptotic Pathways in Tridecylpyrrolidine-Diol Derivative-Induced Apoptosis In Vitro. International Journal of Molecular Sciences, 24(14), 11696. https://doi.org/10.3390/ijms241411696







