Elucidating the Racemization Mechanism of Aliphatic and Aromatic Amino Acids by In Silico Tools
Abstract
1. Introduction
2. Results
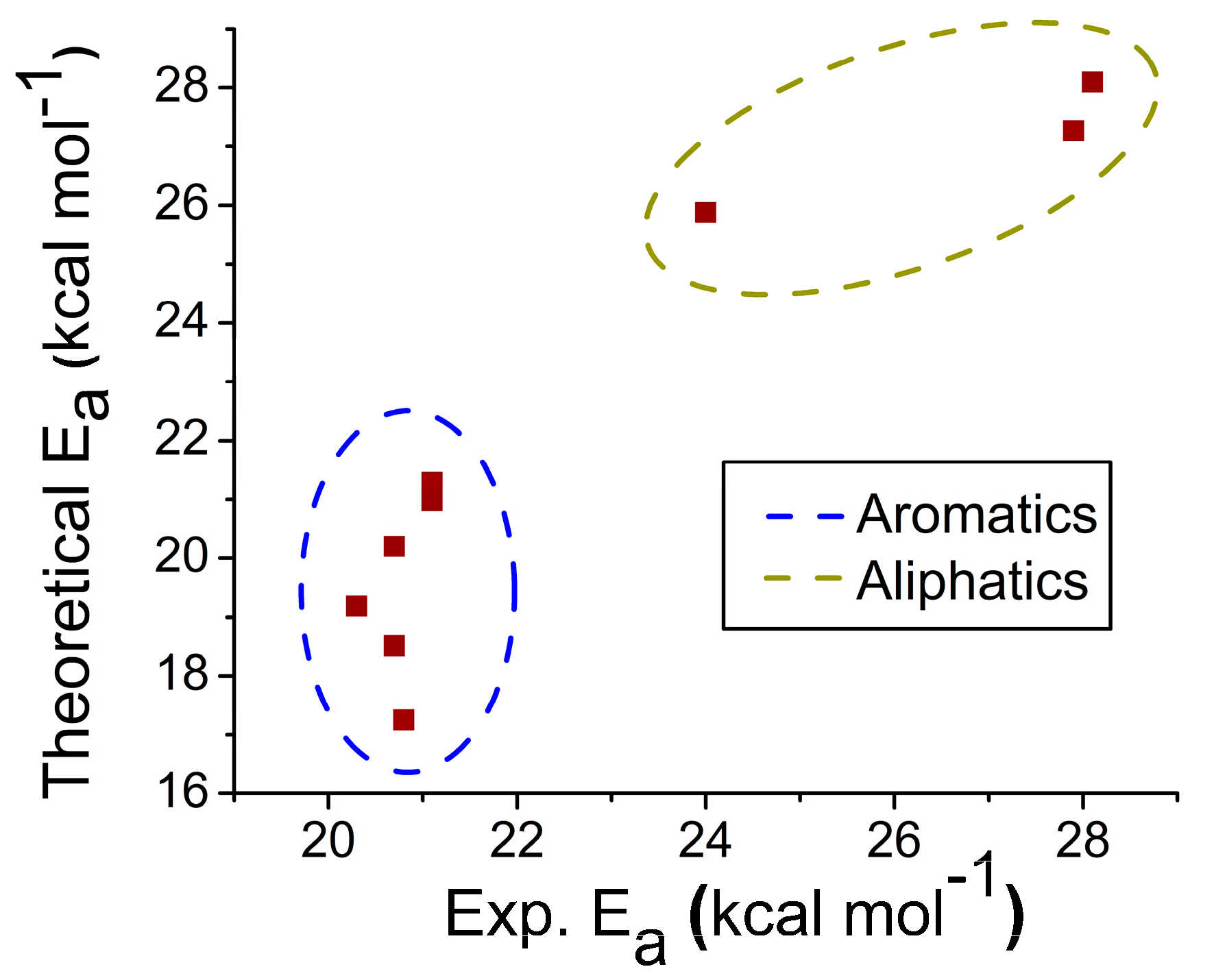
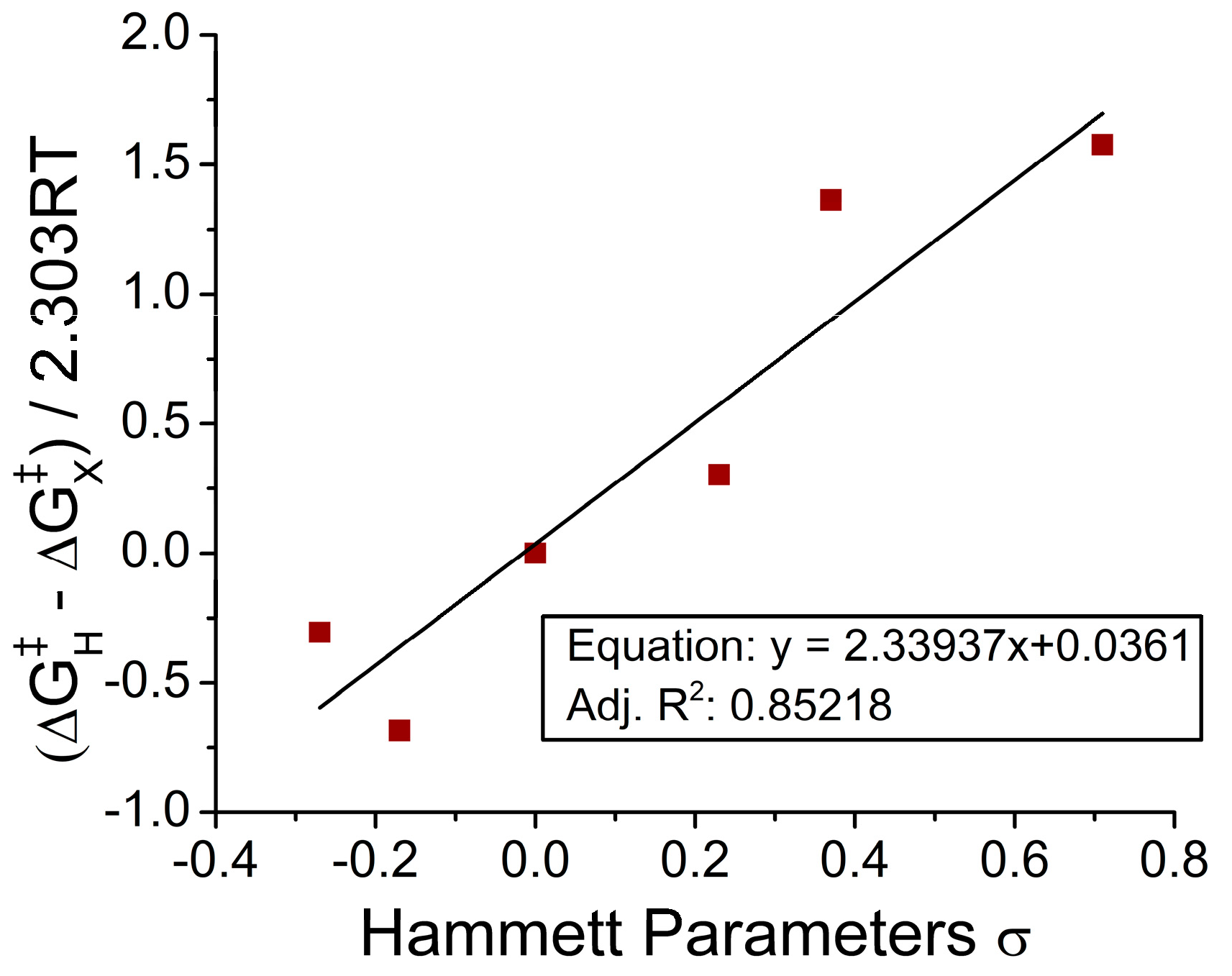
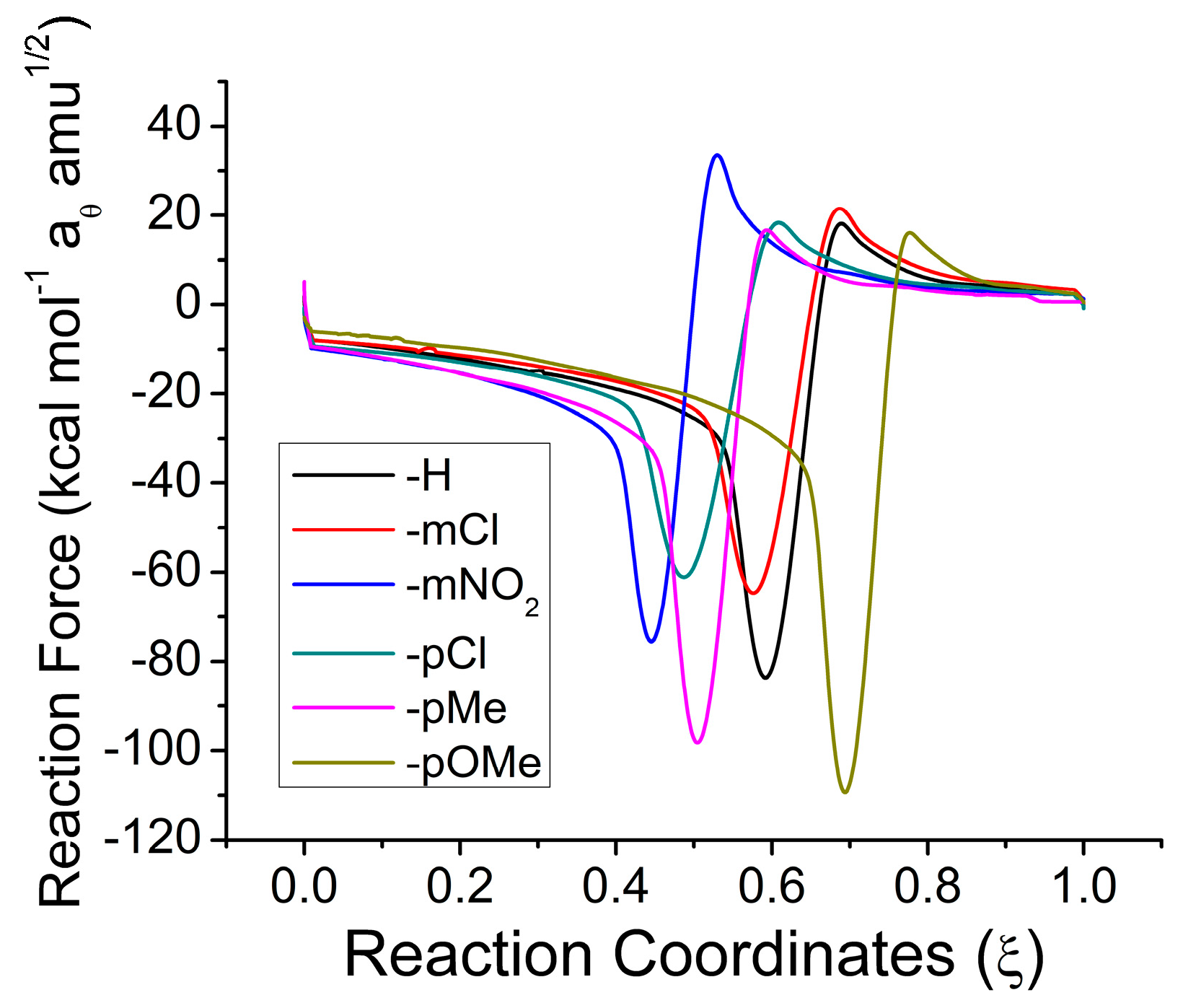
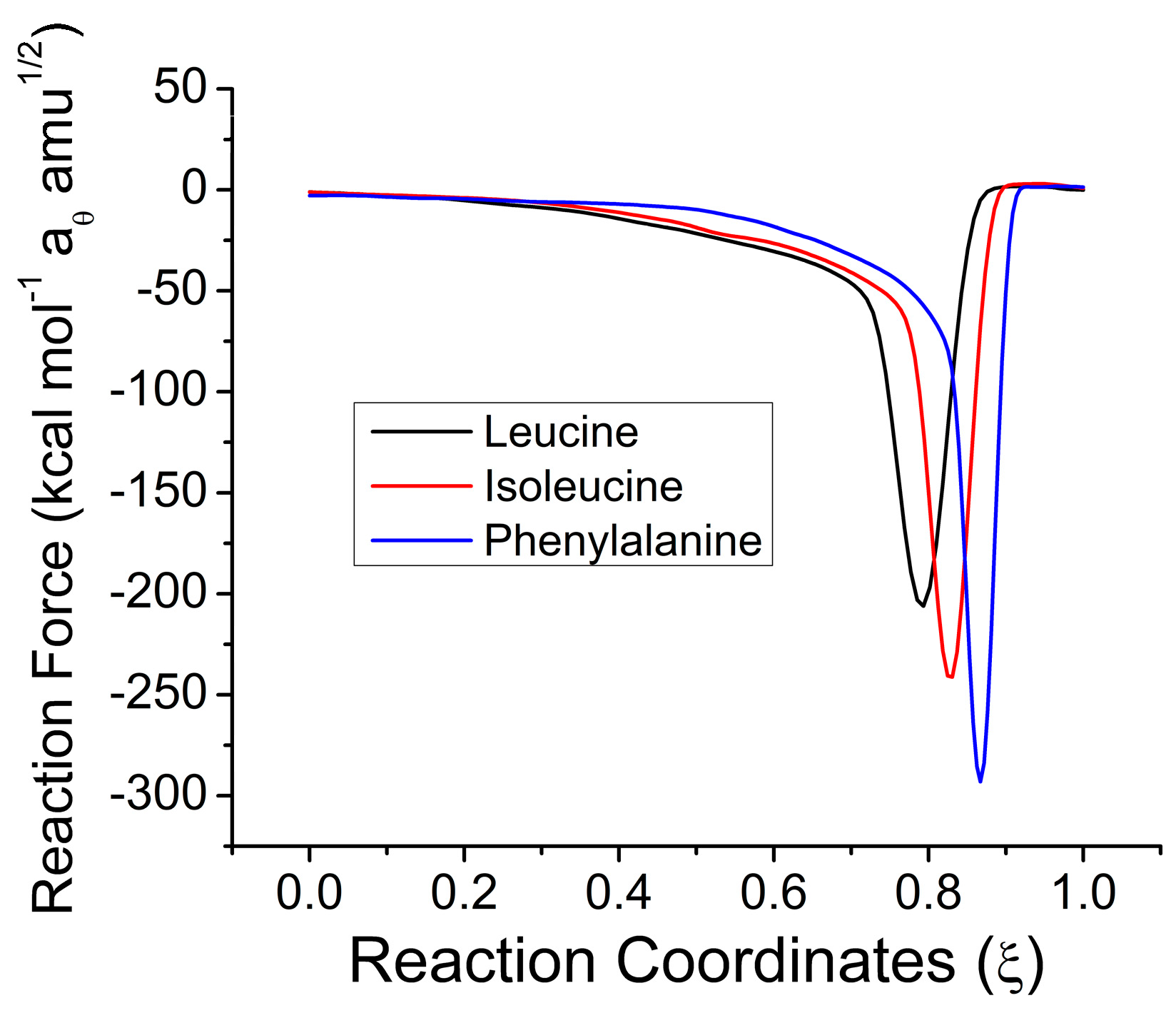
2.1. Reaction Energy Results
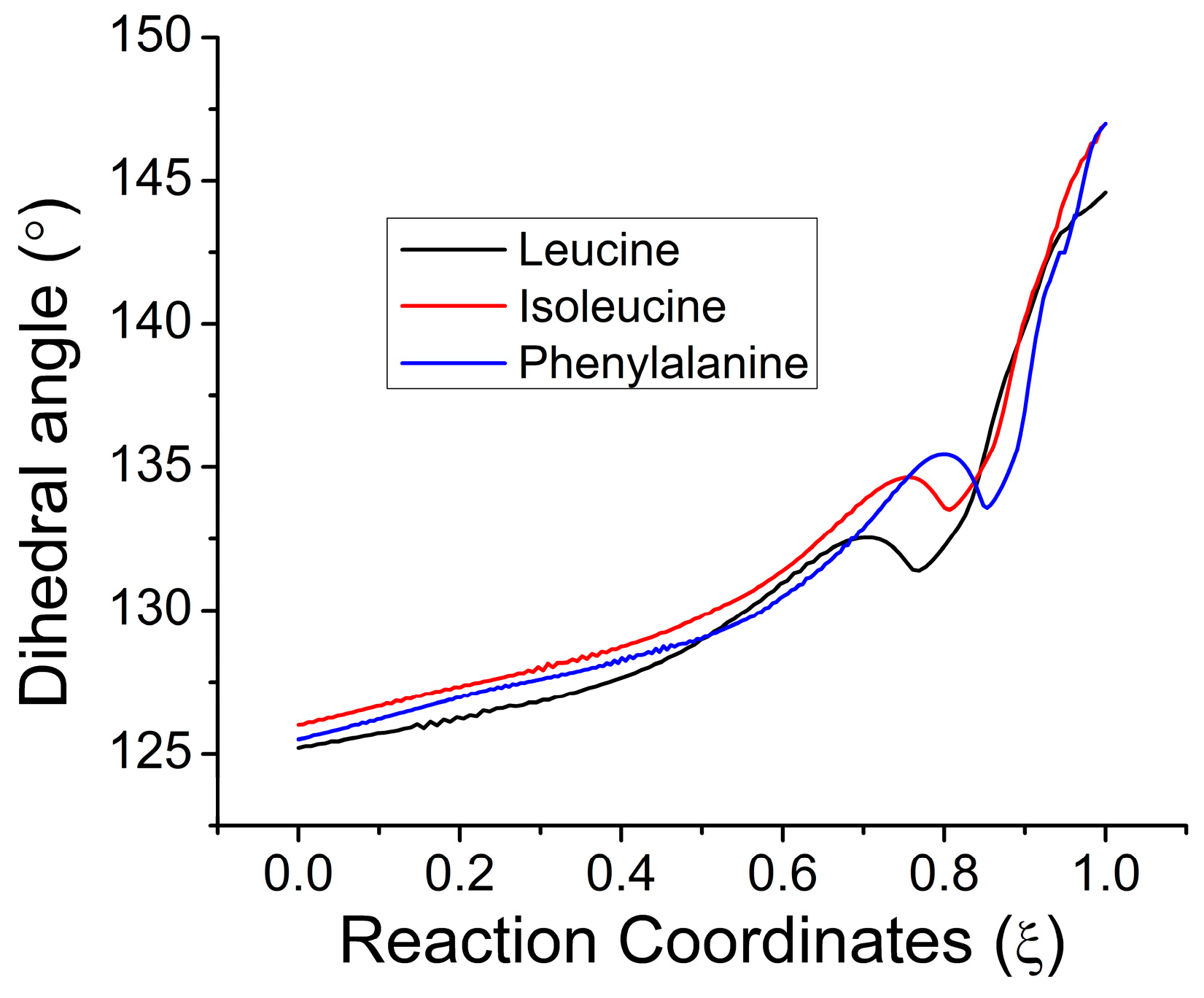
2.2. Geometric Results
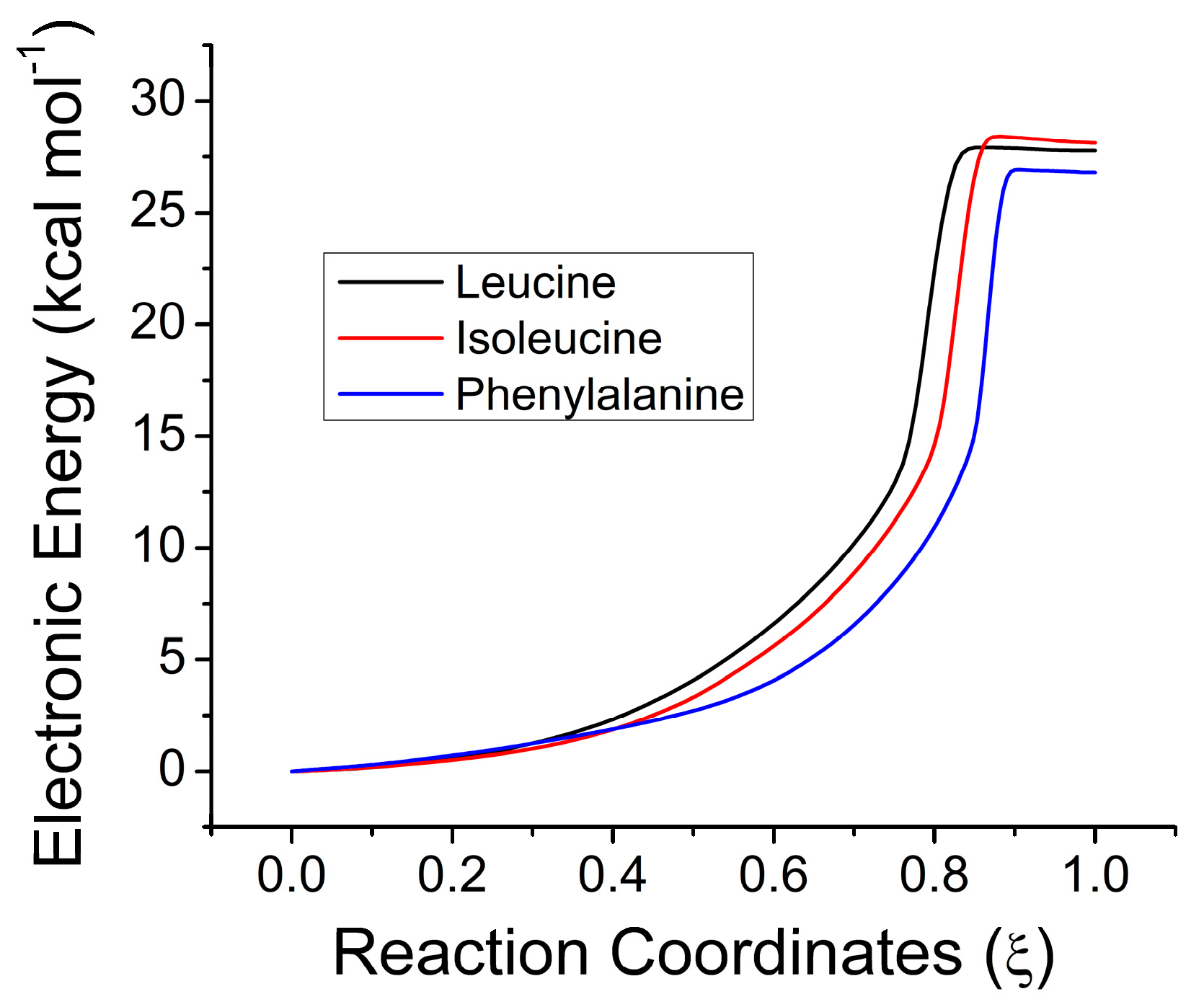
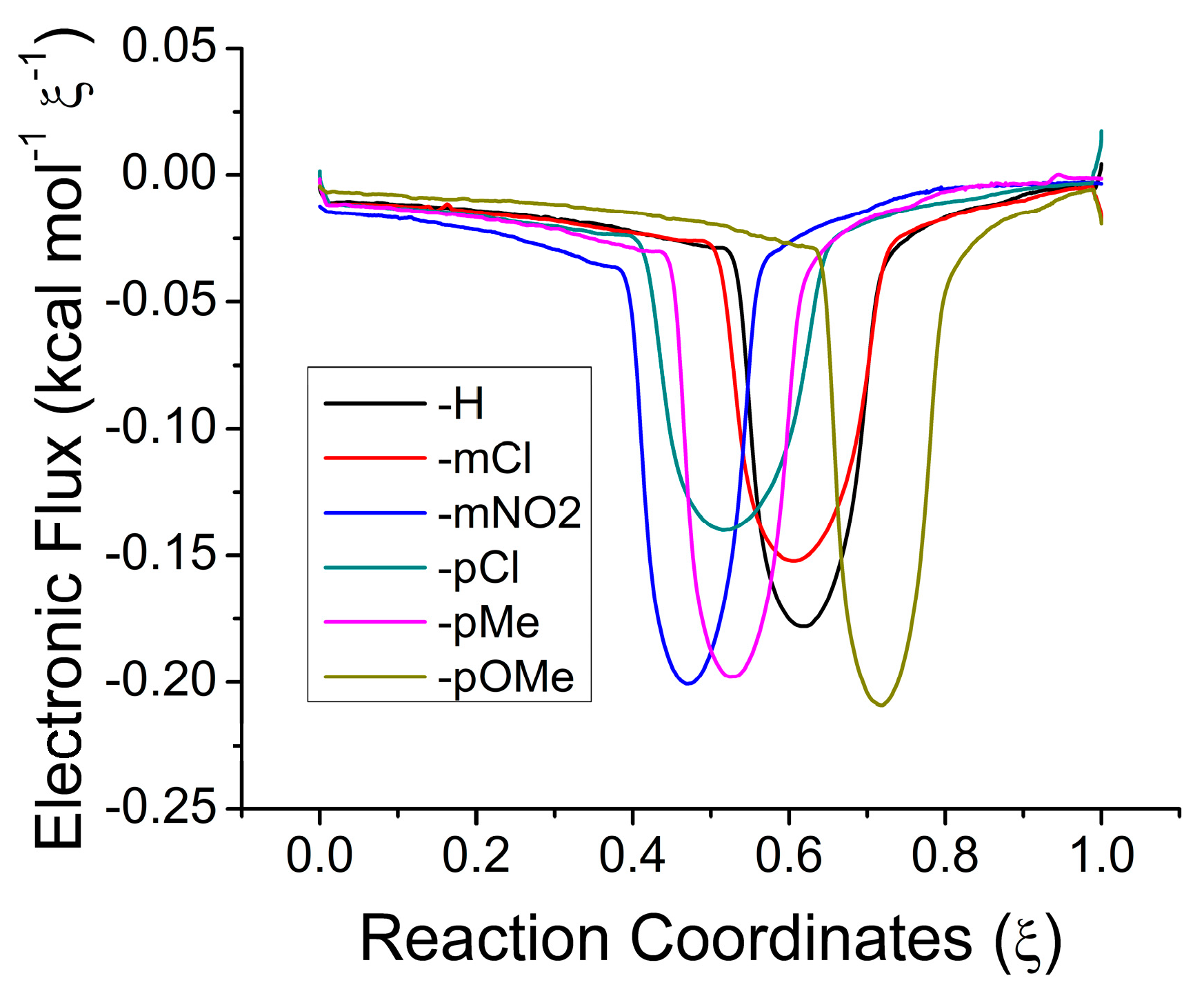
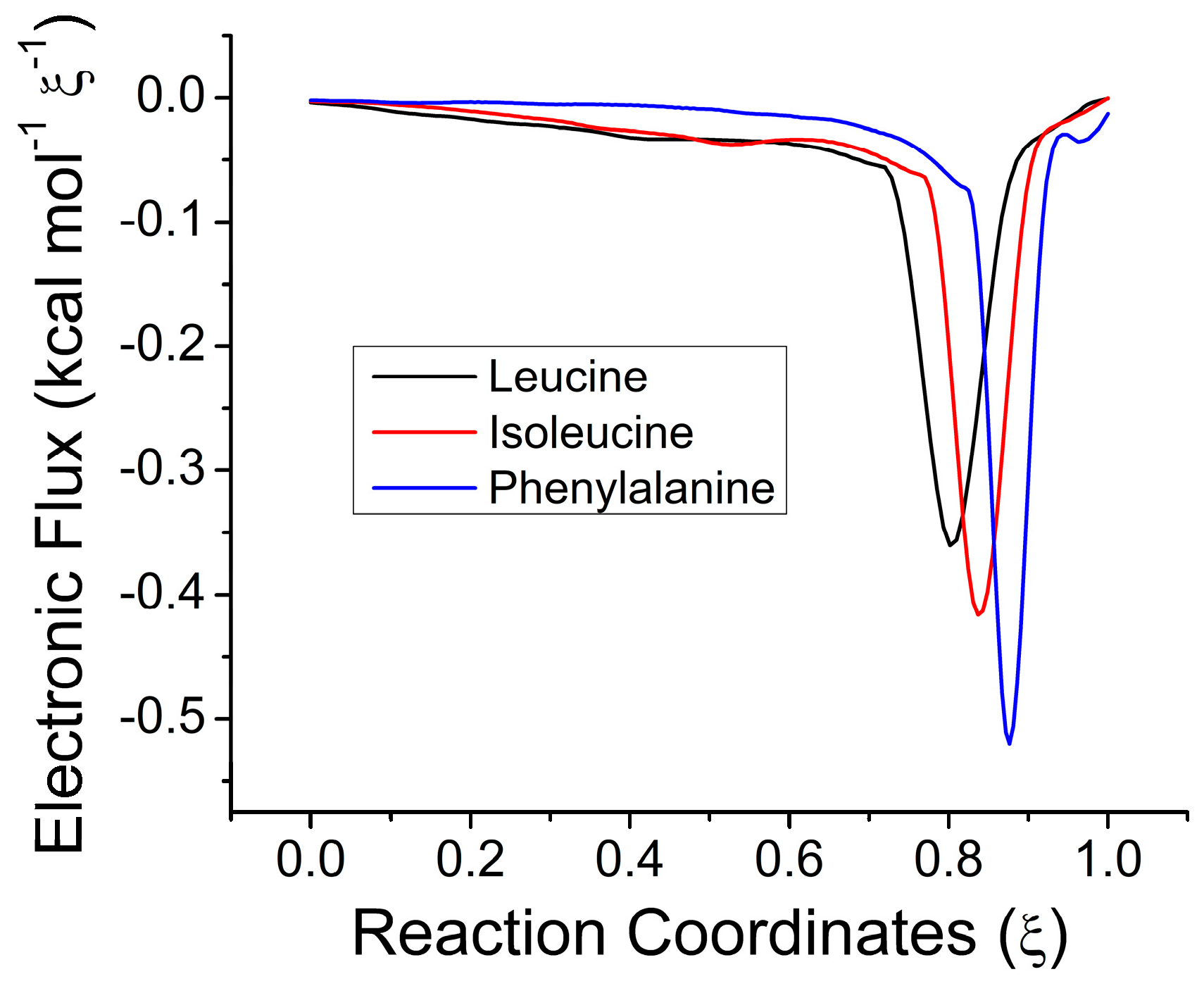
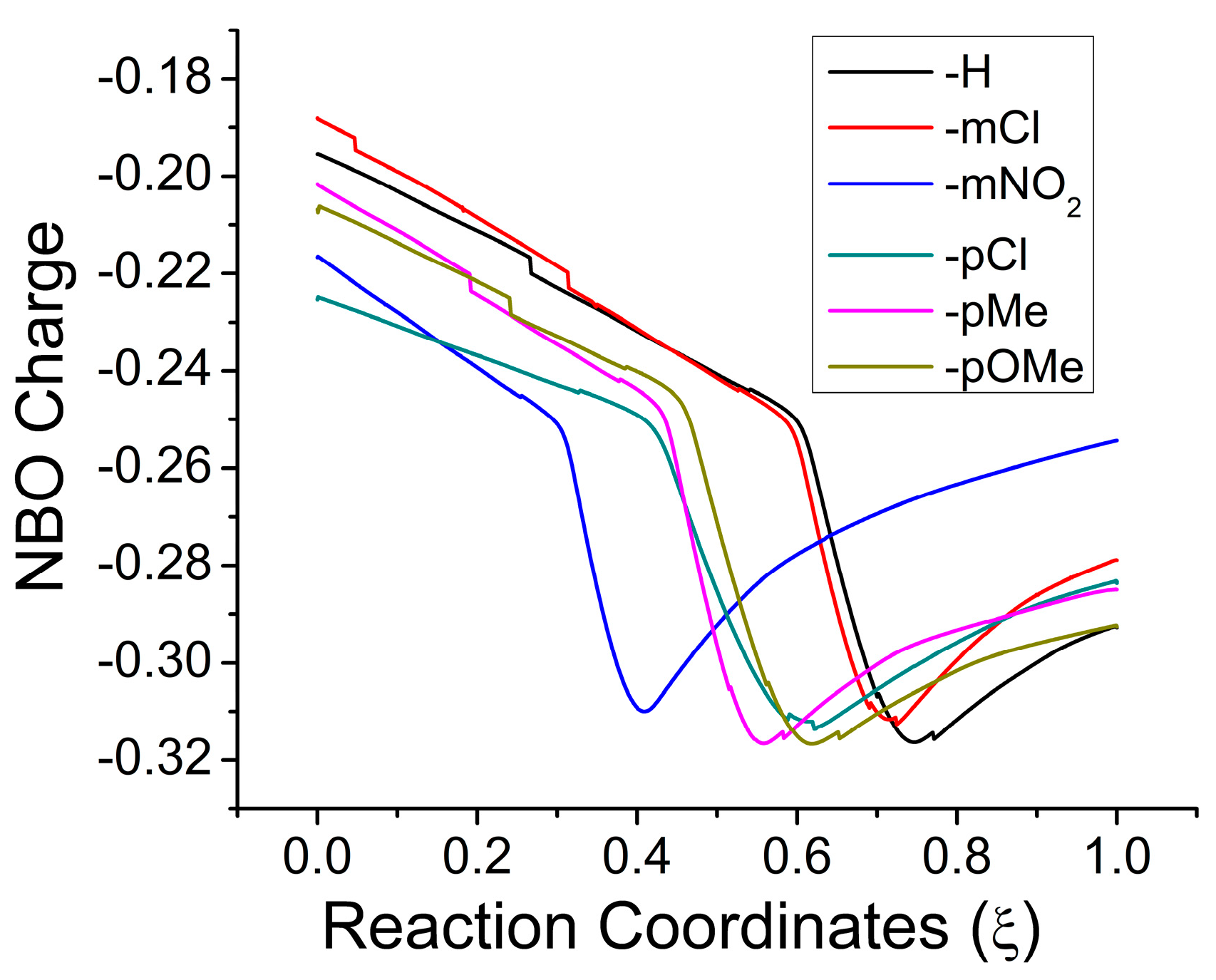
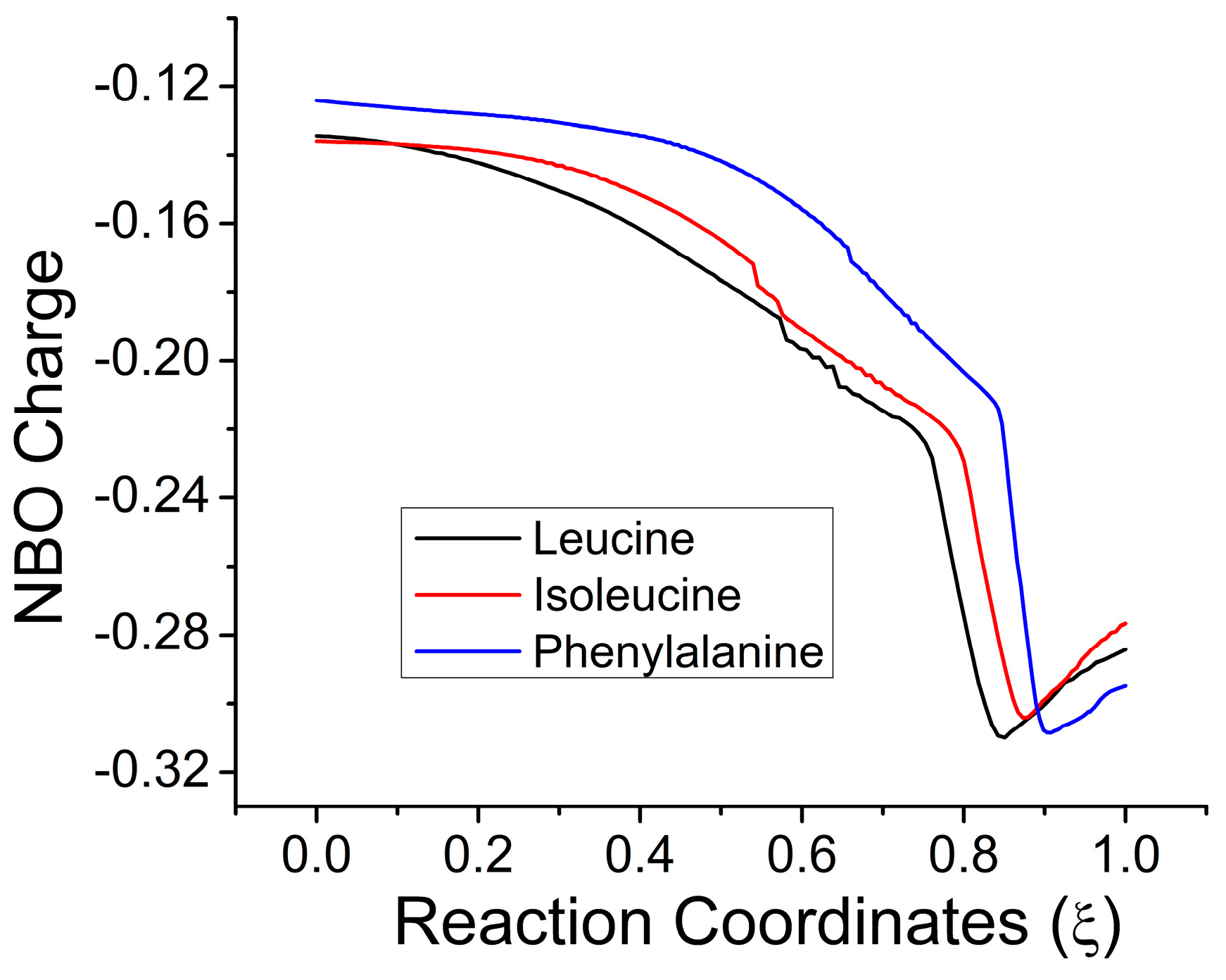
2.3. Electronic Results
3. Discussion
4. Materials and Methods
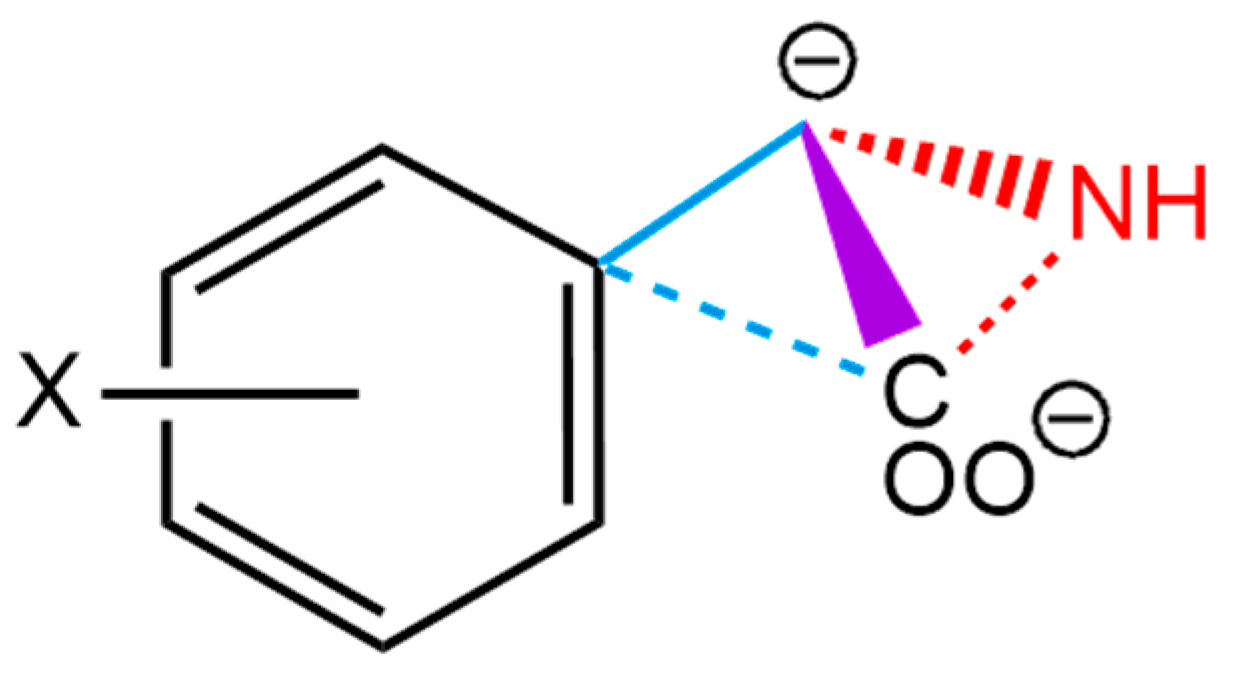
4.1. Data Set: Aromatic and Aliphatic Amino Acids
4.2. Computational Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballard, A.; Narduolo, S.; Ahmad, H.O.; Cosgrove, D.A.; Leach, A.G.; Buurma, N.J. The problem of racemization in drug discovery and tools to predict it. Expert Opin. Drug Discov. 2019, 14, 527–539. [Google Scholar] [CrossRef]
- Marcone, G.L.; Rosini, E.; Crespi, E.; Pollegioni, L. D-amino acids in foods. Appl. Microbiol. Biotechnol. 2020, 104, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Leinisch, F.; Greco, I.; Zhang, W.; Shu, N.; Chuang, C.Y.; Lund, M.N.; Davies, M.J. Characterisation and quantification of protein oxidative modifications and amino acid racemisation in powdered infant milk formula. Free Radic. Res. 2019, 53, 68–81. [Google Scholar] [CrossRef]
- Smith, S.W. Chiral toxicology: It’s the same thing only different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef]
- Bastings, J.J.A.J.; van Eijk, H.M.; Damink, S.W.O.; Rensen, S.S. D-amino acids in health and disease: A focus on cancer. Nutrients 2019, 11, 2205. [Google Scholar] [CrossRef]
- Mann, L.; Lang, M.; Schulze, P.; Halz, J.H.; Csuk, R.; Hoenke, S.; Seidel, R.W.; Richter, A. Racemization-free synthesis of Nα-2-thiophenoyl-phenylalanine-2-morpholinoanilide enantiomers and their antimycobacterial activity. Amino Acids 2021, 53, 1187–1196. [Google Scholar] [CrossRef]
- Yan, T.; Feringa, B.L.; Barta, K. Direct catalytic N-alkylation of α-amino acid esters and amides using alcohols with high retention of stereochemistry. ChemSusChem 2021, 14, 2303–2307. [Google Scholar] [CrossRef]
- Hättasch, T.; Schmuck, C.; Niemeyer, J. Triazole groups as biomimetic amide groups in peptides can trigger racemization. Arkivoc 2021, 2021, 185–196. [Google Scholar] [CrossRef]
- Harriehausen, I.; Bollmann, J.; Carneiro, T.; Bettenbrock, K.; Seidel-Morgenstern, A. Characterization of an immobilized amino acid racemase for potential application in enantioselective chromatographic resolution processes. Catalysts 2021, 11, 726. [Google Scholar] [CrossRef]
- Huang, H.; Jin, Y.; Shirbhate, M.E.; Kang, D.; Choi, M.; Chen, Q.; Kim, Y.; Kim, S.J.; Byun, I.S.; Wang, M.; et al. Enantioselective extraction of unprotected amino acids coupled with racemization. Nat. Commun. 2021, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Dyakin, V.V.; Wisniewski, T.M.; Lajtha, A. Racemization in post-translational modifications relevance to protein aging, aggregation and neurodegeneration: Tip of the iceberg. Symmetry 2021, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, S.A.; Rincón, L.; Torres, F.J.; Rodríguez, V.; Mora, J.R. A computational study of the reaction mechanism involved in the fast cleavage of an unconstrained amide bond assisted by an amine intramolecular nucleophilic attack. J. Comput. Chem. 2021, 42, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Meinard, C.; Bruneau, P.; Perronnet, J. High-performance liquid chromatograph coupled with two detectors: A UV spectrometer and a polarimeter. Example in the field of pyrethroids: Identification of enantiomers. J. Chromatogr. 1985, 349, 109–116. [Google Scholar] [CrossRef]
- Broberg, A.; Nord, C.; Levenfors, J.J.; Bjerketorp, J.; Guss, B.; Öberg, B. In-peptide amino acid racemization via inter-residue oxazoline intermediates during acidic hydrolysis. Amino Acids 2021, 53, 323–331. [Google Scholar] [CrossRef]
- Brewer, G.; Brewer, C.; Butcher, R.J.; Zemba, M. Structural evidence of a ketimine as the product of an amino acid with an aldehyde. An intermediate in the racemization and transamination of amino acids. Inorg. Chem. Commun. 2016, 64, 35–38. [Google Scholar] [CrossRef]
- Smith, G.G.; Sivakua, T. Mechanism of the racemization of amino acids. Kinetics of racemization of arylglycines. J. Org. Chem. 1983, 48, 627–634. [Google Scholar] [CrossRef]
- Dudek, W.M.; Ostrowski, S.; Dobrowolski, J.C. On aromaticity of the aromatic α-amino acids and tuning of the NICS indices to find the aromaticity order. J. Phys. Chem. A 2022, 126, 3433–3444. [Google Scholar] [CrossRef]
- Toussaint, F.C.; Defrance, T.; Decouvreur, S.; Carly, N.; Merschaert, A. Intensification of free-radical racemization for a non-activated amine in a continuous flow reactor. Synthesis 2020, 52, 3389–3396. [Google Scholar] [CrossRef]
- Ruszczycky, M.W.; Liu, H.W. Distinguishing concerted versus stepwise mechanisms using isotope effects on isotope effects. Biochemistry 2021, 60, 3416–3418. [Google Scholar] [CrossRef]
- Dougherty, D.A. Cation-p interactions involving aromatic amino acids. J. Nutr. 2007, 137, 1504S–1508S. [Google Scholar] [CrossRef]
- Cotzias, G.C.; Van Woert, M.H.; Schiffer, L.M. Aromatic amino acids and modification of parkinsonism. J. Med. 1967, 276, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Himmelreich, N.; Montioli, R.; Bertoldi, M.; Carducci, C.; Leuzzi, V.; Gemperle, C.; Berner, T.; Hyland, K.; Thöny, B.; Hoffmann, G.F.; et al. Aromatic amino acid decarboxylase deficiency: Molecular and metabolic basis and therapeutic outlook. Mol. Genet. Metab. 2019, 127, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Xue, B.; Rehak, P.; Jacoby, G.; Ji, W.; Shimon, L.J.W.; Beck, R.; Král, P.; Cao, Y.; Gazit, E. Self-assembly of aromatic amino acid enantiomers into supramolecular materials of high rigidity. ACS Nano 2020, 14, 1694–1706. [Google Scholar] [CrossRef]
- Planas, F.; McLeish, M.J.; Himo, F. Enzymatic stetter reaction: Computational study of the reaction mechanism of MenD. ACS Catal. 2021, 11, 12355–12366. [Google Scholar] [CrossRef]
- Javierre, G.; Fortrie, R.; Jean, M.; Moraleda, D.; Naubron, J.V.; Fotiadu, F. Racemization and transesterification of alkyl hydrogeno-phenylphosphinates. J. Mol. Model. 2017, 23, 168. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, B.; Wang, M. Estimation of the racemization rate constants for α-amino acids using density functional theory. Comput. Theor. Chem. 2017, 1104, 43–46. [Google Scholar] [CrossRef]
- Pokluda, J.; Černý, M.; Šob, M.; Umeno, Y. Ab initio calculations of mechanical properties: Methods and applications. Prog. Mater. Sci. 2015, 73, 127–158. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; He, Z.; Xu, Y.; Yu, W. Theoretical investigations on charge transport properties of tetrabenzo[a,d,j,m]coronene derivatives using different density functional theory functionals (B3LYP, M06-2X, and WB97XD). J. Chem. Res. 2019, 48, 293–303. [Google Scholar] [CrossRef]
- Cuesta, S.A.; Torres, F.J.; Rincón, L.; Paz, J.L.; Márquez, E.A.; Mora, J.R. Effect of the nucleophile’s nature on chloroacetanilide herbicides cleavage reaction mechanism. A dft study. Int. J. Mol. Sci. 2021, 22, 6876. [Google Scholar] [CrossRef]
- Inostroza-Rivera, R.; Herrera, B.; Toro-Labbé, A. Using the reaction force and the reaction electronic flux on the proton transfer of formamide derived systems. Phys. Chem. Chem. Phys. 2014, 16, 14489–14495. [Google Scholar] [CrossRef]
- Cuesta, S.A.; Mora, J.R.; Meneses, L.M.; Márquez, E.A.; Flores-Morales, V.; Rincón, L.; Torres, F.J.; Zambrano, C.H. Unveiling the structure-reactivity relationship involved in the reaction mechanism of the HCl-catalyzed alkyl t-butyl ethers thermal decomposition. A computational study. Int. J. Quantum Chem. 2022, 122, e26915. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Goerigk, L.; Sharma, R. The INV24 test set: How well do quantum-chemical methods describe inversion and racemization barriers? Can. J. Chem. 2016, 94, 1133–1143. [Google Scholar] [CrossRef]
- Takahashi, O.; Kirikoshi, R.; Manabe, N. Racemization of the succinimide intermediate formed in proteins and peptides: A computational study of the mechanism catalyzed by dihydrogen phosphate ion. Int. J. Mol. Sci. 2016, 17, 1698. [Google Scholar] [CrossRef]
- Prabhu, G.; Basavaprabhu; Narendra, N.; Vishwanatha, T.M.; Sureshbabu, V.V. Amino acid chlorides: A journey from instability and racemization toward broader utility in organic synthesis including peptides and their mimetics. Tetrahedron 2015, 71, 2785–2832. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Performance of SM6, SM8, and SMD on the SAMPL1 test set for the prediction of small-molecule solvation free energies. J. Phys. Chem. B 2009, 113, 4538–4543. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Cuesta, S.A.; Mora, J.; Márquez, E. Gas phase decomposition of t-butyl methyl ether catalyzed by different hydrogen halides: A dft study. infoANALÍTICA 2022, 10, 65–79. [Google Scholar] [CrossRef]
- Türker, L.; Atalar, T.; Gümüş, S.; Çamur, Y. A dft study on nitrotriazines. J. Hazard. Mater. 2009, 167, 440–448. [Google Scholar] [CrossRef]
- Da, L.G.; He, J.; Hu, L.F. DFT Study on the structure and racemization mechanism of 2-amino-2′-hydroxy-1,1′-binaphthyl. J. Phys. Org. Chem. 2019, 32, e3900. [Google Scholar] [CrossRef]
- De Souza, M.A.F.; Ventura, E.; Do Monte, S.A.; Riveros, J.M.; Longo, R.L. Revisiting the concept of the (a)synchronicity of diels-alder reactions based on the dynamics of quasiclassical trajectories. J. Comput. Chem. 2016, 37, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Novikov, A.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Theory of the formation and decomposition of N-heterocyclic aminooxycarbenes through metal-assisted [2+3]-dipolar cycloaddition/retro- cycloaddition. Chem.—A Eur. J. 2013, 19, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Novikov, A.S.; Kuznetsov, M.L. Theoretical study of Re(IV) and Ru(II) bis-isocyanide complexes and their reactivity in cycloaddition reactions with nitrones. Inorg. Chim. Acta 2012, 380, 78–89. [Google Scholar] [CrossRef]
- Novikov, A.S.; Kuznetsov, M.L.; Pombeiro, A.J.L.; Bokach, N.A.; Shul’Pin, G.B. Generation of HO• radical from hydrogen peroxide catalyzed by aqua complexes of the group III metals [M(H2O)n]3+ (M = Ga, In, Sc, Y, or La): A theoretical study. ACS Catal. 2013, 3, 1195–1208. [Google Scholar] [CrossRef]







| This Work | Experimental * | |||||
|---|---|---|---|---|---|---|
| X | Ea (kcal/mol) | ΔH‡ (kcal/mol) | ΔG‡ (kcal/mol) | ΔS‡ (cal/mol K) | Ea (kcal/mol) | |
| Aromatic | H | 20.20 | 18.67 | 24.35 | −14.80 | 20.7 |
| mCl | 18.51 | 16.98 | 21.96 | −12.97 | 20.7 | |
| mNO2 | 17.25 | 15.72 | 21.58 | −15.28 | 20.8 | |
| pCl | 19.19 | 17.66 | 23.82 | −16.05 | 20.3 | |
| pMe | 20.98 | 19.46 | 25.55 | −15.90 | 21.1 | |
| pOMe | 21.29 | 19.76 | 24.88 | −13.36 | 21.1 | |
| Aliphatic | Leucine | 27.27 | 26.57 | 33.10 | −17.04 | 27.9 |
| Isoleucine | 28.09 | 25.74 | 32.35 | −16.43 | 28.1 | |
| Phenylalanine | 25.87 | 24.35 | 30.39 | −15.75 | 24.0 | |
| X | W1 | W2 | W3 | W4 | W14 | W23 | |
|---|---|---|---|---|---|---|---|
| Aromatic | H | 6.9307 | 2.9724 | 0.5943 | 4.3796 | 11.3104 | 3.5667 |
| mCl | 5.4205 | 2.3746 | 0.8609 | 4.9446 | 10.3651 | 3.2354 | |
| mNO2 | 4.9695 | 2.0323 | 1.0508 | 8.1109 | 13.0804 | 3.0831 | |
| pCl | 4.4025 | 2.4017 | 0.8704 | 6.0197 | 10.4222 | 3.2721 | |
| pMe | 6.7781 | 3.2663 | 0.5146 | 5.6673 | 12.4454 | 3.7809 | |
| pOMe | 9.2743 | 3.7475 | 0.3897 | 2.6983 | 11.9726 | 4.1373 | |
| Aliphatic | Leu | 21.0125 | 7.4794 | 0.0588 | 0.0694 | 21.0819 | 7.5381 |
| IsoLeu | 21.6972 | 6.6868 | 0.1123 | 0.1332 | 21.8304 | 6.7990 | |
| PheAla | 20.2607 | 6.4456 | 0.0742 | 0.0489 | 20.3096 | 6.5198 |
| Compound | Phenylglycine | mCl-Phenylglycine | mNO2-Phenylglycine | |||
|---|---|---|---|---|---|---|
| Bond | C1-H5 | H5-O6 | C1-H5 | H5-O6 | C1-H5 | H5-O6 |
| Reactant | 0.8114 | 0.0446 | 0.7943 | 0.0544 | 0.7899 | 0.0566 |
| T. State | 0.3396 | 0.4344 | 0.3546 | 0.4171 | 0.3675 | 0.4059 |
| Product | 0.0809 | 0.6602 | 0.0782 | 0.6639 | 0.0549 | 0.6878 |
| %Ev | 64.59 | 63.32 | 61.40 | 59.51 | 57.47 | 55.34 |
| Sy | 0.9901 | 0.9843 | 0.9811 | |||
| Compound | pCl-Phenylglycine | pMe-Phenylglycine | pOMe-Phenylglycine | |||
| Bond | C1-H5 | H5-O6 | C1-H5 | H5-O6 | C1-H5 | H5-O6 |
| Reactant | 0.7751 | 0.0666 | 0.8092 | 0.0463 | 0.8408 | 0.0278 |
| T. State | 0.3505 | 0.4203 | 0.3305 | 0.4424 | 0.3254 | 0.4472 |
| Product | 0.0781 | 0.6625 | 0.0787 | 0.6619 | 0.0891 | 0.6521 |
| %Ev | 60.92 | 59.36 | 65.53 | 64.34 | 68.56 | 67.18 |
| Sy | 0.9870 | 0.9909 | 0.9898 | |||
| Compound | Leucine | Isoleucine | Phenylalanine | |||
|---|---|---|---|---|---|---|
| Bond | C1-H5 | H5-O6 | C1-H5 | H5-O6 | C1-H5 | H5-O6 |
| Reactant | 0.8856 | 0.0058 | 0.8874 | 0.0055 | 0.8967 | 0.0040 |
| T. State | 0.2220 | 0.5332 | 0.2394 | 0.5112 | 0.2453 | 0.5213 |
| Product | 0.1113 | 0.6239 | 0.1060 | 0.6285 | 0.1206 | 0.6271 |
| %Ev | 85.70 | 85.33 | 82.93 | 81.17 | 83.93 | 83.02 |
| Sy | 0.9978 | 0.9893 | 0.9945 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andino, M.S.; Mora, J.R.; Paz, J.L.; Márquez, E.A.; Perez-Castillo, Y.; Agüero-Chapin, G. Elucidating the Racemization Mechanism of Aliphatic and Aromatic Amino Acids by In Silico Tools. Int. J. Mol. Sci. 2023, 24, 11877. https://doi.org/10.3390/ijms241511877
Andino MS, Mora JR, Paz JL, Márquez EA, Perez-Castillo Y, Agüero-Chapin G. Elucidating the Racemization Mechanism of Aliphatic and Aromatic Amino Acids by In Silico Tools. International Journal of Molecular Sciences. 2023; 24(15):11877. https://doi.org/10.3390/ijms241511877
Chicago/Turabian StyleAndino, Mateo S., José R. Mora, José L. Paz, Edgar A. Márquez, Yunierkis Perez-Castillo, and Guillermin Agüero-Chapin. 2023. "Elucidating the Racemization Mechanism of Aliphatic and Aromatic Amino Acids by In Silico Tools" International Journal of Molecular Sciences 24, no. 15: 11877. https://doi.org/10.3390/ijms241511877
APA StyleAndino, M. S., Mora, J. R., Paz, J. L., Márquez, E. A., Perez-Castillo, Y., & Agüero-Chapin, G. (2023). Elucidating the Racemization Mechanism of Aliphatic and Aromatic Amino Acids by In Silico Tools. International Journal of Molecular Sciences, 24(15), 11877. https://doi.org/10.3390/ijms241511877







