Translocation of Oocytic HES1 into Surrounding Cumulus Cells in Bovine: Mechanism of Cellular Interaction during IVM?
Abstract
1. Introduction
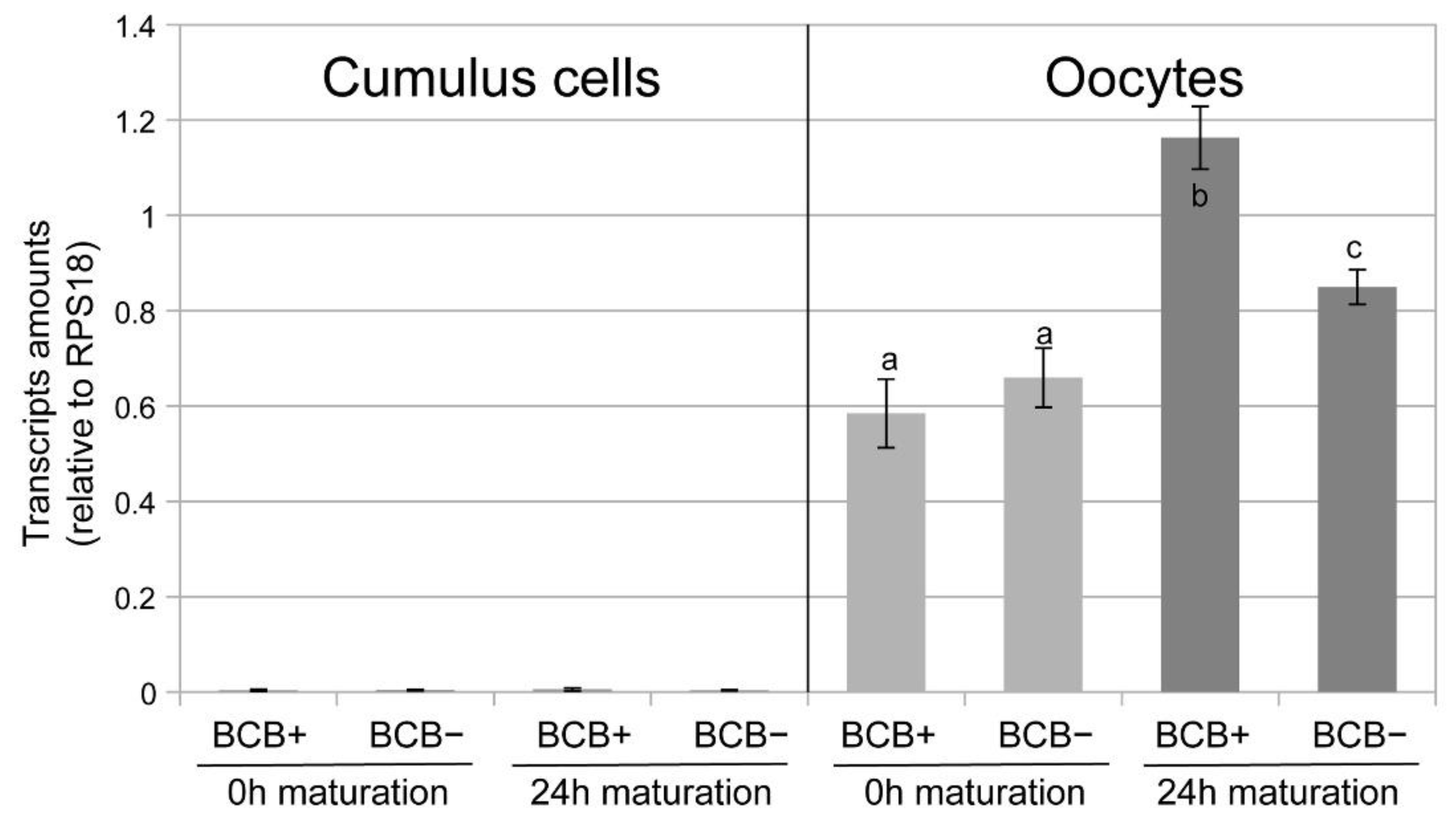
2. Results
3. Discussion
4. Materials and Methods
4.1. Oocyte Recovery, Selection, and In Vitro Maturation
4.2. DNA and RNA Preparation, cDNA Synthesis, and Real-Time Quantitative PCR
4.3. Immunofluorescence Staining and Confocal Microscopy
4.4. Expession of a HES1/GFP Fusion Protein and Fluorescence Recovery after Photo Bleaching (FRAP)
4.5. Statistic
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eppig, J.J. Oocyte-Somatic Cell Interactions During Oocyte Growth and Maturation in the Mammal. In Oogenesis; Browder, L.W., Ed.; Springer: Bosten, MA, USA, 1985. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Burns, K.H.; Viveiros, M.M.; Eppig, J.J. Intercellular communication in the mammalian ovary: Oocytes carry the conversation. Science 2002, 296, 2178–2180. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Reproduction: Oocytes call, granulosa cells connect. Curr. Biol. 2018, 28, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Vanorny, D.A.; Prasasya, R.D.; Chalpe, A.J.; Kilen, S.M.; Mayo, K.E. Notch Signaling Regulates Ovarian Follicle Formation and Coordinates Follicular Growth. Mol. Endocrinol. 2014, 28, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Vanorny, D.A.; Mayo, K.E. The role of Notch signaling in the mammalian ovary. Reproduction 2017, 153, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Trombly, D.J.; Woodruff, T.K.; Mayo, K.E. Suppression of Notch Signaling in the Neonatal Mouse Ovary Decreases Primordial Follicle Formation. Endocrinology 2009, 150, 1014–1024. [Google Scholar] [CrossRef]
- Chen, C.L.; Fu, X.F.; Wang, L.Q.; Wang, J.J.; Ma, H.G.; Cheng, S.F.; Hou, Z.M.; Ma, J.M.; Quan, G.B.; Shen, W.; et al. Primordial follicle assembly was regulated by notch signaling pathway in mice. Mol. Biol. Rep. 2014, 41, 1891–1899. [Google Scholar] [CrossRef]
- Johnson, J.; Espinoza, T.; McGaughey, R.W.; Rawls, A.; Wilson-Rawls, J. Notch pathway genes are expressed in mammalian ovarian follicles. Mech. Dev. 2001, 109, 355–361. [Google Scholar] [CrossRef]
- Zhang, C.P.; Yang, J.L.; Zhang, J.; Li, L.; Huang, L.; Ji, S.Y.; Hu, Z.Y.; Gao, F.; Liu, Y.X. Notch Signaling Is Involved in Ovarian Follicle Development by Regulating Granulosa Cell Proliferation. Endocrinology 2011, 152, 2437–2447. [Google Scholar] [CrossRef]
- Terauchi, K.J.; Shigeta, Y.; Iguchi, T.; Sato, T. Role of Notch signaling in granulosa cell proliferation and polyovular follicle induction during folliculogenesis in mouse ovary. Cell Tissue Res. 2016, 365, 197–208. [Google Scholar] [CrossRef]
- Prasasya, R.D.; Mayo, K.E. Notch signaling regulates differentiation and steroidogenesis in female mouse ovarian granulosa cells. Endocrinology 2018, 159, 184–198. [Google Scholar] [CrossRef]
- Koike, H.; Harada, M.; Kusamoto, A.; Kunitomi, C.; Xu, Z.; Tanaka, T.; Urata, Y.; Nose, E.; Takahashi, N.; Wada-Hiraike, O.; et al. Notch Signaling Induced by Endoplasmic Reticulum Stress Regulates Cumulus-Oocyte Complex Expansion in Polycystic Ovary Syndrome. Biomolecules 2022, 12, 1037. [Google Scholar] [CrossRef]
- Murta, D.; Batista, M.; Silva, E.; Trindade, A.; Mateus, L.; Duarte, A.; Lopes da Costa, L. Differential expression of Notch component and effector genes during ovarian follicle and corpus luteum development during the oestrous cycle. Reprod. Fertil. Dev. 2014, 27, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, N.; Prasasya, R.D.; Mayo, K.E. Activation of Notch Signaling by Oocytes and Jag1 in Mouse Ovarian Granulosa Cells. Endocrinology 2019, 160, 2863–2876. [Google Scholar] [CrossRef] [PubMed]
- Iso, T.; Kedes, L.; Hamamori, Y. HES and HERP families: Multiple effectors of the Notch signaling pathway. J. Cell. Physiol. 2003, 194, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, R.; Ohtsuka, T.; Kobayashi, T. The Hes gene family: Repressors and oscillators that orchestrate embryogenesis. Development 2007, 134, 1243–1251. [Google Scholar] [CrossRef]
- Manosalva, I.; González, A.; Kageyama, R. Hes1 in the somatic cells of the murine ovary is necessary for oocyte survival and maturation. Dev. Biol. 2013, 375, 140–151. [Google Scholar] [CrossRef]
- Alm, H.; Torner, H.; Lohrke, B.; Viergutz, T.; Ghoneim, I.M.; Kanitz, W. Bovine blastocyst Springer development rate in vitro is influenced by selection of oocytes by brillant cresyl blue staining before IVM as indicator for glucose-6-phosphate dehydrogenase activity. Theriogenology 2005, 63, 2194–2205. [Google Scholar] [CrossRef]
- Bhojwani, S.; Alm, H.; Torner, H.; Kanitz, W.; Pöhland, R. Selection of developmentally competent oocytes through brilliant cresyl blue stain enhances blastocyst development rate after bovine nuclear transfer. Theriogenology 2007, 67, 341–345. [Google Scholar] [CrossRef]
- Marello, K.; LaRovere, J.; Sommerville, J. Binding of Xenopus oocyte masking proteins to mRNA sequenzes. Nucleic Acids Res. 1992, 20, 5593–5600. [Google Scholar] [CrossRef]
- Verrotti, A.C.; Strickland, S. Oocyte selection of mutations affecting cytoplasmic polyadenylation of maternal mRNAs. Mol. Reprod. Dev. 1997, 46, 482–488. [Google Scholar] [CrossRef]
- Tomek, W.; Torner, H.; Kanitz, W. Comparative Analysis of Protein Synthesis, Transcription and Cytoplasmic Polyadenylation of mRNA during Maturation of Bovine Oocytes in vitro. Reprod. Domest. Anim. 2002, 37, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Legge, M. Oocyte and zygote zona pellucida permeability to macromolecules. J. Exp. Zool. 1995, 271, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zhang, Y.; Ai, J.; Li, K. Application of Single-Cell RNA Sequencing in Ovarian Development. Biomolecules 2023, 13, 47. [Google Scholar] [CrossRef]
- Pöhland, R.; Tomek, W.; Becker, F.; Kurth, J.; Kanitz, W.; Bhojwani, S. Qualitative and quantitative differences of cytoskeleton proteins in embryos produced in vitro, in vivo, and by somatic nuclear transfer. Mol. Reprod. Dev. 2008, 75, 1109–1119. [Google Scholar] [CrossRef] [PubMed]


| Name | Sequence | Bp |
|---|---|---|
| HES1F | TCTACACCAGCAACAGCGGGA | 100 |
| HES2R | TTCCGCCACGGTCTCCACAT | 100 |
| RPS18 forward | GAGGTGGAACGTGTGATCACCATT | |
| RPS18 reverse | TGTATTTCCCGTCCTTCACGTCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pöhland, R.; Vanselow, J.; Sterza, F.M. Translocation of Oocytic HES1 into Surrounding Cumulus Cells in Bovine: Mechanism of Cellular Interaction during IVM? Int. J. Mol. Sci. 2023, 24, 11932. https://doi.org/10.3390/ijms241511932
Pöhland R, Vanselow J, Sterza FM. Translocation of Oocytic HES1 into Surrounding Cumulus Cells in Bovine: Mechanism of Cellular Interaction during IVM? International Journal of Molecular Sciences. 2023; 24(15):11932. https://doi.org/10.3390/ijms241511932
Chicago/Turabian StylePöhland, Ralf, Jens Vanselow, and Fabiana Melo Sterza. 2023. "Translocation of Oocytic HES1 into Surrounding Cumulus Cells in Bovine: Mechanism of Cellular Interaction during IVM?" International Journal of Molecular Sciences 24, no. 15: 11932. https://doi.org/10.3390/ijms241511932
APA StylePöhland, R., Vanselow, J., & Sterza, F. M. (2023). Translocation of Oocytic HES1 into Surrounding Cumulus Cells in Bovine: Mechanism of Cellular Interaction during IVM? International Journal of Molecular Sciences, 24(15), 11932. https://doi.org/10.3390/ijms241511932






