Hmgb1 Silencing in the Amygdala Inhibits Pain-Related Behaviors in a Rat Model of Neuropathic Pain
Abstract
:1. Introduction
2. Results
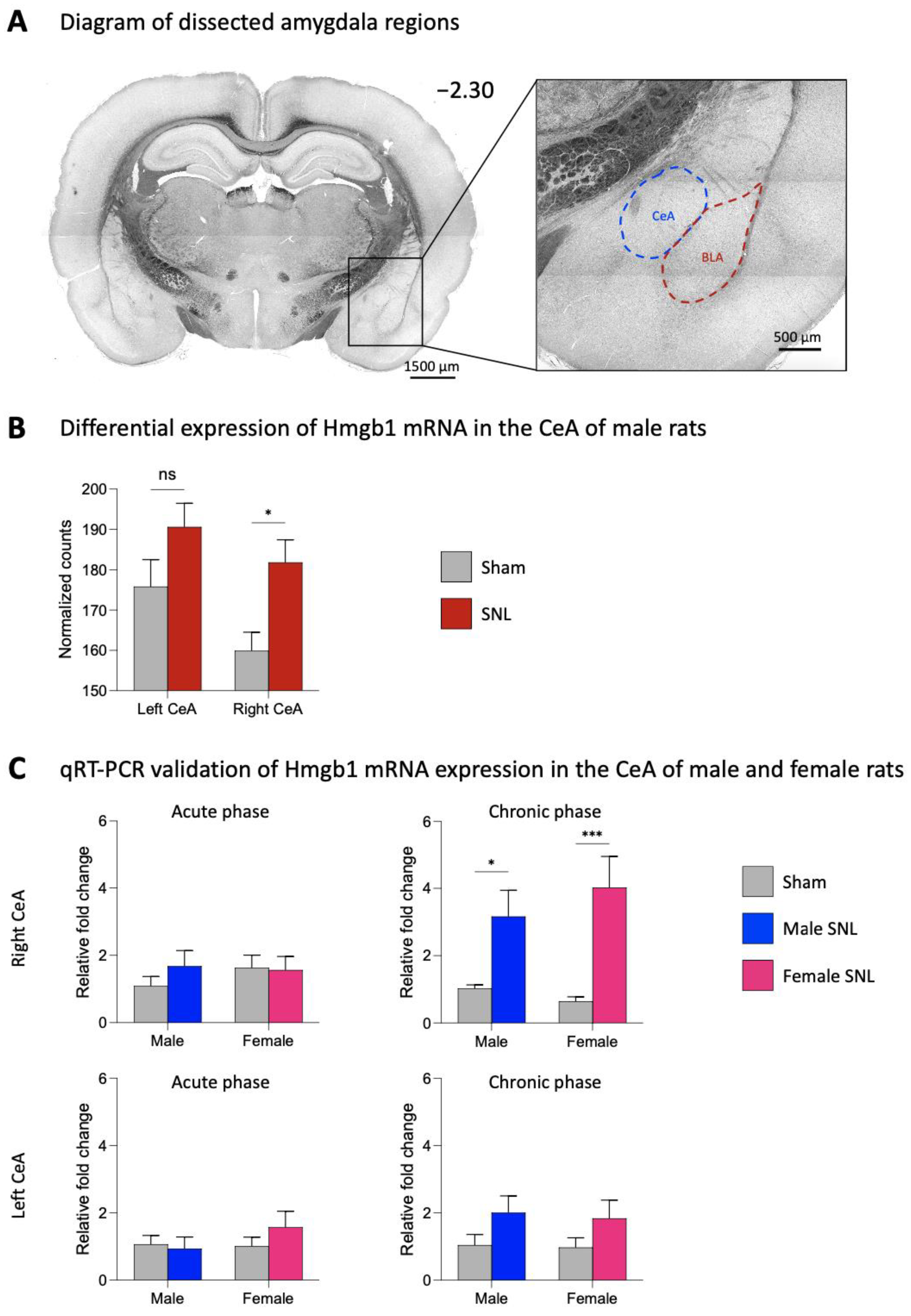
2.1. Expression Levels of Hmgb1 in the Left and Right CeA in Neuropathic Pain
2.2. Effects of Hmgb1 siRNA Pre- and Post-Treatment in the CeA on Hmgb1 Expression
2.3. Effects of Hmgb1 siRNA Pre-Treatment in the Right CeA on Chronic Neuropathic Pain Behaviors
2.4. Effects of Hmgb1 siRNA Post-Treatment in the Right CeA on Chronic Neuropathic Pain Behaviors
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Neuropathic Pain Model
4.3. Experimental Protocol
4.4. Gene Expression Using Bulk RNA Sequencing
4.5. qRT-PCR and Immunohistochemistry
4.6. Behaviors
4.6.1. Mechanosensitivity
4.6.2. Emotional-Affective Responses
4.6.3. Anxiety-Like Behaviors
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of Chronic Pain in the UK: A Systematic Review and Meta-Analysis of Population Studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Eisenberg, E.; O’Brien, T. The Individual and Societal Burden of Chronic Pain in Europe: The Case for Strategic Prioritisation and Action to Improve Knowledge and Availability of Appropriate Care. BMC Public Health 2013, 13, 1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 1001. [Google Scholar] [CrossRef]
- Attal, N. Pharmacological Treatments of Neuropathic Pain: The Latest Recommendations. Rev. Neurol. 2019, 175, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019, 20, S2–S12. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Kuner, T.; Kuner, R. Synaptic Plasticity in Pathological Pain. Trends Neurosci. 2014, 37, 343–355. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Loggia, M.L.; Chonde, D.B.; Akeju, O.; Arabasz, G.; Catana, C.; Edwards, R.R.; Hill, E.; Hsu, S.; Izquierdo-Garcia, D.; Ji, R.R.; et al. Evidence for Brain Glial Activation in Chronic Pain Patients. Brain 2015, 138, 604–615. [Google Scholar] [CrossRef]
- Albrecht, D.S.; Forsberg, A.; Sandström, A.; Bergan, C.; Kadetoff, D.; Protsenko, E.; Lampa, J.; Lee, Y.C.; Höglund, C.O.; Catana, C.; et al. Brain Glial Activation in Fibromyalgia—A Multi-Site Positron Emission Tomography Investigation. Brain. Behav. Immun. 2019, 75, 72–83. [Google Scholar] [CrossRef]
- Veinante, P.; Yalcin, I.; Barrot, M. The Amygdala between Sensation and Affect: A Role in Pain. J. Mol. Psychiatry 2013, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Neugebauer, V. Amygdala Pain Mechanisms. Handb. Exp. Pharmacol. 2015, 227, 261–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neugebauer, V. Amygdala Physiology in Pain. Handb. Behav. Neurosci. 2020, 26, 101–113. [Google Scholar] [CrossRef]
- Neugebauer, V.; Mazzitelli, M.; Cragg, B.; Ji, G.; Navratilova, E.; Porreca, F. Amygdala, Neuropeptides, and Chronic Pain-Related Affective Behaviors. Neuropharmacology 2020, 170, 108052. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.N.; Bobnar, H.J.; Kolber, B.J. Left and Right Hemispheric Lateralization of the Amygdala in Pain. Prog. Neurobiol. 2021, 196, 101891. [Google Scholar] [CrossRef] [PubMed]
- Corder, G.; Ahanonu, B.; Grewe, B.F.; Wang, D.; Schnitzer, M.J.; Scherrer, G. An Amygdalar Neural Ensemble That Encodes the Unpleasantness of Pain. Science 2019, 363, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Rouwette, T.; Vanelderen, P.; Roubos, E.W.; Kozicz, T.; Vissers, K. The Amygdala, a Relay Station for Switching on and off Pain. Eur. J. Pain 2012, 16, 782–792. [Google Scholar] [CrossRef]
- Kato, F.; Sugimura, Y.K.; Takahashi, Y. Pain-Associated Neural Plasticity in the Parabrachial to Central Amygdala Circuit. Adv. Exp. Med. Biol. 2018, 1099, 157–166. [Google Scholar] [CrossRef]
- Neugebauer, V.; Li, W.; Bird, G.C.; Han, J.S. The Amygdala and Persistent Pain. Neuroscientist 2004, 10, 221–234. [Google Scholar] [CrossRef]
- Neugebauer, V.; Presto, P.; Yakhnitsa, V.; Antenucci, N.; Mendoza, B.; Ji, G. Pain-Related Cortico-Limbic Plasticity and Opioid Signaling. Neuropharmacology 2023, 231, 109510. [Google Scholar] [CrossRef]
- Deberry, J.J.; Robbins, M.T.; Ness, T.J. The Amygdala Central Nucleus Is Required for Acute Stress-Induced Bladder Hyperalgesia in a Rat Visceral Pain Model. Brain Res. 2015, 1606, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.J.; Carrasquillo, Y. Projections, Where Art Thou: The State and Future of the Central Amygdala. J. Physiol. 2019, 597, 365–366. [Google Scholar] [CrossRef]
- Liu, J.; Hu, T.; Zhang, M.-Q.; Xu, C.-Y.; Yuan, M.-Y.; Li, R.-X. Differential Efferent Projections of GABAergic Neurons in the Basolateral and Central Nucleus of Amygdala in Mice. Neurosci. Lett. 2021, 745, 135621. [Google Scholar] [CrossRef] [PubMed]
- Weera, M.M.; Shackett, R.S.; Kramer, H.M.; Middleton, J.W.; Gilpin, N.W. Central Amygdala Projections to Lateral Hypothalamus Mediate Avoidance Behavior in Rats. J. Neurosci. 2021, 41, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, S.; Sullivan, R.K.P.; Ianno, D.J.; Sah, P. Functional Properties and Projections of Neurons in the Medial Amygdala. J. Neurosci. 2014, 34, 8699–8715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Moutal, A.; Navratilova, E.; Kopruszinski, C.; Yue, X.; Ikegami, M.; Chow, M.; Kanazawa, I.; Bellampalli, S.S.; Xie, J.; et al. The Prolactin Receptor Long Isoform Regulates Nociceptor Sensitization and Opioid-Induced Hyperalgesia Selectively in Females. Sci. Transl. Med. 2020, 12, 7550. [Google Scholar] [CrossRef]
- Gauriau, C.; Bernard, J.F. Pain Pathways and Parabrachial Circuits in the Rat. Exp. Physiol. 2002, 87, 251–258. [Google Scholar] [CrossRef]
- Wilson, T.D.; Valdivia, S.; Khan, A.; Ahn, H.S.; Adke, A.P.; Gonzalez, S.M.; Sugimura, Y.K.; Carrasquillo, Y. Dual and Opposing Functions of the Central Amygdala in the Modulation of Pain. Cell Rep. 2019, 29, 332–346.e5. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.M.; Neugebauer, V. Cortico-Limbic Pain Mechanisms. Neurosci. Lett. 2019, 702, 15–23. [Google Scholar] [CrossRef]
- Lange, S.S.; Mitchell, D.L.; Vasquez, K.M. High Mobility Group Protein B1 Enhances DNA Repair and Chromatin Modification after DNA Damage. Proc. Natl. Acad. Sci. USA 2008, 105, 10320. [Google Scholar] [CrossRef]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.G.; Yan, Z.; et al. HMGB1 in Health and Disease. Mol. Aspects Med. 2014, 40, 1–116. [Google Scholar] [CrossRef] [Green Version]
- Ugrinova, I.; Pasheva, E. HMGB1 Protein: A Therapeutic Target Inside and Outside the Cell. Adv. Protein Chem. Struct. Biol. 2017, 107, 37–76. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Yang, H.; Harris, H. Extracellular HMGB1 as a Therapeutic Target in Inflammatory Diseases. Expert Opin. Ther. Targets 2018, 22, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Tracey, K.J. HMGB1 Is a Therapeutic Target for Sterile Inflammation and Infection. Annu. Rev. Immunol. 2011, 29, 139–162. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Kwak, M.S.; Lee, B.; Shin, J.M.; Aum, S.; Park, I.H.; Lee, M.G.; Shin, J.S. Secretory Autophagy Machinery and Vesicular Trafficking Are Involved in HMGB1 Secretion. Autophagy 2021, 17, 2345–2362. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Zeh, H.J., III; Lotze, M.T. High-Mobility Group Box 1 [HMGB1] and Cancer. Biochim. Biophys. Acta 2010, 1799, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.; Cao, L.; Khanabdali, R.; Kalionis, B.; Tai, X.; Xia, S. The Emerging Role of HMGB1 in Neuropathic Pain: A Potential Therapeutic Target for Neuroinflammation. J. Immunol. Res. 2016, 2016, 6430423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, T.; Ozaki, M.; Kobayashi, Y.; Kiguchi, N.; Kishioka, S. HMGB1 as a Potential Therapeutic Target for Neuropathic Pain. J. Pharmacol. Sci. 2013, 123, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Agalave, N.M.; Svensson, C.I. Extracellular High-Mobility Group Box 1 Protein (HMGB1) as a Mediator of Persistent Pain. Mol. Med. 2014, 20, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Zhang, L.; Teng, J.; Miao, W. HMGB1 Mediates Microglia Activation via the TLR4/NF-ΚB Pathway in Coriaria Lactone Induced Epilepsy. Mol. Med. Rep. 2018, 17, 5125–5131. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.M.; Zhou, H.; Zhang, F.; Wilson, B.C.; Kam, W.; Hong, J.S. HMGB1 Acts on Microglia Mac1 to Mediate Chronic Neuroinflammation That Drives Progressive Neurodegeneration. J. Neurosci. 2011, 31, 1081–1092. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Xu, C.L.; Yu, J.; Liu, K.; Lin, S.D.; Hu, T.T.; Qu, F.H.; Guo, F.; Lou, G.D.; Nishibori, M.; et al. HMGB1 in the MPFC Governs Comorbid Anxiety in Neuropathic Pain. J. Headache Pain 2022, 23, 102. [Google Scholar] [CrossRef]
- Feldman, P.; Due, M.R.; Ripsch, M.S.; Khanna, R.; White, F.A. The Persistent Release of HMGB1 Contributes to Tactile Hyperalgesia in a Rodent Model of Neuropathic Pain. J. Neuroinflamm. 2012, 9, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allette, Y.M.; Due, M.R.; Wilson, S.M.; Feldman, P.; Ripsch, M.S.; Khanna, R.; White, F.A. Identification of a Functional Interaction of HMGB1 with Receptor for Advanced Glycation End-Products in a Model of Neuropathic Pain. Brain. Behav. Immun. 2014, 42, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasquillo, Y.; Gereau IV, R.W. Hemispheric Lateralization of a Molecular Signal for Pain Modulation in the Amygdala. Mol. Pain 2008, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.; Neugebauer, V. Hemispheric Lateralization of Pain Processing by Amygdala Neurons. J. Neurophysiol. 2009, 102, 2253–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wang, H.; Chavan, S.S.; Andersson, U. High Mobility Group Box Protein 1 (HMGB1): The Prototypical Endogenous Danger Molecule. Mol. Med. 2015, 21, S6–S12. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Vetreno, R.P. Neuroimmune Basis of Alcoholic Brain Damage. Int. Rev. Neurobiol. 2014, 118, 315–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magna, M.; Pisetsky, D.S. The Role of HMGB1 in the Pathogenesis of Inflammatory and Autoimmune Diseases. Mol. Med. 2014, 20, 138–146. [Google Scholar] [CrossRef]
- Klune, J.R.; Dhupar, R.; Cardinal, J.; Billiar, T.R.; Tsung, A. HMGB1: Endogenous Danger Signaling. Mol. Med. 2008, 14, 476–484. [Google Scholar] [CrossRef]
- Yakhnitsa, V.; Ji, G.; Hein, M.; Presto, P.; Griffin, Z.; Ponomareva, O.; Navratilova, E.; Porreca, F.; Neugebauer, V. Kappa Opioid Receptor Blockade in the Amygdala Mitigates Pain Like-Behaviors by Inhibiting Corticotropin Releasing Factor Neurons in a Rat Model of Functional Pain. Front. Pharmacol. 2022, 13, 903978. [Google Scholar] [CrossRef]
- Hein, M.; Ji, G.; Tidwell, D.; D’Souza, P.; Kiritoshi, T.; Yakhnitsa, V.; Navratilova, E.; Porreca, F.; Neugebauer, V. Kappa Opioid Receptor Activation in the Amygdala Disinhibits CRF Neurons to Generate Pain-like Behaviors. Neuropharmacology 2021, 185, 108456. [Google Scholar] [CrossRef]
- Mazzitelli, M.; Yakhnitsa, V.; Neugebauer, B.; Neugebauer, V. Optogenetic Manipulations of CeA-CRF Neurons Modulate Pain- and Anxiety-like Behaviors in Neuropathic Pain and Control Rats. Neuropharmacology 2022, 210, 109031. [Google Scholar] [CrossRef]
- Ji, G.; Neugebauer, V. Kappa Opioid Receptors in the Central Amygdala Modulate Spinal Nociceptive Processing through an Action on Amygdala CRF Neurons. Mol. Brain 2020, 13, 128. [Google Scholar] [CrossRef]
- Presto, P.; Ji, G.; Junell, R.; Griffin, Z.; Neugebauer, V. Fear Extinction-Based Inter-Individual and Sex Differences in Pain-Related Vocalizations and Anxiety-like Behaviors but Not Nocifensive Reflexes. Brain Sci. 2021, 11, 1339. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Gu, K.; Sun, X.; Gu, J.; Li, C.; Wang, G. Lidocaine Alleviates Neuropathic Pain and Neuroinflammation by Inhibiting HMGB1 Expression to Mediate MIP-1α/CCR1 Pathway. J. Neuroimmune Pharmacol. 2021, 16, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Song, T.; Wang, W.; Wang, H.; Zhang, Z. MiR-129-5p Alleviates Neuropathic Pain through Regulating HMGB1 Expression in CCI Rat Models. J. Mol. Neurosci. 2020, 70, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Raver, C.; Uddin, O.; Ji, Y.; Li, Y.; Cramer, N.; Jenne, C.; Morales, M.; Masri, R.; Keller, A. An Amygdalo-Parabrachial Pathway Regulates Pain Perception and Chronic Pain. J. Neurosci. 2020, 40, 3424–3442. [Google Scholar] [CrossRef]
- Baliki, M.N.; Chialvo, D.R.; Geha, P.Y.; Levy, R.M.; Harden, R.N.; Parrish, T.B.; Apkarian, A.V. Chronic Pain and the Emotional Brain: Specific Brain Activity Associated with Spontaneous Fluctuations of Intensity of Chronic Back Pain. J. Neurosci. 2006, 26, 12165–12173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliki, M.N.; Geha, P.Y.; Jabakhanji, R.; Harden, N.; Schnitzer, T.J.; Apkarian, A.V. A Preliminary FMRI Study of Analgesic Treatment in Chronic Back Pain and Knee Osteoarthritis. Mol. Pain 2008, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Mao, C.P.; Yang, H.J.; Yang, Q.X.; Sun, H.H.; Zhang, G.R.; Zhang, Q.J. Altered Amygdala-Prefrontal Connectivity in Chronic Nonspecific Low Back Pain: Resting-State FMRI and Dynamic Causal Modelling Study. Neuroscience 2022, 482, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Geha, P.Y.; Baliki, M.N.; Chialvo, D.R.; Harden, R.N.; Paice, J.A.; Apkarian, A.V. Brain Activity for Spontaneous Pain of Postherpetic Neuralgia and Its Modulation by Lidocaine Patch Therapy. Pain 2007, 128, 88–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, B.; Bentley, D.E.; Elliott, R.; Julyan, P.J.; Boger, E.; Watson, A.; Boyle, Y.; El-Deredy, W.; Jones, A.K.P. Arthritic Pain Is Processed in Brain Areas Concerned with Emotions and Fear. Arthritis Rheum. 2007, 56, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Ohara, S.; Franaszczuk, P.; Zagzoog, N.; Gallagher, M.; Lenz, F.A. Painful Stimuli Evoke Potentials Recorded from the Medial Temporal Lobe in Humans. Neuroscience 2010, 165, 1402–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vachon-Presseau, E.; Roy, M.; Martel, M.O.; Albouy, G.; Chen, J.; Budell, L.; Sullivan, M.J.; Jackson, P.L.; Rainville, P. Neural Processing of Sensory and Emotional-Communicative Information Associated with the Perception of Vicarious Pain. Neuroimage 2012, 63, 54–62. [Google Scholar] [CrossRef]
- Vachon-Presseau, E.; Centeno, M.V.; Ren, W.; Berger, S.E.; Tétreault, P.; Ghantous, M.; Baria, A.; Farmer, M.; Baliki, M.N.; Schnitzer, T.J.; et al. The Emotional Brain as a Predictor and Amplifier of Chronic Pain. J. Dent. Res. 2016, 95, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Simons, L.E.; Moulton, E.A.; Linnman, C.; Carpino, E.; Becerra, L.; Borsook, D. The Human Amygdala and Pain: Evidence from Neuroimaging. Hum. Brain Mapp. 2014, 35, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zhang, D.; Wang, P.; Mao, C.; Miao, Z.; Liu, C.; Xu, C.; Yin, X.; Wu, X. Altered Amygdala Effective Connectivity in Migraine without Aura: Evidence from Resting-state FMRI with Granger Causality Analysis. J. Headache Pain 2021, 22, 25. [Google Scholar] [CrossRef]
- Gandhi, W.; Rosenek, N.R.; Harrison, R.; Salomons, T.V. Functional Connectivity of the Amygdala Is Linked to Individual Differences in Emotional Pain Facilitation. Pain 2020, 161, 300–307. [Google Scholar] [CrossRef]
- Adedoyin, M.O.; Vicini, S.; Neale, J.H. Endogenous N-Acetylaspartylglutamate (NAAG) Inhibits Synaptic Plasticity/Transmission in the Amygdala in a Mouse Inflammatory Pain Model. Mol. Pain 2010, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Sugimura, Y.K.; Takahashi, Y.; Watabe, A.M.; Kato, F. Synaptic and Network Consequences of Monosynaptic Nociceptive Inputs of Parabrachial Nucleus Origin in the Central Amygdala. J. Neurophysiol. 2016, 115, 2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinohara, K.; Watabe, A.M.; Nagase, M.; Okutsu, Y.; Takahashi, Y.; Kurihara, H.; Kato, F. Essential Role of Endogenous Calcitonin Gene-related Peptide in Pain-associated Plasticity in the Central Amygdala. Eur. J. Neurosci. 2017, 46, 2149–2160. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, Y.; Takahashi, Y.; Watabe, A.M.; Kato, F. Predominant Synaptic Potentiation and Activation in the Right Central Amygdala Are Independent of Bilateral Parabrachial Activation in the Hemilateral Trigeminal Inflammatory Pain Model of Rats. Mol. Pain 2018, 14, 1744806918807102. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Takahashi, Y.; Touj, S.; Hotta, H.; Leblond, H.; Kato, F.; Piché, M. Attenuation of Widespread Hypersensitivity to Noxious Mechanical Stimuli by Inhibition of GABAergic Neurons of the Right Amygdala in a Rat Model of Chronic Back Pain. Eur. J. Pain 2022, 26, 911–928. [Google Scholar] [CrossRef]
- Cheng, S.J.; Chen, C.C.; Yang, H.W.; Chang, Y.T.; Bai, S.W.; Chen, C.C.; Yen, C.T.; Min, M.Y. Role of Extracellular Signal-Regulated Kinase in Synaptic Transmission and Plasticity of a Nociceptive Input on Capsular Central Amygdaloid Neurons in Normal and Acid-Induced Muscle Pain Mice. J. Neurosci. 2011, 31, 2258–2270. [Google Scholar] [CrossRef]
- Neugebauer, V.; Li, W. Differential Sensitization of Amygdala Neurons to Afferent Inputs in a Model of Arthritic Pain. J. Neurophysiol. 2003, 89, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Palazzo, E.; Maione, S.; Neugebauer, V. Differential Effects of MGluR7 and MGluR8 Activation on Pain-Related Synaptic Activity in the Amygdala. Neuropharmacology 2011, 61, 1334–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, W.; Kiritoshi, T.; Grégoire, S.; Ji, G.; Guerrini, R.; Calo, G.; Neugebauer, V. Neuropeptide S: A Novel Regulator of Pain-Related Amygdala Plasticity and Behaviors. J. Neurophysiol. 2013, 110, 1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, G.C.; Lash, L.L.; Han, J.S.; Zou, X.; Willis, W.D.; Neugebauer, V. Protein Kinase A-Dependent Enhanced NMDA Receptor Function in Pain-Related Synaptic Plasticity in Rat Amygdala Neurones. J. Physiol. 2005, 564, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S.; Li, W.; Neugebauer, V. Critical Role of Calcitonin Gene-Related Peptide 1 Receptors in the Amygdala in Synaptic Plasticity and Pain Behavior. J. Neurosci. 2005, 25, 10717–10728. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Neugebauer, V. Differential Changes of Group II and Group III MGluR Function in Central Amygdala Neurons in a Model of Arthritic Pain. J. Neurophysiol. 2006, 96, 1803–1815. [Google Scholar] [CrossRef]
- Ji, G.; Neugebauer, V. Differential Effects of CRF1 and CRF2 Receptor Antagonists on Pain-Related Sensitization of Neurons in the Central Nucleus of the Amygdala. J. Neurophysiol. 2007, 97, 3893–3904. [Google Scholar] [CrossRef]
- Fu, Y.; Neugebauer, V. Differential Mechanisms of CRF1 and CRF2 Receptor Functions in the Amygdala in Pain-Related Synaptic Facilitation and Behavior. J. Neurosci. 2008, 28, 3861–3876. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Han, J.; Ishola, T.; Scerbo, M.; Adwanikar, H.; Ramsey, C.; Neugebauer, V. PKA and ERK, but Not PKC, in the Amygdala Contribute to Pain-Related Synaptic Plasticity and Behavior. Mol. Pain 2008, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.; Horváth, C.; Neugebauer, V. NR2B Receptor Blockade Inhibits Pain-Related Sensitization of Amygdala Neurons. Mol. Pain 2009, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Neugebauer, V. Pain-Related Increase of Excitatory Transmission and Decrease of Inhibitory Transmission in the Central Nucleus of the Amygdala Are Mediated by MGluR1. Mol. Pain 2010, 6, 93. [Google Scholar] [CrossRef] [Green Version]
- Han, J.S.; Neugebauer, V. Synaptic Plasticity in the Amygdala in a Visceral Pain Model in Rats. Neurosci. Lett. 2004, 361, 254–257. [Google Scholar] [CrossRef]
- Crock, L.W.; Kolber, B.J.; Morgan, C.D.; Sadler, K.E.; Vogt, S.K.; Bruchas, M.R.; Gereau, R.W. Central Amygdala Metabotropic Glutamate Receptor 5 in the Modulation of Visceral Pain. J. Neurosci. 2012, 32, 14217–14226. [Google Scholar] [CrossRef] [Green Version]
- Allen, H.N.; Chaudhry, S.; Hong, V.M.; Lewter, L.A.; Sinha, G.P.; Carrasquillo, Y.; Taylor, B.K.; Kolber, B.J. A Parabrachial-to-Amygdala Circuit That Determines Hemispheric Lateralization of Somatosensory Processing. Biol. Psychiatry 2023, 93, 370–381. [Google Scholar] [CrossRef]
- Baktay, J.; Neilan, R.M.; Behun, M.; McQuaid, N.; Kolber, B. Modeling Neural Behavior and Pain during Bladder Distention Using an Agent-Based Model of the Central Nucleus of the Amygdala. Spora J. Biomath. 2019, 5, 1. [Google Scholar] [CrossRef]
- Ji, G.; Zhang, W.; Mahimainathan, L.; Narasimhan, M.; Kiritoshi, T.; Fan, X.; Wang, J.; Green, T.A.; Neugebauer, V. 5-HT2C Receptor Knockdown in the Amygdala Inhibits Neuropathic-Pain-Related Plasticity and Behaviors. J. Neurosci. 2017, 37, 1378–1393. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, R.; Takahashi, Y.; Inoue, K.; Kato, F. NMDA Receptor-Independent Synaptic Plasticity in the Central Amygdala in the Rat Model of Neuropathic Pain. Pain 2007, 127, 161–172. [Google Scholar] [CrossRef]
- Gonçalves, L.; Dickenson, A.H. Asymmetric Time-Dependent Activation of Right Central Amygdala Neurones in Rats with Peripheral Neuropathy and Pregabalin Modulation. Eur. J. Neurosci. 2012, 36, 3204–3213. [Google Scholar] [CrossRef]
- Nakao, A.; Takahashi, Y.; Nagase, M.; Ikeda, R.; Kato, F. Role of Capsaicin-Sensitive C-Fiber Afferents in Neuropathic Pain-Induced Synaptic Potentiation in the Nociceptive Amygdala. Mol. Pain 2012, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- Oliva, C.A.; Stehberg, J.; Barra, R.; Mariqueo, T. Neuropathic Pain Induces Interleukin-1β Sensitive Bimodal Glycinergic Activity in the Central Amygdala. Int. J. Mol. Sci. 2022, 23, 7356. [Google Scholar] [CrossRef]
- Torres-Rodriguez, J.M.; Wilson, T.D.; Singh, S.; Chaudhry, S.; Adke, A.P.; Becker, J.J.; Lin, J.L.; Gonzalez, S.M.; Soler-Cedeño, O.; Carrasquillo, Y. The Parabrachial to Central Amygdala Circuit Is a Key Mediator of Injury-Induced Pain Sensitization. bioRxiv 2023. bioRxiv:2023.02.08.527340. [Google Scholar] [CrossRef]
- Kolber, B.J.; Montana, M.C.; Carrasquillo, Y.; Xu, J.; Heinemann, S.F.; Muglia, L.J.; Gereau IV, R.W. Activation of Metabotropic Glutamate Receptor 5 in the Amygdala Modulates Pain-Like Behavior. J. Neurosci. 2010, 30, 8203–8213. [Google Scholar] [CrossRef] [Green Version]
- Palazzo, E.; Marabese, I.; Soukupova, M.; Luongo, L.; Boccella, S.; Giordano, C.; De Novellis, V.; Rossi, F.; Maione, S. Metabotropic Glutamate Receptor Subtype 8 in the Amygdala Modulates Thermal Threshold, Neurotransmitter Release, and Rostral Ventromedial Medulla Cell Activity in Inflammatory Pain. J. Neurosci. 2011, 31, 4687–4697. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, M.; Takahashi, Y.; Sugimura, Y.K.; Tokunaga, R.; Yajima, M.; Kato, F. Active Role of the Central Amygdala in Widespread Mechanical Sensitization in Rats with Facial Inflammatory Pain. Pain 2021, 162, 2273–2286. [Google Scholar] [CrossRef]
- Chen, W.H.; Lien, C.C.; Chen, C.C. Neuronal Basis for Pain-like and Anxiety-like Behaviors in the Central Nucleus of the Amygdala. Pain 2022, 163, E463–E475. [Google Scholar] [CrossRef]
- Ji, G.; Fu, Y.; Ruppert, K.A.; Neugebauer, V. Pain-Related Anxiety-like Behavior Requires CRF1 Receptors in the Amygdala. Mol. Pain 2007, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Thompson, J.; Ji, G.; Ganapathy, V.; Neugebauer, V. Monomethyl Fumarate (MMF) Inhibits Pain Behaviors and Amygdala Activity in a Rat Arthritis Model. Pain 2017, 158, 2376–2385. [Google Scholar] [CrossRef]
- Mazzitelli, M.; Neugebauer, V. Amygdala Group II MGluRs Mediate the Inhibitory Effects of Systemic Group II MGluR Activation on Behavior and Spinal Neurons in a Rat Model of Arthritis Pain. Neuropharmacology 2019, 158, 107706. [Google Scholar] [CrossRef]
- Mazzitelli, M.; Marshall, K.; Pham, A.; Ji, G.; Neugebauer, V. Optogenetic Manipulations of Amygdala Neurons Modulate Spinal Nociceptive Processing and Behavior under Normal Conditions and in an Arthritis Pain Model. Front. Pharmacol. 2021, 12, 25. [Google Scholar] [CrossRef]
- Han, J.S.; Neugebauer, V. MGluR1 and MGluR5 Antagonists in the Amygdala Inhibit Different Components of Audible and Ultrasonic Vocalizations in a Model of Arthritic Pain. Pain 2005, 113, 211–222. [Google Scholar] [CrossRef]
- Ji, G.; Sun, H.; Fu, Y.; Li, Z.; Pais-Vieira, M.; Galhardo, V.; Neugebauer, V. Cognitive Impairment in Pain through Amygdala-Driven Prefrontal Cortical Deactivation. J. Neurosci. 2010, 30, 5451–5464. [Google Scholar] [CrossRef] [Green Version]
- Palazzo, E.; Fu, Y.; Ji, G.; Maione, S.; Neugebauer, V. Group III MGluR7 and MGluR8 in the Amygdala Differentially Modulate Nocifensive and Affective Pain Behaviors. Neuropharmacology 2008, 55, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Grégoire, S.; Neugebauer, V. 5-HT2CR Blockade in the Amygdala Conveys Analgesic Efficacy to SSRIs in a Rat Model of Arthritis Pain. Mol. Pain 2013, 9, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, G.; Ji, G.; Grégoire, S.; Neugebauer, V. Nasal Application of Neuropeptide S Inhibits Arthritis Pain-Related Behaviors through an Action in the Amygdala. Mol. Pain 2014, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.M.; Ji, G.; Neugebauer, V. Small-Conductance Calcium-Activated Potassium (SK) Channels in the Amygdala Mediate Pain-Inhibiting Effects of Clinically Available Riluzole in a Rat Model of Arthritis Pain. Mol. Pain 2015, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Yajima, M.; Sugimoto, M.; Sugimura, Y.K.; Takahashi, Y.; Kato, F. Acetaminophen and Pregabalin Attenuate Central Sensitization in Rodent Models of Nociplastic Widespread Pain. Neuropharmacology 2022, 210, 109029. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, D.; Huang, J.; Cao, J.; Cai, G.; Guo, Y.; Wang, G.; Zhao, S.; Wang, X.; Wu, S. Glutamatergic Neurons in the Amygdala Are Involved in Paclitaxel-Induced Pain and Anxiety. Front. Psychiatry 2022, 13, 869544. [Google Scholar] [CrossRef]
- Presto, P.; Neugebauer, V. Sex Differences in CGRP Regulation and Function in the Amygdala in a Rat Model of Neuropathic Pain. Front. Mol. Neurosci. 2022, 15, 928587. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.H.; Scheel-Krüger, J.; Blackburn-Munro, G. Amygdala GABA-A Receptor Involvement in Mediating Sensory-Discriminative and Affective-Motivational Pain Responses in a Rat Model of Peripheral Nerve Injury. Pain 2007, 127, 17–26. [Google Scholar] [CrossRef]
- Ansah, O.B.; Bourbia, N.; Gonçalves, L.; Almeida, A.; Pertovaara, A. Influence of Amygdaloid Glutamatergic Receptors on Sensory and Emotional Pain-Related Behavior in the Neuropathic Rat. Behav. Brain Res. 2010, 209, 174–178. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Fang, D.; Kong, L.Y.; Jin, Z.R.; Cai, J.; Kang, X.J.; Wan, Y.; Xing, G.G. Sensitization of Neurons in the Central Nucleus of the Amygdala via the Decreased GABAergic Inhibition Contributes to the Development of Neuropathic Pain-Related Anxiety-like Behaviors in Rats. Mol. Brain 2014, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seno, M.D.J.; Assis, D.V.; Gouveia, F.; Antunes, G.F.; Kuroki, M.; Oliveira, C.C.; Santos, L.C.T.; Pagano, R.L.; Martinez, R.C.R. The Critical Role of Amygdala Subnuclei in Nociceptive and Depressive-like Behaviors in Peripheral Neuropathy. Sci. Rep. 2018, 8, 13608. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D. The Parabrachial Nucleus: CGRP Neurons Function as a General Alarm. Trends Neurosci. 2018, 41, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Kruger, L.; Sternini, C.; Brecha, N.C.; Mantyh, P.W. Distribution of Calcitonin Gene-Related Peptide Immunoreactivity in Relation to the Rat Central Somatosensory Projection. J. Comp. Neurol. 1988, 273, 149–162. [Google Scholar] [CrossRef]
- Shimada, S.; Inagaki, S.; Kubota, Y.; Kito, S.; Funaki, H.; Takagi, H. Light and Electron Microscopic Studies of Calcitonin Gene-Related Peptide-like Immunoreactive Terminals in the Central Nucleus of the Amygdala and the Bed Nucleus of the Stria Terminalis of the Rat. Exp. Brain Res. 1989, 77, 217–220. [Google Scholar] [CrossRef]
- D’Hanis, W.; Linke, R.; Yilmazer-Hanke, D.M. Topography of Thalamic and Parabrachial Calcitonin Gene-Related Peptide (CGRP) Immunoreactive Neurons Projecting to Subnuclei of the Amygdala and Extended Amygdala. J. Comp. Neurol. 2007, 505, 268–291. [Google Scholar] [CrossRef]
- Han, J.S.; Adwanikar, H.; Li, Z.; Ji, G.; Neugebauer, V. Facilitation of Synaptic Transmission and Pain Responses by CGRP in the Amygdala of Normal Rats. Mol. Pain 2010, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Lundeberg, T.; Wang, Y.T.; Li, Y.; Yu, L.C. Antinociceptive Effect of Calcitonin Gene-Related Peptide in the Central Nucleus of Amygdala: Activating Opioid Receptors through Amygdala–Periaqueductal Gray Pathway. Neuroscience 2003, 118, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Datta-Chaudhuri, T.; George, S.J.; Haider, B.; Wong, J.; Hepler, T.D.; Andersson, U.; Brines, M.; Tracey, K.J.; Chavan, S.S. High-Frequency Electrical Stimulation Attenuates Neuronal Release of Inflammatory Mediators and Ameliorates Neuropathic Pain. Bioelectron. Med. 2022, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Daston, M.M.; Ratner, N. Expression of P30, a Protein with Adhesive Properties, in Schwann Cells and Neurons of the Developing and Regenerating Peripheral Nerve. J. Cell Biol. 1991, 112, 1229–1239. [Google Scholar] [CrossRef]
- Enokido, Y.; Yoshitake, A.; Ito, H.; Okazawa, H. Age-Dependent Change of HMGB1 and DNA Double-Strand Break Accumulation in Mouse Brain. Biochem. Biophys. Res. Commun. 2008, 376, 128–133. [Google Scholar] [CrossRef]
- Fan, H.; Tang, H.B.; Chen, Z.; Wang, H.Q.; Zhang, L.; Jiang, Y.; Li, T.; Yang, C.F.; Wang, X.Y.; Li, X.; et al. Inhibiting HMGB1-RAGE Axis Prevents pro-Inflammatory Macrophages/Microglia Polarization and Affords Neuroprotection after Spinal Cord Injury. J. Neuroinflamm. 2020, 17, 295. [Google Scholar] [CrossRef]
- Agalave, N.M.; Larsson, M.; Abdelmoaty, S.; Su, J.; Baharpoor, A.; Lundbäck, P.; Palmblad, K.; Andersson, U.; Harris, H.; Svensson, C.I. Spinal HMGB1 Induces TLR4-Mediated Long-Lasting Hypersensitivity and Glial Activation and Regulates Pain-like Behavior in Experimental Arthritis. Pain 2014, 155, 1802–1813. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Y.; Shan, X.; Li, L.; Xia, B.; Wang, H. Anti-Depressive Effectiveness of Baicalin In Vitro and In Vivo. Molecules 2019, 24, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Zhao, Z.; Lu, L.; Liu, J.; Sun, J.; Dong, J. Icariin and Icaritin Ameliorated Hippocampus Neuroinflammation via Mediating HMGB1 Expression in Social Defeat Model in Mice. Int. Immunopharmacol. 2019, 75, 105799. [Google Scholar] [CrossRef]
- Xie, W.; Zhu, T.; Dong, X.; Nan, F.; Meng, X.; Zhou, P.; Sun, G.; Sun, X. HMGB1-Triggered Inflammation Inhibition of Notoginseng Leaf Triterpenes against Cerebral Ischemia and Reperfusion Injury via MAPK and NF-ΚB Signaling Pathways. Biomolecules 2019, 9, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Zhou, G.M.; Wang, L.H.; Zhu, L.; Liu, J.M.; Wang, X.D.; Li, H.T.; Chen, L. Inhibiting High-Mobility Group Box 1 (HMGB1) Attenuates Inflammatory Cytokine Expression and Neurological Deficit in Ischemic Brain Injury Following Cardiac Arrest in Rats. Inflammation 2016, 39, 1594–1602. [Google Scholar] [CrossRef]
- Khatoon, S.; Agarwal, N.B.; Samim, M.; Alam, O. Neuroprotective Effect of Fisetin Through Suppression of IL-1R/TLR Axis and Apoptosis in Pentylenetetrazole-Induced Kindling in Mice. Front. Neurol. 2021, 12, 689069. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Fonken, L.K.; Annis, J.L.; Watkins, L.R.; Maier, S.F. Stress Disinhibits Microglia via Down-Regulation of CD200R: A Mechanism of Neuroinflammatory Priming. Brain. Behav. Immun. 2018, 69, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Antón, M.; Alén, F.; de Heras, R.G.; Serrano, A.; Pavón, F.J.; Leza, J.C.; García-Bueno, B.; de Fonseca, R.F.; Orio, L. Oleoylethanolamide Prevents Neuroimmune HMGB1/TLR4/NF-KB Danger Signaling in Rat Frontal Cortex and Depressive-like Behavior Induced by Ethanol Binge Administration. Addict. Biol. 2017, 22, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Qu, Y.; Huang, J.; Zhu, T.; Zhao, F.; Li, S.; Mu, D. Role of HMGB1 Translocation to Neuronal Nucleus in Rat Model with Septic Brain Injury. Neurosci. Lett. 2017, 645, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, C.; Yang, L.D.; Yu, W.; Tian, D.D.; Gao, M.R.; Wang, W.J.; Li, X.B.; Wu, Y.M.; Wang, M. Paeonol Ameliorates CFA-Induced Inflammatory Pain by Inhibiting HMGB1/TLR4/NF-ΚB P65 Pathway. Metab. Brain Dis. 2021, 36, 273–283. [Google Scholar] [CrossRef]
- Masson, G.S.; Nair, A.R.; Soares, P.P.S.; Michelini, L.C.; Francis, J. Aerobic Training Normalizes Autonomic Dysfunction, HMGB1 Content, Microglia Activation and Inflammation in Hypothalamic Paraventricular Nucleus of SHR. Am. J. Physiol.-Hear. Circ. Physiol. 2015, 309, H1115–H1122. [Google Scholar] [CrossRef] [Green Version]
- Dange, R.B.; Agarwal, D.; Teruyama, R.; Francis, J. Toll-like Receptor 4 Inhibition within the Paraventricular Nucleus Attenuates Blood Pressure and Inflammatory Response in a Genetic Model of Hypertension. J. Neuroinflamm. 2015, 12, 31. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.L.; Yu, X.J.; Zhao, J.Q.; Du, Y.; Xia, W.J.; Su, Q.; Du, M.M.; Yang, Q.; Qi, J.; Li, Y.; et al. Calcitriol Ameliorated Autonomic Dysfunction and Hypertension by Down-Regulating Inflammation and Oxidative Stress in the Paraventricular Nucleus of SHR. Toxicol. Appl. Pharmacol. 2020, 394, 114950. [Google Scholar] [CrossRef]
- Tian, J.; Dai, H.; Deng, Y.; Zhang, J.; Li, Y.; Zhou, J.; Zhao, M.; Zhao, M.; Zhang, C.; Zhang, Y.; et al. The Effect of HMGB1 on Sub-Toxic Chlorpyrifos Exposure-Induced Neuroinflammation in Amygdala of Neonatal Rats. Toxicology 2015, 338, 95–103. [Google Scholar] [CrossRef]
- Lai, S.; Wu, G.; Jiang, Z. Glycyrrhizin Treatment Facilitates Extinction of Conditioned Fear Responses after a Single Prolonged Stress Exposure in Rats. Cell. Physiol. Biochem. 2018, 45, 2529–2539. [Google Scholar] [CrossRef]
- Shibasaki, M.; Sasaki, M.; Miura, M.; Mizukoshi, K.; Ueno, H.; Hashimoto, S.; Tanaka, Y.; Amaya, F. Induction of High Mobility Group Box-1 in Dorsal Root Ganglion Contributes to Pain Hypersensitivity after Peripheral Nerve Injury. Pain 2010, 149, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Morioka, N.; Harano, S.; Nakamura, Y.; Liu, K.; Nishibori, M.; Hisaoka-Nakashima, K.; Nakata, Y. Perineural Expression of High-Mobility Group Box-1 Contributes to Long-Lasting Mechanical Hypersensitivity via Matrix Metalloprotease-9 up-Regulation in Mice with Painful Peripheral Neuropathy. J. Neurochem. 2016, 136, 837–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Morioka, N.; Abe, H.; Zhang, F.F.; Hisaoka-Nakashima, K.; Liu, K.; Nishibori, M.; Nakata, Y. Neuropathic Pain in Rats with a Partial Sciatic Nerve Ligation Is Alleviated by Intravenous Injection of Monoclonal Antibody to High Mobility Group Box-1. PLoS ONE 2013, 8, e73640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, X.; Huang, Y.; Gu, H.F.; Zu, X.Y.; Zou, W.Y.; Song, Z.B.; Guo, Q.L. Effects of Intrathecal Epigallocatechin Gallate, an Inhibitor of Toll-like Receptor 4, on Chronic Neuropathic Pain in Rats. Eur. J. Pharmacol. 2012, 676, 51–56. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Guo, Q.; Xiao, M.; He, C.; Zou, W. Intrathecal Lentivirus-Mediated Transfer of Interleukin-10 Attenuates Chronic Constriction Injury-Induced Neuropathic Pain through Modulation of Spinal High-Mobility Group Box 1 in Rats. Pain Physician 2013, 16, E615–E625. [Google Scholar]
- Rudjito, R.; Agalave, N.M.; Farinotti, A.B.; Lundbäck, P.; Szabo-Pardi, T.A.; Price, T.J.; Harris, H.E.; Burton, M.D.; Svensson, C.I. Sex- and Cell-Dependent Contribution of Peripheral High Mobility Group Box 1 and TLR4 in Arthritis-Induced Pain. Pain 2021, 162, 459–470. [Google Scholar] [CrossRef]
- Chacur, M.; Milligan, E.D.; Gazda, L.S.; Armstrong, C.; Wang, H.; Tracey, K.J.; Maier, S.F.; Watkins, L.R. A New Model of Sciatic Inflammatory Neuritis (SIN): Induction of Unilateral and Bilateral Mechanical Allodynia following Acute Unilateral Peri-Sciatic Immune Activation in Rats. Pain 2001, 94, 231–244. [Google Scholar] [CrossRef]
- Tanaka, J.; Seki, Y.; Ishikura, H.; Tsubota, M.; Sekiguchi, F.; Yamaguchi, K.; Murai, A.; Umemura, T.; Kawabata, A. Recombinant Human Soluble Thrombomodulin Prevents Peripheral HMGB1-Dependent Hyperalgesia in Rats. Br. J. Pharmacol. 2013, 170, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, K.A.; Hansen, M.K.; Pugh, C.R.; Deak, M.M.; Biedenkapp, J.C.; Milligan, E.D.; Johnson, J.D.; Wang, H.; Maier, S.F.; Tracey, K.J.; et al. Further Characterization of High Mobility Group Box 1 (HMGB1) as a Proinflammatory Cytokine: Central Nervous System Effects. Cytokine 2003, 24, 254–265. [Google Scholar] [CrossRef]
- Otoshi, K.I.; Kikuchi, S.I.; Kato, K.; Sekiguchi, M.; Konno, S.I. Anti-HMGB1 Neutralization Antibody Improves Pain-Related Behavior Induced by Application of Autologous Nucleus Pulposus onto Nerve Roots in Rats. Spine 2011, 36, E692–E698. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zeng, Q.; Silverman, H.A.; Gunasekaran, M.; George, S.J.; Devarajan, A.; Addorisio, M.E.; Li, J.; Tsaava, T.; Shah, V.; et al. HMGB1 Released from Nociceptors Mediates Inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2102034118. [Google Scholar] [CrossRef]
- Ren, P.C.; Zhang, Y.; Zhang, X.D.; An, L.J.; Lv, H.G.; He, J.; Gao, C.J.; Sun, X. De High-Mobility Group Box 1 Contributes to Mechanical Allodynia and Spinal Astrocytic Activation in a Mouse Model of Type 2 Diabetes. Brain Res. Bull. 2012, 88, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Tsubota, M.; Ishikura, H.; Sekiguchi, F.; Terada, Y.; Tsujiuchi, T.; Liu, K.; Nishibori, M.; Kawabata, A. Macrophage-Derived HMGB1 as a Pain Mediator in the Early Stage of Acute Pancreatitis in Mice: Targeting RAGE and CXCL12/CXCR4 Axis. J. Neuroimmune Pharmacol. 2017, 12, 693–707. [Google Scholar] [CrossRef]
- Tong, W.; Wang, W.; Huang, J.; Ren, N.; Wu, S.X.; Li, Y.Q. Spinal High-Mobility Group Box 1 Contributes to Mechanical Allodynia in a Rat Model of Bone Cancer Pain. Biochem. Biophys. Res. Commun. 2010, 395, 572–576. [Google Scholar] [CrossRef]
- Tanaka, J.; Yamaguchi, K.; Ishikura, H.; Tsubota, M.; Sekiguchi, F.; Seki, Y.; Tsujiuchi, T.; Murai, A.; Umemura, T.; Kawabata, A. Bladder Pain Relief by HMGB1 Neutralization and Soluble Thrombomodulin in Mice with Cyclophosphamide-Induced Cystitis. Neuropharmacology 2014, 79, 112–118. [Google Scholar] [CrossRef]
- Thakur, V.; Sadanandan, J.; Chattopadhyay, M. High-Mobility Group Box 1 Protein Signaling in Painful Diabetic Neuropathy. Int. J. Mol. Sci. 2020, 21, 881. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zeng, H.; Wang, Q.; Yu, Q.; Wu, J.; Feng, Y.; Deng, P.; Zhang, H. Glycyrrhizin Ameliorates Inflammatory Pain by Inhibiting Microglial Activation-Mediated Inflammatory Response via Blockage of the HMGB1-TLR4-NF-KB Pathway. Exp. Cell Res. 2018, 369, 112–119. [Google Scholar] [CrossRef]
- Nishida, T.; Tsubota, M.; Kawaishi, Y.; Yamanishi, H.; Kamitani, N.; Sekiguchi, F.; Ishikura, H.; Liu, K.; Nishibori, M.; Kawabata, A. Involvement of High Mobility Group Box 1 in the Development and Maintenance of Chemotherapy-Induced Peripheral Neuropathy in Rats. Toxicology 2016, 365, 48–58. [Google Scholar] [CrossRef]
- Liu, C.; Yang, J.; Liu, H.; Xia, T.; Zhang, F. miR-300 Mitigates Cancer-Induced Bone Pain through Targeting HMGB1 in Rat Models. Genes Genom. 2020, 42, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Guo, Y.; Chen, Q.; Xiong, Q.; Min, S. MicroRNA-193a Downregulates HMGB1 to Alleviate Diabetic Neuropathic Pain in a Mouse Model. Neuroimmunomodulation 2019, 26, 250–257. [Google Scholar] [CrossRef]
- Zhan, L.Y.; Lei, S.Q.; Zhang, B.H.; Li, W.L.; Wang, H.X.; Zhao, B.; Cui, S.S.; Ding, H.; Huang, Q.M. Overexpression of MiR-381 Relieves Neuropathic Pain Development via Targeting HMGB1 and CXCR4. Biomed. Pharmacother. 2018, 107, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Fan, X.; Bu, H.; Ma, L.; Kong, C.; Huang, C.; Xu, Y. Downregulation of LncRNA FIRRE Relieved the Neuropathic Pain of Female Mice by Suppressing HMGB1 Expression. Mol. Cell. Biochem. 2021, 476, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mou, J.; Cao, L.; Zhen, S.; Huang, H.; Bao, H. MicroRNA-142-3p Relieves Neuropathic Pain by Targeting High Mobility Group Box 1. Int. J. Mol. Med. 2018, 41, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hisaoka-Nakashima, K.; Tomimura, Y.; Yoshii, T.; Ohata, K.; Takada, N.; Zhang, F.F.; Nakamura, Y.; Liu, K.; Wake, H.; Nishibori, M.; et al. High-Mobility Group Box 1-Mediated Microglial Activation Induces Anxiodepressive-like Behaviors in Mice with Neuropathic Pain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, M.; Raucci, A.; Muñoz, L.M.; Livoti, E.; Celona, B.; Venereau, E.; Apuzzo, T.; De Marchis, F.; Pedotti, M.; Bachi, A.; et al. HMGB1 Promotes Recruitment of Inflammatory Cells to Damaged Tissues by Forming a Complex with CXCL12 and Signaling via CXCR4. J. Exp. Med. 2012, 209, 551–563. [Google Scholar] [CrossRef]
- Tsung, A.; Klune, J.R.; Zhang, X.; Jeyabalan, G.; Cao, Z.; Peng, X.; Stolz, D.B.; Geller, D.A.; Rosengart, M.R.; Billiar, T.R. HMGB1 Release Induced by Liver Ischemia Involves Toll-like Receptor 4–Dependent Reactive Oxygen Species Production and Calcium-Mediated Signaling. J. Exp. Med. 2007, 204, 2913–2923. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of Chromatin Protein HMGB1 by Necrotic Cells Triggers Inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a Late Mediator of Endotoxin Lethality in Mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef]
- Müller, S.; Ronfani, L.; Bianchi, M.E. Regulated Expression and Subcellular Localization of HMGB1, a Chromatin Protein with a Cytokine Function. J. Intern. Med. 2004, 255, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Gardella, S.; Andrei, C.; Ferrera, D.; Lotti, L.V.; Torrisi, M.R.; Bianchi, M.E.; Rubartelli, A. The Nuclear Protein HMGB1 Is Secreted by Monocytes via a Non-Classical, Vesicle-Mediated Secretory Pathway. EMBO Rep. 2002, 3, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; Manfredi, A.A.; et al. Toll-like Receptor 4 and High-Mobility Group Box-1 Are Involved in Ictogenesis and Can Be Targeted to Reduce Seizures. Nat. Med. 2010, 16, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Karatas, H.; Erdener, S.E.; Gursoy-Ozdemir, Y.; Lule, S.; Eren-Koçak, E.; Sen, Z.D.; Dalkara, T. Spreading Depression Triggers Headache by Activating Neuronal Panx1 Channels. Science 2013, 339, 621. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, Q.; Zhou, Y.; Gou, X.; Hou, L.; Chen, S.; Zhu, Z.; Xiong, L. Ethyl Pyruvate Attenuates Spinal Cord Ischemic Injury with a Wide Therapeutic Window through Inhibiting High-Mobility Group Box 1 Release in Rabbits. Anesthesiology 2009, 110, 1279–1286. [Google Scholar] [CrossRef] [Green Version]
- Faraco, G.; Fossati, S.; Bianchi, M.E.; Patrone, M.; Pedrazzi, M.; Sparatore, B.; Moroni, F.; Chiarugi, A. High Mobility Group Box 1 Protein Is Released by Neural Cells upon Different Stresses and Worsens Ischemic Neurodegeneration In Vitro and In Vivo. J. Neurochem. 2007, 103, 590–603. [Google Scholar] [CrossRef]
- Meacham, K.; Shepherd, A.; Mohapatra, D.P.; Haroutounian, S. Neuropathic Pain: Central vs. Peripheral Mechanisms. Curr. Pain Headache Rep. 2017, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.S.; Hulsebosch, C.E. Neuronal Hyperexcitability: A Substrate for Central Neuropathic Pain after Spinal Cord Injury. Curr. Pain Headache Rep. 2011, 15, 215–222. [Google Scholar] [CrossRef]
- Kang, J.; Cho, S.S.; Kim, H.Y.; Lee, B.H.; Cho, H.J.; Gwak, Y.S. Regional Hyperexcitability and Chronic Neuropathic Pain Following Spinal Cord Injury. Cell. Mol. Neurobiol. 2020, 40, 861–878. [Google Scholar] [CrossRef]
- Woolf, C.J. Central Sensitization: Implications for the Diagnosis and Treatment of Pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- GRAY, T.S. Amygdaloid CRF Pathways: Role in Autonomic, Neuroendocrine, and Behavioral Responses to Stress. Ann. N. Y. Acad. Sci. 1993, 697, 53–60. [Google Scholar] [CrossRef]
- Andreoli, M.; Marketkar, T.; Dimitrov, E. Contribution of Amygdala CRF Neurons to Chronic Pain. Exp. Neurol. 2017, 298, 1–12. [Google Scholar] [CrossRef]
- Agalave, N.M.; Rudjito, R.; Farinotti, A.B.; Khoonsari, P.E.; Sandor, K.; Nomura, Y.; Szabo-Pardi, T.A.; Urbina, C.M.; Palada, V.; Price, T.J.; et al. Sex-Dependent Role of Microglia in Disulfide High Mobility Group Box 1 Protein-Mediated Mechanical Hypersensitivity. Pain 2021, 162, 446–458. [Google Scholar] [CrossRef]
- Presto, P.; Mazzitelli, M.; Junell, R.; Griffin, Z.; Neugebauer, V. Sex Differences in Pain along the Neuraxis. Neuropharmacology 2022, 210, 109030. [Google Scholar] [CrossRef]
- Ho Kim, S.; Mo Chung, J. An Experimental Model for Peripheral Neuropathy Produced by Segmental Spinal Nerve Ligation in the Rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Thompson, J.M.; Yakhnitsa, V.; Ji, G.; Neugebauer, V. Small Conductance Calcium Activated Potassium (SK) Channel Dependent and Independent Effects of Riluzole on Neuropathic Pain-Related Amygdala Activity and Behaviors in Rats. Neuropharmacology 2018, 138, 219–231. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Kisby, B.R.; Farris, S.P.; McManus, M.M.; Varodayan, F.P.; Roberto, M.; Harris, R.A.; Ponomarev, I. Alcohol Dependence in Rats Is Associated with Global Changes in Gene Expression in the Central Amygdala. Brain Sci. 2021, 11, 1149. [Google Scholar] [CrossRef]
- Wan, G.; Yang, K.; Lim, Q.E.; Zhou, L.; He, B.P.; Wong, H.K.; Too, H.P. Identification and Validation of Reference Genes for Expression Studies in a Rat Model of Neuropathic Pain. Biochem. Biophys. Res. Commun. 2010, 400, 575–580. [Google Scholar] [CrossRef]
- Koltzenburg, M.; Lundberg, L.E.R.; Torebjörk, H.E. Dynamic and Static Components of Mechanical Hyperalgesia in Human Hairy Skin. Pain 1992, 51, 207–219. [Google Scholar] [CrossRef]
- Ochoa, J.L.; Yarnitsky, D. Mechanical Hyperalgesias in Neuropathic Pain Patients: Dynamic and Static Subtypes. Ann. Neurol. 1993, 33, 465–472. [Google Scholar] [CrossRef]
- La, J.H.; Chung, J.M. Peripheral Afferents and Spinal Inhibitory System in Dynamic and Static Mechanical Allodynia. Pain 2017, 158, 2285. [Google Scholar] [CrossRef]
- Ji, G.; Yakhnitsa, V.; Kiritoshi, T.; Presto, P.; Neugebauer, V. Fear Extinction Learning Ability Predicts Neuropathic Pain Behaviors and Amygdala Activity in Male Rats. Mol. Pain 2018, 14, 1744806918804441. [Google Scholar] [CrossRef] [Green Version]
- Prut, L.; Belzung, C. The Open Field as a Paradigm to Measure the Effects of Drugs on Anxiety-like Behaviors: A Review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef] [Green Version]
- Walf, A.A.; Frye, C.A. The Use of the Elevated plus Maze as an Assay of Anxiety-Related Behavior in Rodents. Nat. Protoc. 2007, 2, 322. [Google Scholar] [CrossRef] [Green Version]
- Šidák, Z. Rectangular Confidence Regions for the Means of Multivariate Normal Distributions. J. Am. Stat. Assoc. 2012, 62, 626–633. [Google Scholar] [CrossRef]
- Pomrenze, M.B.; Millan, E.Z.; Hopf, F.W.; Keiflin, R.; Maiya, R.; Blasio, A.; Dadgar, J.; Kharazia, V.; De Guglielmo, G.; Crawford, E.; et al. A Transgenic Rat for Investigating the Anatomy and Function of Corticotrophin Releasing Factor Circuits. Front. Neurosci. 2015, 9, 487. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presto, P.; Ji, G.; Ponomareva, O.; Ponomarev, I.; Neugebauer, V. Hmgb1 Silencing in the Amygdala Inhibits Pain-Related Behaviors in a Rat Model of Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 11944. https://doi.org/10.3390/ijms241511944
Presto P, Ji G, Ponomareva O, Ponomarev I, Neugebauer V. Hmgb1 Silencing in the Amygdala Inhibits Pain-Related Behaviors in a Rat Model of Neuropathic Pain. International Journal of Molecular Sciences. 2023; 24(15):11944. https://doi.org/10.3390/ijms241511944
Chicago/Turabian StylePresto, Peyton, Guangchen Ji, Olga Ponomareva, Igor Ponomarev, and Volker Neugebauer. 2023. "Hmgb1 Silencing in the Amygdala Inhibits Pain-Related Behaviors in a Rat Model of Neuropathic Pain" International Journal of Molecular Sciences 24, no. 15: 11944. https://doi.org/10.3390/ijms241511944
APA StylePresto, P., Ji, G., Ponomareva, O., Ponomarev, I., & Neugebauer, V. (2023). Hmgb1 Silencing in the Amygdala Inhibits Pain-Related Behaviors in a Rat Model of Neuropathic Pain. International Journal of Molecular Sciences, 24(15), 11944. https://doi.org/10.3390/ijms241511944






