Navigating the Blood–Brain Barrier: Challenges and Therapeutic Strategies in Breast Cancer Brain Metastases
Abstract
1. Introduction
Triple Negative Breast Cancer (TNBC)
2. Brain Metastasis and Breast Cancer Subtypes
3. Brain Metastasis: Mechanisms and Pathophysiology
3.1. Dissemination of BC Cells to the Brain
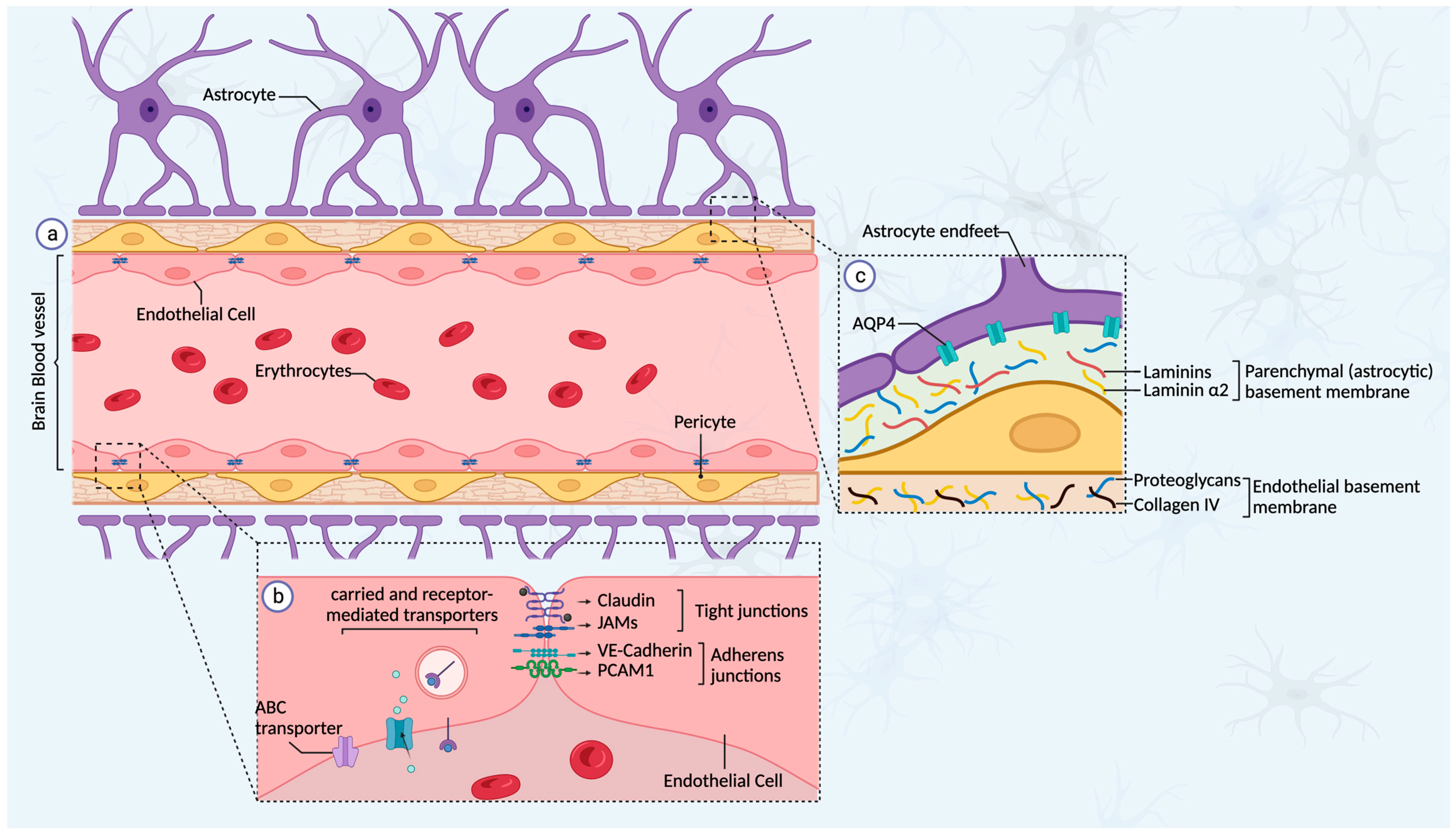
3.2. The Blood–Brain Barrier
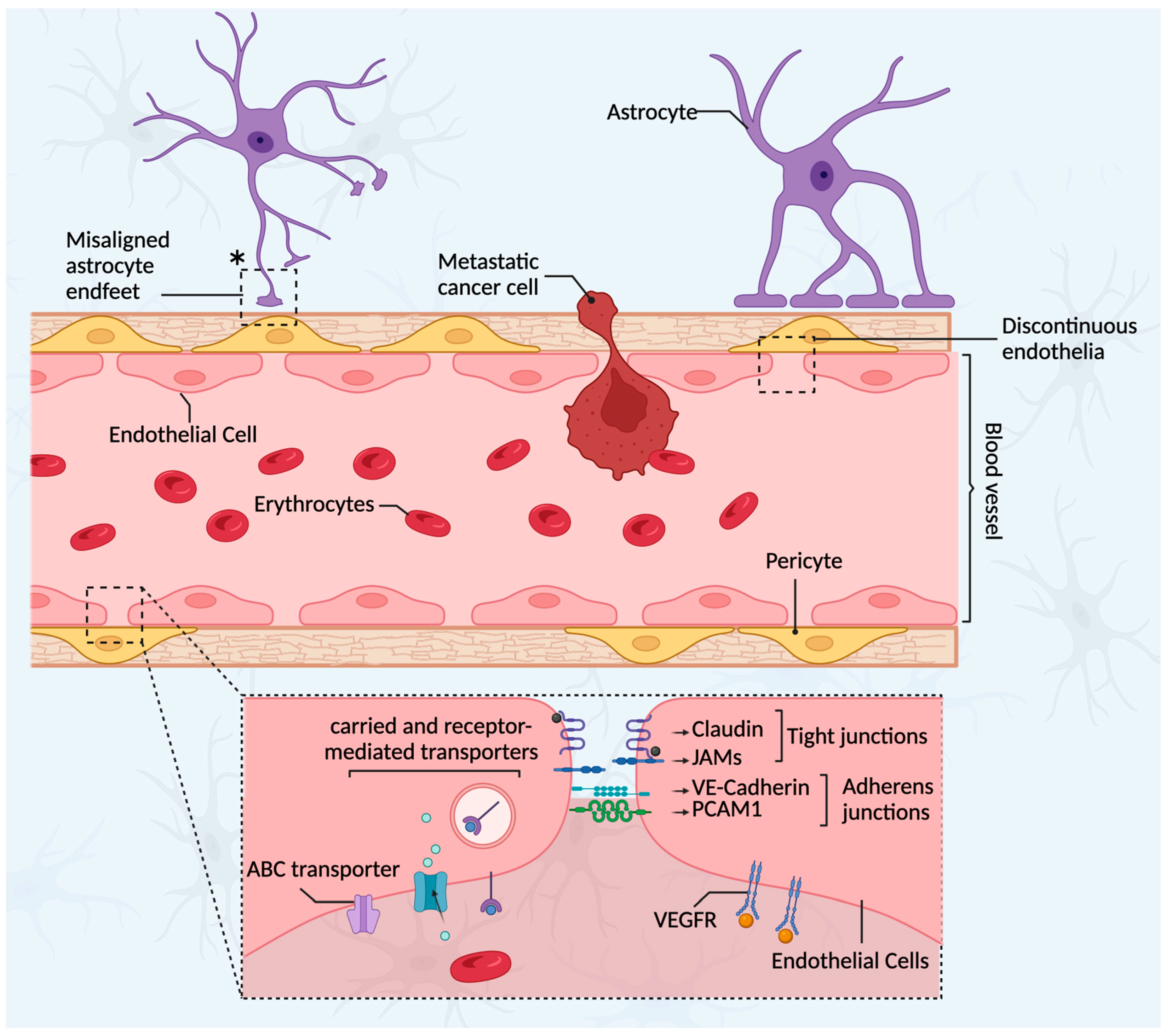
3.3. The Blood–Tumor Barrier (BTB)
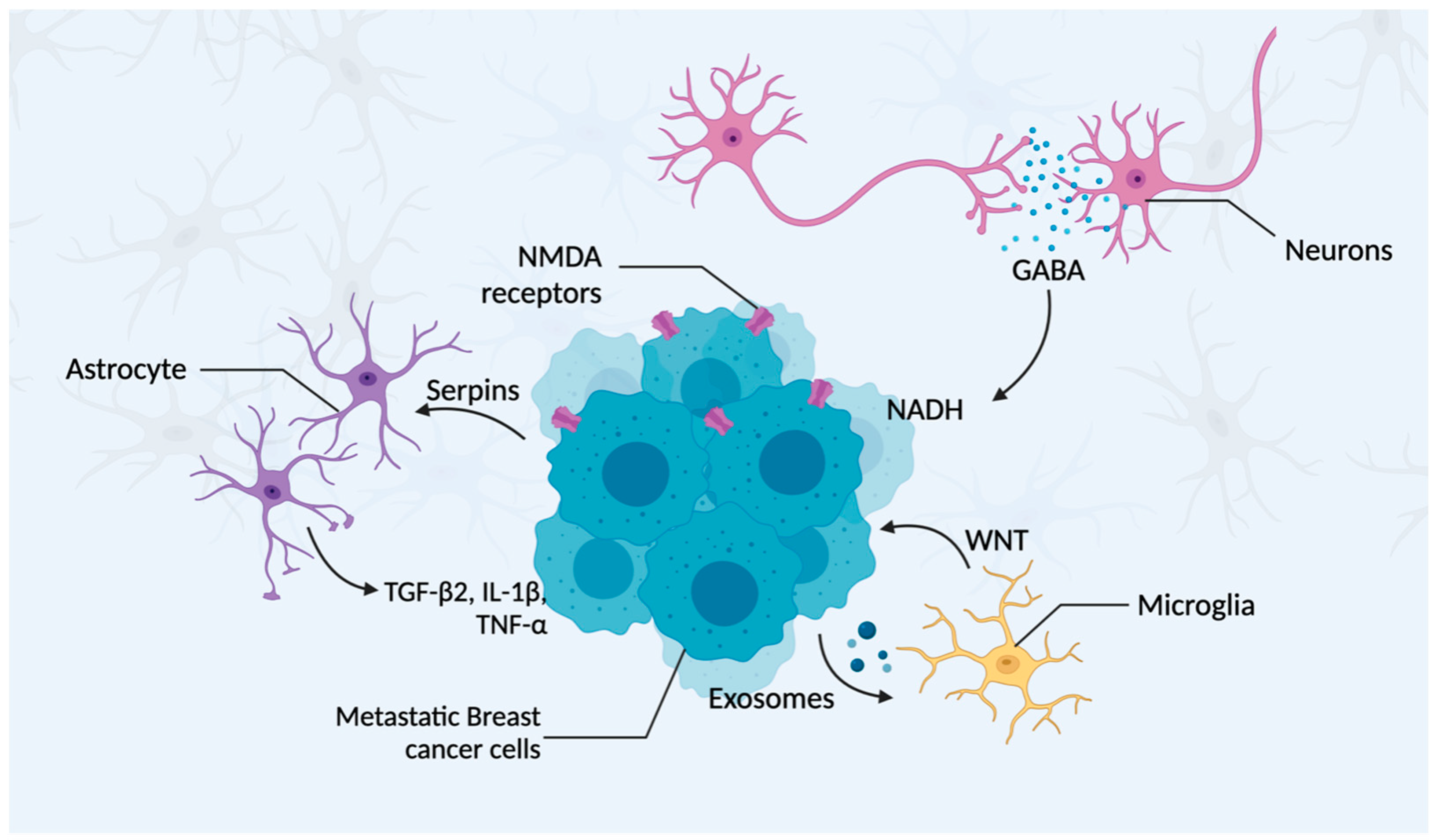
3.4. The Brain Tumor Microenvironment Cellular Composition
3.4.1. Neurons
3.4.2. Astrocytes
3.4.3. Microglia
4. Signaling Pathways Involved in BCBM
5. Current Treatments and Therapies for BCBM
5.1. Utilization of New Anticancer Drugs
5.2. Utilization of Immunotherapy
5.2.1. T-cell-Focused Immunotherapies
5.2.2. Vaccinations
5.2.3. Immune Checkpoint Inhibitors (ICIs) Targeted Therapies
5.2.4. Tumor-Associated Macrophages (TAMs) and Microglia-Targeted Therapies
5.3. Additional Therapies
6. Future Perspectives
6.1. Addressing the BBB
6.2. Accessing the Blood–Brain Barrier
6.3. Personalized Medicine
6.4. Targeting the EMT Process
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Bellon, J.R.; Winer, E.P. CNS Metastases in Breast Cancer. J. Clin. Oncol. 2004, 22, 3608–3617. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 16 May 2023).
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast Cancer-Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomás, N.; Balducci, K.; Abar, L.; Aune, D.; Cariolou, M.; Greenwood, D.C.; Markozannes, G.; Nanu, N.; Vieira, R.; Giovannucci, E.L.; et al. Postdiagnosis Dietary Factors, Supplement Use and Breast Cancer Prognosis: Global Cancer Update Programme (CUP Global) Systematic Literature Review and Meta-Analysis. Int. J. Cancer 2023, 152, 616–634. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Goh, E.L.K.; He, J.; Li, Y.; Fan, Z.; Yu, Z.; Yuan, P.; Liu, D.-X. Emerging Intrinsic Therapeutic Targets for Metastatic Breast Cancer. Biology 2023, 12, 697. [Google Scholar] [CrossRef]
- Edechi, C.; Ikeogu, N.; Terceiro, L.; Uzonna, J.; Myal, Y. Metastasis: A Bane of Breast Cancer Therapy. Eur. Med. J. 2020, 5, 55–62. [Google Scholar] [CrossRef]
- Chen, W.; Hoffmann, A.D.; Liu, H.; Liu, X. Organotropism: New Insights into Molecular Mechanisms of Breast Cancer Metastasis. npj Precis. Onc 2018, 2, 4. [Google Scholar] [CrossRef]
- Koniali, L.; Hadjisavvas, A.; Constantinidou, A.; Christodoulou, K.; Christou, Y.; Demetriou, C.; Panayides, A.S.; Pitris, C.; Pattichis, C.S.; Zamba-Papanicolaou, E.; et al. Risk Factors for Breast Cancer Brain Metastases: A Systematic Review. Oncotarget 2020, 11, 650–669. [Google Scholar] [CrossRef]
- Zaha, D.C. Significance of Immunohistochemistry in Breast Cancer. World J. Clin. Oncol. 2014, 5, 382–392. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Engel, J.M.; Greenlee, R.T.; Mukesh, B.N. Breast Cancer Subtypes Based on ER/PR and Her2 Expression: Comparison of Clinicopathologic Features and Survival. Clin. Med. Res. 2009, 7, 4–13. [Google Scholar] [CrossRef]
- Won, K.-A.; Spruck, C. Triple-Negative Breast Cancer Therapy: Current and Future Perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Darlix, A.; Louvel, G.; Fraisse, J.; Jacot, W.; Brain, E.; Debled, M.; Mouret-Reynier, M.A.; Goncalves, A.; Dalenc, F.; Delaloge, S.; et al. Impact of Breast Cancer Molecular Subtypes on the Incidence, Kinetics and Prognosis of Central Nervous System Metastases in a Large Multicentre Real-Life Cohort. Br. J. Cancer 2019, 121, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Heitz, F.; Rochon, J.; Harter, P.; Lueck, H.-J.; Fisseler-Eckhoff, A.; Barinoff, J.; Traut, A.; Lorenz-Salehi, F.; du Bois, A. Cerebral Metastases in Metastatic Breast Cancer: Disease-Specific Risk Factors and Survival. Ann. Oncol. 2011, 22, 1571–1581. [Google Scholar] [CrossRef]
- Niikura, N.; Hayashi, N.; Masuda, N.; Takashima, S.; Nakamura, R.; Watanabe, K.; Kanbayashi, C.; Ishida, M.; Hozumi, Y.; Tsuneizumi, M.; et al. Treatment Outcomes and Prognostic Factors for Patients with Brain Metastases from Breast Cancer of Each Subtype: A Multicenter Retrospective Analysis. Breast Cancer Res. Treat. 2014, 147, 103–112. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Effect of Tumor Subtype on Survival and the Graded Prognostic Assessment for Patients with Breast Cancer and Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Heitz, F.; Harter, P.; Lueck, H.-J.; Fissler-Eckhoff, A.; Lorenz-Salehi, F.; Scheil-Bertram, S.; Traut, A.; du Bois, A. Triple-Negative and HER2-Overexpressing Breast Cancers Exhibit an Elevated Risk and an Earlier Occurrence of Cerebral Metastases. Eur. J. Cancer 2009, 45, 2792–2798. [Google Scholar] [CrossRef]
- Nieder, C.; Oehlke, O.; Hintz, M.; Grosu, A.L. The Challenge of Durable Brain Control in Patients with Brain-Only Metastases from Breast Cancer. Springerplus 2015, 4, 585. [Google Scholar] [CrossRef]
- Niwińska, A.; Pogoda, K.; Murawska, M.; Niwiński, P. Factors Influencing Survival in Patients with Breast Cancer and Single or Solitary Brain Metastasis. Eur. J. Surg. Oncol. 2011, 37, 635–642. [Google Scholar] [CrossRef][Green Version]
- Niwińska, A.; Murawska, M.; Pogoda, K. Breast Cancer Brain Metastases: Differences in Survival Depending on Biological Subtype, RPA RTOG Prognostic Class and Systemic Treatment after Whole-Brain Radiotherapy (WBRT). Ann. Oncol. 2010, 21, 942–948. [Google Scholar] [CrossRef]
- Dawood, S.; Lei, X.; Litton, J.K.; Buchholz, T.A.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Incidence of Brain Metastases as a First Site of Recurrence among Women with Triple Receptor-Negative Breast Cancer. Cancer 2012, 118, 4652–4659. [Google Scholar] [CrossRef]
- Schneider, M.; Heimann, M.; Schaub, C.; Eichhorn, L.; Potthoff, A.-L.; Giordano, F.A.; Güresir, E.; Ko, Y.-D.; Landsberg, J.; Lehmann, F.; et al. Comorbidity Burden and Presence of Multiple Intracranial Lesions Are Associated with Adverse Events after Surgical Treatment of Patients with Brain Metastases. Cancers 2020, 12, 3209. [Google Scholar] [CrossRef]
- Sun, M.-S.; Yun, Y.-Y.; Liu, H.-J.; Yu, Z.-H.; Yang, F.; Xu, L. Brain Metastases in de Novo Breast Cancer: An Updated Population-Level Study from SEER Database. Asian J. Surg. 2022, 45, 2259–2267. [Google Scholar] [CrossRef]
- Brosnan, E.M.; Anders, C.K. Understanding Patterns of Brain Metastasis in Breast Cancer and Designing Rational Therapeutic Strategies. Ann. Transl. Med. 2018, 6, 163. [Google Scholar] [CrossRef]
- Bailleux, C.; Eberst, L.; Bachelot, T. Treatment Strategies for Breast Cancer Brain Metastases. Br. J. Cancer 2021, 124, 142–155. [Google Scholar] [CrossRef]
- Park, M.; Kim, D.; Ko, S.; Kim, A.; Mo, K.; Yoon, H. Breast Cancer Metastasis: Mechanisms and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 6806. [Google Scholar] [CrossRef]
- Kim, M.Y. Breast Cancer Metastasis. Adv. Exp. Med. Biol. 2021, 1187, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, H.; Song, X.; Yang, Q. Metastatic Heterogeneity of Breast Cancer: Molecular Mechanism and Potential Therapeutic Targets. Semin. Cancer Biol. 2020, 60, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Terceiro, L.; Edechi, C.; Ikeogu, N.; Nickel, B.; Hombach-Klonisch, S.; Sharif, T.; Leygue, E.; Myal, Y. The Breast Tumor Microenvironment: A Key Player in Metastatic Spread. Cancers 2021, 13, 4798. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, R.M.S.M.; Mustafa, D.A.; Soffietti, R.; Kros, J.M. Breast Cancer Brain Metastasis: Molecular Mechanisms and Directions for Treatment. Neuro Oncol. 2018, 20, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Role of the Blood-Brain Barrier in the Formation of Brain Metastases—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3565326/ (accessed on 16 May 2023).
- Arshad, F.; Wang, L.; Sy, C.; Avraham, S.; Avraham, H.K. Blood-Brain Barrier Integrity and Breast Cancer Metastasis to the Brain. Pathol. Res. Int. 2010, 2011, 920509. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Brain Endothelial Cell-Cell Junctions: How to “Open” the Blood Brain Barrier. Curr. Neuropharmacol. 2008, 6, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Luissint, A.-C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.-O. Tight Junctions at the Blood Brain Barrier: Physiological Architecture and Disease-Associated Dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, R.; Lampe, J.; Schwaninger, M.; Collin, L. Intracellular Transport and Regulation of Transcytosis across the Blood-Brain Barrier. Cell Mol. Life Sci. 2019, 76, 1081–1092. [Google Scholar] [CrossRef]
- Miller, D.S. Regulation of ABC Transporters at the Blood-Brain Barrier. Clin. Pharmacol. Ther. 2015, 97, 395–403. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.-H.; Chen, Z.-S. Overcoming ABC Transporter-Mediated Multidrug Resistance: Molecular Mechanisms and Novel Therapeutic Drug Strategies. Drug Resist. Updat. 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central Nervous System Pericytes in Health and Disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef]
- Steeg, P.S. The Blood–Tumour Barrier in Cancer Biology and Therapy. Nat. Rev. Clin. Oncol. 2021, 18, 696–714. [Google Scholar] [CrossRef]
- Cabezas, R.; Ávila, M.; Gonzalez, J.; El-Bachá, R.S.; Báez, E.; García-Segura, L.M.; Jurado Coronel, J.C.; Capani, F.; Cardona-Gomez, G.P.; Barreto, G.E. Astrocytic Modulation of Blood Brain Barrier: Perspectives on Parkinson’s Disease. Front. Cell Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–Endothelial Interactions at the Blood–Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Maurya, S.K.; Gupta, S.; Mishra, R. Transcriptional and Epigenetic Regulation of Microglia in Maintenance of Brain Homeostasis and Neurodegeneration. Front. Mol. Neurosci. 2022, 15, 1072046. [Google Scholar] [CrossRef] [PubMed]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and Non-Immune Functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Bos, P.D.; Zhang, X.H.-F.; Nadal, C.; Shu, W.; Gomis, R.R.; Nguyen, D.X.; Minn, A.J.; Van de Vijver, M.; Gerald, W.; Foekens, J.A.; et al. Genes That Mediate Breast Cancer Metastasis to the Brain. Nature 2009, 459, 1005–1009. [Google Scholar] [CrossRef]
- Salmaggi, A.; Maderna, E.; Calatozzolo, C.; Gaviani, P.; Canazza, A.; Milanesi, I.; Silvani, A.; DiMeco, F.; Carbone, A.; Pollo, B. CXCL12, CXCR4 and CXCR7 Expression in Brain Metastases. Cancer Biol. Ther. 2009, 8, 1608–1614. [Google Scholar] [CrossRef]
- Hinton, C.V.; Avraham, S.; Avraham, H.K. Role of the CXCR4/CXCL12 Signaling Axis in Breast Cancer Metastasis to the Brain. Clin. Exp. Metast. 2010, 27, 97–105. [Google Scholar] [CrossRef]
- Helbig, G.; Christopherson, K.W.; Bhat-Nakshatri, P.; Kumar, S.; Kishimoto, H.; Miller, K.D.; Broxmeyer, H.E.; Nakshatri, H. NF-KappaB Promotes Breast Cancer Cell Migration and Metastasis by Inducing the Expression of the Chemokine Receptor CXCR4. J. Biol. Chem. 2003, 278, 21631–21638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-Secreted MiR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, L.; Li, L.; Cao, Y. Exosomes Derived from Brain Metastatic Breast Cancer Cells Destroy the Blood-Brain Barrier by Carrying LncRNA GS1-600G8.5. Biomed. Res. Int. 2020, 2020, 7461727. [Google Scholar] [CrossRef]
- Lorger, M.; Krueger, J.S.; O’Neal, M.; Staflin, K.; Felding-Habermann, B. Activation of Tumor Cell Integrin Alphavbeta3 Controls Angiogenesis and Metastatic Growth in the Brain. Proc. Natl. Acad. Sci. USA 2009, 106, 10666–10671. [Google Scholar] [CrossRef]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous Blood-Tumor Barrier Permeability Determines Drug Efficacy in Experimental Brain Metastases of Breast Cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef]
- Wasilewski, D.; Priego, N.; Fustero-Torre, C.; Valiente, M. Reactive Astrocytes in Brain Metastasis. Front. Oncol. 2017, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.-C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment-Induced PTEN Loss by Exosomal MicroRNA Primes Brain Metastasis Outgrowth. Nature 2015, 527, 100–104. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Watabe, K. The Roles of Microglia/Macrophages in Tumor Progression of Brain Cancer and Metastatic Disease. Front. Biosci. 2017, 22, 1805–1829. [Google Scholar] [CrossRef] [PubMed]
- Pukrop, T.; Dehghani, F.; Chuang, H.-N.; Lohaus, R.; Bayanga, K.; Heermann, S.; Regen, T.; Van Rossum, D.; Klemm, F.; Schulz, M.; et al. Microglia Promote Colonization of Brain Tissue by Breast Cancer Cells in a Wnt-Dependent Way. Glia 2010, 58, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Lyle, L.T.; Lockman, P.R.; Adkins, C.E.; Mohammad, A.S.; Sechrest, E.; Hua, E.; Palmieri, D.; Liewehr, D.J.; Steinberg, S.M.; Kloc, W.; et al. Alterations in Pericyte Subpopulations Are Associated with Elevated Blood-Tumor Barrier Permeability in Experimental Brain Metastasis of Breast Cancer. Clin. Cancer Res. 2016, 22, 5287–5299. [Google Scholar] [CrossRef] [PubMed]
- Terrell-Hall, T.B.; Nounou, M.I.; El-Amrawy, F.; Griffith, J.I.G.; Lockman, P.R. Trastuzumab Distribution in an In-Vivo and in-Vitro Model of Brain Metastases of Breast Cancer. Oncotarget 2017, 8, 83734–83744. [Google Scholar] [CrossRef]
- Neman, J.; Termini, J.; Wilczynski, S.; Vaidehi, N.; Choy, C.; Kowolik, C.M.; Li, H.; Hambrecht, A.C.; Roberts, E.; Jandial, R. Human Breast Cancer Metastases to the Brain Display GABAergic Properties in the Neural Niche. Proc. Natl. Acad. Sci. USA 2014, 111, 984–989. [Google Scholar] [CrossRef]
- Zeng, Q.; Michael, I.P.; Zhang, P.; Saghafinia, S.; Knott, G.; Jiao, W.; McCabe, B.D.; Galván, J.A.; Robinson, H.P.C.; Zlobec, I.; et al. Synaptic Proximity Enables NMDAR Signaling to Promote Brain Metastasis. Nature 2019, 573, 526–531. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Valiente, M.; Obenauf, A.C.; Jin, X.; Chen, Q.; Zhang, X.H.-F.; Lee, D.J.; Chaft, J.E.; Kris, M.G.; Huse, J.T.; Brogi, E.; et al. Serpins Promote Cancer Cell Survival and Vascular Co-Option in Brain Metastasis. Cell 2014, 156, 1002–1016. [Google Scholar] [CrossRef]
- Gong, X.; Hou, Z.; Endsley, M.P.; Gronseth, E.I.; Rarick, K.R.; Jorns, J.M.; Yang, Q.; Du, Z.; Yan, K.; Bordas, M.L.; et al. Interaction of Tumor Cells and Astrocytes Promotes Breast Cancer Brain Metastases through TGF-Β2/ANGPTL4 Axes. NPJ Precis. Oncol. 2019, 3, 24. [Google Scholar] [CrossRef]
- Wang, L.; Cossette, S.M.; Rarick, K.R.; Gershan, J.; Dwinell, M.B.; Harder, D.R.; Ramchandran, R. Astrocytes Directly Influence Tumor Cell Invasion and Metastasis in Vivo. PLoS ONE 2013, 8, e80933. [Google Scholar] [CrossRef]
- Kaverina, N.; Borovjagin, A.V.; Kadagidze, Z.; Baryshnikov, A.; Baryshnikova, M.; Malin, D.; Ghosh, D.; Shah, N.; Welch, D.R.; Gabikian, P.; et al. Astrocytes Promote Progression of Breast Cancer Metastases to the Brain via a KISS1-Mediated Autophagy. Autophagy 2017, 13, 1905–1923. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Boire, A.; Jin, X.; Valiente, M.; Er, E.E.; Lopez-Soto, A.; Jacob, L.; Patwa, R.; Shah, H.; Xu, K.; et al. Carcinoma-Astrocyte Gap Junctions Promote Brain Metastasis by CGAMP Transfer. Nature 2016, 533, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Watters, A.; Cheng, N.; Perry, C.E.; Xu, K.; Alicea, G.M.; Parris, J.L.D.; Baraban, E.; Ray, P.; Nayak, A.; et al. Polyunsaturated Fatty Acids from Astrocytes Activate PPARγ Signaling in Cancer Cells to Promote Brain Metastasis. Cancer Discov. 2019, 9, 1720–1735. [Google Scholar] [CrossRef]
- Zhao, T.; Du, H.; Blum, J.S.; Yan, C. Critical Role of PPARγ in Myeloid-Derived Suppressor Cell-Stimulated Cancer Cell Proliferation and Metastasis. Oncotarget 2016, 7, 1529–1543. [Google Scholar] [CrossRef]
- Dudvarski Stankovic, N.; Teodorczyk, M.; Ploen, R.; Zipp, F.; Schmidt, M.H.H. Microglia-Blood Vessel Interactions: A Double-Edged Sword in Brain Pathologies. Acta Neuropathol. 2016, 131, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Klotz, R.; Thomas, A.; Teng, T.; Han, S.M.; Iriondo, O.; Li, L.; Restrepo-Vassalli, S.; Wang, A.; Izadian, N.; MacKay, M.; et al. Circulating Tumor Cells Exhibit Metastatic Tropism and Reveal Brain Metastasis Drivers. Cancer Discov. 2020, 10, 86–103. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Louie, E.; Chen, X.F.; Coomes, A.; Ji, K.; Tsirka, S.; Chen, E.I. Neurotrophin-3 Modulates Breast Cancer Cells and the Microenvironment to Promote the Growth of Breast Cancer Brain Metastasis. Oncogene 2013, 32, 4064–4077. [Google Scholar] [CrossRef] [PubMed]
- Chhichholiya, Y.; Ruthuparna, M.; Velagaleti, H.; Munshi, A. Brain Metastasis in Breast Cancer: Focus on Genes and Signaling Pathways Involved, Blood-Brain Barrier and Treatment Strategies. Clin. Transl. Oncol. 2023, 25, 1218–1241. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Liu, Y.; Wu, S.-Y.; Wu, K.; Sharma, S.; Mo, Y.-Y.; Feng, J.; Sanders, S.; Jin, G.; Singh, R.; et al. Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal MiRNA to Promote Brain Metastasis. Cancer Res. 2018, 78, 4316–4330. [Google Scholar] [CrossRef]
- Singh, T.; Kaushik, M.; Mishra, L.C.; Behl, C.; Singh, V.; Tuli, H.S. Exosomal MiRNAs as Novel Avenues for Breast Cancer Treatment. Front. Genet. 2023, 14, 1134779. [Google Scholar] [CrossRef] [PubMed]
- Patras, L.; Shaashua, L.; Matei, I.; Lyden, D. Immune Determinants of the Pre-Metastatic Niche. Cancer Cell 2023, 41, 546–572. [Google Scholar] [CrossRef]
- Wnt Signaling in Cancer|Oncogene. Available online: https://www.nature.com/articles/onc2016304 (accessed on 17 May 2023).
- Klemm, F.; Bleckmann, A.; Siam, L.; Chuang, H.N.; Rietkötter, E.; Behme, D.; Schulz, M.; Schaffrinski, M.; Schindler, S.; Trümper, L.; et al. β-Catenin-Independent WNT Signaling in Basal-like Breast Cancer and Brain Metastasis. Carcinogenesis 2011, 32, 434–442. [Google Scholar] [CrossRef]
- Latour, M.; Her, N.-G.; Kesari, S.; Nurmemmedov, E. WNT Signaling as a Therapeutic Target for Glioblastoma. Int. J. Mol. Sci. 2021, 22, 8428. [Google Scholar] [CrossRef]
- Lamb, R.; Ablett, M.P.; Spence, K.; Landberg, G.; Sims, A.H.; Clarke, R.B. Wnt Pathway Activity in Breast Cancer Sub-Types and Stem-Like Cells. PLoS ONE 2013, 8, e67811. [Google Scholar] [CrossRef]
- Nam, D.-H.; Jeon, H.-M.; Kim, S.; Kim, M.H.; Lee, Y.-J.; Lee, M.S.; Kim, H.; Joo, K.M.; Lee, D.-S.; Price, J.E.; et al. Activation of Notch Signaling in a Xenograft Model of Brain Metastasis. Clin. Cancer Res. 2008, 14, 4059–4066. [Google Scholar] [CrossRef]
- Xing, F.; Kobayashi, A.; Okuda, H.; Watabe, M.; Pai, S.K.; Pandey, P.R.; Hirota, S.; Wilber, A.; Mo, Y.-Y.; Moore, B.E.; et al. Reactive Astrocytes Promote the Metastatic Growth of Breast Cancer Stem-like Cells by Activating Notch Signalling in Brain. EMBO Mol. Med. 2013, 5, 384–396. [Google Scholar] [CrossRef]
- McGowan, P.M.; Simedrea, C.; Ribot, E.J.; Foster, P.J.; Palmieri, D.; Steeg, P.S.; Allan, A.L.; Chambers, A.F. Notch1 Inhibition Alters the CD44hi/CD24lo Population and Reduces the Formation of Brain Metastases from Breast Cancer. Mol. Cancer Res. 2011, 9, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Gumuskaya, B.; Alper, M.; Hucumenoglu, S.; Altundag, K.; Uner, A.; Guler, G. EGFR Expression and Gene Copy Number in Triple-Negative Breast Carcinoma. Cancer Genet. Cytogenet. 2010, 203, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Grupka, N.L.; Lear-Kaul, K.C.; Kleinschmidt-DeMasters, B.K.; Singh, M. Epidermal Growth Factor Receptor Status in Breast Cancer Metastases to the Central Nervous System. Comparison with HER-2/Neu Status. Arch. Pathol. Lab. Med. 2004, 128, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Adamo, B.; Deal, A.M.; Burrows, E.; Geradts, J.; Hamilton, E.; Blackwell, K.L.; Livasy, C.; Fritchie, K.; Prat, A.; Harrell, J.C.; et al. Phosphatidylinositol 3-Kinase Pathway Activation in Breast Cancer Brain Metastases. Breast Cancer Res. 2011, 13, R125. [Google Scholar] [CrossRef] [PubMed]
- Gaedcke, J.; Traub, F.; Milde, S.; Wilkens, L.; Stan, A.; Ostertag, H.; Christgen, M.; von Wasielewski, R.; Kreipe, H.H. Predominance of the Basal Type and HER-2/Neu Type in Brain Metastasis from Breast Cancer. Mod. Pathol. 2007, 20, 864–870. [Google Scholar] [CrossRef]
- Ippen, F.M.; Grosch, J.K.; Subramanian, M.; Kuter, B.M.; Liederer, B.M.; Plise, E.G.; Mora, J.L.; Nayyar, N.; Schmidt, S.P.; Giobbie-Hurder, A.; et al. Targeting the PI3K/Akt/MTOR Pathway with the Pan-Akt Inhibitor GDC-0068 in PIK3CA-Mutant Breast Cancer Brain Metastases. Neuro Oncol. 2019, 21, 1401–1411. [Google Scholar] [CrossRef]
- Majd, N.; Weathers, S.-P.; de Groot, J. Are We AKT-Ually Getting Closer to Making Targeted Therapy Successful in Breast Cancer Brain Metastases? Neuro Oncol. 2019, 21, 1344–1345. [Google Scholar] [CrossRef]
- Araki, K.; Miyoshi, Y. Mechanism of Resistance to Endocrine Therapy in Breast Cancer: The Important Role of PI3K/Akt/MTOR in Estrogen Receptor-Positive, HER2-Negative Breast Cancer. Breast Cancer 2018, 25, 392–401. [Google Scholar] [CrossRef]
- Blazquez, R.; Wlochowitz, D.; Wolff, A.; Seitz, S.; Wachter, A.; Perera-Bel, J.; Bleckmann, A.; Beißbarth, T.; Salinas, G.; Riemenschneider, M.J.; et al. PI3K: A Master Regulator of Brain Metastasis-Promoting Macrophages/Microglia. Glia 2018, 66, 2438–2455. [Google Scholar] [CrossRef]
- Lee, J.J.; Loh, K.; Yap, Y.-S. PI3K/Akt/MTOR Inhibitors in Breast Cancer. Cancer Biol. Med. 2015, 12, 342–354. [Google Scholar] [CrossRef]
- Martin, V.; Botta, F.; Zanellato, E.; Molinari, F.; Crippa, S.; Mazzucchelli, L.; Frattini, M. Molecular Characterization of EGFR and EGFR-Downstream Pathways in Triple Negative Breast Carcinomas with Basal like Features. Histol. Histopathol. 2012, 27, 785–792. [Google Scholar] [CrossRef]
- Wikman, H.; Lamszus, K.; Detels, N.; Uslar, L.; Wrage, M.; Benner, C.; Hohensee, I.; Ylstra, B.; Eylmann, K.; Zapatka, M.; et al. Relevance of PTEN Loss in Brain Metastasis Formation in Breast Cancer Patients. Breast Cancer Res. 2012, 14, R49. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, N.; Seeboeck, R.; Hofmann, E.; Eger, A. ErbB Family Signalling: A Paradigm for Oncogene Addiction and Personalized Oncology. Cancers 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Untangling the ErbB Signalling Network|Nature Reviews Molecular Cell Biology. Available online: https://www.nature.com/articles/35052073 (accessed on 18 May 2023).
- Baselga, J.; Swain, S.M. Novel Anticancer Targets: Revisiting ERBB2 and Discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Maurer, C.; Tulpin, L.; Moreau, M.; Dumitrescu, C.; de Azambuja, E.; Paesmans, M.; Nogaret, J.-M.; Piccart, M.J.; Awada, A. Risk Factors for the Development of Brain Metastases in Patients with HER2-Positive Breast Cancer. ESMO Open 2018, 3, e000440. [Google Scholar] [CrossRef]
- Palmieri, D.; Bronder, J.L.; Herring, J.M.; Yoneda, T.; Weil, R.J.; Stark, A.M.; Kurek, R.; Vega-Valle, E.; Feigenbaum, L.; Halverson, D.; et al. Her-2 Overexpression Increases the Metastatic Outgrowth of Breast Cancer Cells in the Brain. Cancer Res. 2007, 67, 4190–4198. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Bartsch, R.; Preusser, M.; Ricken, G.; Steger, G.G.; Bago-Horvath, Z.; Rudas, M.; Streubel, B.; Dubsky, P.; Gnant, M.; et al. Co-Overexpression of HER2/HER3 Is a Predictor of Impaired Survival in Breast Cancer Patients. Breast 2014, 23, 637–643. [Google Scholar] [CrossRef]
- Momeny, M.; Saunus, J.M.; Marturana, F.; McCart Reed, A.E.; Black, D.; Sala, G.; Iacobelli, S.; Holland, J.D.; Yu, D.; Da Silva, L.; et al. Heregulin-HER3-HER2 Signaling Promotes Matrix Metalloproteinase-Dependent Blood-Brain-Barrier Transendothelial Migration of Human Breast Cancer Cell Lines. Oncotarget 2015, 6, 3932–3946. [Google Scholar] [CrossRef]
- Lower, E.E.; Drosick, D.R.; Blau, R.; Brennan, L.; Danneman, W.; Hawley, D.K. Increased Rate of Brain Metastasis with Trastuzumab Therapy Not Associated with Impaired Survival. Clin. Breast Cancer 2003, 4, 114–119. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative Radiotherapy in the Treatment of Single Metastases to the Brain: A Randomized Trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef]
- Soffietti, R.; Kocher, M.; Abacioglu, U.M.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. A European Organisation for Research and Treatment of Cancer Phase III Trial of Adjuvant Whole-Brain Radiotherapy versus Observation in Patients with One to Three Brain Metastases from Solid Tumors after Surgical Resection or Radiosurgery: Quality-of-Life Results. J. Clin. Oncol. 2013, 31, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Serizawa, T.; Higuchi, Y.; Sato, Y.; Kawagishi, J.; Yamanaka, K.; Shuto, T.; Akabane, A.; Jokura, H.; Yomo, S.; et al. A Multi-Institutional Prospective Observational Study of Stereotactic Radiosurgery for Patients With Multiple Brain Metastases (JLGK0901 Study Update): Irradiation-Related Complications and Long-Term Maintenance of Mini-Mental State Examination Scores. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 31–40. [Google Scholar] [CrossRef]
- Gao, C.; Wang, F.; Suki, D.; Strom, E.; Li, J.; Sawaya, R.; Hsu, L.; Raghavendra, A.; Tripathy, D.; Ibrahim, N.K. Effects of Systemic Therapy and Local Therapy on Outcomes of 873 Breast Cancer Patients with Metastatic Breast Cancer to Brain: MD Anderson Cancer Center Experience. Int. J. Cancer 2021, 148, 961–970. [Google Scholar] [CrossRef]
- Liubota, R.; Vereshchako, R.; Anikusko, M.; Liubota, I.; Vakulenko, G. Systemic Chemotherapeutic Treatment of Patients with Breast Cancer Brain Metastases. Exp. Oncol. 2021, 43, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Baine, M.J.; Meza, J.L.; Lin, C. Association of Immunotherapy With Survival Among Patients With Brain Metastases Whose Cancer Was Managed With Definitive Surgery of the Primary Tumor. JAMA Netw. Open 2020, 3, e2015444. [Google Scholar] [CrossRef]
- Nieblas-Bedolla, E.; Nayyar, N.; Singh, M.; Sullivan, R.J.; Brastianos, P.K. Emerging Immunotherapies in the Treatment of Brain Metastases. Oncologist 2021, 26, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Niesel, K.; Schulz, M.; Anthes, J.; Alekseeva, T.; Macas, J.; Salamero-Boix, A.; Möckl, A.; Oberwahrenbrock, T.; Lolies, M.; Stein, S.; et al. The Immune Suppressive Microenvironment Affects Efficacy of Radio-Immunotherapy in Brain Metastasis. EMBO Mol. Med. 2021, 13, e13412. [Google Scholar] [CrossRef]
- Lin, N.U. Breast Cancer Brain Metastases: New Directions in Systemic Therapy. Ecancermedicalscience 2013, 7, 307. [Google Scholar] [CrossRef]
- Bartsch, R.; Berghoff, A.S.; Furtner, J.; Marhold, M.; Bergen, E.S.; Roider-Schur, S.; Starzer, A.M.; Forstner, H.; Rottenmanner, B.; Dieckmann, K.; et al. Trastuzumab Deruxtecan in HER2-Positive Breast Cancer with Brain Metastases: A Single-Arm, Phase 2 Trial. Nat. Med. 2022, 28, 1840–1847. [Google Scholar] [CrossRef]
- Núñez Abad, M.; Calabuig-Fariñas, S.; Lobo de Mena, M.; José Godes Sanz de Bremond, M.; García González, C.; Torres Martínez, S.; García-García, J.Á.; Iranzo González-Cruz, V.; Camps Herrero, C. Update on Systemic Treatment in Early Triple Negative Breast Cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835920986749. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Shao, Z. Fulvestrant in the Treatment of Hormone Receptor-positive/Human Epidermal Growth Factor Receptor 2-negative Advanced Breast Cancer: A Review. Cancer Med. 2019, 8, 1943–1957. [Google Scholar] [CrossRef]
- Miles, D.W. Recent Advances in Systemic Therapy. When HER2 Is Not the Target: Advances in the Treatment of HER2-Negative Metastatic Breast Cancer. Breast Cancer Res. 2009, 11, 208. [Google Scholar] [CrossRef]
- Kim, M.; Kizilbash, S.H.; Laramy, J.K.; Gampa, G.; Parrish, K.E.; Sarkaria, J.N.; Elmquist, W.F. Barriers to Effective Drug Treatment for Brain Metastases: A Multifactorial Problem in the Delivery of Precision Medicine. Pharm. Res. 2018, 35, 177. [Google Scholar] [CrossRef]
- Tsimberidou, A.-M.; Van Morris, K.; Vo, H.H.; Eck, S.; Lin, Y.-F.; Rivas, J.M.; Andersson, B.S. T-Cell Receptor-Based Therapy: An Innovative Therapeutic Approach for Solid Tumors. J. Hematol. Oncol. 2021, 14, 102. [Google Scholar] [CrossRef]
- Grabert, R.C.; Cousens, L.P.; Smith, J.A.; Olson, S.; Gall, J.; Young, W.B.; Davol, P.A.; Lum, L.G. Human T Cells Armed with Her2/Neu Bispecific Antibodies Divide, Are Cytotoxic, and Secrete Cytokines with Repeated Stimulation. Clin. Cancer Res. 2006, 12, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Lum, L.G.; Thakur, A.; Al-Kadhimi, Z.; Colvin, G.A.; Cummings, F.J.; Legare, R.D.; Dizon, D.S.; Kouttab, N.; Maizel, A.; Colaiace, W.; et al. Targeted T Cell Therapy in Stage IV Breast Cancer: A Phase I Clinical Trial. Clin. Cancer Res. 2015, 21, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Priceman, S.J.; Tilakawardane, D.; Jeang, B.; Aguilar, B.; Murad, J.P.; Park, A.K.; Chang, W.-C.; Ostberg, J.R.; Neman, J.; Jandial, R.; et al. Regional Delivery of Chimeric Antigen Receptor-Engineered T Cells Effectively Targets HER2+ Breast Cancer Metastasis to the Brain. Clin. Cancer Res. 2018, 24, 95–105. [Google Scholar] [CrossRef]
- Angelini, G.; Gardella, S.; Ardy, M.; Ciriolo, M.R.; Filomeni, G.; Di Trapani, G.; Clarke, F.; Sitia, R.; Rubartelli, A. Antigen-Presenting Dendritic Cells Provide the Reducing Extracellular Microenvironment Required for T Lymphocyte Activation. Proc. Natl. Acad. Sci. USA 2002, 99, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Sears, A.K.; Perez, S.A.; Clifton, G.T.; Benavides, L.C.; Gates, J.D.; Clive, K.S.; Holmes, J.P.; Shumway, N.M.; Van Echo, D.C.; Carmichael, M.G.; et al. AE37: A Novel T-Cell-Eliciting Vaccine for Breast Cancer. Expert. Opin. Biol. Ther. 2011, 11, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Clifton, G.T.; Holmes, J.P.; Schneble, E.; van Echo, D.; Ponniah, S.; Peoples, G.E. Final Report of the Phase I/II Clinical Trial of the E75 (Nelipepimut-S) Vaccine with Booster Inoculations to Prevent Disease Recurrence in High-Risk Breast Cancer Patients. Ann. Oncol. 2014, 25, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Laureano, R.S.; Sprooten, J.; Vanmeerbeerk, I.; Borras, D.M.; Govaerts, J.; Naulaerts, S.; Berneman, Z.N.; Beuselinck, B.; Bol, K.F.; Borst, J.; et al. Trial Watch: Dendritic Cell (DC)-Based Immunotherapy for Cancer. Oncoimmunology 2022, 11, 2096363. [Google Scholar] [CrossRef] [PubMed]
- Rupp, T.; Genest, L.; Babin, D.; Legrand, C.; Hunault, M.; Froget, G.; Castagné, V. Anti-CTLA-4 and Anti-PD-1 Immunotherapies Repress Tumor Progression in Preclinical Breast and Colon Model with Independent Regulatory T Cells Response. Transl. Oncol. 2022, 20, 101405. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination Nivolumab and Ipilimumab or Nivolumab Alone in Melanoma Brain Metastases: A Multicentre Randomised Phase 2 Study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Margolin, K.; Ernstoff, M.S.; Hamid, O.; Lawrence, D.; McDermott, D.; Puzanov, I.; Wolchok, J.D.; Clark, J.I.; Sznol, M.; Logan, T.F.; et al. Ipilimumab in Patients with Melanoma and Brain Metastases: An Open-Label, Phase 2 Trial. Lancet Oncol. 2012, 13, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Iorgulescu, J.B.; Harary, M.; Zogg, C.K.; Ligon, K.L.; Reardon, D.A.; Hodi, F.S.; Aizer, A.A.; Smith, T.R. Improved Risk-Adjusted Survival for Melanoma Brain Metastases in the Era of Checkpoint Blockade Immunotherapies: Results from a National Cohort. Cancer Immunol. Res. 2018, 6, 1039–1045. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.P.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.M.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef]
- Richardsen, E.; Uglehus, R.D.; Johnsen, S.H.; Busund, L.-T. Macrophage-Colony Stimulating Factor (CSF1) Predicts Breast Cancer Progression and Mortality. Anticancer. Res. 2015, 35, 865–874. [Google Scholar]
- de Gooijer, M.C.; Zhang, P.; Buil, L.C.M.; Çitirikkaya, C.H.; Thota, N.; Beijnen, J.H.; van Tellingen, O. Buparlisib Is a Brain Penetrable Pan-PI3K Inhibitor. Sci. Rep. 2018, 8, 10784. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Saura, C.; Barroso-Sousa, R.; Guo, H.; Ciruelos, E.; Bermejo, B.; Gavilá, J.; Serra, V.; Prat, A.; Paré, L.; et al. Phase 2 Study of Buparlisib (BKM120), a Pan-Class I PI3K Inhibitor, in Patients with Metastatic Triple-Negative Breast Cancer. Breast Cancer Res. 2020, 22, 120. [Google Scholar] [CrossRef]
- O’Reilly, T.; McSheehy, P.M. Biomarker Development for the Clinical Activity of the MTOR Inhibitor Everolimus (RAD001): Processes, Limitations, and Further Proposals. Transl. Oncol. 2010, 3, 65–79. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Van Swearingen, A.E.D.; Siegel, M.B.; Deal, A.M.; Sambade, M.J.; Hoyle, A.; Hayes, D.N.; Jo, H.; Little, P.; Dees, E.C.; Muss, H.; et al. LCCC 1025: A Phase II Study of Everolimus, Trastuzumab, and Vinorelbine to Treat Progressive HER2-Positive Breast Cancer Brain Metastases. Breast Cancer Res. Treat. 2018, 171, 637–648. [Google Scholar] [CrossRef]
- Stanley, E.R.; Chitu, V. CSF-1 Receptor Signaling in Myeloid Cells. Cold Spring Harb. Perspect. Biol. 2014, 6, a021857. [Google Scholar] [CrossRef]
- Rietkötter, E.; Bleckmann, A.; Bayerlová, M.; Menck, K.; Chuang, H.-N.; Wenske, B.; Schwartz, H.; Erez, N.; Binder, C.; Hanisch, U.-K.; et al. Anti-CSF-1 Treatment Is Effective to Prevent Carcinoma Invasion Induced by Monocyte-Derived Cells but Scarcely by Microglia. Oncotarget 2015, 6, 15482–15493. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.C.; Ruffell, B.; Oei, Y.; Bissell, M.J.; Coussens, L.M.; Pryer, N.; Daniel, D. CSF1R Inhibition Delays Cervical and Mammary Tumor Growth in Murine Models by Attenuating the Turnover of Tumor-Associated Macrophages and Enhancing Infiltration by CD8+ T Cells. Oncoimmunology 2013, 2, e26968. [Google Scholar] [CrossRef]
- Sirkisoon, S.R.; Carpenter, R.L.; Rimkus, T.; Doheny, D.; Zhu, D.; Aguayo, N.R.; Xing, F.; Chan, M.; Ruiz, J.; Metheny-Barlow, L.J.; et al. TGLI1 Transcription Factor Mediates Breast Cancer Brain Metastasis via Activating Metastasis-Initiating Cancer Stem Cells and Astrocytes in the Tumor Microenvironment. Oncogene 2020, 39, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-W.; Zhu, H.; Cao, X.; Aldrich, A.; Ali-Osman, F. A Novel Splice Variant of GLI1 That Promotes Glioblastoma Cell Migration and Invasion. Cancer Res. 2009, 69, 6790–6798. [Google Scholar] [CrossRef]
- Carpenter, R.L.; Paw, I.; Zhu, H.; Sirkisoon, S.; Xing, F.; Watabe, K.; Debinski, W.; Lo, H.-W. The Gain-of-Function GLI1 Transcription Factor TGLI1 Enhances Expression of VEGF-C and TEM7 to Promote Glioblastoma Angiogenesis. Oncotarget 2015, 6, 22653–22665. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, C.; Rosa, R.; D’Amato, V.; Ciciola, P.; Servetto, A.; Marciano, R.; Orsini, R.C.; Formisano, L.; De Falco, S.; Cicatiello, V.; et al. Hedgehog Signalling Pathway Orchestrates Angiogenesis in Triple-Negative Breast Cancers. Br. J. Cancer 2017, 116, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.K.; Ke, Y.; Wang, F.; Kayani, M.A.; Malik, M.F.A. Influence of SHH/GLI1 Axis on EMT Mediated Migration and Invasion of Breast Cancer Cells. Sci. Rep. 2019, 9, 6620. [Google Scholar] [CrossRef]
- Doheny, D.; Manore, S.; Sirkisoon, S.R.; Zhu, D.; Aguayo, N.R.; Harrison, A.; Najjar, M.; Anguelov, M.; Cox, A.O.; Furdui, C.M.; et al. An FDA-Approved Antifungal, Ketoconazole, and Its Novel Derivative Suppress TGLI1-Mediated Breast Cancer Brain Metastasis by Inhibiting the DNA-Binding Activity of Brain Metastasis-Promoting Transcription Factor TGLI1. Cancers 2022, 14, 4256. [Google Scholar] [CrossRef]
- Doheny, D.; Manore, S.; Wong, G.L.; Zhu, D.; Sirkisoon, S.; Anguelov, M.; Augayo, N.R.; Regua, A.T.; Cox, A.; Furdui, C.; et al. Abstract 2433: Targeting TGLI1 Pharmacologically as a New Therapeutic Strategy for Breast Cancer Brain Metastases. Cancer Res. 2022, 82, 2433. [Google Scholar] [CrossRef]
- Zhuang, S.; Li, L.; Zang, Y.; Li, G.; Wang, F. RRM2 Elicits the Metastatic Potential of Breast Cancer Cells by Regulating Cell Invasion, Migration and VEGF Expression via the PI3K/AKT Signaling. Oncol. Lett. 2020, 19, 3349–3355. [Google Scholar] [CrossRef] [PubMed]
- Hosonaga, M.; Saya, H.; Arima, Y. Molecular and Cellular Mechanisms Underlying Brain Metastasis of Breast Cancer. Cancer Metast. Rev. 2020, 39, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Ramkissoon, S.H.; Xie, S.; Goel, S.; Stover, D.G.; Guo, H.; Luu, V.; Marco, E.; Ramkissoon, L.A.; Kang, Y.J.; et al. Combination Inhibition of PI3K and MTORC1 Yields Durable Remissions in Mice Bearing Orthotopic Patient-Derived Xenografts of HER2-Positive Breast Cancer Brain Metastases. Nat. Med. 2016, 22, 723–726. [Google Scholar] [CrossRef]
- Zhao, H.-F.; Wang, J.; Shao, W.; Wu, C.-P.; Chen, Z.-P.; To, S.-S.T.; Li, W.-P. Recent Advances in the Use of PI3K Inhibitors for Glioblastoma Multiforme: Current Preclinical and Clinical Development. Mol. Cancer 2017, 16, 100. [Google Scholar] [CrossRef]
- Colardo, M.; Segatto, M.; Di Bartolomeo, S. Targeting RTK-PI3K-MTOR Axis in Gliomas: An Update. Int. J. Mol. Sci. 2021, 22, 4899. [Google Scholar] [CrossRef]
- Leone, J.P.; Trippa, L.; Milisits, L.; Andrews, C.; Ligibel, J.; Parsons, H.; Bi, W.; Zhao, J.; Winer, E.; Lin, N. TRLS-03. Phase II trial of gdc-0084 in combination with trastuzumab for patients with her2-positive breast cancer brain metastases (BCBM). Neuro-Oncol. Adv. 2019, 1, i9. [Google Scholar] [CrossRef]
- Blanchard, A.A.; Zelinski, T.; Xie, J.; Cooper, S.; Penner, C.; Leygue, E.; Myal, Y. Identification of Claudin 1 Transcript Variants in Human Invasive Breast Cancer. PLoS ONE 2016, 11, e0163387. [Google Scholar] [CrossRef]
- Myal, Y.; Leygue, E.; Blanchard, A.A. Claudin 1 in Breast Tumorigenesis: Revelation of a Possible Novel “Claudin High” Subset of Breast Cancers. J. Biomed. Biotechnol. 2010, 2010, 956897. [Google Scholar] [CrossRef]
- Zhou, B.; Moodie, A.; Blanchard, A.A.A.; Leygue, E.; Myal, Y. Claudin 1 in Breast Cancer: New Insights. J. Clin. Med. 2015, 4, 1960–1976. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Syed, N.; Therachiyil, L.; Nisar, S.; Hashem, S.; Macha, M.A.; Yadav, S.K.; Krishnankutty, R.; Muralitharan, S.; Al-Naemi, H.; et al. Claudin-1, A Double-Edged Sword in Cancer. Int. J. Mol. Sci. 2020, 21, 569. [Google Scholar] [CrossRef] [PubMed]
- Sladojevic, N.; Stamatovic, S.M.; Johnson, A.M.; Choi, J.; Hu, A.; Dithmer, S.; Blasig, I.E.; Keep, R.F.; Andjelkovic, A.V. Claudin-1-Dependent Destabilization of the Blood–Brain Barrier in Chronic Stroke. J. Neurosci. 2019, 39, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Basivireddy, J.; Kollar, A.; Biron, K.E.; Reickmann, P.; Jefferies, W.A.; McQuaid, S. Blood-Brain Barrier Disruption and Enhanced Vascular Permeability in the Multiple Sclerosis Model EAE. J. Neuroimmunol. 2010, 229, 180–191. [Google Scholar] [CrossRef]
- Izraely, S.; Sagi-Assif, O.; Klein, A.; Meshel, T.; Ben-Menachem, S.; Zaritsky, A.; Ehrlich, M.; Prieto, V.G.; Bar-Eli, M.; Pirker, C.; et al. The Metastatic Microenvironment: Claudin-1 Suppresses the Malignant Phenotype of Melanoma Brain Metastasis. Int. J. Cancer 2015, 136, 1296–1307. [Google Scholar] [CrossRef]
- Sun, H.; Xu, J.; Dai, S.; Ma, Y.; Sun, T. Breast Cancer Brain Metastasis: Current Evidence and Future Directions. Cancer Med. 2022, 12, 1007–1024. [Google Scholar] [CrossRef]
- Gambardella, V.; Tarazona, N.; Cejalvo, J.M.; Lombardi, P.; Huerta, M.; Roselló, S.; Fleitas, T.; Roda, D.; Cervantes, A. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers 2020, 12, 1009. [Google Scholar] [CrossRef]
- Morgan, A.J.; Giannoudis, A.; Palmieri, C. The Genomic Landscape of Breast Cancer Brain Metastases: A Systematic Review. Lancet Oncol. 2021, 22, e7–e17. [Google Scholar] [CrossRef]
- Bertucci, F.; Ng, C.K.Y.; Patsouris, A.; Droin, N.; Piscuoglio, S.; Carbuccia, N.; Soria, J.C.; Dien, A.T.; Adnani, Y.; Kamal, M.; et al. Genomic Characterization of Metastatic Breast Cancers. Nature 2019, 569, 560–564. [Google Scholar] [CrossRef]
- Dienstmann, R.; Jang, I.S.; Bot, B.; Friend, S.; Guinney, J. Database of Genomic Biomarkers for Cancer Drugs and Clinical Targetability in Solid Tumors. Cancer Discov. 2015, 5, 118–123. [Google Scholar] [CrossRef]
- Wong, K.K. DNMT1: A Key Drug Target in Triple-Negative Breast Cancer. Semin. Cancer Biol. 2021, 72, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Joe, N.S.; Hodgdon, C.; Kraemer, L.; Redmond, K.J.; Stearns, V.; Gilkes, D.M. A Common Goal to CARE: Cancer Advocates, Researchers, and Clinicians Explore Current Treatments and Clinical Trials for Breast Cancer Brain Metastases. npj Breast Cancer 2021, 7, 121. [Google Scholar] [CrossRef]
- Margarido, A.S.; Uceda-Castro, R.; Hahn, K.; de Bruijn, R.; Kester, L.; Hofland, I.; Lohuis, J.; Seinstra, D.; Broeks, A.; Jonkers, J.; et al. Epithelial-to-Mesenchymal Transition Drives Invasiveness of Breast Cancer Brain Metastases. Cancers 2022, 14, 3115. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Stoupis, G.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Circulating Tumor Cells with Stemness and Epithelial-to-Mesenchymal Transition Features Are Chemoresistant and Predictive of Poor Outcome in Metastatic Breast Cancer. Mol. Cancer Ther. 2019, 18, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Bryan, S.; Witzel, I.; Borgmann, K.; Oliveira-Ferrer, L. Molecular Mechanisms Associated with Brain Metastases in HER2-Positive and Triple Negative Breast Cancers. Cancers 2021, 13, 4137. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Yamashita, D.; Minata, M.; Ibrahim, A.N.; Yamaguchi, S.; Coviello, V.; Bernstock, J.D.; Harada, S.; Cerione, R.A.; Tannous, B.A.; La Motta, C.; et al. Identification of ALDH1A3 as a Viable Therapeutic Target in Breast Cancer Metastasis-Initiating Cells. Mol. Cancer Ther. 2020, 19, 1134–1147. [Google Scholar] [CrossRef]
- Wu, W.; Schecker, J.; Würstle, S.; Schneider, F.; Schönfelder, M.; Schlegel, J. Aldehyde Dehydrogenase 1A3 (ALDH1A3) Is Regulated by Autophagy in Human Glioblastoma Cells. Cancer Lett. 2018, 417, 112–123. [Google Scholar] [CrossRef]
| Subtype | Molecular Marker | Ki-67 | Incidence of CNS Metastasis | OS Rate after BCBM 1 |
|---|---|---|---|---|
| Luminal A and B | ER+, PR+, HER2− | Low or high | 14% | 7.1–9.3 |
| HER2-positive | ER+, PR+, HER2+ | High | 33% | 11.5–18.9 |
| TNBC | ER−, PR−, HER2− | High | 50% | 4.4–4.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terceiro, L.E.L.; Ikeogu, N.M.; Lima, M.F.; Edechi, C.A.; Nickel, B.E.; Fischer, G.; Leygue, E.; McManus, K.J.; Myal, Y. Navigating the Blood–Brain Barrier: Challenges and Therapeutic Strategies in Breast Cancer Brain Metastases. Int. J. Mol. Sci. 2023, 24, 12034. https://doi.org/10.3390/ijms241512034
Terceiro LEL, Ikeogu NM, Lima MF, Edechi CA, Nickel BE, Fischer G, Leygue E, McManus KJ, Myal Y. Navigating the Blood–Brain Barrier: Challenges and Therapeutic Strategies in Breast Cancer Brain Metastases. International Journal of Molecular Sciences. 2023; 24(15):12034. https://doi.org/10.3390/ijms241512034
Chicago/Turabian StyleTerceiro, Lucas E. L., Nnamdi M. Ikeogu, Matheus F. Lima, Chidalu A. Edechi, Barbara E. Nickel, Gabor Fischer, Etienne Leygue, Kirk J. McManus, and Yvonne Myal. 2023. "Navigating the Blood–Brain Barrier: Challenges and Therapeutic Strategies in Breast Cancer Brain Metastases" International Journal of Molecular Sciences 24, no. 15: 12034. https://doi.org/10.3390/ijms241512034
APA StyleTerceiro, L. E. L., Ikeogu, N. M., Lima, M. F., Edechi, C. A., Nickel, B. E., Fischer, G., Leygue, E., McManus, K. J., & Myal, Y. (2023). Navigating the Blood–Brain Barrier: Challenges and Therapeutic Strategies in Breast Cancer Brain Metastases. International Journal of Molecular Sciences, 24(15), 12034. https://doi.org/10.3390/ijms241512034





