Increased Drop in Activity of Alkaline Phosphatase in Plasma from Patients with Endocarditis
Abstract
:1. Introduction
2. Results
2.1. Patient Population and Cut-off Values
2.2. Preoperative Characteristics
2.3. Procedural Data
2.4. Laboratory Data
2.5. Adverse Events and Mortality
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Laboratory Data
4.3. Follow-Ups
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pettersson, G.B.; Coselli, J.S.; Writing, C.; Pettersson, G.B.; Coselli, J.S.; Hussain, S.T.; Griffin, B.; Blackstone, E.H.; Gordon, S.M.; LeMaire, S.A.; et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J. Thorac. Cardiovasc. Surg. 2017, 153, 1241–1258.e29. [Google Scholar] [CrossRef] [Green Version]
- Pierce, D.; Calkins, B.C.; Thornton, K. Infectious endocarditis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 981–986. [Google Scholar] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Iung, B. 2015 ESC Guidelines on the management of infective endocarditis: A big step forward for an old disease. Heart 2016, 102, 992–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, T.L.; Baddour, L.M.; Bayer, A.S.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr. Infective endocarditis. Nat. Rev. Dis. Primers 2016, 2, 16059. [Google Scholar] [CrossRef] [Green Version]
- Gagneux-Brunon, A.; Pouvaret, A.; Maillard, N.; Berthelot, P.; Lutz, M.F.; Cazorla, C.; Tulane, C.; Fuzellier, J.F.; Verhoeven, P.O.; Fresard, A.; et al. Acute kidney injury in infective endocarditis: A retrospective analysis. Med. Mal. Infect. 2019, 49, 527–533. [Google Scholar] [CrossRef]
- Peters, E.; Heemskerk, S.; Masereeuw, R.; Pickkers, P. Alkaline phosphatase: A possible treatment for sepsis-associated acute kidney injury in critically ill patients. Am. J. Kidney Dis. 2014, 63, 1038–1048. [Google Scholar] [CrossRef]
- Hummeke-Oppers, F.; Hemelaar, P.; Pickkers, P. Innovative Drugs to Target Renal Inflammation in Sepsis: Alkaline Phosphatase. Front. Pharmacol. 2019, 10, 919. [Google Scholar] [CrossRef]
- Koyama, I.; Matsunaga, T.; Harada, T.; Hokari, S.; Komoda, T. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin. Biochem. 2002, 35, 455–461. [Google Scholar] [CrossRef]
- Poelstra, K.; Bakker, W.W.; Klok, P.A.; Kamps, J.A.; Hardonk, M.J.; Meijer, D.K. Dephosphorylation of endotoxin by alkaline phosphatase in vivo. Am. J. Pathol. 1997, 151, 1163–1169. [Google Scholar]
- Kerner, A.; Avizohar, O.; Sella, R.; Bartha, P.; Zinder, O.; Markiewicz, W.; Levy, Y.; Brook, G.J.; Aronson, D. Association between elevated liver enzymes and C-reactive protein: Possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arter. Thromb. Vasc. Biol. 2005, 25, 193–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, C.B.; Tung, C.F.; Yang, D.Y.; Hu, W.H.; Hung, D.Z.; Peng, Y.C.; Chang, C.S. Extremely high levels of alkaline phosphatase in adult patients as a manifestation of bacteremia. Hepatogastroenterology 2005, 52, 1347–1350. [Google Scholar] [PubMed]
- Katasako, A.; Sasaki, S.; Raita, Y.; Yamamoto, S.; Tochitani, K.; Murakami, M.; Nishioka, R.; Fujisaki, K. Association between serum alkaline phosphatase and bacteraemia in haemodialysis outpatients: A multicentre retrospective cross-sectional study. BMJ Open 2022, 12, e058666. [Google Scholar] [CrossRef]
- Fawley, J.; Gourlay, D.M. Intestinal alkaline phosphatase: A summary of its role in clinical disease. J. Surg. Res. 2016, 202, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickkers, P.; Heemskerk, S.; Schouten, J.; Laterre, P.F.; Vincent, J.L.; Beishuizen, A.; Jorens, P.G.; Spapen, H.; Bulitta, M.; Peters, W.H.; et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: A prospective randomized double-blind placebo-controlled trial. Crit. Care 2012, 16, R14. [Google Scholar] [CrossRef] [Green Version]
- Al-Fares, A.; Pettenuzzo, T.; Del Sorbo, L. Extracorporeal life support and systemic inflammation. Intensive Care Med. Exp. 2019, 7 (Suppl. S1), 46. [Google Scholar] [CrossRef]
- Giacinto, O.; Satriano, U.; Nenna, A.; Spadaccio, C.; Lusini, M.; Mastroianni, C.; Nappi, F.; Chello, M. Inflammatory Response and Endothelial Dysfunction Following Cardiopulmonary Bypass: Pathophysiology and Pharmacological Targets. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 158–173. [Google Scholar] [CrossRef]
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1682–1690. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Takedachi, M.; Thompson, L.F.; Wilder, T.F.; Fernandez, P.; Cronstein, B.N. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5′-nucleotidase: Findings in a study of ecto-5′-nucleotidase gene-deficient mice. Arthritis Rheum. 2007, 56, 1440–1445. [Google Scholar] [CrossRef]
- Neuhof, C.; Wendling, J.; Dapper, F.; Bauer, J.; Zickmann, B.; Jochum, M.; Tillmanns, H.; Neuhoft, H. Endotoxemia and cytokine generation in cardiac surgery in relation to flow mode and duration of cardiopulmonary bypass. Shock 2001, 16 (Suppl. S1), 39–43. [Google Scholar] [CrossRef] [PubMed]
- Paparella, D.; Yau, T.M.; Young, E. Cardiopulmonary bypass induced inflammation: Pathophysiology and treatment. An update. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2002, 21, 232–244. [Google Scholar] [CrossRef] [Green Version]
- Riddington, D.W.; Venkatesh, B.; Boivin, C.M.; Bonser, R.S.; Elliott, T.S.; Marshall, T.; Mountford, P.J.; Bion, J.F. Intestinal permeability, gastric intramucosal pH, and systemic endotoxemia in patients undergoing cardiopulmonary bypass. JAMA 1996, 275, 1007–1012. [Google Scholar] [CrossRef]
- Davidson, J.; Tong, S.; Hauck, A.; Lawson, D.S.; Jaggers, J.; Kaufman, J.; da Cruz, E. Alkaline phosphatase activity after cardiothoracic surgery in infants and correlation with post-operative support and inflammation: A prospective cohort study. Crit. Care 2012, 16, R160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, J.A.; Urban, T.T.; Tong, S.; Maddux, A.; Hill, G.; Frank, B.S.; Watson, J.D.; Jaggers, J.; Simoes, E.A.F.; Wischmeyer, P. Alkaline Phosphatase Activity and Endotoxemia After Infant Cardiothoracic Surgery. Shock 2019, 51, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Poschner, T.; Schaefer, A.K.; Hutschala, D.; Goliasch, G.; Riebandt, J.; Distelmaier, K.; Bernardi, M.H.; Andreas, M.; Brands, R.; Aref, T.; et al. Impact of Venoarterial Extracorporeal Membrane Oxygenation on Alkaline Phosphatase Metabolism after Cardiac Surgery. Biomolecules 2021, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.K.; Hutschala, D.; Andreas, M.; Bernardi, M.H.; Brands, R.; Shabanian, S.; Laufer, G.; Wiedemann, D. Decrease in serum alkaline phosphatase and prognostic relevance in adult cardiopulmonary bypass. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 383–390. [Google Scholar] [CrossRef]
- Biancari, F.; Dalen, M.; Fiore, A.; Ruggieri, V.G.; Saeed, D.; Jonsson, K.; Gatti, G.; Zipfel, S.; Perrotti, A.; Bounader, K.; et al. Multicenter study on postcardiotomy venoarterial extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2020, 159, 1844–1854.e46. [Google Scholar] [CrossRef]
- Khorsandi, M.; Dougherty, S.; Bouamra, O.; Pai, V.; Curry, P.; Tsui, S.; Clark, S.; Westaby, S.; Al-Attar, N.; Zamvar, V. Extra-corporeal membrane oxygenation for refractory cardiogenic shock after adult cardiac surgery: A systematic review and meta-analysis. J. Cardiothorac. Surg. 2017, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Vongpatanasin, W.; Hillis, L.D.; Lange, R.A. Prosthetic heart valves. N. Engl. J. Med. 1996, 335, 407–416. [Google Scholar] [CrossRef]
- Wang, A.; Athan, E.; Pappas, P.A.; Fowler, V.G., Jr.; Olaison, L.; Pare, C.; Almirante, B.; Munoz, P.; Rizzi, M.; Naber, C.; et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007, 297, 1354–1361. [Google Scholar] [CrossRef]
- Doenst, T.; Borger, M.A.; Weisel, R.D.; Yau, T.M.; Maganti, M.; Rao, V. Relation between aortic cross-clamp time and mortality--not as straightforward as expected. Eur. J. Cardiothorac. Surg. 2008, 33, 660–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissinen, J.; Biancari, F.; Wistbacka, J.O.; Peltola, T.; Loponen, P.; Tarkiainen, P.; Virkkila, M.; Tarkka, M. Safe time limits of aortic cross-clamping and cardiopulmonary bypass in adult cardiac surgery. Perfusion 2009, 24, 297–305. [Google Scholar] [CrossRef]
- Coleman, J.E. Structure and mechanism of alkaline phosphatase. Annu. Rev. Biophys. Biomol. Struct. 1992, 21, 441–483. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.A.; Urban, T.T.; Baird, C.; Tong, S.; Woodruff, A.; Twite, M.; Jaggers, J.; Simoes, E.A.F.; Wischmeyer, P. Alkaline Phosphatase in Infant Cardiopulmonary Bypass: Kinetics and Relationship to Organ Injury and Major Cardiovascular Events. J. Pediatr. 2017, 190, 49–55.e2. [Google Scholar] [CrossRef] [PubMed]
- Tuin, A. Detoxification of LPS by alkaline phosphatase: Application of a new concept in sepsis and inflammatory bowel disease. 2007, 184.
- Verpooten, G.F.; Nouwen, E.J.; Hoylaerts, M.F.; Hendrix, P.G.; de Broe, M.E. Segment-specific localization of intestinal-type alkaline phosphatase in human kidney. Kidney Int. 1989, 36, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Coux, G.; Trumper, L.; Elias, M.M. Renal function and cortical (Na++K+)-ATPase activity, abundance and distribution after ischaemia-reperfusion in rats. Biochim. Biophys. Acta 2002, 1586, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Khundmiri, S.J.; Asghar, M.; Khan, F.; Salim, S.; Yusufi, A.N. Effect of reversible and irreversible ischemia on marker enzymes of BBM from renal cortical PT subpopulations. Am. J. Physiol. 1997, 273, F849–F856. [Google Scholar] [CrossRef]
- Corredor, C.; Thomson, R.; Al-Subaie, N. Long-Term Consequences of Acute Kidney Injury after Cardiac Surgery: A Systematic Review and Meta-Analysis. J. Cardiothorac. Vasc. Anesth. 2016, 30, 69–75. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zhang, H.H.; Zhao, S.L.; Wu, H.Y.; Li, H.N.; Li, W.; Yang, J. Clinical value of alkaline phosphatase on the surface membrane of neutrophils for prediction of bacteremia in patients with systemic inflammatory response syndrome. Diagn. Microbiol. Infect. Dis. 2021, 100, 114105. [Google Scholar] [CrossRef]
- Singh, S.B.; Lin, H.C. Role of Intestinal Alkaline Phosphatase in Innate Immunity. Biomolecules 2021, 11, 1784. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Gioannini, T.L.; Weiss, J.P. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol. Res. 2007, 39, 249–260. [Google Scholar] [CrossRef]
- Clarke, T.B.; Francella, N.; Huegel, A.; Weiser, J.N. Invasive bacterial pathogens exploit TLR-mediated downregulation of tight junction components to facilitate translocation across the epithelium. Cell Host Microbe 2011, 9, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Pawar, R.D.; Castrezana-Lopez, L.; Allam, R.; Kulkarni, O.P.; Segerer, S.; Radomska, E.; Meyer, T.N.; Schwesinger, C.M.; Akis, N.; Grone, H.J.; et al. Bacterial lipopeptide triggers massive albuminuria in murine lupus nephritis by activating Toll-like receptor 2 at the glomerular filtration barrier. Immunology 2009, 128, e206–e221. [Google Scholar] [CrossRef] [PubMed]
- Hamarneh, S.R.; Mohamed, M.M.; Economopoulos, K.P.; Morrison, S.A.; Phupitakphol, T.; Tantillo, T.J.; Gul, S.S.; Gharedaghi, M.H.; Tao, Q.; Kaliannan, K.; et al. A novel approach to maintain gut mucosal integrity using an oral enzyme supplement. Ann. Surg. 2014, 260, 706–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kats, S.; Brands, R.; Hamad, M.A.; Seinen, W.; Scharnhorst, V.; Wulkan, R.W.; Schonberger, J.P.; Oeveren, W. Prophylactic treatment with alkaline phosphatase in cardiac surgery induces endogenous alkaline phosphatase release. Int. J. Artif. Organs 2012, 35, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Presbitero, A.; Mancini, E.; Brands, R.; Krzhizhanovskaya, V.V.; Sloot, P.M.A. Supplemented Alkaline Phosphatase Supports the Immune Response in Patients Undergoing Cardiac Surgery: Clinical and Computational Evidence. Front. Immunol. 2018, 9, 2342. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Schumann, G.; Klauke, R.; Canalias, F.; Bossert-Reuther, S.; Franck, P.F.; Gella, F.J.; Jorgensen, P.J.; Kang, D.; Lessinger, J.M.; Panteghini, M.; et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. Part 9: Reference procedure for the measurement of catalytic concentration of alkaline phosphatase International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Scientific Division, Committee on Reference Systems of Enzymes (C-RSE) (1)). Clin. Chem. Lab. Med. 2011, 49, 1439–1446. [Google Scholar] [CrossRef] [Green Version]


| Overall n = 314 | AP Drop < 30% n = 135 | AP Drop ≥ 30% n = 179 | p-Value ª | ||
|---|---|---|---|---|---|
| Age | 62 (70;48) | 59 (69;45) | 62 (70;49) | 0.230 | |
| Female | 85 (27.1) | 37 (27.4) | 48 (26.8) | 0.907 | |
| BMI | 25.3 (28.9;22.8) | 25.1 (28.9;22.9) | 25.6 (28.7;22.5) † | 0.703 | |
| EuroScore II | 11.7 (26.4;5.5) | 9.1 (15.9;3.5) | 16.1 (34.3;6.4) | 0.000 * | |
| NYHA III | 78 (24.8) | 34 (25.2) | 44 (24.6) | 0.902 | |
| NYHA IV | 89 (28.3) | 35 (25.9) | 54 (30.2) | 0.409 | |
| LVEF | 60 (60;55) | 60 (60;55) | 60 (60;55) | 0.042 * | |
| Hypertension | 200 (63.7) | 79 (58.5) | 121 (67.6) | 0.098 | |
| Atrial fibrillation | 90 (28.7) | 38 (28.1) | 52 (29.1) | 0.861 | |
| IDDM | 15 (4.8) | 6 (4.4) | 9 (5.0) | 0.810 | |
| Preoperative dialysis | 32 (10.2) | 8 (5.9) | 24 (13.4) | 0.030 * | |
| Cancer | 19 (6.1) | 10 (7.4) | 9 (5.0) | 0.381 | |
| h/o stroke | 123 (39.2) | 55 (40.7) | 68 (38.0) | 0.621 | |
| Modified Duke criteria | |||||
| Major criteria | Positive BC | 259 (82.5) | 107 (79.3) | 152 (84.9) | 0.192 |
| Vegetation | 266 (84.7) | 114 (84.4) | 152 (84.9) | 0.908 | |
| Annular abscess | 131 (41.7) | 55 (40.7) | 76 (42.5) | 0.760 | |
| Minor criteria | IV drug abuse | 24 (7.6) | 11 (8.1) | 13 (7.3) | 0.770 |
| Fever > 38 °C | 201 (64.0) | 81 (60) | 120 (67.0) | 0.198 | |
| Vascular phenomena | 161 (51.3) | 67 (49.6) | 94 (52.5) | 0.613 | |
| Immunologic phenomena | 19 (6.1) | 11 (8.1) | 8 (4.5) | 0.176 | |
| PVE | 99 (31.5) | 27 (20.0) | 72 (40.2) | 0.000 * | |
| Preoperative ventilation | 142 (45.2) | 64 (47.4) | 78 (43.6) | 0.499 | |
| Preoperative inotropic support | 88 (28.0) | 32 (23.7) | 56 (31.3) | 0.139 | |
| CPR | 13 (4.1) | 5 (3.7) | 8 (4.5) | 0.736 | |
| Lactate value | 0.9 (1.3;0.7) | 0.8 (1.2;0.7) | 1.0 (1.3;0.7) | 0.013 * |
| Overall n = 314 | AP Drop < 30% n = 135 | AP Drop ≥ 30% n = 179 | p-Value ª | |
|---|---|---|---|---|
| Urgent operation | 262 (83.4) | 118 (87.4) | 144 (80.4) | 0.100 |
| Emergency operation | 49 (15.6) | 16 (11.9) | 33 (18.4) | 0.111 |
| Salvage operation | 3 (1.0) | 1 (0.7) | 2 (1.1) | 0.734 |
| Full sternotomy | 303 (96.5) | 128 (94.8) | 175 (97.8) | 0.159 |
| Re-sternotomy | 102 (32.5) | 29 (21.5) | 73 (40.8) | 0.000 * |
| Isolated AVR | 168 (53.5) | 84 (62.2) | 84 (46.9) | 0.007 * |
| Isolated MVR | 93 (29.6) | 39 (28.9) | 54 (30.2) | 0.806 |
| Double valve replacement | 52 (16.9) | 12 (8.9) | 41 (22.9) | 0.001 * |
| Surgery time | 276 (390;220) | 240 (300;195) | 348 (455;255) | 0.000 * |
| CPB | 139 (204;104) | 110 (155;90) | 162 (237;117) | 0.000 * |
| ACC | 100 (144;70) | 81 (113;62) | 111 (159;79) | 0.000 * |
| Overall n = 314 | AP Drop < 30% n = 135 | AP Drop ≥ 30% n = 179 | p-Value ª | |
|---|---|---|---|---|
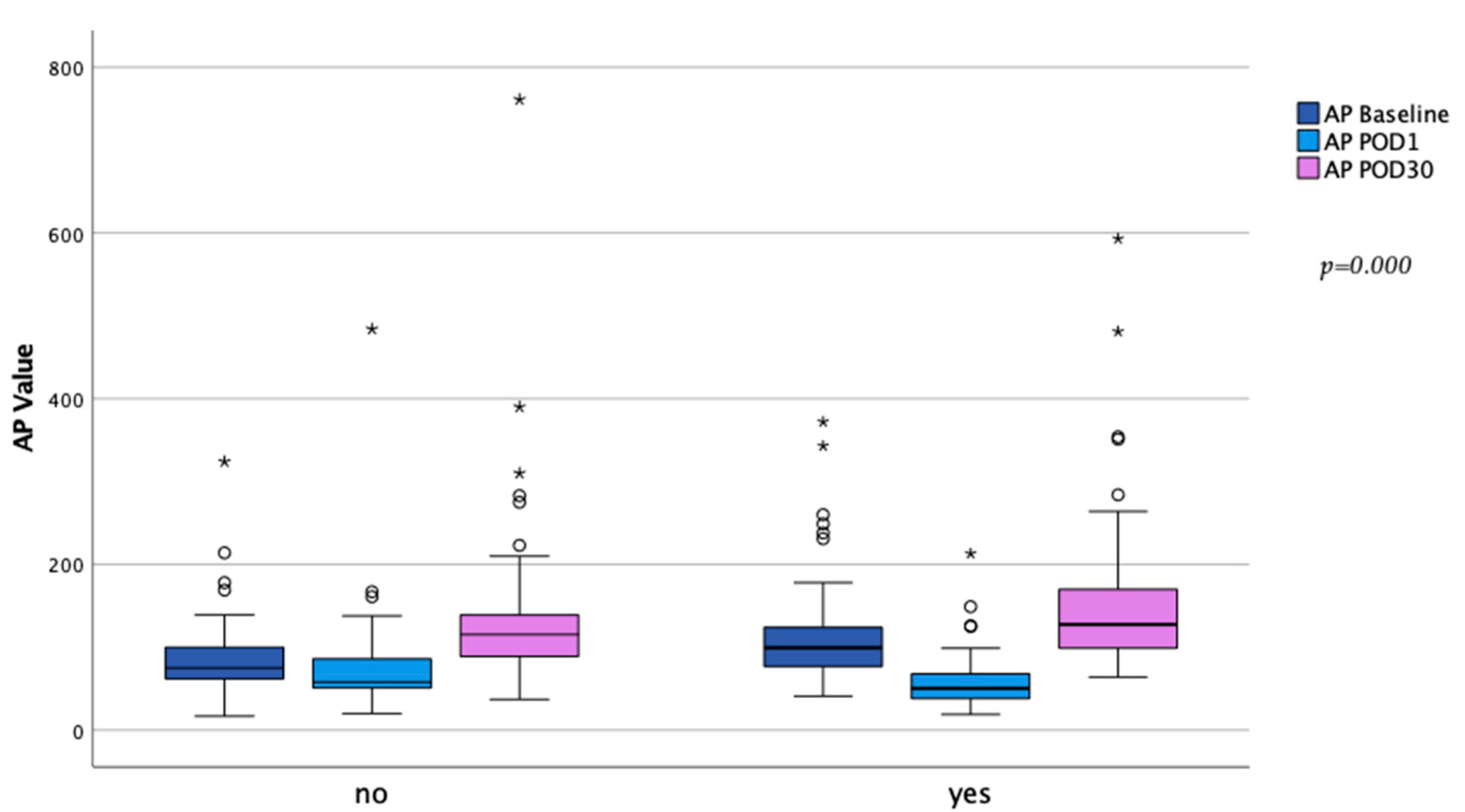
| Baseline AP value | 85 (112;69) | 74 (96;62) | 93 (120;76) | 0.000 * |
| Baseline CRP value | 4.2 (10.7;1.6) | 3.9 (8.3;1.7) | 4.5 (11.7;1.5) | 0.456 |
| AP Drop | 32.5 (45.2;23.0) | 21.4 (25.8;11.5) | 42.7 (54.7;36.4) | 0.000 * |
| First day baseline AP value surpassed å | 5 (7;4) | 4 (6;3) | 6 (7;4) | 0.000 * |
| Baseline within 3 days | 118 (37.6) | 59 (43.7) | 59 (33.0) | 0.052 |
| Baseline within 5 days | 208 (66.2) | 100 (74.1) | 108 (60.3) | 0.011 * |
| 30 day AP value ∫ | 121 (157;95) | 116 (141;89) | 128 (172;99) | 0.089 |
| 30 day CRP value ç | 3.4 (7.8;1.1) | 2.1 (5.7;0.7) | 4.6 (10.6;1.8) | 0.001 * |
| Overall n = 314 | AP Drop < 30% n = 135 | AP Drop ≥ 30% n = 179 | p-Value ª | ||
|---|---|---|---|---|---|
| Bleeding revision | 38 (12.1) | 10 (7.4) | 28 (15.6) | 0.027 * | |
| Need for any renal replacement therapy | 49 (15.6) | 10 (7.4) | 39 (21.8) | 0.001 * | |
| Need for any renal replacement therapy without preoperative | 37 (11.8) | 9 (6.7) | 28 (15.6) | 0.015 * | |
| Need for ECMO | 37 (11.8) | 3 (2.2) | 34 (19.0) | 0.000 * | |
| Prolonged intubation | 107 (34.1) | 38 (28.1) | 69 (38.5) | 0.054 | |
| Prolonged ICU stay | 129 (41.1) | 43 (31.9) | 86 (48.0) | 0.004 * | |
| Prolonged hospital stay | 62 (19.7) | 22 (16.3) | 40 (22.3) | 0.182 | |
| Mortality | |||||
| 30 day | 23 (7.3) | 4 (3.0) | 19 (10.6) | 0.010 * | |
| In-hospital | 39 (12.4) | 8 (5.9) | 31 (17.3) | 0.002 * | |
| 1 year | 65 (20.7) | 19 (14.1) | 46 (25.7) | 0.012* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahrovic, A.; Poschner, T.; Schober, A.; Angleitner, P.; Alajbegovic, L.; Andreas, M.; Hutschala, D.; Brands, R.; Laufer, G.; Wiedemann, D. Increased Drop in Activity of Alkaline Phosphatase in Plasma from Patients with Endocarditis. Int. J. Mol. Sci. 2023, 24, 11728. https://doi.org/10.3390/ijms241411728
Kahrovic A, Poschner T, Schober A, Angleitner P, Alajbegovic L, Andreas M, Hutschala D, Brands R, Laufer G, Wiedemann D. Increased Drop in Activity of Alkaline Phosphatase in Plasma from Patients with Endocarditis. International Journal of Molecular Sciences. 2023; 24(14):11728. https://doi.org/10.3390/ijms241411728
Chicago/Turabian StyleKahrovic, Amila, Thomas Poschner, Anna Schober, Philipp Angleitner, Leila Alajbegovic, Martin Andreas, Doris Hutschala, Ruud Brands, Günther Laufer, and Dominik Wiedemann. 2023. "Increased Drop in Activity of Alkaline Phosphatase in Plasma from Patients with Endocarditis" International Journal of Molecular Sciences 24, no. 14: 11728. https://doi.org/10.3390/ijms241411728






