The P2X7 Receptor, a Multifaceted Receptor in Alzheimer’s Disease
Abstract
1. The Purinergic Receptor P2X7
2. Alzheimer’s Disease
3. The P2X7 Purinergic Receptor and AD
3.1. P2X7 Expression
3.2. P2X7 and Genetic Risk Factors
3.3. P2X7 and Amyloid Plaques
3.4. P2X7 and Tauopathy
3.5. P2X7 and Synaptic Functions
3.6. P2X7 and the Inflammasome NLRP3
3.7. P2X7 and Chemokines
3.8. P2X7 and Microglia
3.9. P2X7 and Astrocytes
3.10. P2X7 as a Therapeutic Target
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCarthy, A.E.; Yoshioka, C.; Mansoor, S.E. Full-Length P2X7 Structures Reveal How Palmitoylation Prevents Channel Desensitization. Cell 2019, 179, 659–670.e13. [Google Scholar] [CrossRef] [PubMed]
- Miras-Portugal, M.T.; Ortega, F.; Gómez-Villafuertes, R.; Gualix, J.; Pérez-Sen, R.; Delicado, E.G. P2X7 Receptors in the Central Nervous System. Biochem. Pharmacol. 2021, 187, 114472. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, S.; Matzinger, P. Danger Signals: SOS to the Immune System. Curr. Opin. Immunol. 2001, 13, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Leeson, H.C.; Kasherman, M.A.; Chan-Ling, T.; Lovelace, M.D.; Brownlie, J.C.; Toppinen, K.M.; Gu, B.J.; Weible, M.W., II. P2X7 Receptors Regulate Phagocytosis and Proliferation in Adult Hippocampal and SVZ Neural Progenitor Cells: Implications for Inflammation in Neurogenesis. Stem Cells 2018, 36, 1764–1777. [Google Scholar] [CrossRef] [PubMed]
- Solle, M.; Labasi, J.; Perregaux, D.G.; Stam, E.; Petrushova, N.; Koller, B.H.; Griffiths, R.J.; Gabel, C.A. Altered Cytokine Production in Mice Lacking P2X7Receptors*. J. Biol. Chem. 2001, 276, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.S.; Sluyter, R.; Gu, B.J.; Stokes, L.; Fuller, S.J. The Human P2X7 Receptor and Its Role in Innate Immunity. Tissue Antigens 2011, 78, 321–332. [Google Scholar] [CrossRef]
- Ferrari, D.; Pizzirani, C.; Adinolfi, E.; Lemoli, R.M.; Curti, A.; Idzko, M.; Panther, E.; Di Virgilio, F. The P2X7 Receptor: A Key Player in IL-1 Processing and Release. J. Immunol. 2006, 176, 3877–3883. [Google Scholar] [CrossRef]
- Kanellopoulos, J.M.; Delarasse, C. Pleiotropic Roles of P2X7 in the Central Nervous System. Front. Cell Neurosci. 2019, 13, 401. [Google Scholar] [CrossRef]
- Suzuki, T.; Hide, I.; Ido, K.; Kohsaka, S.; Inoue, K.; Nakata, Y. Production and Release of Neuroprotective Tumor Necrosis Factor by P2X7 Receptor-Activated Microglia. J. Neurosci. 2004, 24, 1–7. [Google Scholar] [CrossRef]
- Solini, A.; Chiozzi, P.; Morelli, A.; Fellin, R.; Di Virgilio, F. Human Primary Fibroblasts in Vitro Express a Purinergic P2X7 Receptor Coupled to Ion Fluxes, Microvesicle Formation and IL-6 Release. J. Cell Sci. 1999, 112 Pt 3, 297–305. [Google Scholar] [CrossRef]
- Shieh, C.-H.; Heinrich, A.; Serchov, T.; van Calker, D.; Biber, K. P2X7-Dependent, but Differentially Regulated Release of IL-6, CCL2, and TNF-α in Cultured Mouse Microglia. Glia 2014, 62, 592–607. [Google Scholar] [CrossRef]
- Heinrich, A.; Andó, R.D.; Túri, G.; Rózsa, B.; Sperlágh, B. K+ Depolarization Evokes ATP, Adenosine and Glutamate Release from Glia in Rat Hippocampus: A Microelectrode Biosensor Study. Br. J. Pharmacol. 2012, 167, 1003–1020. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.; Vizi, E.S.; Sperlágh, B. Lack of ATP-Evoked GABA and Glutamate Release in the Hippocampus of P2X7 Receptor-/- Mice. Neuroreport 2004, 15, 2387–2391. [Google Scholar] [CrossRef] [PubMed]
- Parvathenani, L.K.; Tertyshnikova, S.; Greco, C.R.; Roberts, S.B.; Robertson, B.; Posmantur, R. P2X7 Mediates Superoxide Production in Primary Microglia and Is Up-Regulated in a Transgenic Mouse Model of Alzheimer’s Disease. J. Biol. Chem. 2003, 278, 13309–13317. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Won, S.M.; Gwag, B.J.; Lee, Y.B. Microglial P2X7 Receptor Expression Is Accompanied by Neuronal Damage in the Cerebral Cortex of the APPswe/PS1dE9 Mouse Model of Alzheimer’s Disease. Exp. Mol. Med. 2011, 43, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Wiley, J.S. P2X7 as a Scavenger Receptor for Innate Phagocytosis in the Brain. Br. J. Pharmacol. 2018, 175, 4195–4208. [Google Scholar] [CrossRef]
- Illes, P. P2X7 Receptors Amplify CNS Damage in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 5996. [Google Scholar] [CrossRef]
- Mattson, M.P. Cellular Actions of Beta-Amyloid Precursor Protein and Its Soluble and Fibrillogenic Derivatives. Physiol. Rev. 1997, 77, 1081–1132. [Google Scholar] [CrossRef]
- Duyckaerts, C.; Bennecib, M.; Grignon, Y.; Uchihara, T.; He, Y.; Piette, F.; Hauw, J.J. Modeling the Relation between Neurofibrillary Tangles and Intellectual Status. Neurobiol. Aging 1997, 18, 267–273. [Google Scholar] [CrossRef]
- Martin, E.; Amar, M.; Dalle, C.; Youssef, I.; Boucher, C.; Le Duigou, C.; Brückner, M.; Prigent, A.; Sazdovitch, V.; Halle, A.; et al. New Role of P2X7 Receptor in an Alzheimer’s Disease Mouse Model. Mol. Psychiatry 2019, 24, 108–125. [Google Scholar] [CrossRef]
- Martínez-Frailes, C.; Di Lauro, C.; Bianchi, C.; de Diego-García, L.; Sebastián-Serrano, Á.; Boscá, L.; Díaz-Hernández, M. Amyloid Peptide Induced Neuroinflammation Increases the P2X7 Receptor Expression in Microglial Cells, Impacting on Its Functionality. Front. Cell Neurosci. 2019, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- McLarnon, J.G.; Ryu, J.K.; Walker, D.G.; Choi, H.B. Upregulated Expression of Purinergic P2X(7) Receptor in Alzheimer Disease and Amyloid-Beta Peptide-Treated Microglia and in Peptide-Injected Rat Hippocampus. J. Neuropathol. Exp. Neurol. 2006, 65, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.; Martin, E.; Ces, A.; Sarrazin, N.; Lagouge-Roussey, P.; Nous, C.; Boucherit, L.; Youssef, I.; Prigent, A.; Faivre, E.; et al. P2X7-Deficiency Improves Plasticity and Cognitive Abilities in a Mouse Model of Tauopathy. Prog. Neurobiol. 2021, 206, 102139. [Google Scholar] [CrossRef]
- Di Lauro, C.; Bianchi, C.; Sebastián-Serrano, Á.; Soria-Tobar, L.; Alvarez-Castelao, B.; Nicke, A.; Díaz-Hernández, M. P2X7 Receptor Blockade Reduces Tau Induced Toxicity, Therapeutic Implications in Tauopathies. Prog. Neurobiol. 2022, 208, 102173. [Google Scholar] [CrossRef]
- Jin, H.; Han, J.; Resing, D.; Liu, H.; Yue, X.; Miller, R.L.; Schoch, K.M.; Miller, T.M.; Perlmutter, J.S.; Egan, T.M.; et al. Synthesis and in Vitro Characterization of a P2X7 Radioligand [123I]TZ6019 and Its Response to Neuroinflammation in a Mouse Model of Alzheimer Disease. Eur. J. Pharmacol. 2018, 820, 8–17. [Google Scholar] [CrossRef]
- Li, Y.; Laws, S.M.; Miles, L.A.; Wiley, J.S.; Huang, X.; Masters, C.L.; Gu, B.J. Genomics of Alzheimer’s Disease Implicates the Innate and Adaptive Immune Systems. Cell Mol. Life Sci. 2021, 78, 7397–7426. [Google Scholar] [CrossRef]
- Bartlett, R.; Stokes, L.; Sluyter, R. The P2X7 Receptor Channel: Recent Developments and the Use of P2X7 Antagonists in Models of Disease. Pharmacol. Rev. 2014, 66, 638–675. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Sluyter, R.; Skarratt, K.K.; Shemon, A.N.; Dao-Ung, L.-P.; Fuller, S.J.; Barden, J.A.; Clarke, A.L.; Petrou, S.; Wiley, J.S. An Arg307 to Gln Polymorphism within the ATP-Binding Site Causes Loss of Function of the Human P2X7 Receptor. J. Biol. Chem. 2004, 279, 31287–31295. [Google Scholar] [CrossRef]
- Wiley, J.S.; Dao-Ung, L.-P.; Li, C.; Shemon, A.N.; Gu, B.J.; Smart, M.L.; Fuller, S.J.; Barden, J.A.; Petrou, S.; Sluyter, R. An Ile-568 to Asn Polymorphism Prevents Normal Trafficking and Function of the Human P2X7 Receptor. J. Biol. Chem. 2003, 278, 17108–17113. [Google Scholar] [CrossRef]
- Stokes, L.; Fuller, S.J.; Sluyter, R.; Skarratt, K.K.; Gu, B.J.; Wiley, J.S. Two Haplotypes of the P2X(7) Receptor Containing the Ala-348 to Thr Polymorphism Exhibit a Gain-of-Function Effect and Enhanced Interleukin-1beta Secretion. FASEB J. 2010, 24, 2916–2927. [Google Scholar] [CrossRef]
- Gu, B.J.; Field, J.; Dutertre, S.; Ou, A.; Kilpatrick, T.J.; Lechner-Scott, J.; Scott, R.; Lea, R.; Taylor, B.V.; Stankovich, J.; et al. A Rare P2X7 Variant Arg307Gln with Absent Pore Formation Function Protects against Neuroinflammation in Multiple Sclerosis. Hum. Mol. Genet. 2015, 24, 5644–5654. [Google Scholar] [CrossRef]
- Gu, B.J.; Zhang, W.; Worthington, R.A.; Sluyter, R.; Dao-Ung, P.; Petrou, S.; Barden, J.A.; Wiley, J.S. A Glu-496 to Ala Polymorphism Leads to Loss of Function of the Human P2X7 Receptor. J. Biol. Chem. 2001, 276, 11135–11142. [Google Scholar] [CrossRef] [PubMed]
- Cabrini, G.; Falzoni, S.; Forchap, S.L.; Pellegatti, P.; Balboni, A.; Agostini, P.; Cuneo, A.; Castoldi, G.; Baricordi, O.R.; Di Virgilio, F. A His-155 to Tyr Polymorphism Confers Gain-of-Function to the Human P2X7 Receptor of Human Leukemic Lymphocytes. J. Immunol. 2005, 175, 82–89. [Google Scholar] [CrossRef]
- Boldt, W.; Klapperstück, M.; Büttner, C.; Sadtler, S.; Schmalzing, G.; Markwardt, F. Glu496Ala Polymorphism of Human P2X7 Receptor Does Not Affect Its Electrophysiological Phenotype. Am. J. Physiol. Cell Physiol. 2003, 284, C749–C756. [Google Scholar] [CrossRef]
- Wiley, J.S.; Dao-Ung, L.P.; Gu, B.J.; Sluyter, R.; Shemon, A.N.; Li, C.; Taper, J.; Gallo, J.; Manoharan, A. A Loss-of-Function Polymorphic Mutation in the Cytolytic P2X7 Receptor Gene and Chronic Lymphocytic Leukaemia: A Molecular Study. Lancet 2002, 359, 1114–1119. [Google Scholar] [CrossRef]
- Li, C.M.; Campbell, S.J.; Kumararatne, D.S.; Bellamy, R.; Ruwende, C.; McAdam, K.P.W.J.; Hill, A.V.S.; Lammas, D.A. Association of a Polymorphism in the P2X7 Gene with Tuberculosis in a Gambian Population. J. Infect. Dis. 2002, 186, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.M.; Falzoni, S.; Rizzo, R.; Cipollone, F.; Zuliani, G.; Di Virgilio, F. Possible Protective Role of the 489C>T P2X7R Polymorphism in Alzheimer’s Disease. Exp. Gerontol. 2014, 60, 117–119. [Google Scholar] [CrossRef]
- Gu, B.J.; Baird, P.N.; Vessey, K.A.; Skarratt, K.K.; Fletcher, E.L.; Fuller, S.J.; Richardson, A.J.; Guymer, R.H.; Wiley, J.S. A Rare Functional Haplotype of the P2RX4 and P2RX7 Genes Leads to Loss of Innate Phagocytosis and Confers Increased Risk of Age-Related Macular Degeneration. FASEB J. 2013, 27, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, X.; Fowler, C.; Lim, Y.Y.; Laws, S.M.; Faux, N.; Doecke, J.D.; Trounson, B.; Pertile, K.; Rumble, R.; et al. Identification of Leukocyte Surface P2X7 as a Biomarker Associated with Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 7867. [Google Scholar] [CrossRef]
- Sanz, J.M.; Falzoni, S.; Morieri, M.L.; Passaro, A.; Zuliani, G.; Di Virgilio, F. Association of Hypomorphic P2X7 Receptor Genotype With Age. Front. Mol. Neurosci. 2020, 13, 8. [Google Scholar] [CrossRef]
- Delarasse, C.; Auger, R.; Gonnord, P.; Fontaine, B.; Kanellopoulos, J.M. The Purinergic Receptor P2X7 Triggers Alpha-Secretase-Dependent Processing of the Amyloid Precursor Protein. J. Biol. Chem. 2011, 286, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Darmellah, A.; Rayah, A.; Auger, R.; Cuif, M.-H.; Prigent, M.; Arpin, M.; Alcover, A.; Delarasse, C.; Kanellopoulos, J.M. Ezrin/Radixin/Moesin Are Required for the Purinergic P2X7 Receptor (P2X7R)-Dependent Processing of the Amyloid Precursor Protein. J. Biol. Chem. 2012, 287, 34583–34595. [Google Scholar] [CrossRef] [PubMed]
- León-Otegui, M.; Gómez-Villafuertes, R.; Díaz-Hernández, J.I.; Díaz-Hernández, M.; Miras-Portugal, M.T.; Gualix, J. Opposite Effects of P2X7 and P2Y2 Nucleotide Receptors on α-Secretase-Dependent APP Processing in Neuro-2a Cells. FEBS Lett. 2011, 585, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Hernandez, J.I.; Gomez-Villafuertes, R.; León-Otegui, M.; Hontecillas-Prieto, L.; Del Puerto, A.; Trejo, J.L.; Lucas, J.J.; Garrido, J.J.; Gualix, J.; Miras-Portugal, M.T.; et al. In Vivo P2X7 Inhibition Reduces Amyloid Plaques in Alzheimer’s Disease through GSK3β and Secretases. Neurobiol. Aging 2012, 33, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Delpech, J.-C.; Venkatesan Kalavai, S.; Van Enoo, A.A.; Hu, J.; Ikezu, S.; Ikezu, T. P2RX7 Inhibitor Suppresses Exosome Secretion and Disease Phenotype in P301S Tau Transgenic Mice. Mol. Neurodegener. 2020, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Alvarez-Castelao, B.; Sebastián-Serrano, Á.; Di Lauro, C.; Soria-Tobar, L.; Nicke, A.; Engel, T.; Díaz-Hernández, M. P2X7 Receptor Inhibition Ameliorates Ubiquitin-Proteasome System Dysfunction Associated with Alzheimer’s Disease. Alzheimers Res. Ther. 2023, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 Inflammasome Is Involved in the Innate Immune Response to Amyloid-Beta. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 Is Activated in Alzheimer’s Disease and Contributes to Pathology in APP/PS1 Mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Rampe, D.; Wang, L.; Ringheim, G.E. P2X7 Receptor Modulation of Beta-Amyloid- and LPS-Induced Cytokine Secretion from Human Macrophages and Microglia. J. Neuroimmunol. 2004, 147, 56–61. [Google Scholar] [CrossRef]
- Hu, S.J.; Calippe, B.; Lavalette, S.; Roubeix, C.; Montassar, F.; Housset, M.; Levy, O.; Delarasse, C.; Paques, M.; Sahel, J.-A.; et al. Upregulation of P2RX7 in Cx3cr1-Deficient Mononuclear Phagocytes Leads to Increased Interleukin-1β Secretion and Photoreceptor Neurodegeneration. J. Neurosci. 2015, 35, 6987–6996. [Google Scholar] [CrossRef]
- Sanz, J.M.; Chiozzi, P.; Ferrari, D.; Colaianna, M.; Idzko, M.; Falzoni, S.; Fellin, R.; Trabace, L.; Di Virgilio, F. Activation of Microglia by Amyloid {beta} Requires P2X7 Receptor Expression. J. Immunol. 2009, 182, 4378–4385. [Google Scholar] [CrossRef]
- Martin, E.; Delarasse, C. Complex Role of Chemokine Mediators in Animal Models of Alzheimer’s Disease. Biomed. J. 2018, 41, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Boucher, C.; Fontaine, B.; Delarasse, C. Distinct Inflammatory Phenotypes of Microglia and Monocyte-Derived Macrophages in Alzheimer’s Disease Models: Effects of Aging and Amyloid Pathology. Aging Cell 2017, 16, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Dorothée, G.; Hunot, S.; Martin, E.; Monnet, Y.; Duchamp, M.; Dong, Y.; Légeron, F.-P.; Leboucher, A.; Burnouf, S.; et al. Hippocampal T Cell Infiltration Promotes Neuroinflammation and Cognitive Decline in a Mouse Model of Tauopathy. Brain 2017, 140, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.Q.; Qin, S.X.; Wu, L.J.; Mackay, C.R.; Hyman, B.T. Immunohistochemical Study of the Beta-Chemokine Receptors CCR3 and CCR5 and Their Ligands in Normal and Alzheimer’s Disease Brains. Am. J. Pathol. 1998, 153, 31–37. [Google Scholar] [CrossRef]
- Passos, G.F.; Figueiredo, C.P.; Prediger, R.D.S.; Pandolfo, P.; Duarte, F.S.; Medeiros, R.; Calixto, J.B. Role of the Macrophage Inflammatory Protein-1alpha/CC Chemokine Receptor 5 Signaling Pathway in the Neuroinflammatory Response and Cognitive Deficits Induced by Beta-Amyloid Peptide. Am. J. Pathol. 2009, 175, 1586–1597. [Google Scholar] [CrossRef]
- Marciniak, E.; Faivre, E.; Dutar, P.; Alves Pires, C.; Demeyer, D.; Caillierez, R.; Laloux, C.; Buée, L.; Blum, D.; Humez, S. The Chemokine MIP-1α/CCL3 Impairs Mouse Hippocampal Synaptic Transmission, Plasticity and Memory. Sci. Rep. 2015, 5, 15862. [Google Scholar] [CrossRef]
- Panenka, W.; Jijon, H.; Herx, L.M.; Armstrong, J.N.; Feighan, D.; Wei, T.; Yong, V.W.; Ransohoff, R.M.; MacVicar, B.A. P2X7-like Receptor Activation in Astrocytes Increases Chemokine Monocyte Chemoattractant Protein-1 Expression via Mitogen-Activated Protein Kinase. J. Neurosci. 2001, 21, 7135–7142. [Google Scholar] [CrossRef]
- Kataoka, A.; Tozaki-Saitoh, H.; Koga, Y.; Tsuda, M.; Inoue, K. Activation of P2X7 Receptors Induces CCL3 Production in Microglial Cells through Transcription Factor NFAT. J. Neurochem. 2009, 108, 115–125. [Google Scholar] [CrossRef]
- Man, S.-M.; Ma, Y.-R.; Shang, D.-S.; Zhao, W.-D.; Li, B.; Guo, D.-W.; Fang, W.-G.; Zhu, L.; Chen, Y.-H. Peripheral T Cells Overexpress MIP-1alpha to Enhance Its Transendothelial Migration in Alzheimer’s Disease. Neurobiol. Aging 2007, 28, 485–496. [Google Scholar] [CrossRef]
- Miras-Portugal, M.T.; Sebastián-Serrano, Á.; de Diego García, L.; Díaz-Hernández, M. Neuronal P2X7 Receptor: Involvement in Neuronal Physiology and Pathology. J. Neurosci. 2017, 37, 7063–7072. [Google Scholar] [CrossRef] [PubMed]
- Illes, P.; Khan, T.M.; Rubini, P. Neuronal P2X7 Receptors Revisited: Do They Really Exist? J. Neurosci. 2017, 37, 7049–7062. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.W.; Walser, S.M.; Aprile-Garcia, F.; Dedic, N.; Chen, A.; Holsboer, F.; Arzt, E.; Wurst, W.; Deussing, J.M. Genetically Dissecting P2rx7 Expression within the Central Nervous System Using Conditional Humanized Mice. Purinergic Signal 2017, 13, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Hajek, K.; Zhang, J.; Kopp, R.; Grosche, A.; Rissiek, B.; Saul, A.; Bruzzone, S.; Engel, T.; Jooss, T.; Krautloher, A.; et al. Re-Evaluation of Neuronal P2X7 Expression Using Novel Mouse Models and a P2X7-Specific Nanobody. eLife 2018, 7, e36217. [Google Scholar] [CrossRef]
- Weisman, G.A.; Camden, J.M.; Peterson, T.S.; Ajit, D.V.; Woods, L.T.; Erb, L. P2 Receptors for Extracellular Nucleotides in the Central Nervous System: Role of P2X7 and P2Y2 Receptor Interactions in Neuroinflammation. Mol. Neurobiol. 2012, 46, 96–113. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Huang, L.; Tang, Y. The P2X7 Receptor: A New Therapeutic Target in Alzheimer’s Disease. Expert Opin. Ther. Targets 2019, 23, 165–176. [Google Scholar] [CrossRef]
- Gu, B.J.; Saunders, B.M.; Petrou, S.; Wiley, J.S. P2X(7) Is a Scavenger Receptor for Apoptotic Cells in the Absence of Its Ligand, Extracellular ATP. J. Immunol. 2011, 187, 2365–2375. [Google Scholar] [CrossRef]
- Gu, B.J.; Saunders, B.M.; Jursik, C.; Wiley, J.S. The P2X7-Nonmuscle Myosin Membrane Complex Regulates Phagocytosis of Nonopsonized Particles and Bacteria by a Pathway Attenuated by Extracellular ATP. Blood 2010, 115, 1621–1631. [Google Scholar] [CrossRef]
- Ni, J.; Wang, P.; Zhang, J.; Chen, W.; Gu, L. Silencing of the P2X(7) Receptor Enhances Amyloid-β Phagocytosis by Microglia. Biochem. Biophys. Res. Commun. 2013, 434, 363–369. [Google Scholar] [CrossRef]
- Beltran-Lobo, P.; Reid, M.J.; Jimenez-Sanchez, M.; Verkhratsky, A.; Perez-Nievas, B.G.; Noble, W. Astrocyte Adaptation in Alzheimer’s Disease: A Focus on Astrocytic P2X7R. Essays Biochem. 2023, 67, 119–130. [Google Scholar] [CrossRef]
- Sperlágh, B.; Köfalvi, A.; Deuchars, J.; Atkinson, L.; Milligan, C.J.; Buckley, N.J.; Vizi, E.S. Involvement of P2X7 Receptors in the Regulation of Neurotransmitter Release in the Rat Hippocampus. J. Neurochem. 2002, 81, 1196–1211. [Google Scholar] [CrossRef] [PubMed]
- Fellin, T.; Pozzan, T.; Carmignoto, G. Purinergic Receptors Mediate Two Distinct Glutamate Release Pathways in Hippocampal Astrocytes. J. Biol. Chem. 2006, 281, 4274–4284. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Deussing, J.; Tang, Y.; Illes, P. Astrocytic Rather than Neuronal P2X7 Receptors Modulate the Function of the Tri-Synaptic Network in the Rodent Hippocampus. Brain Res. Bull. 2019, 151, 164–173. [Google Scholar] [CrossRef]
- Francistiová, L.; Bianchi, C.; Di Lauro, C.; Sebastián-Serrano, Á.; de Diego-García, L.; Kobolák, J.; Dinnyés, A.; Díaz-Hernández, M. The Role of P2X7 Receptor in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 94. [Google Scholar] [CrossRef]
- Keystone, E.C.; Wang, M.M.; Layton, M.; Hollis, S.; McInnes, I.B.; on behalf of the D1520C00001 Study Team. Clinical Evaluation of the Efficacy of the P2X7 Purinergic Receptor Antagonist AZD9056 on the Signs and Symptoms of Rheumatoid Arthritis in Patients with Active Disease despite Treatment with Methotrexate or Sulphasalazine. Ann. Rheum. Dis. 2012, 71, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Stock, T.C.; Bloom, B.J.; Wei, N.; Ishaq, S.; Park, W.; Wang, X.; Gupta, P.; Mebus, C.A. Efficacy and Safety of CE-224,535, an Antagonist of P2X7 Receptor, in Treatment of Patients with Rheumatoid Arthritis Inadequately Controlled by Methotrexate. J. Rheumatol. 2012, 39, 720–727. [Google Scholar] [CrossRef]
- Eser, A.; Colombel, J.-F.; Rutgeerts, P.; Vermeire, S.; Vogelsang, H.; Braddock, M.; Persson, T.; Reinisch, W. Safety and Efficacy of an Oral Inhibitor of the Purinergic Receptor P2X7 in Adult Patients with Moderately to Severely Active Crohn’s Disease: A Randomized Placebo-Controlled, Double-Blind, Phase IIa Study. Inflamm. Bowel Dis. 2015, 21, 2247–2253. [Google Scholar] [CrossRef]
- Jiang, L.-H.; Mackenzie, A.B.; North, R.A.; Surprenant, A. Brilliant Blue G Selectively Blocks ATP-Gated Rat P2X7 Receptors. Mol. Pharmacol. 2000, 58, 82–88. [Google Scholar] [CrossRef]
- Jo, S.; Bean, B.P. Inhibition of Neuronal Voltage-Gated Sodium Channels by Brilliant Blue G. Mol. Pharmacol. 2011, 80, 247–257. [Google Scholar] [CrossRef]
- Lee, S.; Ha, H.; Jang, J.; Byun, Y. Recent Advances in the Development of Antidepressants Targeting the Purinergic P2X7 Receptor. Curr. Med. Chem. 2023, 30, 164–177. [Google Scholar] [CrossRef]
- Territo, P.R.; Zarrinmayeh, H. P2X7 Receptors in Neurodegeneration: Potential Therapeutic Applications From Basic to Clinical Approaches. Front. Cell Neurosci. 2021, 15, 617036. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Espinoza, C.; Guillou, C.; Rissiek, B.; Wilmes, M.; Javidi, E.; Schwarz, N.; Junge, M.; Haag, F.; Liaukouskaya, N.; Wanner, N.; et al. Effective Targeting of Microglial P2X7 Following Intracerebroventricular Delivery of Nanobodies and Nanobody-Encoding AAVs. Front. Pharmacol. 2022, 13, 1029236. [Google Scholar] [CrossRef] [PubMed]
- Wilmes, M.; Pinto Espinoza, C.; Ludewig, P.; Stabernack, J.; Liesz, A.; Nicke, A.; Gelderblom, M.; Gerloff, C.; Falzoni, S.; Tolosa, E.; et al. Blocking P2X7 by Intracerebroventricular Injection of P2X7-Specific Nanobodies Reduces Stroke Lesions. J. Neuroinflamm. 2022, 19, 256. [Google Scholar] [CrossRef] [PubMed]
| SNP | rs Number | Amino Acid Change | Affected Region of P2X7 Protein | Change in Function | Associated AD Risk | Reference |
|---|---|---|---|---|---|---|
| 1513A>C | rs3751143 | Glu496Ala | Carboxyl-terminal tail | Loss of function | Decreased risk of AD | [32] |
| 489C>T | rs208294 | His155Tyr | Extracellular loop | Gain of function | Increased risk of AD | [33] |
| 474G>A | rs28360447 | Gly150Arg | Extracellular loop | Loss of function | Increased risk of AD | [30] |
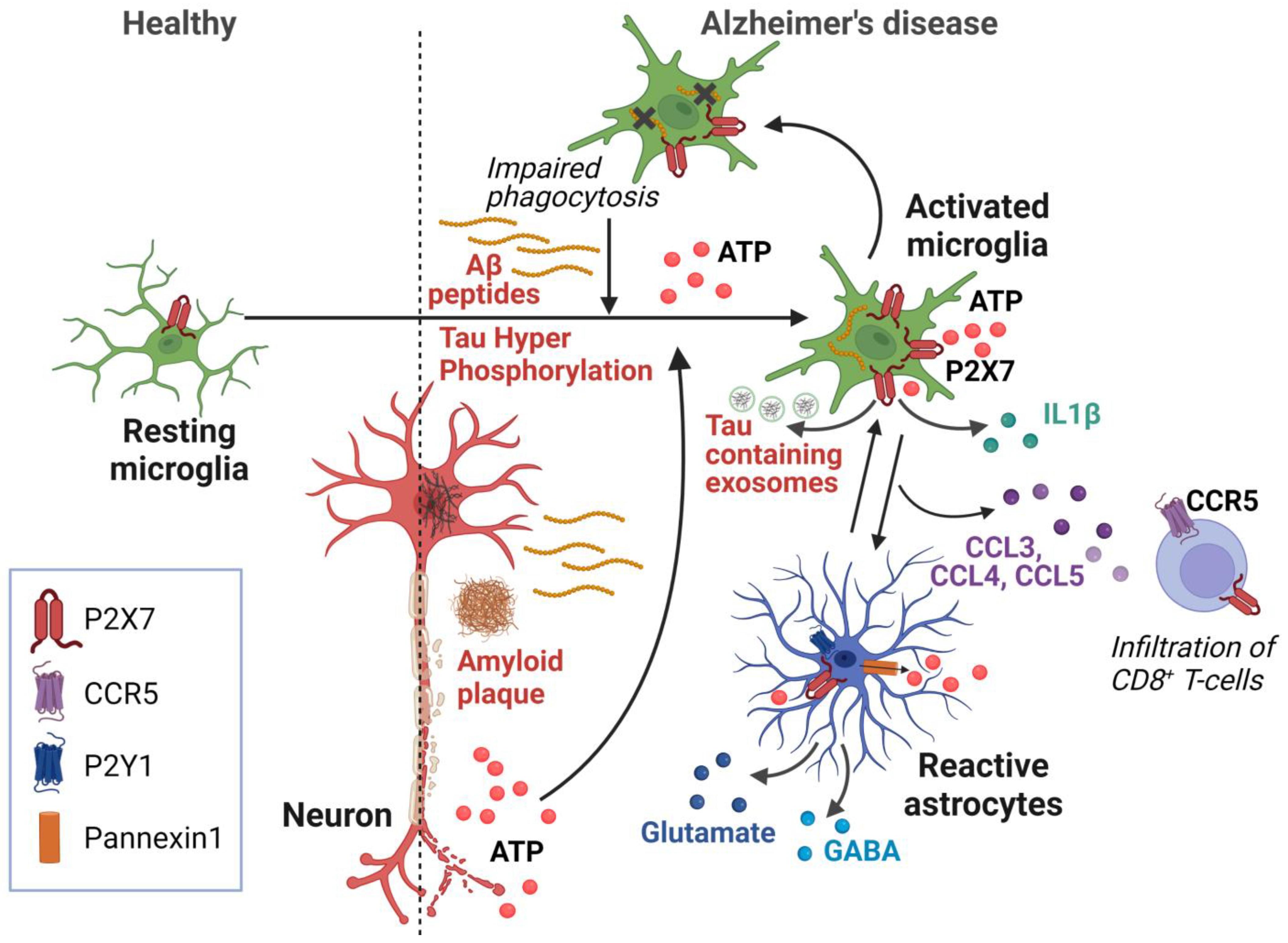
| Role | Cells Involved | References |
|---|---|---|
| Aβ phagocytosis | Microglia | [16,24,69] |
| Release of Tau containing exosomes | Microglia | [45] |
| Release of IL1β | Microglia | [24,44,49,51] |
| Release of chemokines | Microglia | [20,23,59] |
| Release of chemokines | Astrocyte | [20,23,58] |
| Release of glutamate and GABA | Astrocyte | [70,71,72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronning, K.E.; Déchelle-Marquet, P.-A.; Che, Y.; Guillonneau, X.; Sennlaub, F.; Delarasse, C. The P2X7 Receptor, a Multifaceted Receptor in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 11747. https://doi.org/10.3390/ijms241411747
Ronning KE, Déchelle-Marquet P-A, Che Y, Guillonneau X, Sennlaub F, Delarasse C. The P2X7 Receptor, a Multifaceted Receptor in Alzheimer’s Disease. International Journal of Molecular Sciences. 2023; 24(14):11747. https://doi.org/10.3390/ijms241411747
Chicago/Turabian StyleRonning, Kaitryn E., Paul-Alexandre Déchelle-Marquet, Yueshen Che, Xavier Guillonneau, Florian Sennlaub, and Cécile Delarasse. 2023. "The P2X7 Receptor, a Multifaceted Receptor in Alzheimer’s Disease" International Journal of Molecular Sciences 24, no. 14: 11747. https://doi.org/10.3390/ijms241411747
APA StyleRonning, K. E., Déchelle-Marquet, P.-A., Che, Y., Guillonneau, X., Sennlaub, F., & Delarasse, C. (2023). The P2X7 Receptor, a Multifaceted Receptor in Alzheimer’s Disease. International Journal of Molecular Sciences, 24(14), 11747. https://doi.org/10.3390/ijms241411747







