Engineering of Shikimate Pathway and Terminal Branch for Efficient Production of L-Tryptophan in Escherichia coli
Abstract
:1. Introduction
2. Results and Discussion
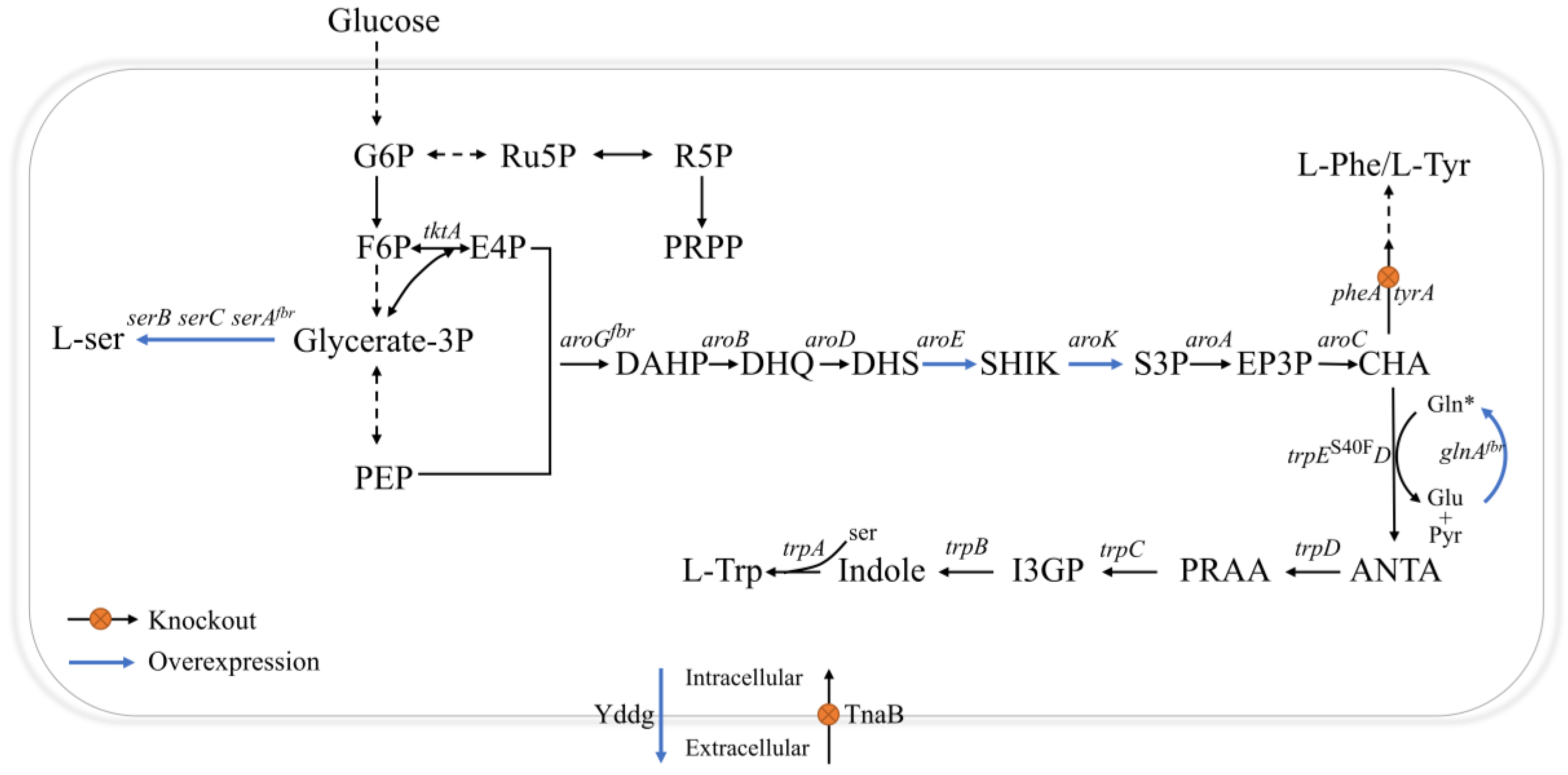
2.1. Identifying and Relieving Rate-Limiting Steps in the Shikimate Pathway
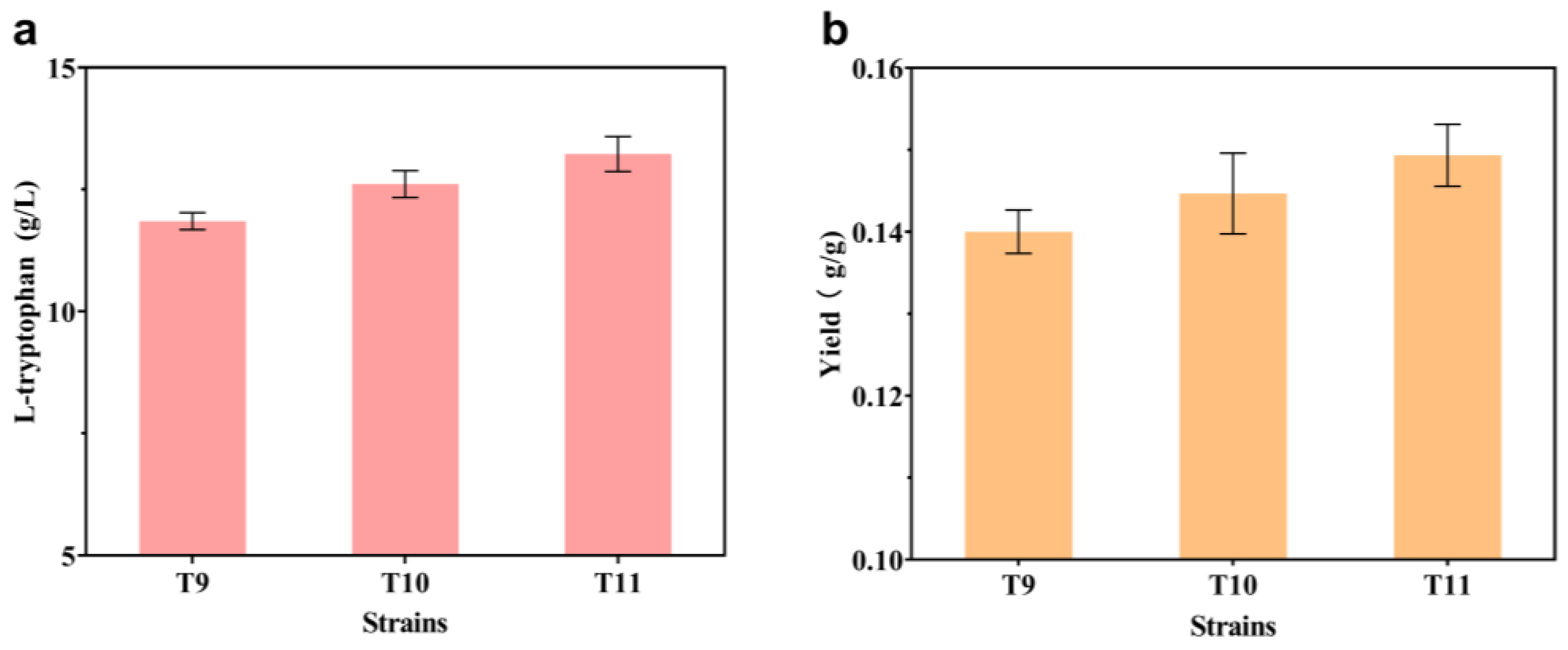
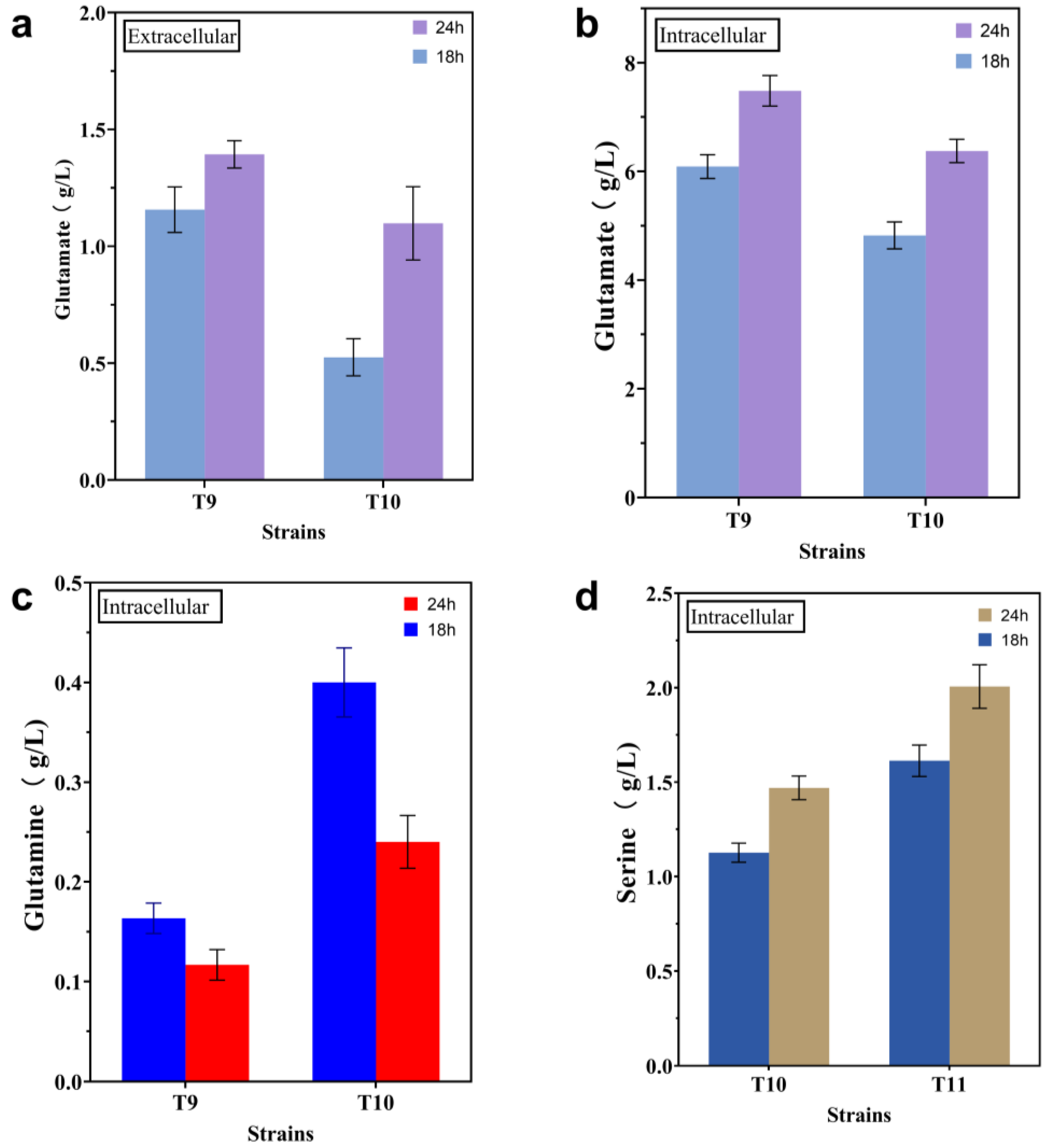
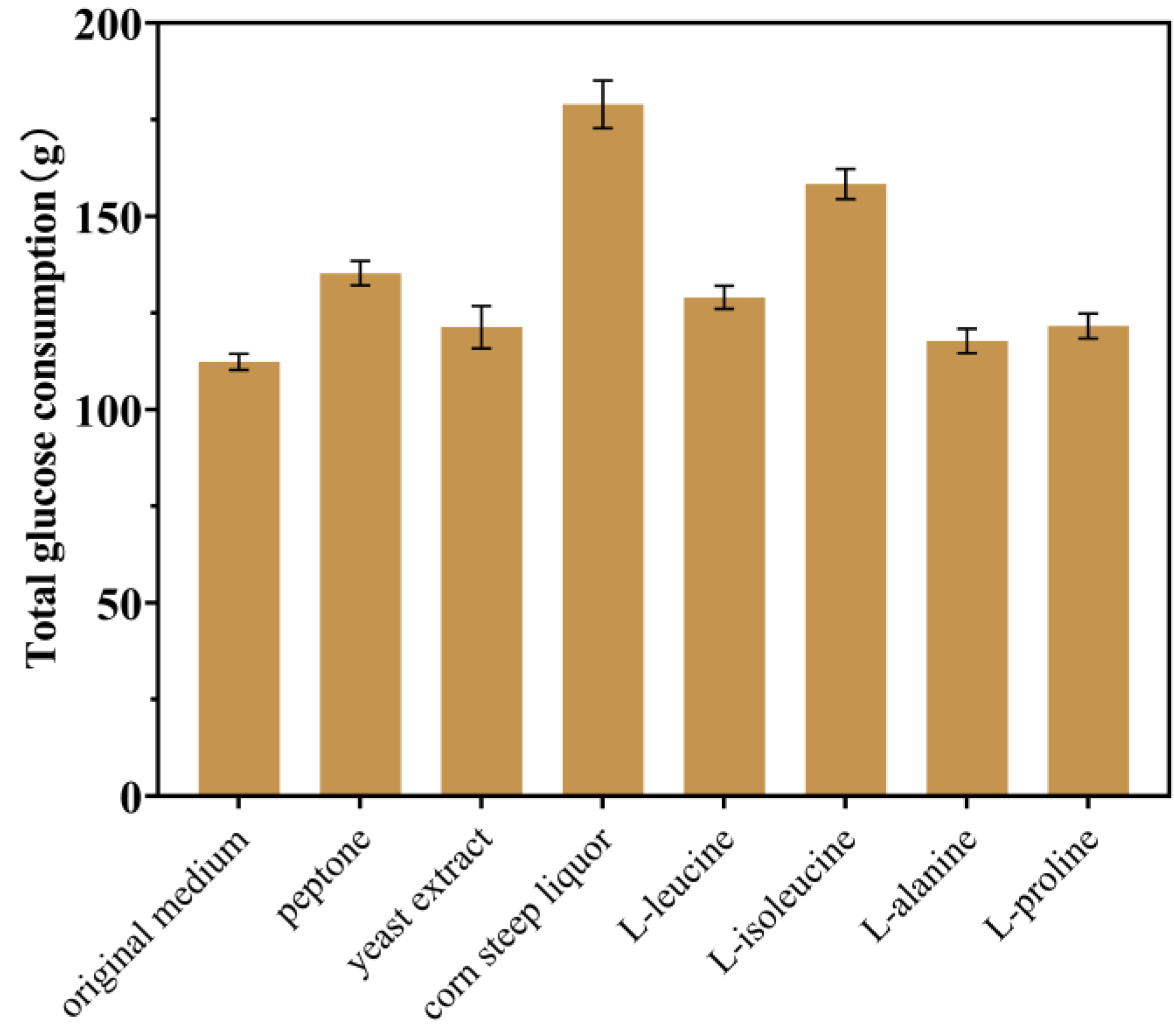
2.2. Enhancing Intracellular Availability of L-gln and L-ser
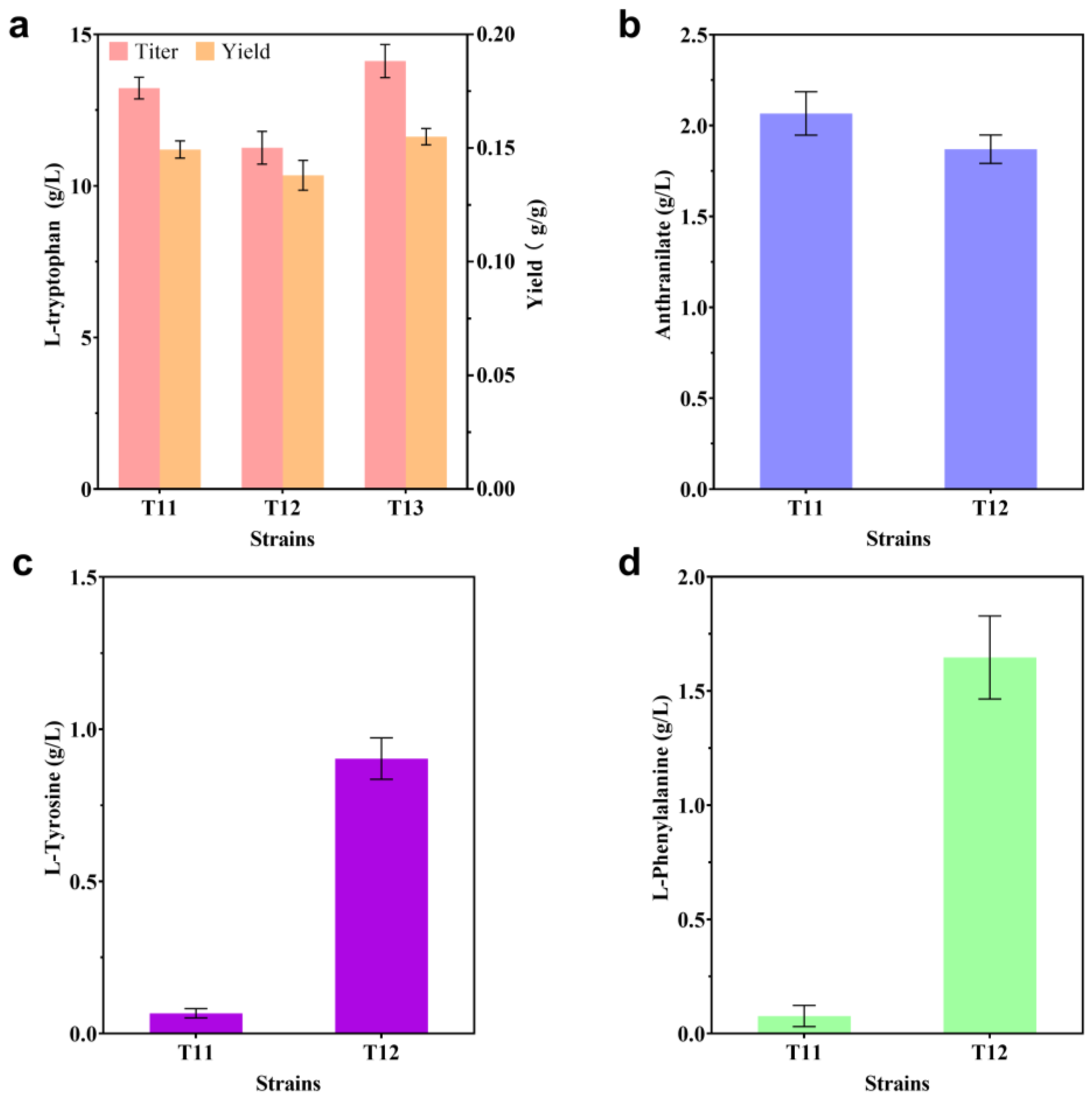
2.3. Modification of the L-trp Transport System
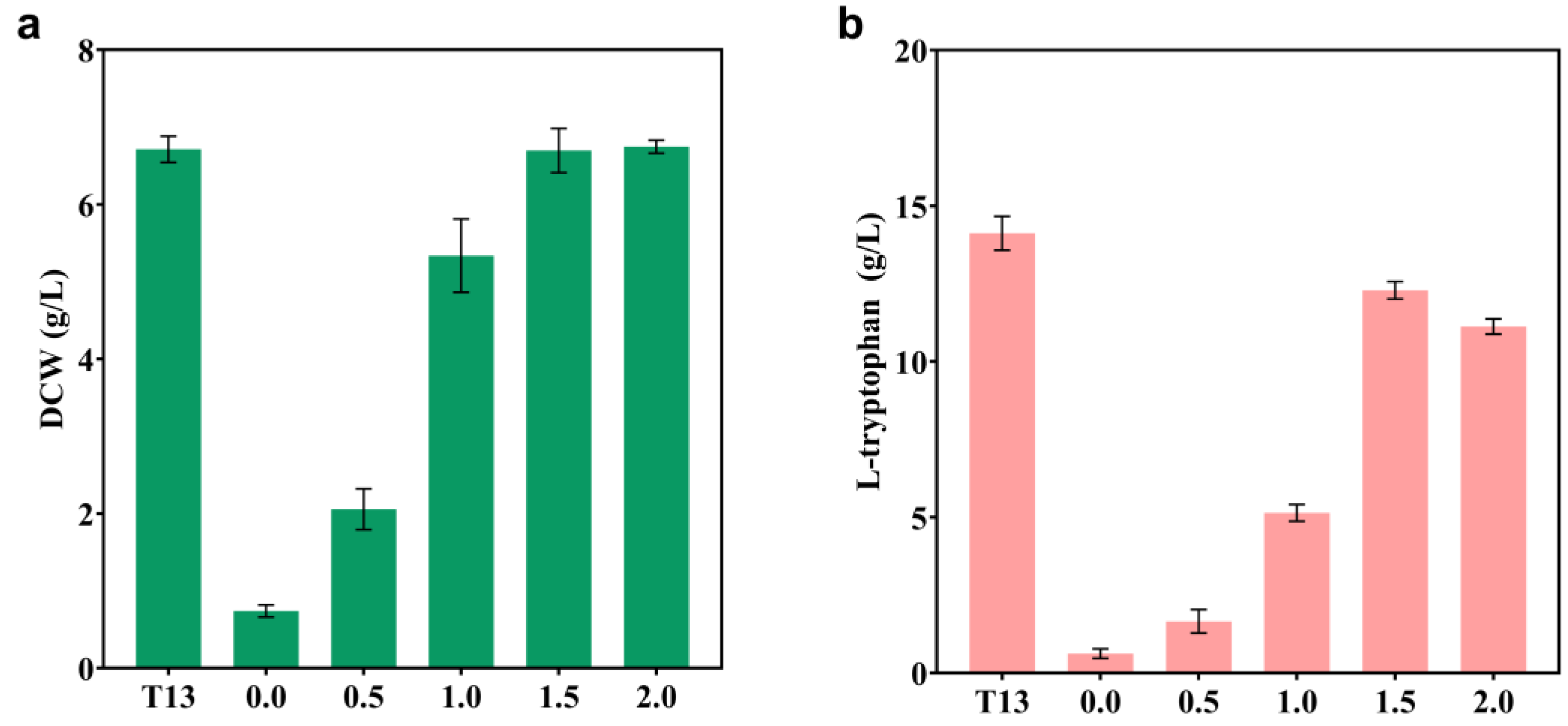
2.4. Blocking Competing Pathways
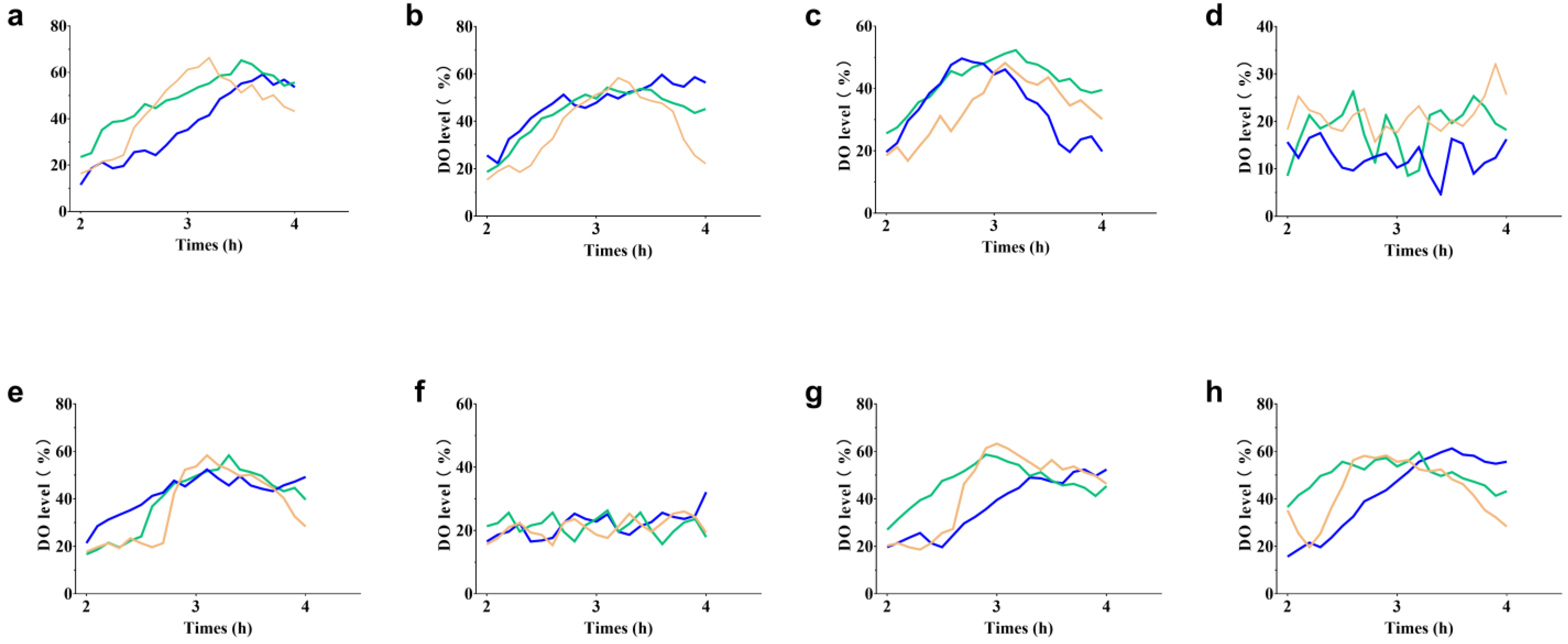
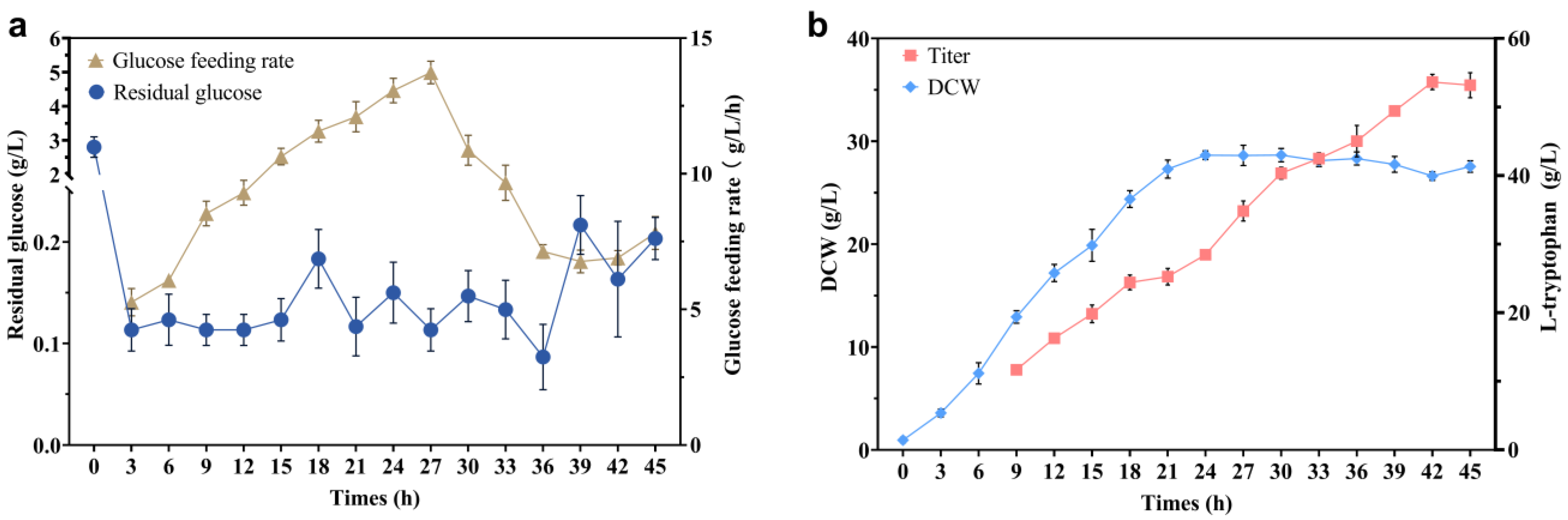
2.5. Optimization of L-trp Production in a 30 L Triple Fermenter
3. Materials and Methods
3.1. Strains and Plasmids
3.2. Gene Editing Using CRISPR/Cas9
3.3. Shake-Flask Fermentation
3.4. Fed-Batch Fermentation in a 30 L Bioreactor
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Zeng, A.-P. Rational design and metabolic analysis of Escherichia coli for effective production of L-tryptophan at high concentration. Appl. Microbiol. Biotechnol. 2017, 101, 559–568. [Google Scholar] [CrossRef]
- Chen, L.; Chen, M.; Ma, C.; Zeng, A.P. Discovery of feed-forward regulation in L-tryptophan biosynthesis and its use in metabolic engineering of E. coli for efficient tryptophan bioproduction. Metab. Eng. 2018, 47, 434–444. [Google Scholar] [CrossRef]
- Rose, J.K.; Squires, C.L.; Yanofsky, C.; Yang, H.L.; Zubay, G. Regulation of in vitro transcription of the tryptophan operon by purified RNA polymerase in the presence of partially purified repressor and tryptophan. Nat. New Biol. 1973, 245, 133–137. [Google Scholar] [CrossRef]
- Merino, E.; Jensen, R.A.; Yanofsky, C. Evolution of bacterial trp operons and their regulation. Curr. Opin. Microbiol. 2008, 11, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Ding, S.; Liu, Y.; Gao, C.; Hu, G.; Song, W.; Liu, J.; Chen, X.; Liu, L. Enhancing tryptophan production by balancing precursors in Escherichia coli. Biotechnol. Bioeng. 2022, 119, 983–993. [Google Scholar] [CrossRef]
- Xiong, B.; Zhu, Y.; Tian, D.; Jiang, S.; Fan, X.; Ma, Q.; Wu, H.; Xie, X. Flux redistribution of central carbon metabolism for efficient production of l-tryptophan in Escherichia coli. Biotechnol. Bioeng. 2021, 118, 1393–1404. [Google Scholar] [CrossRef]
- Zhao, C.; Cheng, L.; Xu, Q.; Wang, J.; Shen, Z.; Chen, N. Improvement of the production of L-tryptophan in Escherichia coli by application of a dissolved oxygen stage control strategy. Ann. Microbiol. 2016, 66, 843–854. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, Y.; Xie, X.; Xu, Q.; Chen, N. Modification of tryptophan transport system and its impact on production of L-tryptophan in Escherichia coli. Bioresour. Technol. 2012, 114, 549–554. [Google Scholar] [CrossRef]
- Varma, A.; Boesch, B.W.; Palsson, B.O. Biochemical production capabilities of Escherichia coli. Biotechnol. Bioeng. 1993, 42, 59–73. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.-C.; Tsai, H.-L.; Chen, S.-L.; Mou, D.-G. Amplification of the tryptophan operon gene in Escherichia coli chromosome to increase l-tryptophan biosynthesis. Appl. Microbiol. Biotechnol. 1993, 40, 301–305. [Google Scholar] [CrossRef]
- Berry, A. Improving production of aromatic compounds in Escherichia coli by metabolic engineering. Trends Biotechnol. 1996, 14, 250–256. [Google Scholar] [CrossRef]
- Snell, K.D.; Draths, K.M.; Frost, J.W. Synthetic Modification of the Escherichia coli Chromosome: Enhancing the Biocatalytic Conversion of Glucose into Aromatic Chemicals. J. Am. Chem Soc. 1996, 118, 5605–5614. [Google Scholar] [CrossRef]
- Dell, K.A.; Frost, J.W. Identification and removal of impediments to biocatalytic synthesis of aromatics from D-glucose: Rate-limiting enzymes in the common pathway of aromatic amino acid biosynthesis. J. Am. Chem. Soc. 1993, 115, 11581–11589. [Google Scholar] [CrossRef]
- Li, Z.; Ding, D.; Wang, H.; Liu, L.; Fang, H.; Chen, T.; Zhang, D. Engineering Escherichia coli to improve tryptophan production via genetic manipulation of precursor and cofactor pathways. Synth. Syst. Biotechnol. 2020, 5, 200–205. [Google Scholar] [CrossRef]
- Minliang, C.; Chengwei, M.; Lin, C.; Zeng, A.-P. Integrated laboratory evolution and rational engineering of GalP/Glk-dependent Escherichia coli for higher yield and productivity of L-tryptophan biosynthesis. Metab. Eng. Commun. 2021, 12, e00167. [Google Scholar] [CrossRef]
- Jing, K.; Tang, Y.; Yao, C.; Del Rio-Chanona, E.A.; Ling, X.; Zhang, D. Overproduction of L-tryptophan via simultaneous feed of glucose and anthranilic acid from recombinant Escherichia coli W3110: Kinetic modeling and process scale-up. Biotechnol. Bioeng. 2018, 115, 371–381. [Google Scholar] [CrossRef]
- Zhao, C.; Fang, H.; Wang, J.; Zhang, S.; Zhao, X.; Li, Z.; Lin, C.; Shen, Z.; Cheng, L. Application of fermentation process control to increase l-tryptophan production in Escherichia coli. Biotechnol. Prog. 2020, 36, e2944. [Google Scholar] [CrossRef]
- Commichau, F.M.; Forchhammer, K.; Stülke, J. Regulatory links between carbon and nitrogen metabolism. Curr. Opin. Microbiol. 2006, 9, 167–172. [Google Scholar] [CrossRef]
- Gunka, K.; Commichau, F.M. Control of glutamate homeostasis in Bacillus subtilis: A complex interplay between ammonium assimilation, glutamate biosynthesis and degradation. Mol. Microbiol. 2012, 85, 213–224. [Google Scholar] [CrossRef]
- Wray, L.V.; Fisher, S.H. Functional roles of the conserved Glu304 loop of Bacillus subtilis glutamine synthetase. J. Bacteriol. 2010, 192, 5018–5025. [Google Scholar] [CrossRef] [Green Version]
- Pearson, J.T.; Dabrowski, M.J.; Kung, I.; Atkins, W.M. The central loop of Escherichia coli glutamine synthetase is flexible and functionally passive. Arch. Biochem. Biophys. 2005, 436, 397–405. [Google Scholar] [CrossRef]
- Zhao, Z.-J.; Zou, C.; Zhu, Y.-X.; Dai, J.; Chen, S.; Wu, D.; Wu, J.; Chen, J. Development of L-tryptophan production strains by defined genetic modification in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2011, 38, 1921–1929. [Google Scholar] [CrossRef]
- Gu, P.; Yang, F.; Li, F.; Liang, Q.; Qi, Q. Knocking out analysis of tryptophan permeases in Escherichia coli for improving L-tryptophan production. Appl. Microbiol. Biotechnol. 2013, 97, 6677–6683. [Google Scholar] [CrossRef]
- Gu, P.; Yang, F.; Su, T.; Wang, Q.; Liang, Q.; Qi, Q. A rapid and reliable strategy for chromosomal integration of gene(s) with multiple copies. Sci. Rep. 2015, 5, 9684. [Google Scholar] [CrossRef] [Green Version]
- Tröndle, J.; Schoppel, K.; Bleidt, A.; Trachtmann, N.; Sprenger, G.A.; Weuster-Botz, D. Metabolic control analysis of L-tryptophan production with Escherichia coli based on data from short-term perturbation experiments. J. Biotechnol. 2020, 307, 15–28. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, B.; Duan, C.; Sun, B.; Yang, J.; Yang, S. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl. Environ. Microbiol. 2015, 81, 2506–2514. [Google Scholar] [CrossRef] [Green Version]
- Hama, H.; Kayahara, T.; Tsuda, M.; Tsuchiya, T. Inhibition of homoserine dehydrogenase I by L-serine in Escherichia coli. J. Biochem. 1991, 109, 604–608. [Google Scholar] [CrossRef]
- Malla, S.; van der Helm, E.; Darbani, B.; Wieschalka, S.; Förster, J.; Borodina, I.; Sommer, M.O.A. A Novel Efficient L-Lysine Exporter Identified by Functional Metagenomics. Front. Microbiol. 2022, 13, 855736. [Google Scholar] [CrossRef]
- Tsyrenzhapova, I.S.; Doroshenko, V.G.; Airich, L.G.; Mironov, A.S.; Mashko, S.V. Gene yddG of Escherichia coli encoding the putative exporter of aromatic amino acids: Constitutive transcription and dependence of the expression on the cell growth rate. Russ. J. Genet. 2009, 45, 525–532. [Google Scholar] [CrossRef]
- Doroshenko, V.; Airich, L.; Vitushkina, M.; Kolokolova, A.; Livshits, V.; Mashko, S. YddG from Escherichia coli promotes export of aromatic amino acids. FEMS Microbiol. Lett. 2007, 275, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Trötschel, C.; Deutenberg, D.; Bathe, B.; Burkovski, A.; Krämer, R. Characterization of methionine export in Corynebacterium glutamicum. J. Bacteriol. 2005, 187, 3786–3794. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cao, G.; Xu, D.; Fan, L.; Wu, X.; Ni, X.; Zhao, S.; Zheng, P.; Sun, J.; Ma, Y. A Novel Corynebacterium glutamicum l-Glutamate Exporter. Appl. Environ. Microbiol. 2018, 84, e02691-17. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.S.; Lauersen, K.J.; Ikram, S.; Li, C. Efflux Transporters’ Engineering and Their Application in Microbial Production of Heterologous Metabolites. ACS Synth. Biol. 2021, 10, 646–669. [Google Scholar] [CrossRef]
- Krämer, R. Systems and mechanisms of amino acid uptake and excretion in prokaryotes. Arch. Microbiol. 1994, 162, 1–13. [Google Scholar] [CrossRef]
- Martínez, J.A.; Bolívar, F.; Escalante, A. Shikimic Acid Production in Escherichia coli: From Classical Metabolic Engineering Strategies to Omics Applied to Improve Its Production. Front. Bioeng. Biotechnol. 2015, 3, 145. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Hu, M.; Tian, L.; Liu, F.; Wang, Q.; Xu, M.; Rao, Z. Enhancing l-glutamine production in Corynebacterium glutamicum by rational metabolic engineering combined with a two-stage pH control strategy. Bioresour. Technol. 2021, 341, 125799. [Google Scholar] [CrossRef]
- Wang, L.; Li, N.; Yu, S.; Zhou, J. Enhancing caffeic acid production in Escherichia coli by engineering the biosynthesis pathway and transporter. Bioresour. Technol. 2022, 368, 128320. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, S.; Wu, D.; Wu, J.; Chen, J. Effect of gene knockouts of l-tryptophan uptake system on the production of l-tryptophan in Escherichia coli. Process Biochem. 2012, 47, 340–344. [Google Scholar] [CrossRef]
- Tazuya-Murayama, K.; Aramaki, H.; Mishima, M.; Saito, K.; Ishida, S.; Yamada, K.; Tazuya-Murayama, K.; Aramaki, H.; Mishima, M.; Saito, K.; et al. Effect of L-serine on the biosynthesis of aromatic amino acids in Escherichia coli. J. Nutr. Sci. Vitaminol. 2006, 52, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, L.; Elsaraf, M.; Mindt, M.; Wendisch, V.F. l-Serine Biosensor-Controlled Fermentative Production of l-Tryptophan Derivatives by Corynebacterium glutamicum. Biology 2022, 11, 744. [Google Scholar] [CrossRef]
- Bennett, B.D.; Yuan, J.; Kimball, E.H.; Rabinowitz, J.D. Absolute quantitation of intracellular metabolite concentrations by an isotope ratio-based approach. Nat. Protoc. 2008, 3, 1299–1311. [Google Scholar] [CrossRef] [Green Version]


| Strains | Carbon Source | Culturing Methods | Titer (g/L) | Yield (g/g) | Reference |
|---|---|---|---|---|---|
| SX11 | glucose | 5 L bioreactor Fed-batch | 41.7 | 0.227 | [6] |
| TRP12 | glucose | 5 L bioreactor Fed-batch | 52.1 | 0.171 | [5] |
| TS-10 | glucose | shake-flask | 1.71 | NS | [14] |
| S028AA | glucose | 1.5 L bioreactor Fed-batch | 48.3 | 0.185 | [15] |
| W3650/trpA-EBR/tnaA | glucose | 100 L bioreactor Fed-batch * | >45 ** | 0.224 | [16] |
| Dmtr/pta-Y | glucose | 30 L bioreactor Fed-batch | 52.6 | 0.202 | [17] |
| Strains/Plasmids | Characteristics | Source |
|---|---|---|
| Strains | ||
| E. coli jm109 | the cloning host | This lab |
| T8 | W3110, ΔlacI, tyrR::Ptrc-aroGS180F, trpR::Ptrc-serAH344A,N364A, trpE::Ptrc-trpES40F, ΔpykF, tktA::Ptrc-tktA, ΔptsH, yjiV::PJ23105-UTRWP1-galP, ylbE::PJ23114-UTRMK3-glk, PrpsT p1-ppc, Prrnc p1-gltA | This lab |
| T8U | T8, pSB4K5 | This study |
| T8B | T8, pSB4K5-aroB | This study |
| T8D | T8, pSB4K5-aroD | This study |
| T8E | T8, pSB4K5-aroE | This study |
| T8K | T8, pSB4K5-aroK | This study |
| T8A | T8, pSB4K5-aroA | This study |
| T8C | T8, pSB4K5-aroC | This study |
| T8KE | ycjv::Ptrc-aroKE | This study |
| T9 (T8EK) | ycjv::Ptrc-aroEK | This study |
| T10 | T9, mbhA::Ptrc-glnAL159I,E304A | This study |
| T11 | T10, yeeP::PserB-serB, PserC-serC | This study |
| T12 | T11, yddG::Ptrc- yddG | This study |
| T13 | T11, ΔTnaB | This study |
| T14 | T13, ΔpheA, ΔtyrA | This study |
| T15 | T13, ΔyeeP::PserB-serB, PserC-serC | This study |
| Plasmids | ||
| pCas9 | repA101(Ts), Pcas-cas9, kan, lacIq, ParaB-Red, Ptrc-sgRNA-pMB1 | [26] |
| pTarget | pMB1, aadA, sgRNA | [26] |
| Name | Content (mg/g) | Name | Content (mg/g) |
|---|---|---|---|
| Leucine | 9.25 ± 0.21 | Tryptophan | 0.58 ± 0.19 |
| Isoleucine | 13.98 ± 0.31 | Phenylalanine | 7.12 ± 0.54 |
| arginine | 8.69 ± 0.18 | Glutamate | 6.35 ± 0.28 |
| Alanine | 12.56 ± 0.41 | Lysine | 6.85 ± 0.51 |
| Histidine | 3.92 ± 0.61 | serine | 3.32 ± 0.32 |
| Glycine | 1.36 ± 0.29 | Valine | 7.25 ± 0.41 |
| Methionine | 1.25 ± 0.51 | Aspartate | 3.96 ± 0.12 |
| Asparagine | 4.32 ± 0.31 | proline | 8.91 ± 0.19 |
| threonine | 4.58 ± 0.51 |
| Name | Sequence |
|---|---|
| AroE-1 | ctagctcccagtagacgaagttcgcgcac |
| AroE-2 | gttcctcctactggatggcctgattcacg |
| AroE-3 | cgtctactgggagctagagctgtcagacca |
| AroE-4 | ccatccagtaggaggaactacactgtctgcag |
| aroK-1 | cagagagcggagctagagctgtcagacc |
| aroK-2 | ggtacagtaaggaggaactacactgtctgcag |
| aroK-3 | ctagctccgctctctgagcgaagcg |
| aroK-4 | agttcctccttactgtacccgcagacgagtg |
| N20-mbhA-F | gccaaaacctgccaccgtgggttttagagctagaaatagcaag |
| N20-mbhA-R | ccacggtggcaggttttggcactagtattatacctaggactgag |
| trc-glnA-F | ttgacaattaatcatccggctcgtataatgtgtggaaagaggaggaattttaccaaatgg |
| trc-glnA-R | ctgacacttttagtgccaagg |
| serBC-1 | atgcggtataacgatgtcagcagccagc |
| serBC-2 | gacatcgttataccgcatcaggcgct |
| serBC-3 | tccattgccctgtcgtgactgatgccc |
| serBC-4 | cgacagggcaatggatacaaggtagcctcatg |
| trcY-F | cctcaatagcggtagaattgacaattaatcatccggctcgtataatg |
| trcY-R | ggatgattaattgtcaattctaccgctattgaggtaggtcaattt |
| N20-tnaB-F | gccggtgcctggtttttctggttttagagctagaaatagcaag |
| N20-tnaB-R | cagaaaaaccaggcaccggcactagtattatacctaggactgag |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wang, B.-B.; Xu, J.-Z.; Zhang, W.-G. Engineering of Shikimate Pathway and Terminal Branch for Efficient Production of L-Tryptophan in Escherichia coli. Int. J. Mol. Sci. 2023, 24, 11866. https://doi.org/10.3390/ijms241411866
Liu S, Wang B-B, Xu J-Z, Zhang W-G. Engineering of Shikimate Pathway and Terminal Branch for Efficient Production of L-Tryptophan in Escherichia coli. International Journal of Molecular Sciences. 2023; 24(14):11866. https://doi.org/10.3390/ijms241411866
Chicago/Turabian StyleLiu, Shuai, Bing-Bing Wang, Jian-Zhong Xu, and Wei-Guo Zhang. 2023. "Engineering of Shikimate Pathway and Terminal Branch for Efficient Production of L-Tryptophan in Escherichia coli" International Journal of Molecular Sciences 24, no. 14: 11866. https://doi.org/10.3390/ijms241411866





