Esaxerenone Inhibits Renal Angiogenesis and Endothelial-Mesenchymal Transition via the VEGFA and TGF-β1 Pathways in Aldosterone-Infused Mice
Abstract
:1. Introduction
2. Results
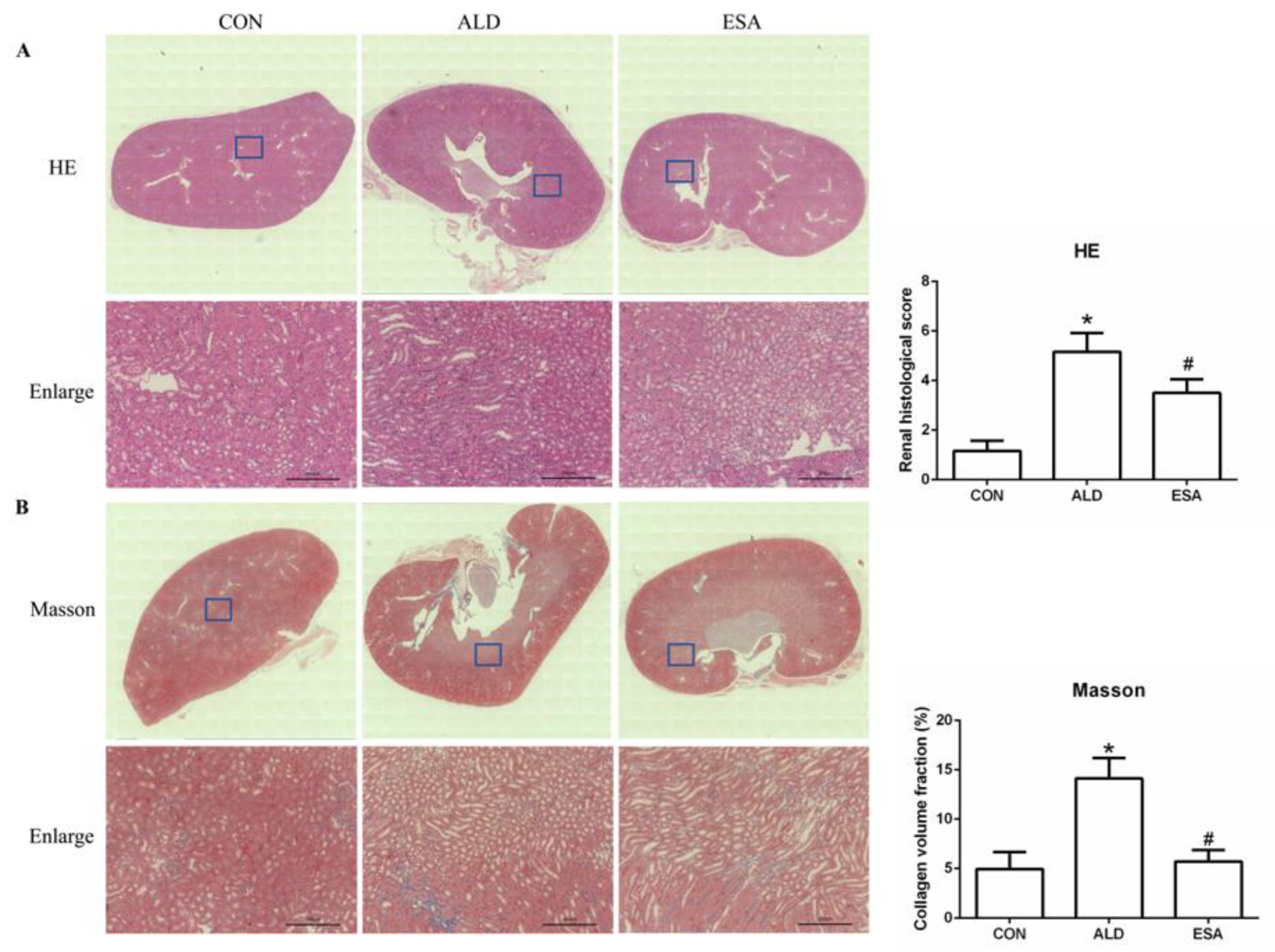
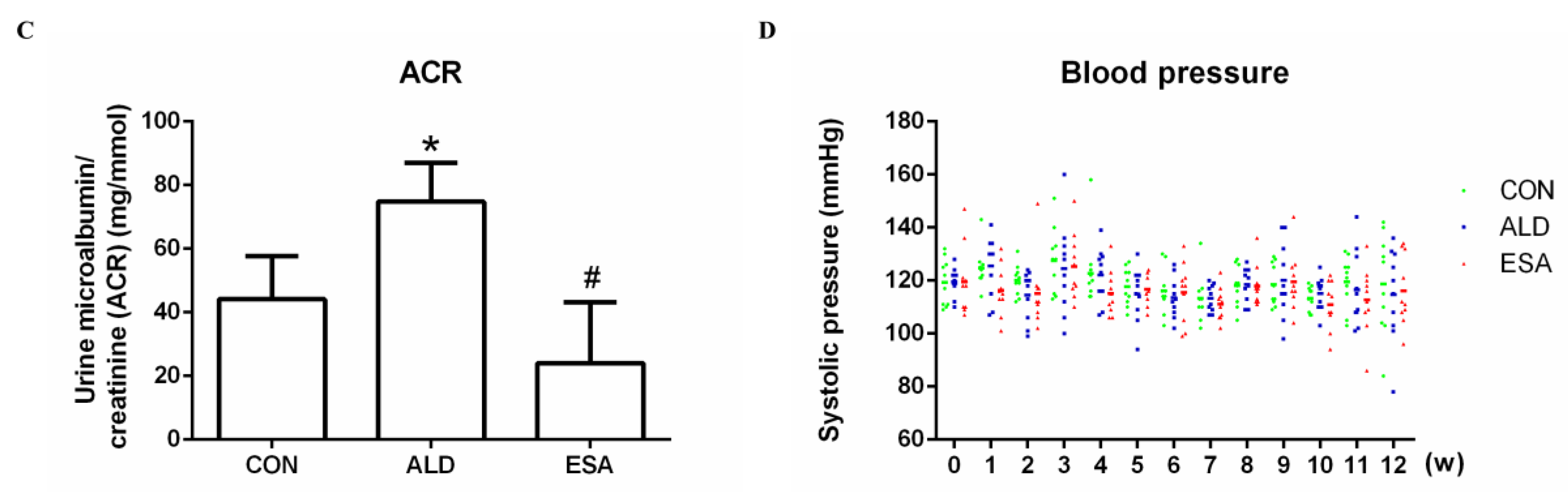
2.1. Esaxerenone Reverses Renal Injury in Aldosterone-Treated Mice
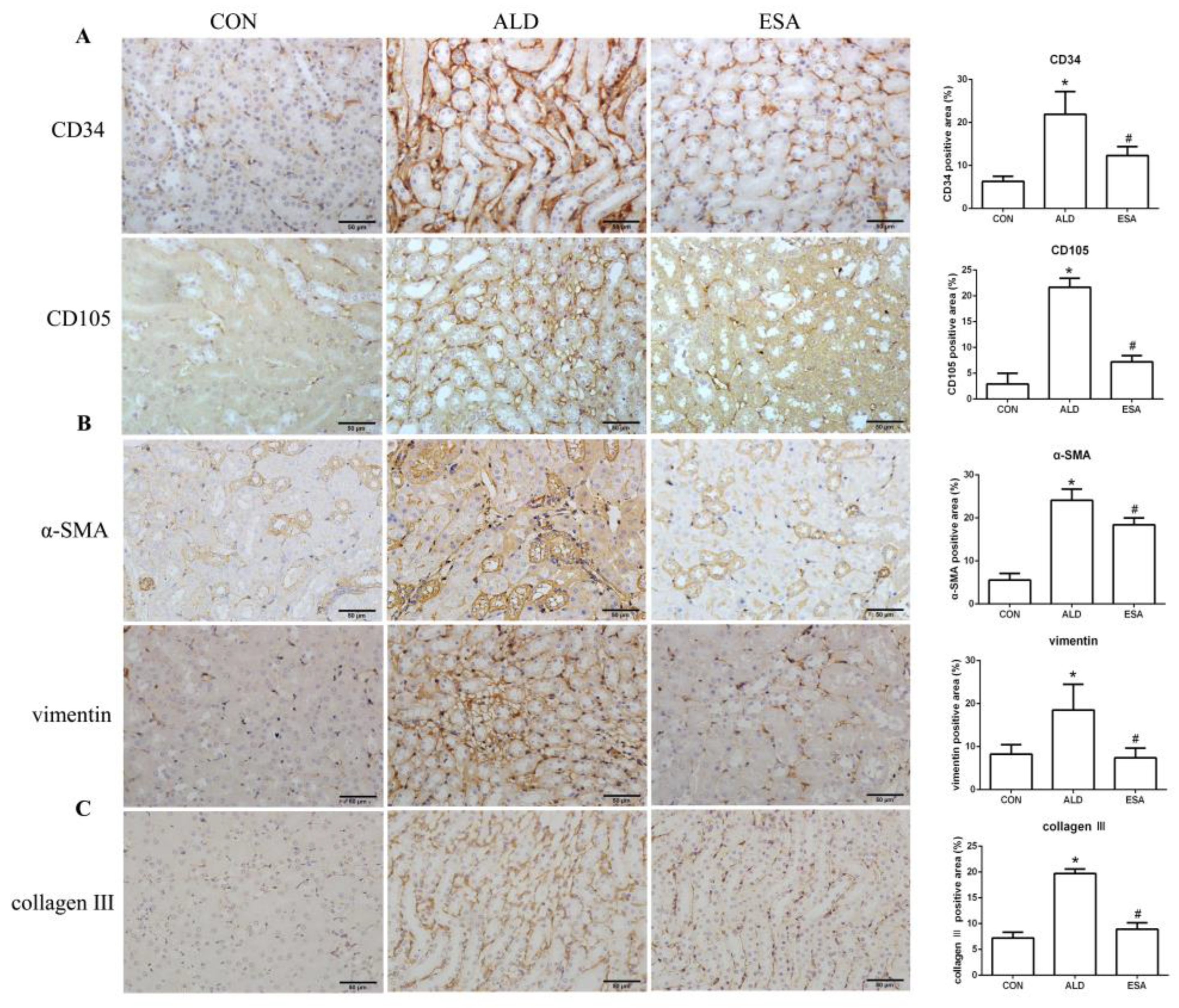
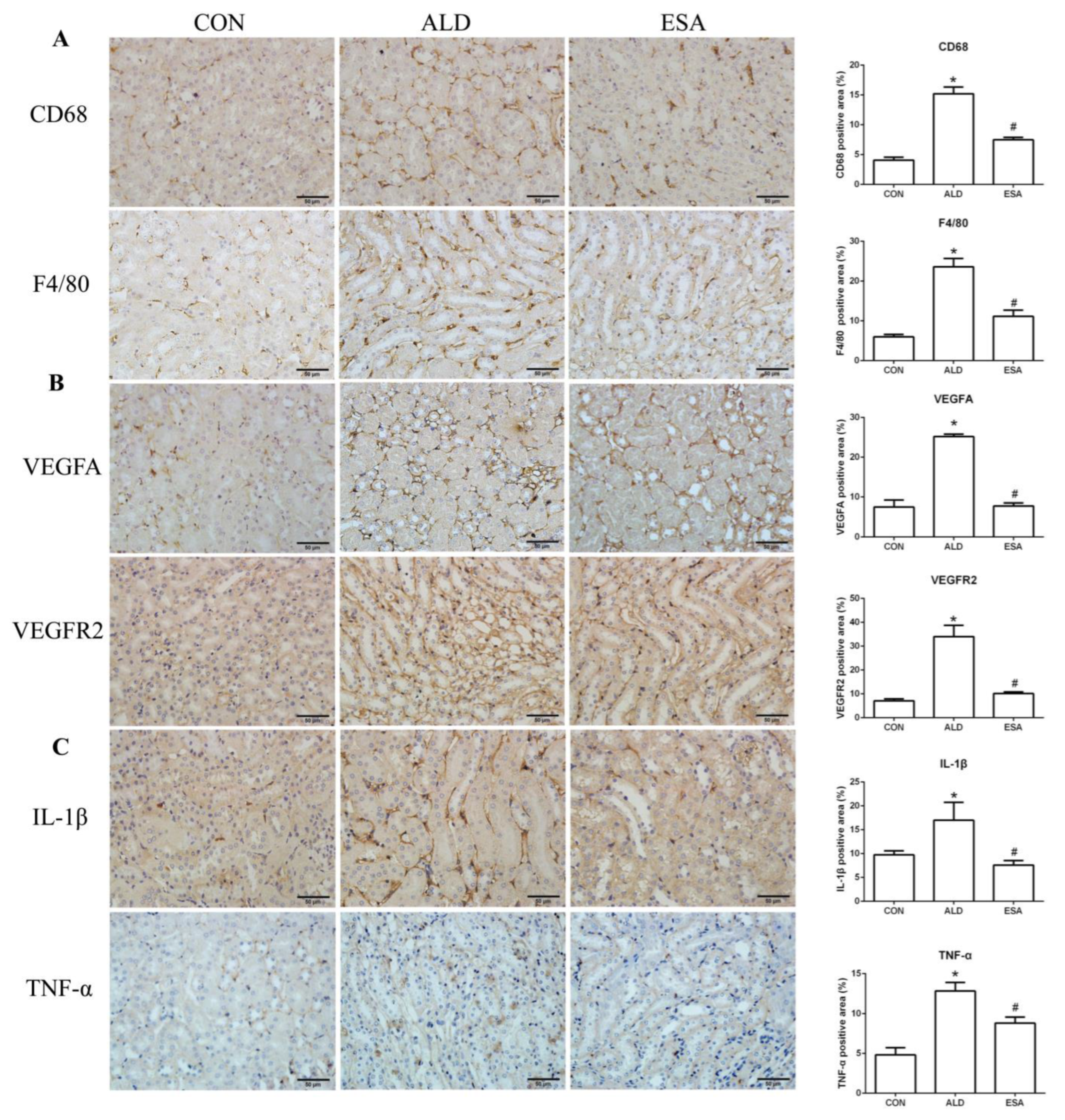
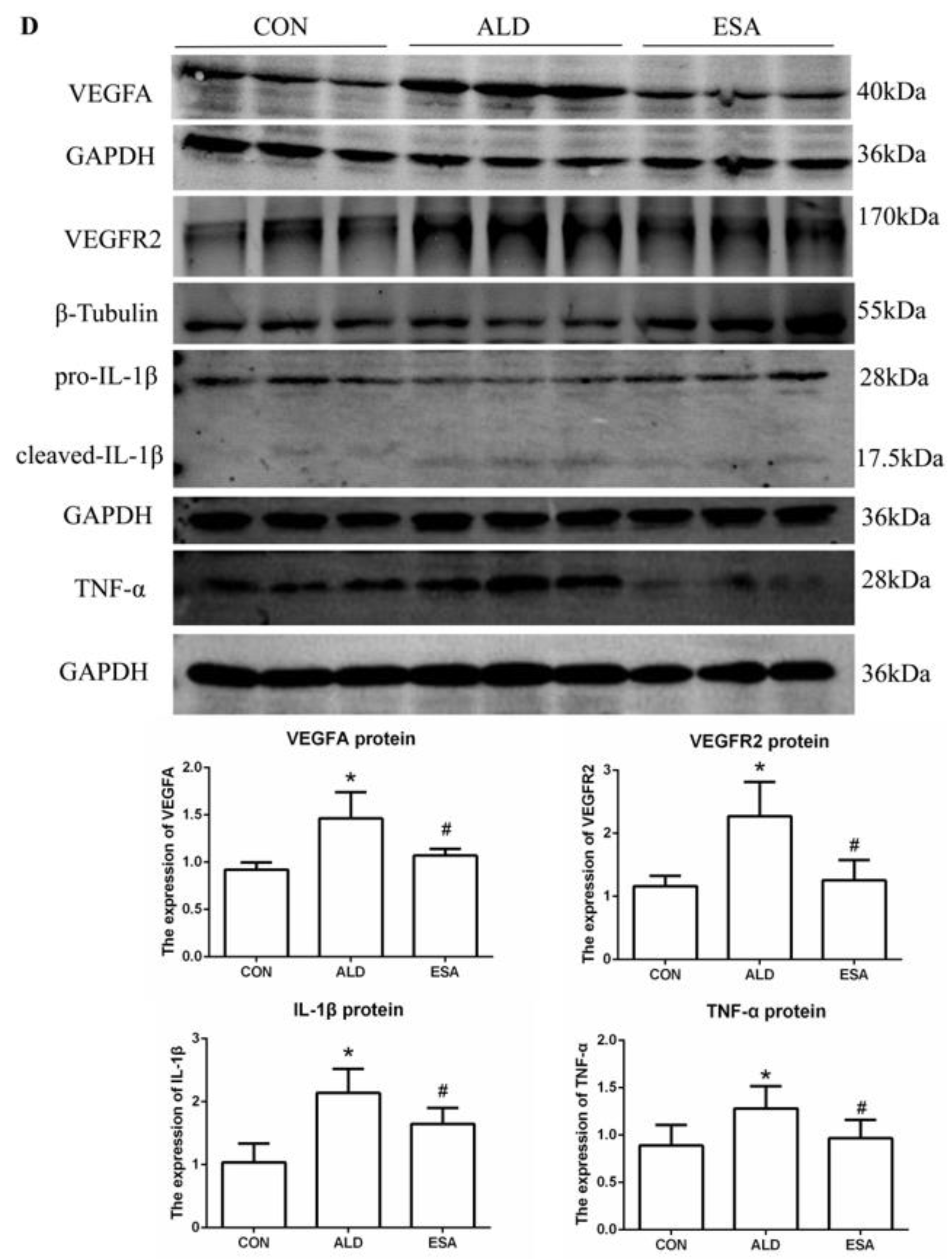
2.2. Esaxerenone Inhibits Renal Angiogenesis in Aldosterone-Treated Mice
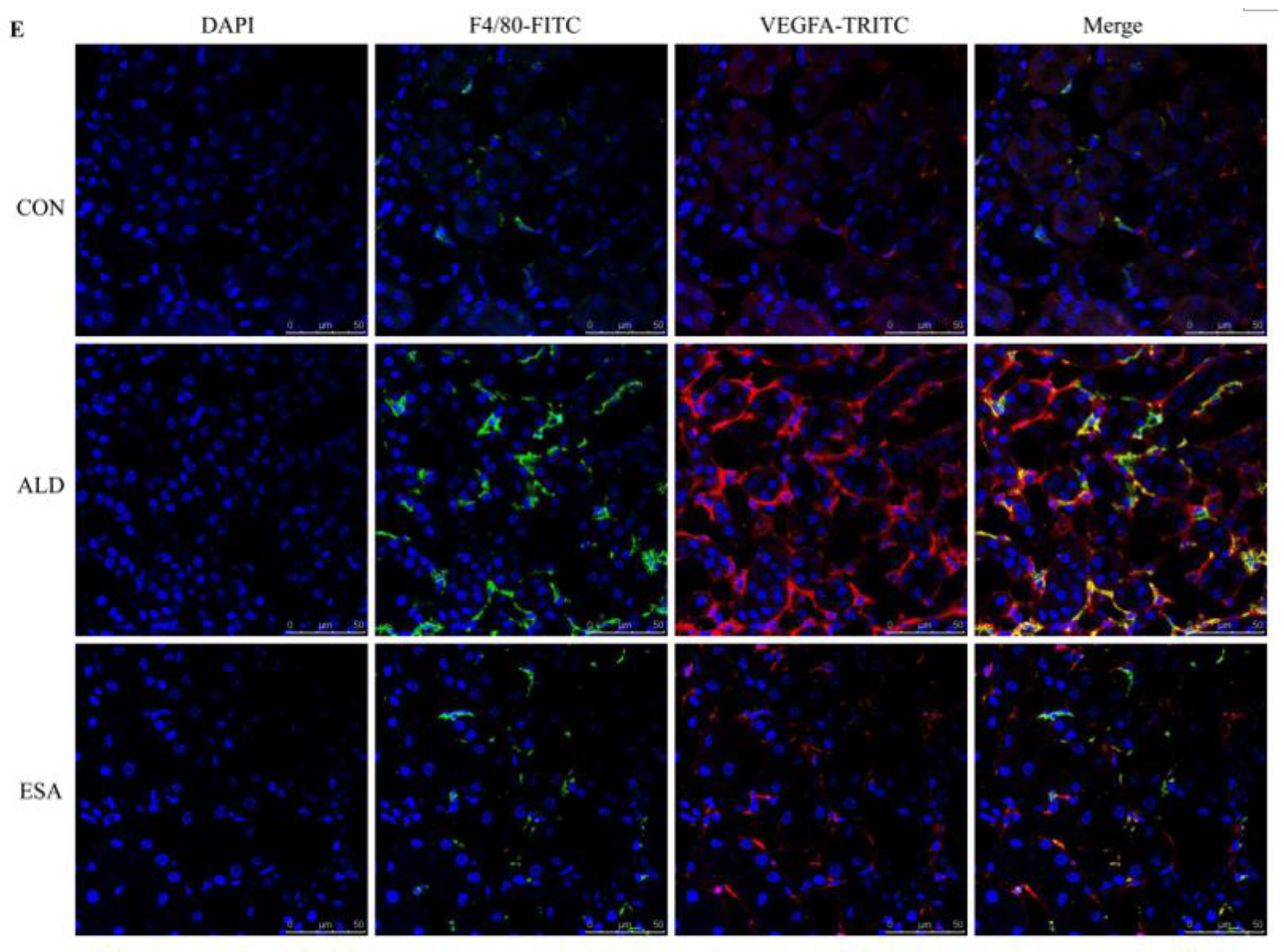
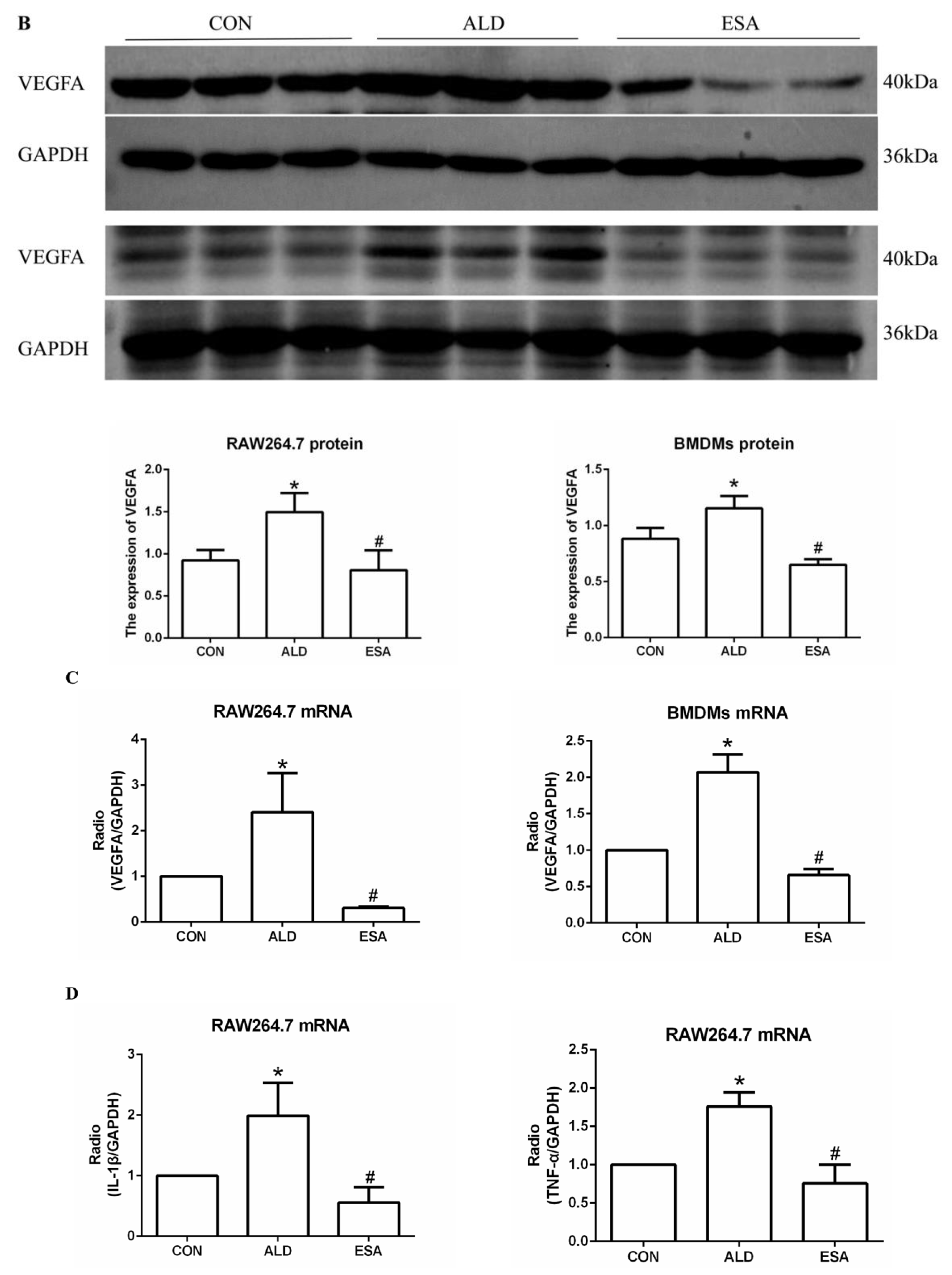
2.3. Esaxerenone Alleviates the Secretion of Vascular Endothelial Growth Factor A (VEGFA) by Macrophages in Aldosterone-Treated Mice
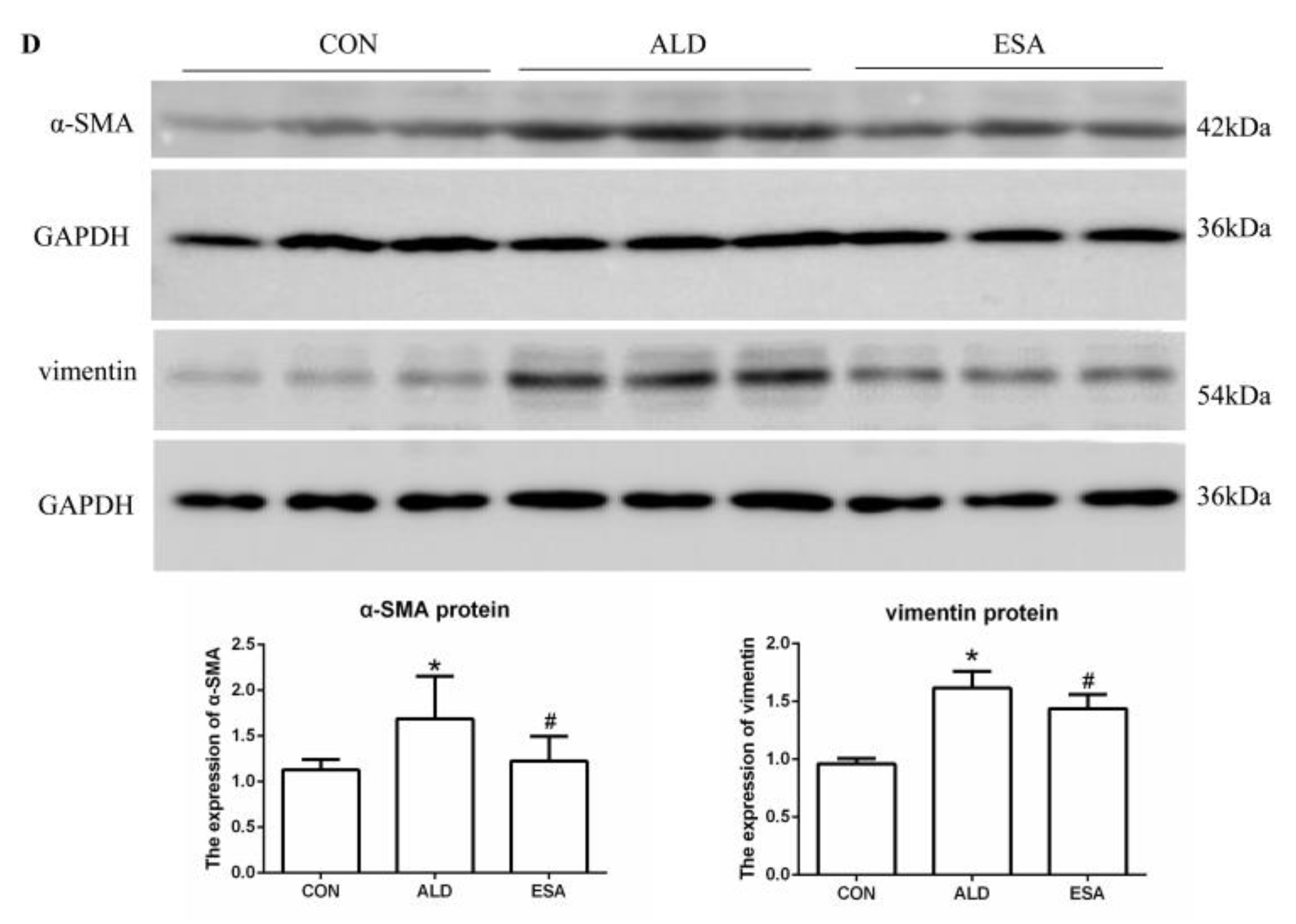
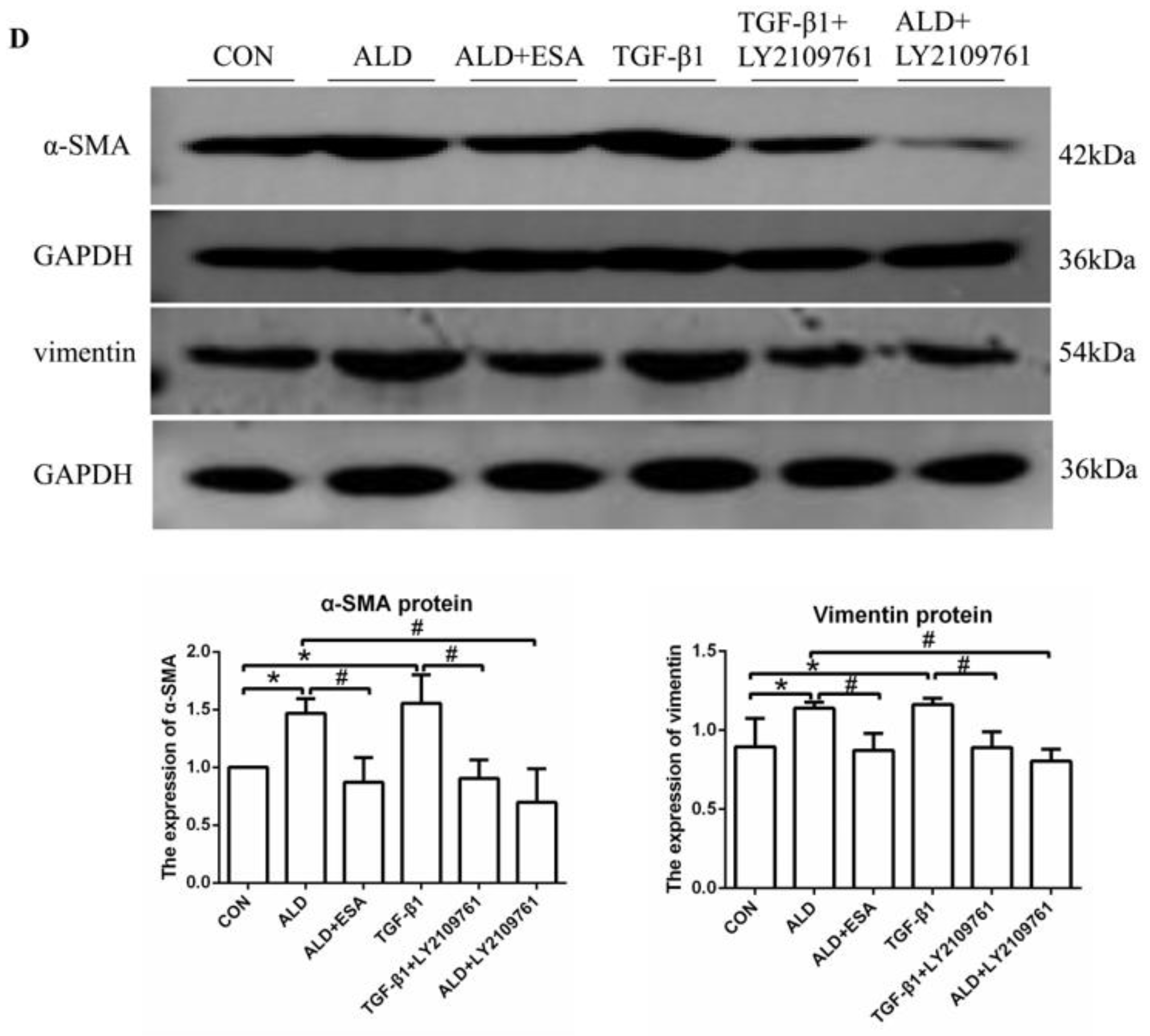
2.4. Esaxerenone Alleviates EndMT in the Kidneys of Aldosterone-Treated Mice
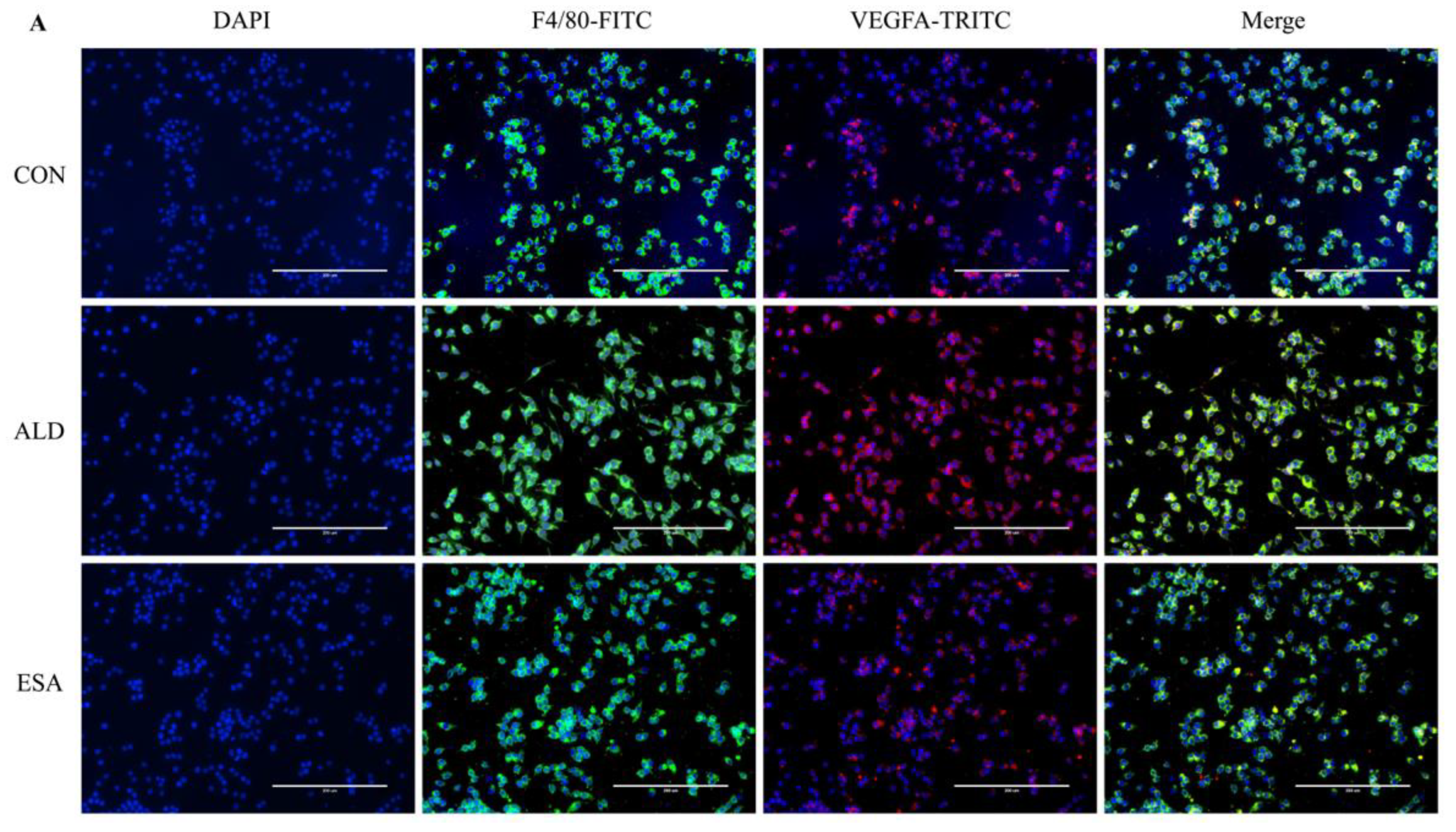
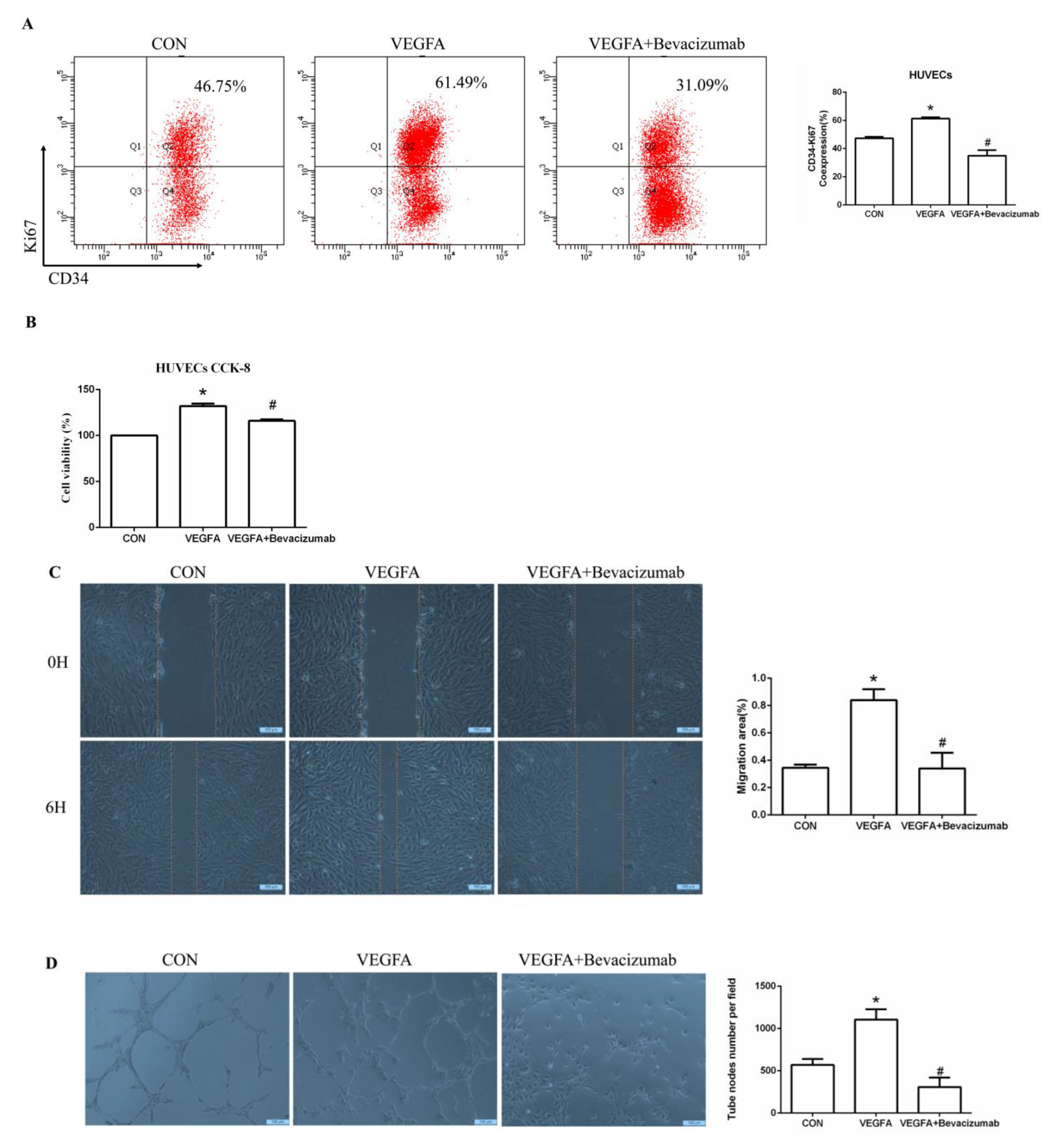
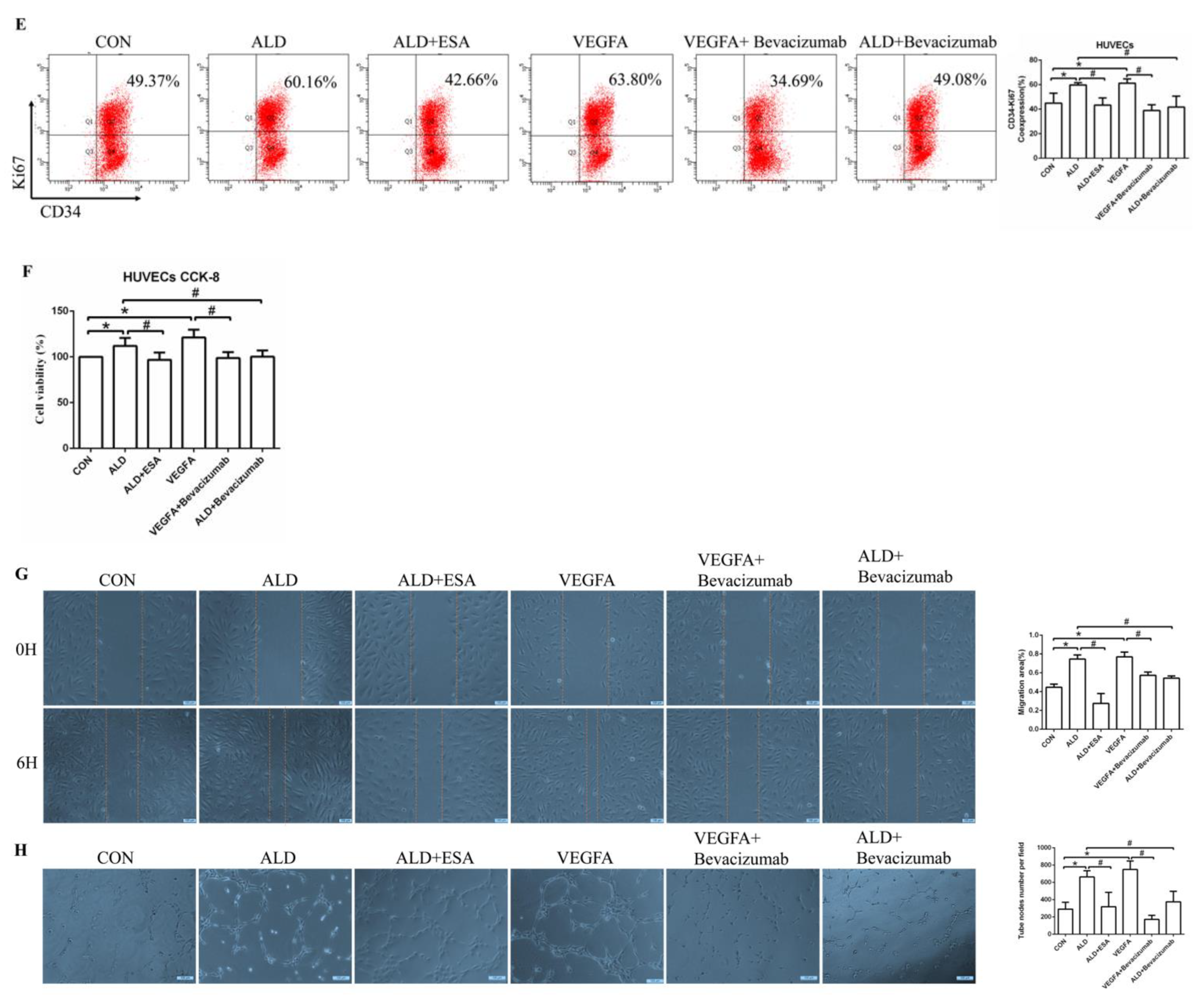
2.5. Esaxerenone Inhibits Angiogenesis by Inhibiting the Secretion of VEGFA from Macrophages In Vitro
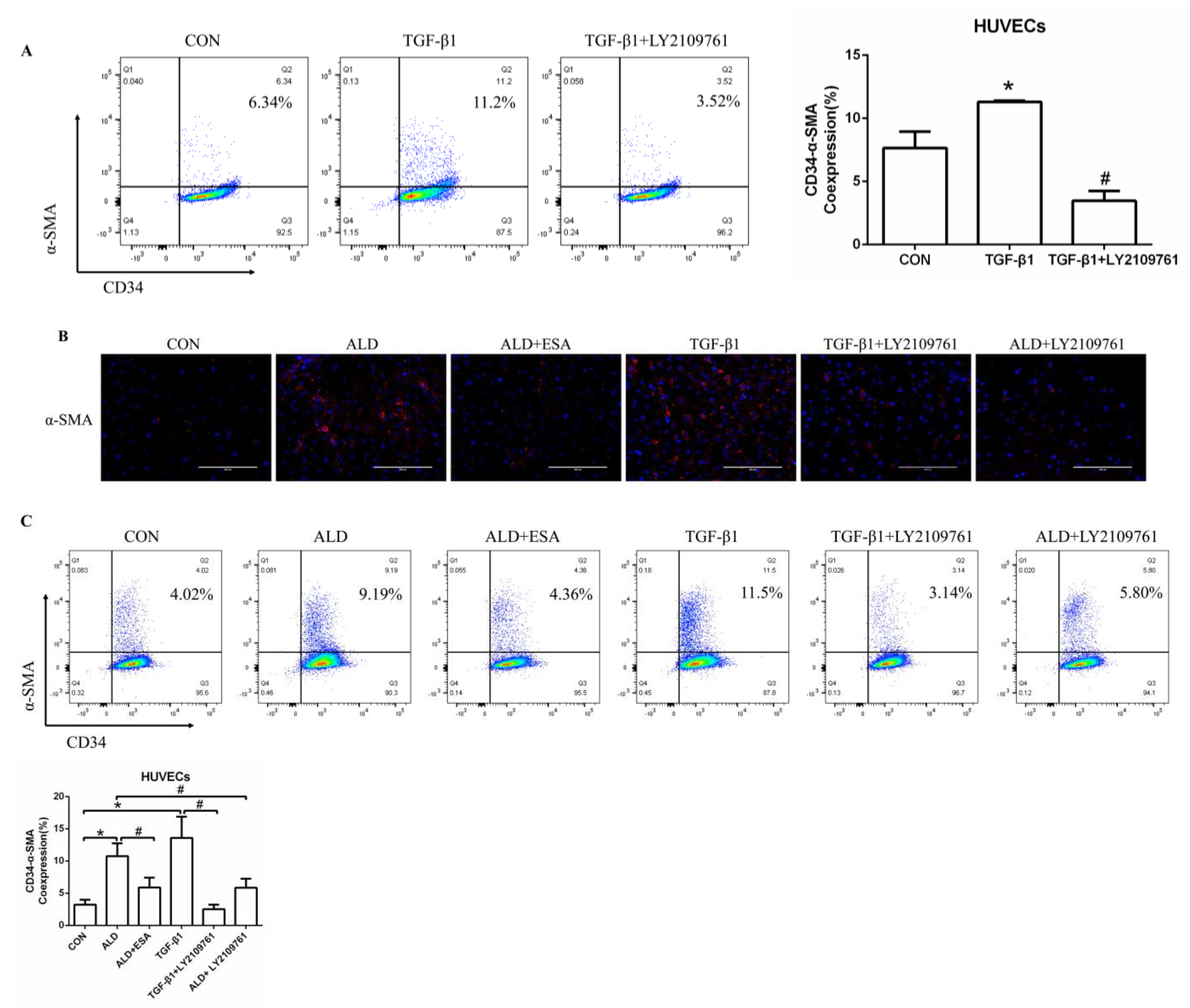
2.6. The MR/TGF-β1 Pathway Is Involved in EndMT In Vitro
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Models
4.2. Blood Pressure and Biochemical Parameter Analysis
4.3. Histological Analysis, Immunohistochemistry, and Immunofluorescence Analysis
4.4. Protein Extraction and Western Blot Analysis
4.5. Reverse Transcription and Quantitative Real-Time PCR
4.6. In Vitro Cell Culture Assays
4.6.1. Flow Cytometry
4.6.2. CCK8 Assay
4.6.3. Immunofluorescence Cell Staining
4.6.4. Scratch Wound Assay
4.6.5. Endothelial Tube Formation Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Ballermann, B.J.; Obeidat, M. Tipping the balance from angiogenesis to fibrosis I n CKD. Kidney Int. Suppl. 2014, 4, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Szewczyk, G.; Rak, J.; Ruth, J.H. Inflammatory mediators of angiogenesis. Mediat. Inflamm. 2013, 2013, 610543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Chang, Y.H.; Yang, S.Y.; Wu, K.D.; Chu, T.S. Update of pathophysiology and management of diabetic kidney disease. J. Formos. Med. Assoc. 2018, 117, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Ben, Y.; Li, H.; Xiong, Y.; Chen, G.; Hao, J.; Ma, X.; Gao, X.; Qiang, P.; Shimosawa, T.; et al. Eplerenone Prevents Cardiac Fibrosis by Inhibiting Angiogenesis in Unilateral Urinary Obstruction Rats. J. Renin. Angiotensin Aldosterone Syst. 2022, 2022, 1283729. [Google Scholar] [CrossRef]
- Rudnicki, M.; Perco, P.; Enrich, J.; Eder, S.; Heininger, D.; Bernthaler, A.; Wiesinger, M.; Sarkozi, R.; Noppert, S.J.; Schramek, H.; et al. Hypoxia response and VEGF-A expression in human proximal tubular epithelial cells in stable and progressive renal disease. Lab. Investig. 2009, 89, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimke, H.; Sparks, M.A.; Thomson, B.R.; Frische, S.; Coffman, T.M.; Quaggin, S.E. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J. Am. Soc. Nephrol. 2015, 26, 1027–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinnikova, M.G.; Bakarev, M.A.; Nikityuk, D.B.; Lushnikova, E.L. Immunohistochemical Study of the Expression of Vascular Endothelial Growth Factor Receptor-2 (KDR/Flk-1) during Myocardial Infarction. Bull. Exp. Biol. Med. 2017, 163, 500–505. [Google Scholar] [CrossRef]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Miyata, K.N.; Nast, C.C.; LaPage, J.A.; Mahoney, M.; Nguyen, S.; Khan, K.; Wu, Q.; Adler, S.G.; Dai, T. Dual therapy with an angiotensin receptor blocker and a JAK1/2 inhibitor attenuates dialysate-induced angiogenesis and preserves peritoneal membrane structure and function in an experimental CKD rat model. Perit. Dial. Int. 2023, 43, 159–167. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, H.; Chen, J.; He, L.; Chen, Y. Role of VEGF-A and LRG1 in Abnormal Angiogenesis Associated with Diabetic Nephropathy. Front. Physiol. 2020, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Morimoto, M.; Kitajima, S.; Koike, T.; Yu, Y.; Shiiki, H.; Nagata, M.; Watanabe, T.; Fan, J. Increased expression of vascular endothelial growth factor in kidney leads to progressive impairment of glomerular functions. J. Am. Soc. Nephrol. 2007, 18, 2094–2104. [Google Scholar] [CrossRef] [Green Version]
- Leonard, E.C.; Friedrich, J.L.; Basile, D.P. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am. J. Physiol. Renal. Physiol. 2008, 295, F1648–F1657. [Google Scholar] [CrossRef] [Green Version]
- Basile, D.P.; Friedrich, J.L.; Spahic, J.; Knipe, N.; Mang, H.; Leonard, E.C.; Changizi-Ashtiyani, S.; Bacallao, R.L.; Molitoris, B.A.; Sutton, T.A. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am. J. Physiol. Renal. Physiol. 2011, 300, F721–F733. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Jin, C.; Ma, H.; Huang, M.; Shi, G.P.; Wang, J.; Xiang, M. EphrinB2/EphB4 pathway in postnatal angiogenesis: A potential therapeutic target for ischemic cardiovascular disease. Angiogenesis 2016, 19, 297–309. [Google Scholar] [CrossRef]
- Wu, X.; Reboll, M.R.; Korf-Klingebiel, M.; Wollert, K.C. Angiogenesis after acute myocardial infarction. Cardiovasc. Res. 2021, 117, 1257–1273. [Google Scholar] [CrossRef]
- Zou, J.; Fei, Q.; Xiao, H.; Wang, H.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X.; Wang, K.; Wang, N. VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J. Cell Physiol. 2019, 234, 17690–17703. [Google Scholar] [CrossRef]
- Feng, J.; Wang, C.; Liu, T.; Li, J.; Wu, L.; Yu, Q.; Li, S.; Zhou, Y.; Zhang, J.; Chen, J.; et al. Procyanidin B2 inhibits the activation of hepatic stellate cells and angiogenesis via the Hedgehog pathway during liver fibrosis. J. Cell Mol. Med. 2019, 23, 6479–6493. [Google Scholar] [CrossRef] [Green Version]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef] [Green Version]
- Amano, K.; Okigaki, M.; Adachi, Y.; Fujiyama, S.; Mori, Y.; Kosaki, A.; Iwasaka, T.; Matsubara, H. Mechanism for IL-1 beta-mediated neovascularization unmasked by IL-1 beta knock-out mice. J. Mol. Cell Cardiol. 2004, 36, 469–480. [Google Scholar] [CrossRef]
- Ogami, K.; Yamaguchi, R.; Imoto, S.; Tamada, Y.; Araki, H.; Print, C.; Miyano, S. Computational gene network analysis reveals TNF-induced angiogenesis. BMC Syst. Biol. 2012, 6 (Suppl. 2), S12. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.C.; Liu, C.J.; Lin, T.J.; Mar, A.C.; Wang, H.H.; Chen, C.W.; Hong, Z.X.; Lee, T.C. Blocking TNF-alpha inhibits angiogenesis and growth of IFIT2-depleted metastatic oral squamous cell carcinoma cells. Cancer Lett. 2016, 370, 207–215. [Google Scholar] [CrossRef]
- Zhao, T.; Zhao, W.; Chen, Y.; Ahokas, R.A.; Sun, Y. Vascular endothelial growth factor (VEGF)-A: Role on cardiac angiogenesis following myocardial infarction. Microvasc. Res. 2010, 80, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, G.Y.; Sun, H.Y.; Meng, T.; Cheng, C.C.; Zhao, H.P.; Luo, X.L.; Yang, M.M. Dioscin Reduces Vascular Damage in the Retina of db/db Mice by Inhibiting the VEGFA Signaling Pathway. Front. Pharmacol. 2021, 12, 811897. [Google Scholar] [CrossRef]
- Lu, Y.; Qin, T.; Li, J.; Wang, L.; Zhang, Q.; Jiang, Z.; Mao, J. MicroRNA-140-5p inhibits invasion and angiogenesis through targeting VEGF-A in breast cancer. Cancer Gene Ther. 2017, 24, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Ma, Y.; Zhan, S.; Zhang, G.; Cao, L.; Zhang, X.; Shi, T.; Chen, W. B7-H3 promotes colorectal cancer angiogenesis through activating the NF-kappaB pathway to induce VEGFA expression. Cell Death Dis. 2020, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Yamazaki, Y.; Tezuka, Y.; Omata, K.; Ono, Y.; Morimoto, R.; Nakamura, Y.; Suzuki, T.; Satoh, F.; Sasano, H. Pathology of Aldosterone Biosynthesis and its Action. Tohoku J. Exp. Med. 2021, 254, 1–15. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Tostes, R.C.; Paradis, P.; Schiffrin, E.L. Aldosterone, Inflammation, Immune System, and Hypertension. Am. J. Hypertens. 2021, 34, 15–27. [Google Scholar] [CrossRef]
- Han, H.I.; Skvarca, L.B.; Espiritu, E.B.; Davidson, A.J.; Hukriede, N.A. The role of macrophages during acute kidney injury: Destruction and repair. Pediatr. Nephrol. 2019, 34, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Yan, L.; He, Q.; Xie, H.; Chen, M. Resveratrol Ameliorates Cardiac Remodeling in a Murine Model of Heart Failure With Preserved Ejection Fraction. Front. Pharmacol. 2021, 12, 646240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Kalish, F.S.; Wong, R.J.; Stevenson, D.K. Hypoxia regulates placental angiogenesis via alternatively activated macrophages. Am. J. Reprod. Immunol. 2018, 80, e12989. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Akahori, H.; Harari, E.; Smith, S.L.; Polavarapu, R.; Karmali, V.; Otsuka, F.; Gannon, R.L.; Braumann, R.E.; Dickinson, M.H.; et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J. Clin. Investig. 2018, 128, 1106–1124. [Google Scholar] [CrossRef]
- Zhang, Q.; Cunha, A.P.D.; Li, S.; Hao, Q.; Kainz, V.; Huang, Q.; Wu, H.Y. IL-27 regulates HIF-1alpha-mediated VEGFA response in macrophages of diabetic retinopathy patients and healthy individuals. Cytokine 2019, 113, 238–247. [Google Scholar] [CrossRef]
- Yamauchi, T.; Doi, S.; Nakashima, A.; Doi, T.; Sohara, E.; Uchida, S.; Masaki, T. Na+-Cl− cotransporter-mediated chloride uptake contributes to hypertension and renal damage in aldosterone-infused rats. Am. J. Physiol. Renal. Physiol. 2018, 315, F300–F312. [Google Scholar] [CrossRef] [Green Version]
- Stravodimos, K.G.; Koritsiadis, G.; Lazaris, A.C.; Agrogiannis, G.; Koutalellis, G.; Constantinides, C.; Patsouris, E.; Kapetanakis, T.; Zervas, A. Hydronephrosis promotes expression of hypoxia-inducible factor 1 alpha. Urol. Int. 2009, 82, 38–42. [Google Scholar] [CrossRef]
- de Castro Bras, L.E.; Frangogiannis, N.G. Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix Biol. 2020, 91–92, 176–187. [Google Scholar] [CrossRef]
- Falke, L.L.; Gholizadeh, S.; Goldschmeding, R.; Kok, R.J.; Nguyen, T.Q. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat. Rev. Nephrol. 2015, 11, 233–244. [Google Scholar] [CrossRef]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Chen, X.; Chen, X.; Chen, L.; Zheng, G.; Zhou, H.; Zhou, X. Anti-Fibrosis Effect of Relaxin and Spironolactone Combined on Isoprenaline-Induced Myocardial Fibrosis in Rats via Inhibition of Endothelial-Mesenchymal Transition. Cell Physiol. Biochem. 2017, 41, 1167–1178. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Han, J.S.; Choi, B.S.; Yang, C.W.; Kim, Y.S. Aldosterone-induced TGF-beta1 expression is regulated by mitogen-activated protein kinases and activator protein-1 in mesangial cells. J. Korean Med. Sci. 2009, 24 (Suppl. 1), S195–S203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Han, Z.; Tao, J.; Wang, J.; Liu, X.; Zhou, W.; Xu, Z.; Zhao, C.; Wang, Z.; Tan, R.; et al. Role of endothelial-to-mesenchymal transition induced by TGF-beta1 in transplant kidney interstitial fibrosis. J. Cell Mol. Med. 2017, 21, 2359–2369. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chang, Y.; Hao, J.; Zhang, C.; Yang, F.; Wang, Z.; Liu, Y.; Wang, X.; Mu, S.; Xu, Q. Eplerenone Attenuates Fibrosis in the Contralateral Kidney of UUO Rats by Preventing Macrophage-to-Myofibroblast Transition. Front. Pharmacol. 2021, 12, 620433. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xian, Y.; Gao, X.; Qiang, P.; Hao, J.; Yang, F.; Shimosawa, T.; Chang, Y.; Xu, Q. Eplerenone inhibits the macrophage-to-myofibroblast transition in rats with UUO-induced type 4 cardiorenal syndrome through the MR/CTGF pathway. Int. Immunopharmacol. 2022, 113 Pt A, 109396. [Google Scholar] [CrossRef]
- Qiang, P.; Hao, J.; Yang, F.; Han, Y.; Chang, Y.; Xian, Y.; Xiong, Y.; Gao, X.; Liang, L.; Shimosawa, T.; et al. Esaxerenone inhibits the macrophage-to-myofibroblast transition through mineralocorticoid receptor/TGF-beta1 pathway in mice induced with aldosterone. Front. Immunol. 2022, 13, 948658. [Google Scholar] [CrossRef]
- Kawarazaki, W.; Nagase, M.; Yoshida, S.; Takeuchi, M.; Ishizawa, K.; Ayuzawa, N.; Ueda, K.; Fujita, T. Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J. Am. Soc. Nephrol. 2012, 23, 997–1007. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Chang, J.; Chang, Y.; Fan, L.; Liu, Z.; Zhang, C.; Shimosawa, T.; Yang, F.; Xu, Q. Esaxerenone Inhibits Renal Angiogenesis and Endothelial-Mesenchymal Transition via the VEGFA and TGF-β1 Pathways in Aldosterone-Infused Mice. Int. J. Mol. Sci. 2023, 24, 11766. https://doi.org/10.3390/ijms241411766
Gao X, Chang J, Chang Y, Fan L, Liu Z, Zhang C, Shimosawa T, Yang F, Xu Q. Esaxerenone Inhibits Renal Angiogenesis and Endothelial-Mesenchymal Transition via the VEGFA and TGF-β1 Pathways in Aldosterone-Infused Mice. International Journal of Molecular Sciences. 2023; 24(14):11766. https://doi.org/10.3390/ijms241411766
Chicago/Turabian StyleGao, Xiaomeng, Jingyue Chang, Yi Chang, Lili Fan, Ziqian Liu, Cuijuan Zhang, Tatsuo Shimosawa, Fan Yang, and Qingyou Xu. 2023. "Esaxerenone Inhibits Renal Angiogenesis and Endothelial-Mesenchymal Transition via the VEGFA and TGF-β1 Pathways in Aldosterone-Infused Mice" International Journal of Molecular Sciences 24, no. 14: 11766. https://doi.org/10.3390/ijms241411766





