Dihydrotanshinone Triggers Porimin-Dependent Oncosis by ROS-Mediated Mitochondrial Dysfunction in Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Results
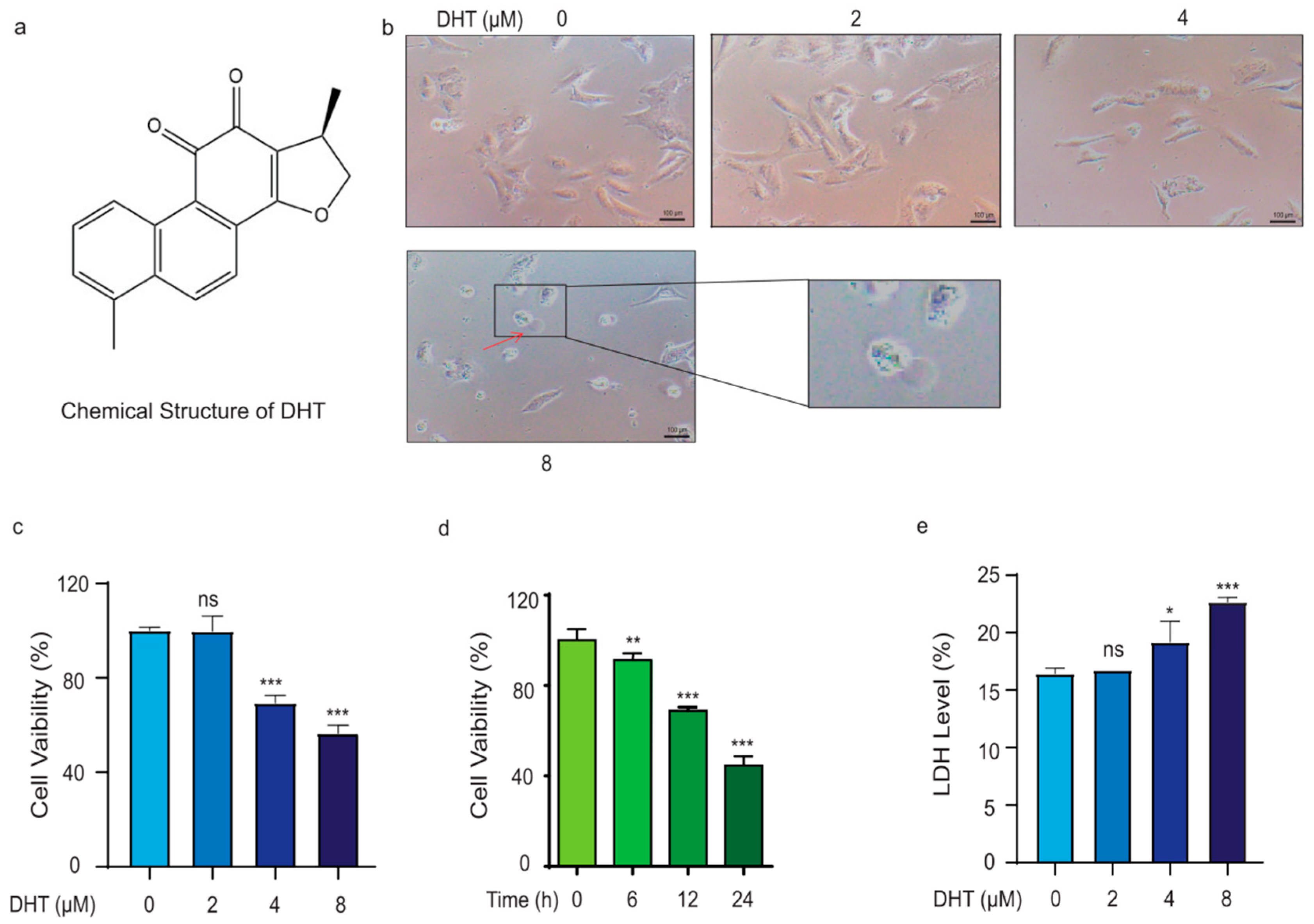
2.1. DHT Triggered A549 Cell Death in a Non-Apoptosis Type
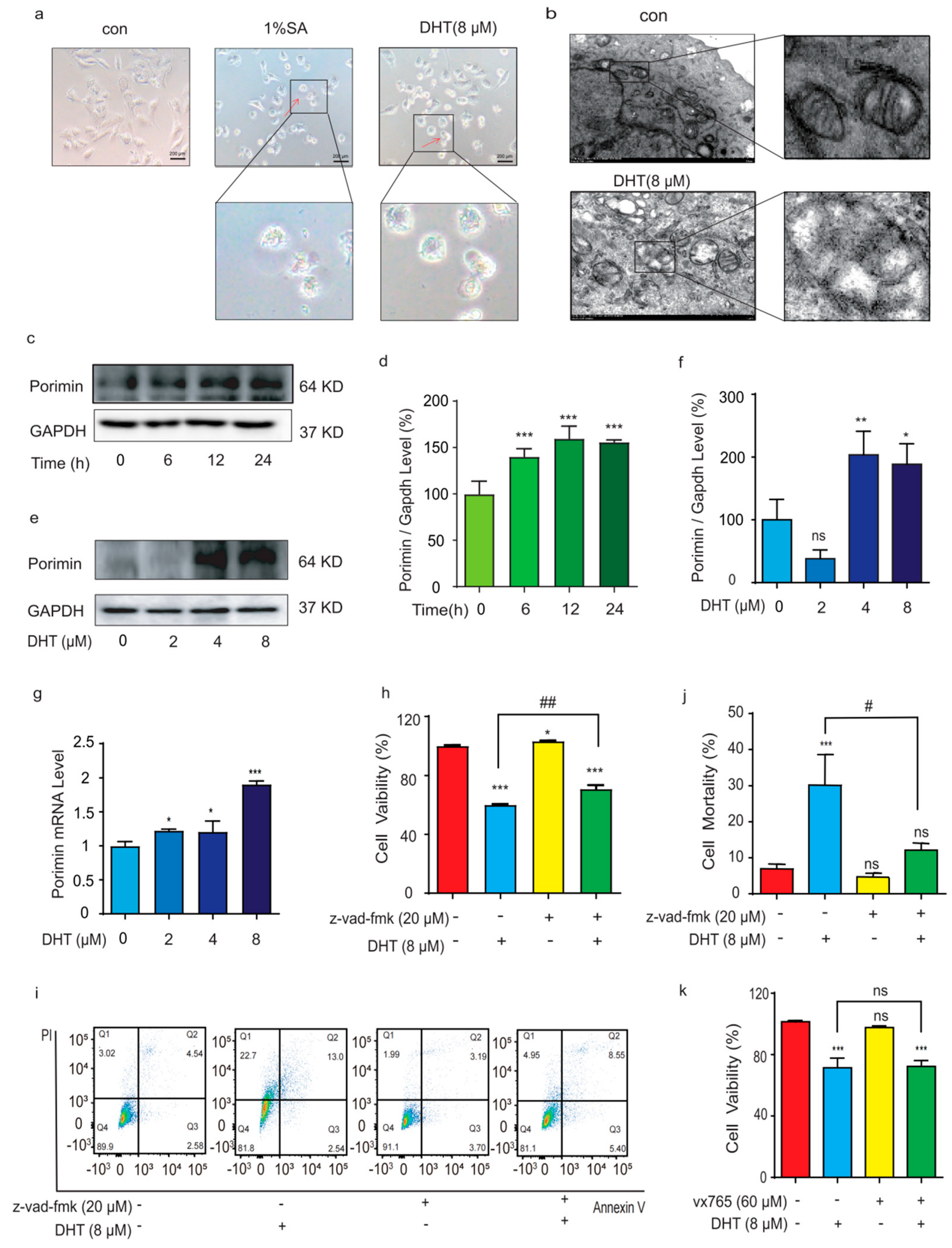
2.2. DHT-Induced, Porimin-Dependent Oncosis in A549 Cells
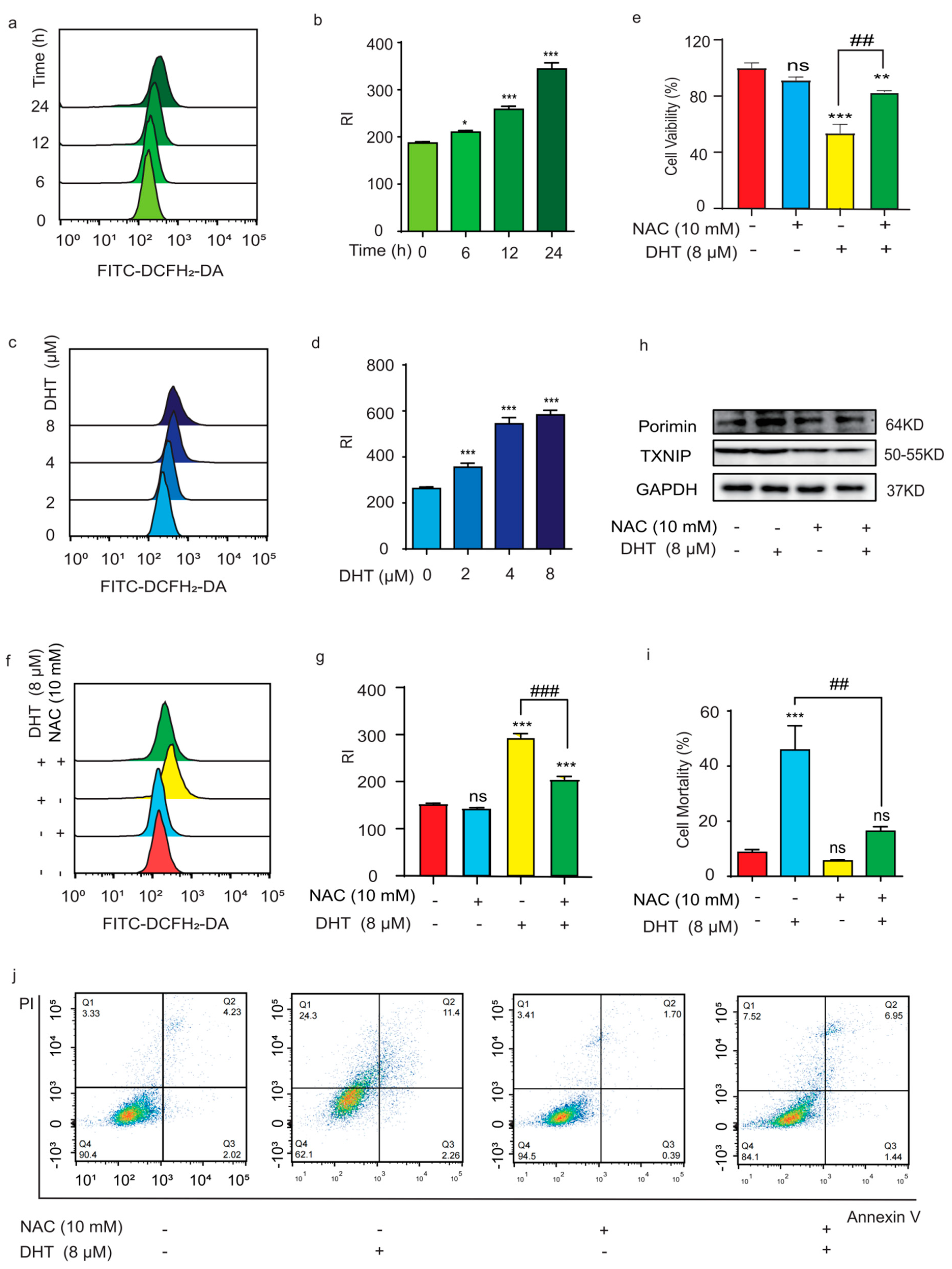
2.3. ROS Regulated DHT-Induced, Porimin-Dependent Oncosis in A549 Cells
2.4. Mitochondria Depletion and Ca2+ Influx Regulated DHT-Induced, Porimin-Dependent Oncosis in A549 Cells
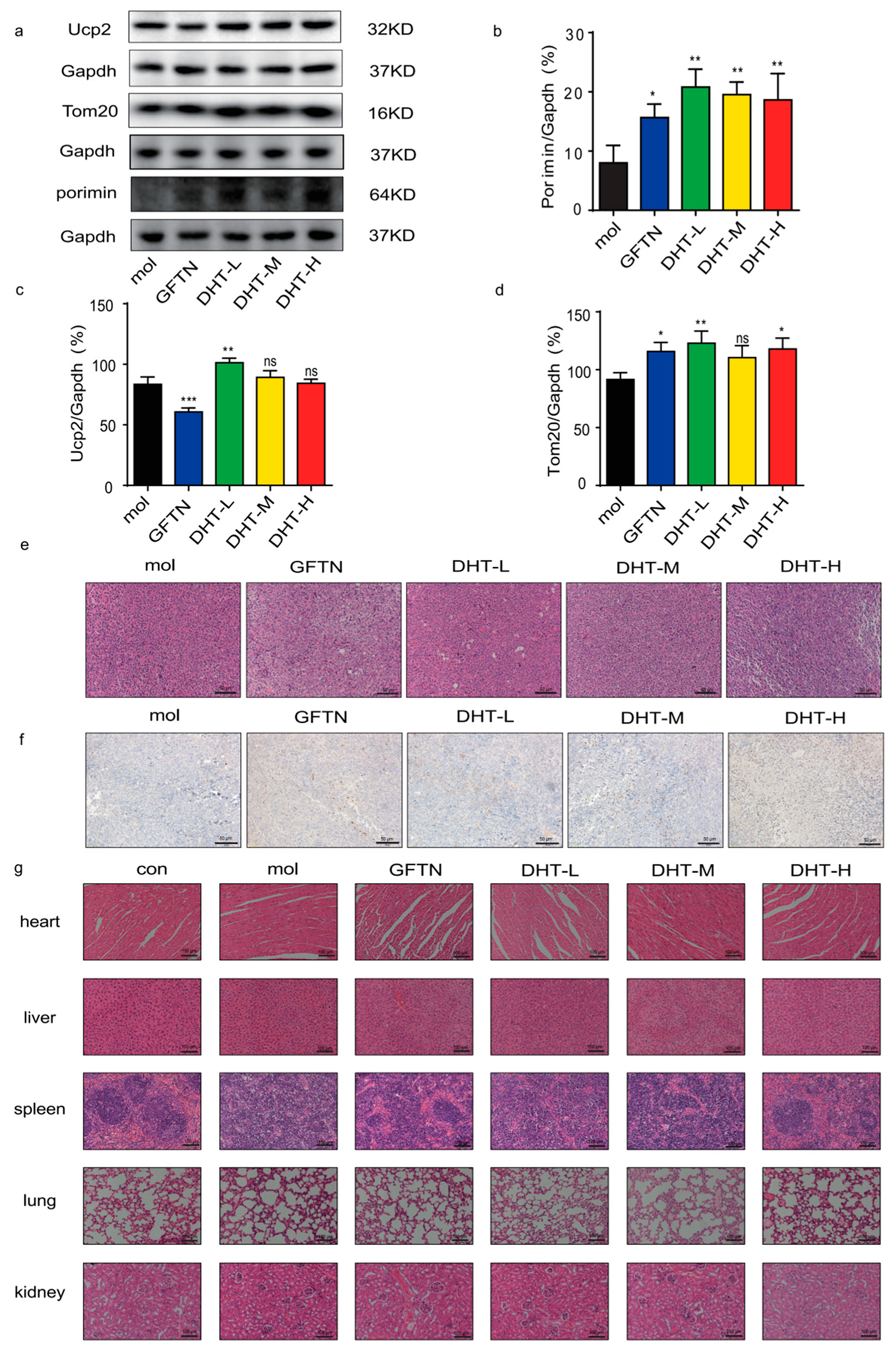
2.5. DHT-Induced Oncosis in LLC Xenograft Mice Model
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cells and Cell Culture
4.3. Cell Viability Assay
4.4. Inhibitors Treatment
4.5. LDH Detection
4.6. Intracellular ATP Detection
4.7. Analysis of Mitochondrial Membrane Potential
4.8. Cell Death Detection
4.9. Flow Cytometry
4.10. Western Blotting Analysis
4.11. qPCR Analysis
- Porimin-F: 5′-GCGGCATCTAATACAACACCAG-3′;
- Porimin-R: 5′-TTGTGGGTTACGGTCATTGTGGATG-3′;
- GAPDH-F: 5′-CATGACCACAGTCCATGCCATCAC-3′;
- GAPDH-R: 5′-TGAGGTCCACCCACCCTGTTGCTGT-3′.
4.12. Transmission Electron Microscopy
4.13. Animal Experiment
4.14. Histopathology Analysis
4.15. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA-Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Nasim, F.; Sabath, B.F.; Eapen, G.A. Lung Cancer. Med. Clin. N. Am. 2019, 103, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.S.; Baldwin, D.R. Recent advances in the management of lung cancer. Clin. Med. 2018, 18 (Suppl. 2), S41–S46. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef]
- Xiao, L.; Lan, X.; Shi, X.; Zhao, K.; Wang, D.; Wang, X.; Li, F.; Huang, H.; Liu, J. Cytoplasmic RAP1 mediates cisplatin resistance of non-small cell lung cancer. Cell Death Dis. 2017, 8, e2803. [Google Scholar] [CrossRef]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar]
- Weerasinghe, P.; Buja, L.M. Oncosis: An important non-apoptotic mode of cell death. Exp. Mol. Pathol. 2012, 93, 302–308. [Google Scholar] [CrossRef]
- Ma, L.S.; Jiang, C.Y.; Cui, M.; Lu, R.; Liu, S.S.; Zheng, B.B.; Li, L.; Li, X. Fluopsin C induces oncosis of human breast adenocarcinoma cells. Acta Pharmacol. Sin. 2013, 34, 1093–1100. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Tan, H.L.; Matsudaira, P.T.; Choo, A. Excess reactive oxygen species production mediates monoclonal antibody-induced human embryonic stem cell death via oncosis. Cell Death Differ. 2017, 24, 546–558. [Google Scholar] [CrossRef]
- Wang, J.; Wong, Y.K.; Liao, F. What has traditional Chinese medicine delivered for modern medicine? Expert Rev. Mol. Med. 2018, 20, e4. [Google Scholar] [CrossRef]
- Jia, Q.; Zhu, R.; Tian, Y.; Chen, B.; Li, R.; Li, L.; Wang, L.; Che, Y.; Zhao, D.; Mo, F.; et al. Salvia miltiorrhiza in diabetes: A review of its pharmacology, phytochemistry, and safety. Phytomed. Int. J. Phytother. Phytopharm. 2019, 58, 152871. [Google Scholar] [CrossRef]
- Petitjean, S.J.L.; Lecocq, M.; Lelong, C.; Denis, R.; Defrère, S.; Mariage, P.A.; Alsteens, D.; Pilette, C. Salvia miltiorrhiza Bunge as a Potential Natural Compound against COVID-19. Cells 2022, 11, 1311. [Google Scholar] [CrossRef]
- Liu, H.; He, Z.; Germič, N.; Ademi, H.; Frangež, Ž.; Felser, A.; Peng, S.; Riether, C.; Djonov, V.; Nuoffer, J.M.; et al. ATG12 deficiency leads to tumor cell oncosis owing to diminished mitochondrial biogenesis and reduced cellular bioenergetics. Cell Death Differ. 2020, 27, 1965–1980. [Google Scholar] [CrossRef]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Rosas-Murrieta, N.H.; Millán-Perez-Peña, L.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, R.; Shen, N.; Jin, Y.; Alina, A.; Yang, S.; Lin, S. Sulforaphane reduces apoptosis and oncosis along with protecting liver injury-induced ischemic reperfusion by activating the Nrf2/ARE pathway. Hepatol. Int. 2015, 9, 321–329. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, F.; Qu, K.; Liu, C.; Zhang, J. TXNIP: A Double-Edged Sword in Disease and Therapeutic Outlook. Oxid. Med. Cell Longev. 2022, 2022, 7805115. [Google Scholar] [CrossRef]
- Yoshihara, E.; Masaki, S.; Matsuo, Y.; Chen, Z.; Tian, H.; Yodoi, J. Thioredoxin/Txnip: Redoxisome, as a redox switch for the pathogenesis of diseases. Front. Immunol. 2014, 4, 514. [Google Scholar] [CrossRef]
- Saxena, G.; Chen, J.; Shalev, A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010, 285, 3997–4005. [Google Scholar] [CrossRef]
- Toda, C.; Diano, S. Mitochondrial UCP2 in the central regulation of metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 757–764. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Harper, M.E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radical Bio. Med. 2011, 51, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.M.; Xu, D.; Fergusson, M.M.; Combs, C.A.; Xu, Y.; Finkel, T. Regulation of cellular oncosis by uncoupling protein 2. J. Biol. Chem. 2002, 277, 27385–27392. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Zhu, Y.; Li, N.; Li, W.; Shang, C.; Song, G.; Li, S.; Cong, J.; Li, T.; et al. Apoptin induces pyroptosis of colorectal cancer cells via the GSDME-dependent pathway. Int. J. Biol. Sci. 2022, 18, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Schexnayder, C.; Broussard, K.; Onuaguluchi, D.; Poché, A.; Ismail, M.; McAtee, L.; Llopis, S.; Keizerweerd, A.; McFerrin, H.; Williams, C. Metformin Inhibits Migration and Invasion by Suppressing ROS Production and COX2 Expression in MDA-MB-231 Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3692. [Google Scholar] [CrossRef]
- Chang, J.F.; Yeh, J.C.; Ho, C.T.; Liu, S.H.; Hsieh, C.Y.; Wang, T.M.; Chang, S.W.; Lee, I.T.; Huang, K.Y.; Wang, J.Y.; et al. Targeting ROS and cPLA2/COX2 Expressions Ameliorated Renal Damage in Obese Mice with Endotoxemia. Int. J. Mol. Sci. 2019, 20, 4393. [Google Scholar] [CrossRef]
- Peters, A.A.; Jamaludin, S.Y.N.; Yapa, K.; Chalmers, S.; Wiegmans, A.P.; Lim, H.F.; Milevskiy, M.J.G.; Azimi, I.; Davis, F.M.; Northwood, K.S.; et al. Oncosis and apoptosis induction by activation of an overexpressed ion channel in breast cancer cells. Oncogene 2017, 36, 6490–6500. [Google Scholar] [CrossRef]
- Trump, B.F.; Berezesky, I.K.; Chang, S.H.; Phelps, P.C. The pathways of cell death: Oncosis, apoptosis, and necrosis. Toxicol. Pathol. 1997, 25, 82–88. [Google Scholar] [CrossRef]
- Van Cruchten, S.; Van Den Broeck, W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat. Histol. Embryol. 2002, 31, 214–223. [Google Scholar] [CrossRef]
- Andersson, R.; Wang, X. Patterns of pancreatic cell death: Apoptosis versus oncosis. Pancreas 1998, 17, 281–288. [Google Scholar] [CrossRef]
- Loh, K.Y.; Wang, Z.; Liao, P. Oncotic Cell Death in Stroke. Rev. Physiol. Bioch. Pharmacol. 2019, 176, 37–64. [Google Scholar]
- Miao, E.A.; Rajan, J.V.; Aderem, A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011, 243, 206–214. [Google Scholar] [CrossRef]
- Ryoke, T.; Gu, Y.; Ikeda, Y.; Martone, M.E.; Oh, S.S.; Jeon, E.S.; Knowlton, K.U.; Ross, J., Jr. Apoptosis and oncosis in the early progression of left ventricular dysfunction in the cardiomyopathic hamster. Basic Res. Cardiol. 2002, 97, 65–75. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, C.; Prasad, K.V.; Freeman, G.J.; Schlossman, S.F. Molecular cloning of Porimin, a novel cell surface receptor mediating oncotic cell death. Proc. Natl. Acad. Sci. USA 2001, 98, 9778–9783. [Google Scholar] [CrossRef]
- Malhotra, J.; Malvezzi, M.; Negri, E.; La Vecchia, C.; Boffetta, P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016, 48, 889–902. [Google Scholar] [CrossRef]
- Sun, C.; Gao, W.; Liu, J.; Cheng, H.; Hao, J. FGL1 regulates acquired resistance to Gefitinib by inhibiting apoptosis in non-small cell lung cancer. Resp. Res. 2020, 21, 210. [Google Scholar] [CrossRef]
- Guan, R.; Chen, Y.; Zeng, L.; Rees, T.W.; Jin, C.; Huang, J.; Chen, Z.S., Jr.; Chao, H. Oncosis-inducing cyclometalated iridium(iii) complexes. Chem. Sci. 2018, 9, 5183–5190. [Google Scholar] [CrossRef]
- Wang, L.; Mai, Z.; Zhao, M.; Wang, B.; Yu, S.; Wang, X.; Chen, T. Aspirin induces oncosis in tumor cells. Apoptosis Int. J. Prog. Cell Death 2019, 24, 758–772. [Google Scholar] [CrossRef]
- Akimoto, M.; Hayashi, J.I.; Nakae, S.; Saito, H.; Takenaga, K. Interleukin-33 enhances programmed oncosis of ST2L-positive low-metastatic cells in the tumour microenvironment of lung cancer. Cell Death Dis. 2016, 7, e2057. [Google Scholar] [CrossRef]
- Sun, C.; Han, B.; Zhai, Y.; Zhao, H.; Li, X.; Qian, J.; Hao, X.; Liu, Q.; Shen, J.; Kai, G. Dihydrotanshinone I inhibits ovarian tumor growth by activating oxidative stress through Keap1-mediated Nrf2 ubiquitination degradation. Free Radic. Biol. Med. 2022, 180, 220–235. [Google Scholar] [CrossRef]
- Jiang, X.L.; Deng, B.; Deng, S.H.; Cai, M.; Ding, W.J.; Tan, Z.B.; Chen, R.X.; Xu, Y.C.; Xu, H.L.; Zhang, S.W.; et al. Dihydrotanshinone I inhibits the growth of hepatoma cells by direct inhibition of Src. Phytomed. Int. J. Phytother. Phytopharm. 2022, 95, 153705. [Google Scholar] [CrossRef]
- Zhao, H.; Liang, Y.; Sun, C.; Zhai, Y.; Li, X.; Jiang, M.; Yang, R.; Li, X.; Shu, Q.; Kai, G.; et al. Dihydrotanshinone I Inhibits the Lung Metastasis of Breast Cancer by Suppressing Neutrophil Extracellular Traps Formation. Int. J. Mol. Sci. 2022, 23, 15180. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xiang, Y.; Wang, R.; Zhang, L.; Zhang, H.; Chen, H.; Luan, X.; Chen, L. Dihydrotanshinone I Inhibits the Proliferation and Growth of Oxaliplatin-Resistant Human HCT116 Colorectal Cancer Cells. Molecules 2022, 27, 7774. [Google Scholar] [CrossRef] [PubMed]
- Miholjcic, T.B.S.; Halse, H.; Bonvalet, M.; Bigorgne, A.; Rouanne, M.; Dercle, L.; Shankar, V.; Marabelle, A. Rationale for LDH-targeted cancer immunotherapy. Eur. J. Cancer 2023, 181, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.C.; Mendes, R.; Silva, F.; Mendes, T.F.; Zelaya-Lazo, A.; Halwachs, K.; Purkal, J.J.; Isidro, I.A.; Félix, A.; Boghaert, E.R.; et al. Application of LDH assay for therapeutic efficacy evaluation of ex vivo tumor models. Sci. Rep. 2021, 11, 18571. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Van Vleet, T.; Schnellmann, R.G. The role of calpain in oncotic cell death. Annu. Rev. Pharmacol. 2004, 44, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cui, L.; Ye, J.; Yang, G.; Lu, G.; Fang, X.; Zeng, Z.; Zhou, J. Dioscin facilitates ROS-induced apoptosis via the p38-MAPK/HSP27-mediated pathways in lung squamous cell carcinoma. Int. J. Biol. Sci. 2020, 16, 2883–2894. [Google Scholar] [CrossRef]
- Yang, Y.; Karakhanova, S.; Hartwig, W.; D’Haese, J.G.; Philippov, P.P.; Werner, J.; Bazhin, A.V. Mitochondria and Mitochondrial ROS in Cancer: Novel Targets for Anticancer Therapy. J. Cell Physiol. 2016, 231, 2570–2581. [Google Scholar] [CrossRef]
- Franek, W.R.; Morrow, D.M.; Zhu, H.; Vancurova, I.; Miskolci, V.; Darley-Usmar, K.; Simms, H.H.; Mantell, L.L. NF-kappaB protects lung epithelium against hyperoxia-induced nonapoptotic cell death-oncosis. Free Radic. Biol. Med. 2004, 37, 1670–1679. [Google Scholar] [CrossRef]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cmls 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- Kurebayashi, N.; Murayama, T.; Ota, R.; Suzuki, J.; Kanemaru, K.; Kobayashi, T.; Ohno, S.; Horie, M.; Iino, M.; Yamashita, F.; et al. Cytosolic Ca2+-dependent Ca2+ release activity primarily determines the ER Ca2+ level in cells expressing the CPVT-linked mutant RYR2. J. Gen. Physiol. 2022, 154, e202112869. [Google Scholar] [CrossRef]
- Cummings, B.S.; McHowat, J.; Schnellmann, R.G. Phospholipase A(2)s in cell injury and death. J. Pharmacol. Exp. Ther. 2000, 294, 793–799. [Google Scholar]
- Chun, J.T.; Santella, L. Roles of the actin-binding proteins in intracellular Ca2+ signalling. Acta Physiol. 2009, 195, 61–70. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Yuan, R.; Pan, J.; Fan, Q.; Sun, K.; Xu, Z.; Gao, X.; Wang, Q.; He, J.; Ye, Y.; et al. Dihydrotanshinone Triggers Porimin-Dependent Oncosis by ROS-Mediated Mitochondrial Dysfunction in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2023, 24, 11953. https://doi.org/10.3390/ijms241511953
Zhang D, Yuan R, Pan J, Fan Q, Sun K, Xu Z, Gao X, Wang Q, He J, Ye Y, et al. Dihydrotanshinone Triggers Porimin-Dependent Oncosis by ROS-Mediated Mitochondrial Dysfunction in Non-Small-Cell Lung Cancer. International Journal of Molecular Sciences. 2023; 24(15):11953. https://doi.org/10.3390/ijms241511953
Chicago/Turabian StyleZhang, Dongjie, Renyikun Yuan, Jiaping Pan, Qiumei Fan, Kaili Sun, Zhipeng Xu, Xiang Gao, Qinqin Wang, Jia He, Yaqing Ye, and et al. 2023. "Dihydrotanshinone Triggers Porimin-Dependent Oncosis by ROS-Mediated Mitochondrial Dysfunction in Non-Small-Cell Lung Cancer" International Journal of Molecular Sciences 24, no. 15: 11953. https://doi.org/10.3390/ijms241511953
APA StyleZhang, D., Yuan, R., Pan, J., Fan, Q., Sun, K., Xu, Z., Gao, X., Wang, Q., He, J., Ye, Y., Mu, Z., Leng, J., & Gao, H. (2023). Dihydrotanshinone Triggers Porimin-Dependent Oncosis by ROS-Mediated Mitochondrial Dysfunction in Non-Small-Cell Lung Cancer. International Journal of Molecular Sciences, 24(15), 11953. https://doi.org/10.3390/ijms241511953





