Impact of Lipid Metabolism on Macrophage Polarization: Implications for Inflammation and Tumor Immunity
Abstract
1. Introduction
2. Macrophage Polarization
3. Lipid Metabolism
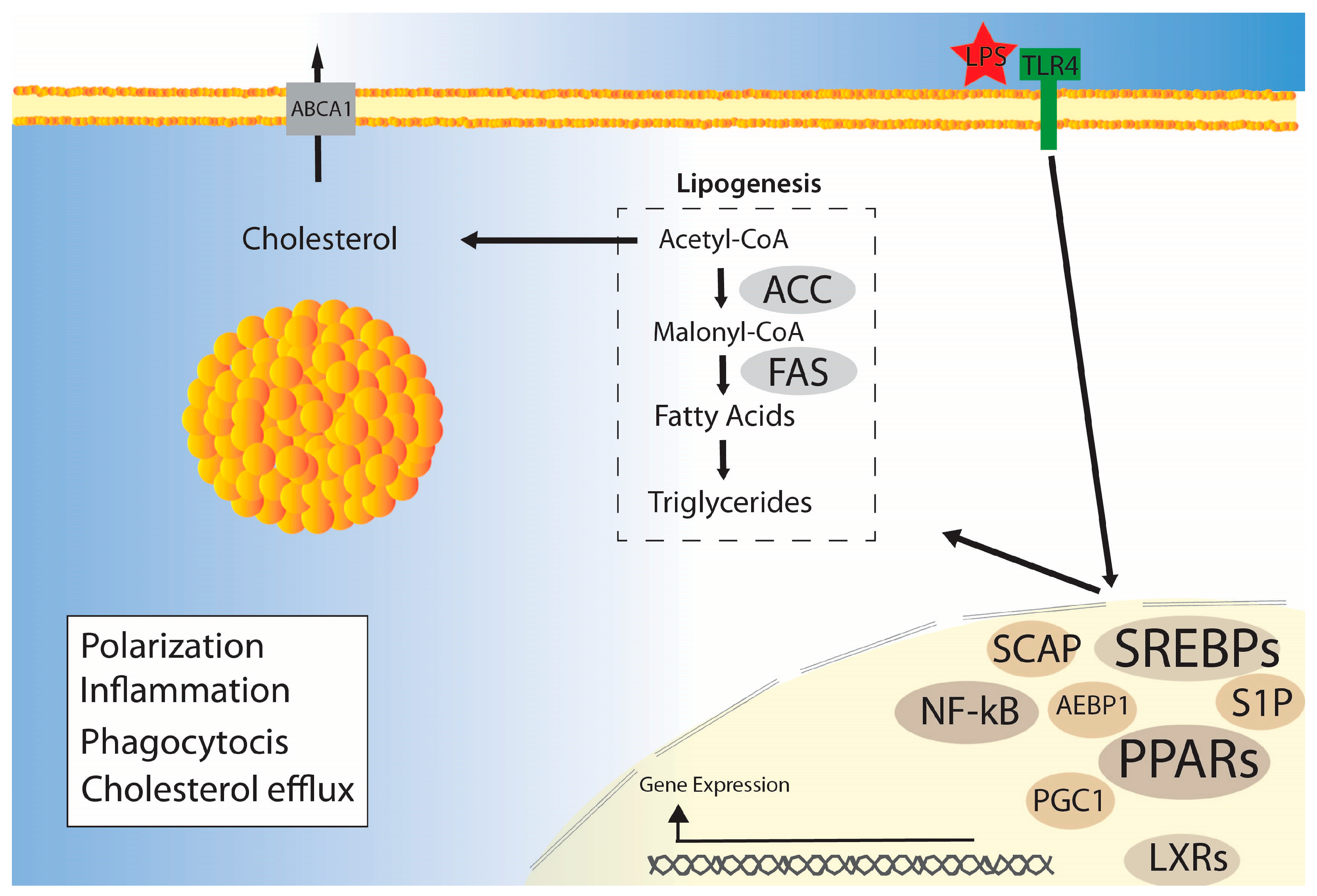
3.1. Lipogenesis
3.2. Cholesterol Metabolism
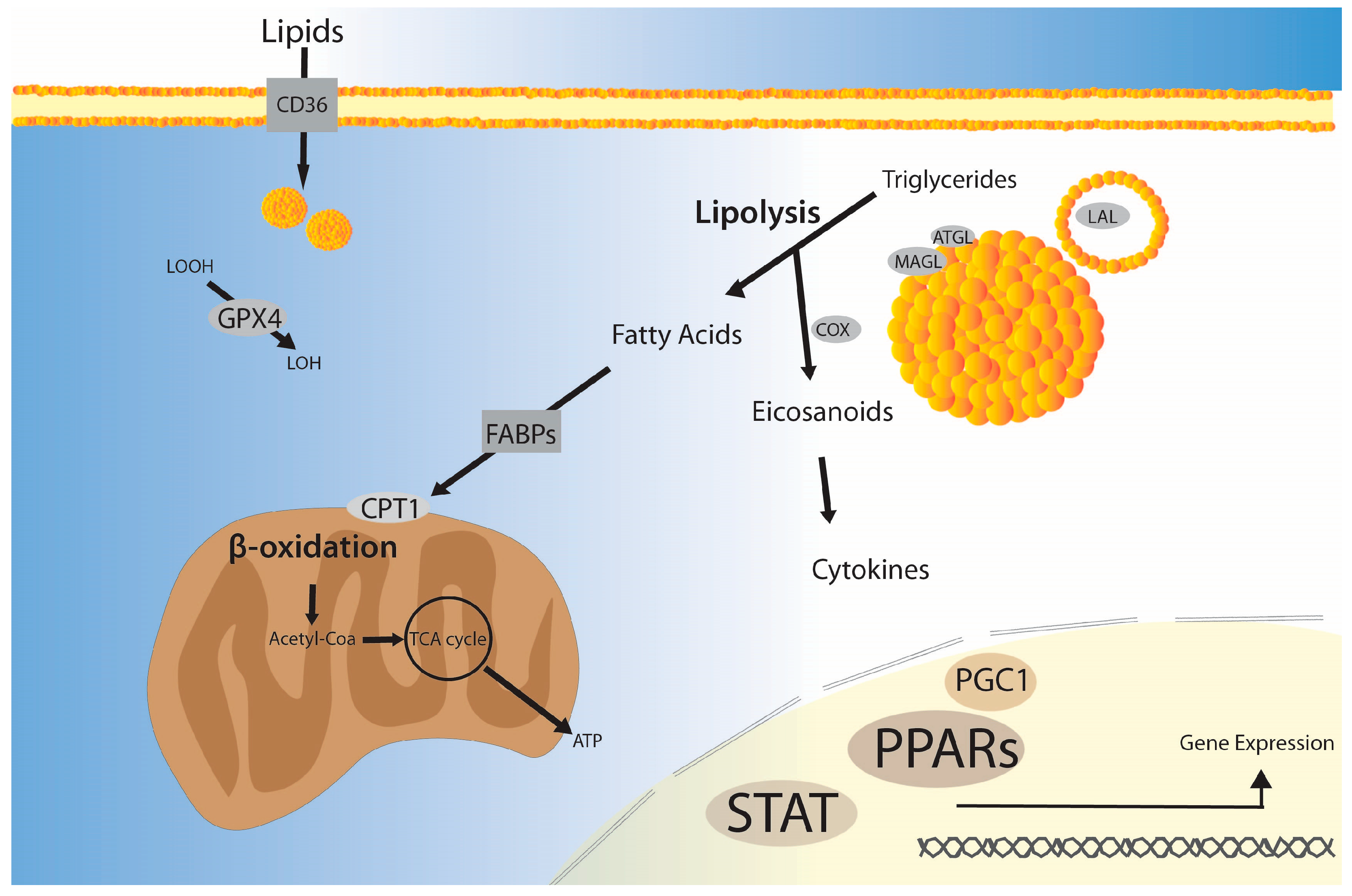
3.3. Fatty Acid β-Oxidation
3.4. Lipolysis
3.5. Lipid Uptake and Transport
4. Macrophages and Cancer
5. Macrophages and Cyclooxygenase Inhibitors
6. Macrophages and Ferroptosis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, X.; Song, H.; Yin, L.; Rizzo, M.G.; Sidhu, R.; Covey, D.F.; Ory, R.S.D.S.; Semenkovich, C.F. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature 2016, 539, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Quintin, J.; Joosten, L.A.B.; van der Meer, J.W.M.; Netea, M.G.; Riksen, N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef]
- Welch, J.S.; Ricote, M.; Akiyama, T.E.; Gonzalez, F.J.; Glass, C.K. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc. Natl. Acad. Sci. USA 2003, 100, 6712–6717. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.; Ro, H.S. LPS-induced suppression of macrophage cholesterol efflux is mediated by adipocyte enhancer-binding protein 1. Int. J. Biochem. Cell Biol. 2009, 41, 1518–1525. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.W.; Kang, M.; Kim, H.Y.; Han, J.; Kang, S.; Lee, J.-R.; Jeong, G.-J.; Kwon, S.P.; Song, S.Y.; Go, S.; et al. M1 Macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano 2018, 12, 8977–8993. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Fu, M.; Xin, H.B. Polarizing macrophages in vitro. Methods Mol. Biol. 2018, 1784, 119–126. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Pireaux, V.; Sauvage, A.; Bihin, B.; Van Steenbrugge, M.; Rousseau, A.; Van Antwerpen, P.; Boudjeltia, K.Z.; Raes, M. Myeloperoxidase-oxidized LDLs enhance an anti-inflammatory M2 and antioxidant phenotype in murine macrophages. Mediat. Inflamm. 2016, 2016, 8249476. [Google Scholar] [CrossRef]
- de la Paz Sánchez-Martínez, M.; Blanco-Favela, F.; Mora-Ruiz, M.D.; Chávez-Rueda, A.K.; Bernabe-García, M.; Chávez-Sánchez, L. IL-17-differentiated macrophages secrete pro-inflammatory cytokines in response to oxidized low-density lipoprotein. Lipids Health Dis. 2017, 16, 196. [Google Scholar] [CrossRef] [PubMed]
- Isa, S.A.; Ruffino, J.S.; Ahluwalia, M.; Thomas, A.W.; Morris, K.; Webb, R. M2 macrophages exhibit higher sensitivity to oxLDL-induced lipotoxicity than other monocyte/macrophage subtypes. Lipids Health Dis. 2011, 10, 229. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Song, Y.; Guo, X.-L.; Miao, R.-Y.; Fu, Y.-Q.; Miao, C.-F.; Zhang, C. Exosomes derived from oxLDL-stimulated macrophages induce neutrophil extracellular traps to drive atherosclerosis. Cell Cycle 2019, 18, 2674–2684. [Google Scholar] [CrossRef]
- Yan, W.; Li, T.; Yin, T.; Hou, Z.; Qu, K.; Wang, N.; Durkan, C.; Dong, L.; Qiu, J.; Gregersen, H.; et al. M2 macrophage-derived exosomes promote the c-KIT phenotype of vascular smooth muscle cells during vascular tissue repair after intravascular stent implantation. Theranostics 2020, 10, 10712–10728. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mai, W.; Li, R.; Deng, S.; Li, L.; Zhou, Y.; Qin, Q.; Zhang, Y.; Zhou, X.; Han, M.; et al. Macrophages evoke autophagy of hepatic stellate cells to promote liver fibrosis in NAFLD mice via the PGE2/EP4 pathway. Cell. Mol. Life Sci. 2022, 79, 303. [Google Scholar] [CrossRef]
- Shan, K.; Feng, N.; Cui, J.; Wang, S.; Qu, H.; Fu, G.; Li, J.; Chen, H.; Wang, X.; Wang, R.; et al. Resolvin D1 and D2 inhibit tumour growth and inflammation via modulating macrophage polarization. J. Cell. Mol. Med. 2020, 24, 8045–8056. [Google Scholar] [CrossRef]
- Zizzo, G.; Cohen, P.L. The PPAR-γ antagonist GW9662 elicits differentiation of M2c-like cells and upregulation of the MerTK/Gas6 axis: A key role for PPAR-γ in human macrophage polarization. J. Inflamm. 2015, 12, 36. [Google Scholar] [CrossRef]
- Lee, J.-H.; Phelan, P.; Shin, M.; Oh, B.-C.; Han, X.; Im, S.-S.; Osborne, T.F. SREBP-1a-stimulated lipid synthesis is required for macrophage phagocytosis downstream of TLR4-directed mTORC1. Proc. Natl. Acad. Sci. USA 2018, 115, E12228–E12234. [Google Scholar] [CrossRef]
- Im, S.-S.; Yousef, L.; Blaschitz, C.; Liu, J.Z.; Edwards, R.A.; Young, S.G.; Raffatellu, M.; Osborne, T.F. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 2011, 13, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Manabe, I.; Hayakawa, S.; Endo, Y.; Oishi, Y. Caspase-11 contributes to site-1 protease cleavage and SREBP1 activation in the inflammatory response of macrophages. Front. Immunol. 2023, 14, 1009973. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Liu, H.M.; Lee, T.Y. Ursodeoxycholic acid regulates hepatic energy homeostasis and white adipose tissue macrophages polarization in leptin-deficiency obese mice. Cells 2019, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Tang, Q.; Qin, W.; Li, Z.; Wang, D.; Zhang, W.; Cao, X.; Lu, Y.; Ge, X.; Sun, H.; et al. Treatment of obesity-related inflammation with a novel synthetic pentacyclic oleanane triterpenoids via modulation of macrophage polarization. EBioMedicine 2019, 45, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Najafi-Shoushtari, S.H.; Kristo, F.; Li, Y.; Shioda, T.; Cohen, D.E.; Gerszten, R.E.; Näär, A.M. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 2010, 328, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Kusnadi, A.; Park, S.H.; Yuan, R.; Pannellini, T.; Giannopoulou, E.; Oliver, D.; Lu, T.; Park-Min, K.-H.; Ivashkiv, L.B. The cytokine TNF promotes transcription factor SREBP activity and binding to inflammatory genes to activate macrophages and limit tissue repair. Immunity 2019, 51, 241–257.e9. [Google Scholar] [CrossRef]
- Ahangari, F.; Price, N.L.; Malik, S.; Chioccioli, M.; Bärnthaler, T.; Adams, T.S.; Kim, J.; Pradeep, S.P.; Ding, S.; Cosmos, C.; et al. microRNA-33 deficiency in macrophages enhances autophagy, improves mitochondrial homeostasis, and protects against lung fibrosis. JCI Insight 2023, 8, e158100. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, S.H.; Lee, E.-H.; Cho, J.-Y.; Song, D.-K.; Lee, Y.J.; Kwon, T.K.; Oh, B.-C.; Cho, K.W.; Osborne, T.F.; et al. SCAP deficiency facilitates obesity and insulin resistance through shifting adipose tissue macrophage polarization. J. Adv. Res. 2023, 45, 1–13. [Google Scholar] [CrossRef]
- Ramírez, C.M.; Torrecilla-Parra, M.; Pardo-Marqués, V.; De-Frutos, M.F.; Pérez-García, A.; Tabraue, C.; de la Rosa, J.V.; Martín-Rodriguez, P.; Díaz-Sarmiento, M.; Nuñez, U.; et al. Crosstalk between LXR and caveolin-1 signaling supports cholesterol efflux and anti-inflammatory pathways in macrophages. Front. Endocrinol. 2021, 12, 635923. [Google Scholar] [CrossRef]
- Li, J.; Scherl, A.; Medina, F.; Frank, P.G.; Kitsis, R.N.; Tanowitz, H.B.; Sotgia, F.; Lisanti, M.P. Impaired phagocytosis in caveolin-1 deficient macrophages. Cell Cycle 2005, 4, 1599–1607. [Google Scholar] [CrossRef]
- Feng, K.; Ma, C.; Liu, Y.; Yang, X.; Yang, Z.; Chen, Y.; Xu, T.; Yang, C.; Zhang, S.; Li, Q.; et al. Encapsulation of LXR ligand by D-Nap-GFFY hydrogel enhances anti-tumorigenic actions of LXR and removes LXR-induced lipogenesis. Theranostics 2021, 11, 2634–2654. [Google Scholar] [CrossRef]
- de la Aleja, A.G.; Herrero, C.; Torres-Torresano, M.; de la Rosa, J.V.; Alonso, B.; Capa-Sardón, E.; Muller, I.B.; Jansen, G.; Puig-Kröger, A.; Vega, M.A.; et al. Activation of LXR nuclear receptors impairs the anti-inflammatory gene and functional profile of M-CSF-dependent human monocyte-derived macrophages. Front. Immunol. 2022, 13, 835478. [Google Scholar] [CrossRef]
- de la Aleja, A.G.; Herrero, C.; Torres-Torresano, M.; Schiaffino, M.T.; del Castillo, A.; Alonso, B.; Vega, M.A.; Puig-Kröger, A.; Castrillo, A.; Corbí, L. Inhibition of LXR controls the polarization of human inflammatory macrophages through upregulation of MAFB. Cell. Mol. Life Sci. 2023, 80, 96. [Google Scholar] [CrossRef]
- Chinetti, G.; Lestavel, S.; Bocher, V.; Remaley, A.T.; Neve, B.; Torra, I.P.; Teissier, E.; Minnich, A.; Jaye, M.; Duverger, N.; et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 2001, 7, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Posokhova, E.N.; Khoshchenko, O.M.; Chasovskikh, M.I.; Pivovarova, E.N.; Dushkin, M.I. Lipid synthesis in macrophages during inflammation in vivo: Effect of agonists of peroxisome proliferator activated receptors alpha and gamma and of retinoid X receptors. Biochemistry 2008, 73, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.; Zhang, L.; Ro, H.S. Adipocyte enhancer-binding protein-1 promotes macrophage inflammatory responsiveness by up-regulating NF-kappaB via IkappaBalpha negative regulation. Mol. Biol. Cell 2007, 18, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Liu, J.; Yu, Z.-X.; Yamazaki, T.; Yan, Y.; Kawagishi, H.; Rovira, I.I.; Liu, C.; Wolfgang, M.J.; Mukouyama, Y.-S.; et al. Macrophage fatty acid oxidation inhibits atherosclerosis progression. J. Mol. Cell. Cardiol. 2019, 127, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Chinetti, G.; Lestavel, S.; Fruchart, J.C.; Clavey, V.; Staels, B. Peroxisome proliferator-activated receptor alpha reduces cholesterol esterification in macrophages. Circ. Res. 2003, 92, 212–217. [Google Scholar] [CrossRef]
- Genoula, M.; Franco, J.L.M.; Maio, M.; Dolotowicz, B.; Ferreyra, M.; Milillo, M.A.; Mascarau, R.; Moraña, E.J.; Palmero, D.; Matteo, M.; et al. Fatty acid oxidation of alternatively activated macrophages prevents foam cell formation, but Mycobacterium tuberculosis counteracts this process via HIF-1α activation. PLOS Pathog. 2020, 16, e1008929. [Google Scholar] [CrossRef]
- Vats, D.; Mukundan, L.; Odegaard, J.I.; Zhang, L.; Smith, K.L.; Morel, C.R.; Wagner, R.A.; Greaves, D.R.; Murray, P.J.; Chawla, A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006, 4, 13–24. [Google Scholar] [CrossRef]
- Liu, P.-S.; Chen, Y.-T.; Li, X.; Hsueh, P.-C.; Tzeng, S.-F.; Chen, H.; Shi, P.-Z.; Xie, X.; Parik, S.; Planque, M.; et al. CD40 signal rewires fatty acid and glutamine metabolism for stimulating macrophage anti-tumorigenic functions. Nat. Immunol. 2023, 24, 452–462. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Mao, C.; Sun, M.; Dominah, G.; Chen, L.; Zhuang, Z. Fatty acid oxidation contributes to IL-1β secretion in M2 macrophages and promotes macrophage-mediated tumor cell migration. Mol. Immunol. 2018, 94, 27–35. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Zhao, J.; Meng, F.; Yao, C.; Bao, E.; Sun, N.; Chen, X.; Cheng, W.; Hua, H.; et al. Mitochondrial STAT3 exacerbates LPS-induced sepsis by driving CPT1a-mediated fatty acid oxidation. Theranostics 2022, 12, 976–998. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Hsieh, W.Y.; Minarrieta, L.; Duong, T.N.; Kim, K.K.; Desousa, B.R.; Andreyev, A.Y.; Bowman, C.E.; Caradonna, K.; Dranka, B.P.; et al. Etomoxir inhibits macrophage polarization by disrupting CoA homeostasis. Cell Metab. 2018, 28, 490–503.e7. [Google Scholar] [CrossRef]
- He, F.; Chen, Y.; He, D.; He, S. USP14-mediated deubiquitination of SIRT1 in macrophage promotes fatty acid oxidation amplification and M2 phenotype polarization. Biochem. Biophys. Res. Commun. 2023, 646, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Calle, P.; Muñoz, A.; Sola, A.; Hotter, G. CPT1a gene expression reverses the inflammatory and anti-phagocytic effect of 7-ketocholesterol in RAW264.7 macrophages. Lipids Health Dis. 2019, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Lammers, B.; Chandak, P.G.; Aflaki, E.; Van Puijvelde, G.H.; Radovic, B.; Hildebrand, R.B.; Meurs, I.; Out, R.; Kuiper, J.; Van Berkel, T.J.; et al. Macrophage adipose triglyceride lipase deficiency attenuates atherosclerotic lesion development in low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 67–73. [Google Scholar] [CrossRef] [PubMed]
- van Dierendonck, X.A.M.H.; Vrieling, F.; Smeehuijzen, L.; Deng, L.; Boogaard, J.P.; Croes, C.-A.; Temmerman, L.; Wetzels, S.; Biessen, E.; Kersten, S.; et al. Triglyceride breakdown from lipid droplets regulates the inflammatory response in macrophages. Proc. Natl. Acad. Sci. USA 2022, 119, e2114739119. [Google Scholar] [CrossRef]
- Chandak, P.G.; Radović, B.; Aflaki, E.; Kolb, D.; Buchebner, M.; Fröhlich, E.; Magnes, C.; Sinner, F.; Haemmerle, G.; Zechner, R.; et al. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 2010, 285, 20192–20201. [Google Scholar] [CrossRef]
- Habib, A.; Chokr, D.; Wan, J.; Hegde, P.; Mabire, M.; Siebert, M.; Ribeiro-Parenti, L.; Le Gall, M.; Lettéron, P.; Pilard, N.; et al. Inhibition of monoacylglycerol lipase, an anti-inflammatory and antifibrogenic strategy in the liver. Gut 2019, 68, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.-C.; Everts, B.; Ivanova, Y.; O’Sullivan, D.; Nascimento, M.; Smith, A.M.; Beatty, W.; Love-Gregory, L.; Lam, W.Y.; O’Neill, C.M.; et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014, 15, 846–855. [Google Scholar] [CrossRef]
- Grabner, G.F.; Zimmermann, R.; Schicho, R.; Taschler, U. Monoglyceride lipase as a drug target: At the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol. Ther. 2017, 175, 35–46. [Google Scholar] [CrossRef]
- Xiang, W.; Shi, R.; Kang, X.; Zhang, X.; Chen, P.; Zhang, L.; Hou, A.; Wang, R.; Zhao, Y.; Zhao, K.; et al. Monoacylglycerol lipase regulates cannabinoid receptor 2-dependent macrophage activation and cancer progression. Nat. Commun. 2018, 9, 2574. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Pan, J.; Deng, Z.; Chen, T.; Xia, J.; Liu, Z.; Zou, C.; Qin, B. Monoacylglycerol lipase regulates macrophage polarization and cancer progression in uveal melanoma and pan-cancer. Front. Immunol. 2023, 14, 1161960. [Google Scholar] [CrossRef]
- Clemetson, K.J.; Pfueller, S.L.; Luscher, E.F.; Jenkins, C.S. Isolation of the membrane glycoproteins of human blood platelets by lectin affinity chromatography. Biochim. Biophys. Acta 1977, 464, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Harmon, C.M.; Abumrad, N.A. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: Isolation and ammo-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J. Membr. Biol. 1993, 133, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.E.; Sun, M.; Zhang, R.; Febbraio, M.; Silverstein, R.; Hazen, S.L. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 2006, 203, 2613–2625. [Google Scholar] [CrossRef]
- Fadok, V.A.; Warner, M.L.; Bratton, D.L.; Henson, P.M. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3). J. Immunol. 1998, 161, 6250–6257. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Nagy, L.; Alvarez, J.G.; Thomazy, V.A.; Evans, R.M. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998, 93, 241–252. [Google Scholar] [CrossRef]
- Huh, H.Y.; Pearce, S.F.; Yesner, L.M.; Schindler, J.L.; Silverstein, R.L. Regulated expression of CD36 during monocyte-to-macrophage differentiation: Potential role of CD36 in foam cell formation. Blood 1996, 87, 2020–2028. [Google Scholar] [CrossRef]
- Grajchen, E.; Wouters, E.; van de Haterd, B.; Haidar, M.; Hardonnière, K.; Dierckx, T.; Van Broeckhoven, J.; Erens, C.; Hendrix, S.; Kerdine-Römer, S.; et al. CD36-mediated uptake of myelin debris by macrophages and microglia reduces neuroinflammation. J. Neuroinflammation 2020, 17, 224. [Google Scholar] [CrossRef]
- Rać, M.E.; Safranow, K.; Poncyljusz, W. Molecular basis of human CD36 gene mutations. Mol. Med. 2007, 13, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Fujiwara, H.; Morimoto, M.; Abe, K.; Yoshida, S.; Takahashi, Y.; Fuda, H.; Hui, S.-P.; Akita, H.; Kobayashi, K.; et al. Phenotype-genotype correlation in CD36 deficiency types I and II. Thromb. Haemost. 2000, 84, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakata, T.; Oka, T.; Ogawa, T.; Okamoto, F.; Kusaka, Y.; Sohmiya, K.; Shimamoto, K.; Itakura, K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J. Lipid Res. 2001, 42, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, D.; Xu, X.; He, H.; Zhu, Y.; Lei, T.; Ou, H. Oxidized high-density lipoprotein promotes CD36 palmitoylation and increases lipid uptake in macrophages. J. Biol. Chem. 2022, 298, 102000. [Google Scholar] [CrossRef]
- Song, Z.; Xia, H.; Yang, L.; Wang, S.; Sun, G. Lowering the n-6/n-3 PUFAs ratio inhibits the formation of THP-1 macrophage-derived foam cell. Lipids Health Dis. 2018, 17, 125. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wei, D.; Zhang, Z.; Guo, H.; Li, S.; Zhang, J.; Zhang, P.; Zhang, L.; Zhao, Y. FABP5 controls macrophage alternative activation and allergic asthma by selectively programming long-chain unsaturated fatty acid metabolism. Cell Rep. 2022, 41, 111668. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.M.; Holt, V.V.; Malpass, L.R.; Hines, I.N.; Wheeler, M.D. Fatty acid-binding protein 5 limits the anti-inflammatory response in murine macrophages. Mol. Immunol 2015, 67, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell 2019, 35, 588–602.e10. [Google Scholar] [CrossRef]
- Su, P.; Wang, Q.; Bi, E.; Ma, X.; Liu, L.; Yang, M.; Qian, J.; Yi, Q. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res. 2020, 80, 1438–1450. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Hanna, A.; Lama-Sherpa, T.; Metge, B.; Kammerud, S.C.; Benavides, G.A.; Kumar, A.; Alsheikh, H.A.; Mota, M.; Chen, D.; et al. Hedgehog signaling regulates metabolism and polarization of mammary tumor-associated macrophages. Cancer Res. 2021, 81, 5425–5437. [Google Scholar] [CrossRef]
- Raines, L.N.; Zhao, H.; Wang, Y.; Chen, H.-Y.; Gallart-Ayala, H.; Hsueh, P.-C.; Cao, W.; Koh, Y.; Alamonte-Loya, A.; Liu, P.-S.; et al. PERK is a critical metabolic hub for immunosuppressive function in macrophages. Nat. Immunol. 2022, 23, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Shrestha, R.; Zhu, X.; Geller, A.E.; Wu, S.; Woeste, M.R.; Li, W.; Wang, H.; Yuan, F.; Xu, R.; et al. Inducing trained immunity in pro-metastatic macrophages to control tumor metastasis. Nat. Immunol. 2023, 24, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.B.; Carpenter, E.S.; Steele, N.G.; Donahue, K.L.; Nwosu, Z.C.; Pacheco, A.; Velez-Delgado, A.; Menjivar, R.E.; Lima, F.; The, S.; et al. Apolipoprotein E promotes immune suppression in pancreatic cancer through NF-κB-mediated production of CXCL1. Cancer Res. 2021, 81, 4305–4318. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chikina, M.; Deshpande, R.; Menk, A.V.; Wang, T.; Tabib, T.; Brunazzi, E.A.; Vignali, K.M.; Sun, M.; Stolz, D.B.; et al. Treg cells promote the SREBP1-dependent metabolic fitness of tumor-promoting macrophages via repression of CD8(+) T cell-derived interferon-γ. Immunity 2019, 51, 381–397.e6. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Henning, S.M.; Guan, J.; Grogan, T.; Elashoff, D.; Cohen, P.; Aronson, W.J. Effect of dietary omega-3 fatty acids on castrate-resistant prostate cancer and tumor-associated macrophages. Prostate Cancer Prostatic Dis. 2020, 23, 127–135. [Google Scholar] [CrossRef]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Cyclooxygenase pathways. Acta Biochim. Pol. 2014, 61, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.; Swain, R.A. NSAIDs. Are there any differences? Arch. Fam. Med. 1993, 2, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Voloshyna, I.; Kasselman, L.J.; Carsons, S.E.; Littlefield, M.J.; Gomolin, I.H.; De Leon, J.; Reiss, A.B. COX-2-dependent and independent effects of COX-2 inhibitors and NSAIDs on proatherogenic changes in human monocytes/macrophages. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2017, 65, 694–704. [Google Scholar] [CrossRef]
- Janjic, J.M.; Vasudeva, K.; Saleem, M.; Stevens, A.; Liu, L.; Patel, S.; Pollock, J.A. Low-dose NSAIDs reduce pain via macrophage targeted nanoemulsion delivery to neuroinflammation of the sciatic nerve in rat. J. Neuroimmunol. 2018, 318, 72–79. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastava, R.; Tripathi, C.; Jain, N.K.; Tewari, B.N.; Lone, M.-U.; Baghel, K.S.; Kumar, V.; Misra, S.; Bhadauria, S.; et al. Cyclooxygenase-2 inhibition attenuates hypoxic cancer cells induced m2-polarization of macrophages. Cell. Mol. Biol. 2014, 60, 10–15. [Google Scholar]
- Javeed, A.; Hou, Y.; Duan, K.; Zhang, B.; Shen, H.; Cao, Y.; Zhao, Y. Aspirin significantly decreases the nonopsonic phagocytosis and immunogenicity of macrophages in mice. Inflamm. Res. 2011, 60, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Mahady, S.E.; Margolis, K.L.; Chan, A.; Polekhina, G.; Woods, R.L.; Wolfe, R.; Nelson, M.R.; Lockery, J.E.; Wood, E.M.; Reid, C.; et al. Major GI bleeding in older persons using aspirin: Incidence and risk factors in the ASPREE randomised controlled trial. Gut 2021, 70, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Pennock, N.; Martinson, H.; Guo, Q.; Betts, C.B.; Jindal, S.; Tsujikawa, T.; Coussens, L.M.; Borges, V.F.; Schedin, P. Ibuprofen supports macrophage differentiation, T cell recruitment, and tumor suppression in a model of postpartum breast cancer. J. ImmunoTherapy Cance 2018, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Vingren, J.L.; Boyett, J.C.; Lee, E.C.; Levitt, D.E.; Luk, H.Y.; McDermott, B.P.; Munoz, C.X.; Ganio, M.S.; Armstrong, L.E.; Hill, D.W. A single dose of ibuprofen impacts IL-10 response to 164-km road cycling in the heat. Res. Q. Exerc. Sport 2023, 94, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by lipid peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef]
- Piattini, F.; Matsushita, M.; Muri, J.; Bretscher, P.; Feng, X.; Freigang, S.; Dalli, J.; Schneider, C.; Kopf, M. Differential sensitivity of inflammatory macrophages and alternatively activated macrophages to ferroptosis. Eur. J. Immunol. 2021, 51, 2417–2429. [Google Scholar] [CrossRef]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Shen, Z.; Lu, Y.; Sun, F.; Shi, H. p53 promotes ferroptosis in macrophages treated with Fe3O4 nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 42791–42803. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-G.; Peng, X.-C.; Yu, T.-T.; Xu, H.-Z.; Han, N.; Yang, X.-X.; Li, Q.-R.; Hu, J.; Liu, B.; Yang, Z.-Y.; et al. Dihydroartemisinin remodels macrophage into an M1 phenotype via ferroptosis-mediated DNA damage. Front. Pharmacol. 2022, 13, 949835. [Google Scholar] [CrossRef]
- Yi, C.; Wu, S.; Duan, Q.; Liu, L.; Li, L.; Luo, Y.; Wang, A. Ferroptosis-dependent breast cancer cell-derived exosomes inhibit migration and invasion of breast cancer cells by suppressing M2 macrophage polarization. PeerJ 2023, 11, e15060. [Google Scholar] [CrossRef]
- Dai, E.; Han, L.; Liu, J.; Xie, Y.; Kroemer, G.; Klionsky, D.J.; Zeh, H.J.; Kang, R.; Wang, J.; Tang, D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy 2020, 16, 2069–2083. [Google Scholar] [CrossRef] [PubMed]
- Cheu, J.W.-S.; Lee, D.; Li, Q.; Goh, C.C.; Bao, M.H.-R.; Yuen, V.W.-H.; Zhang, M.S.; Yang, C.; Chan, C.Y.-K.; Tse, A.P.-W.; et al. Ferroptosis suppressor protein 1 inhibition promotes tumor ferroptosis and anti-tumor immune responses in liver cancer. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 133–159. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassiliou, E.; Farias-Pereira, R. Impact of Lipid Metabolism on Macrophage Polarization: Implications for Inflammation and Tumor Immunity. Int. J. Mol. Sci. 2023, 24, 12032. https://doi.org/10.3390/ijms241512032
Vassiliou E, Farias-Pereira R. Impact of Lipid Metabolism on Macrophage Polarization: Implications for Inflammation and Tumor Immunity. International Journal of Molecular Sciences. 2023; 24(15):12032. https://doi.org/10.3390/ijms241512032
Chicago/Turabian StyleVassiliou, Evros, and Renalison Farias-Pereira. 2023. "Impact of Lipid Metabolism on Macrophage Polarization: Implications for Inflammation and Tumor Immunity" International Journal of Molecular Sciences 24, no. 15: 12032. https://doi.org/10.3390/ijms241512032
APA StyleVassiliou, E., & Farias-Pereira, R. (2023). Impact of Lipid Metabolism on Macrophage Polarization: Implications for Inflammation and Tumor Immunity. International Journal of Molecular Sciences, 24(15), 12032. https://doi.org/10.3390/ijms241512032






