Interaction, Insensitivity and Thermal Conductivity of CL-20/TNT-Based Polymer-Bonded Explosives through Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Results and Discussion
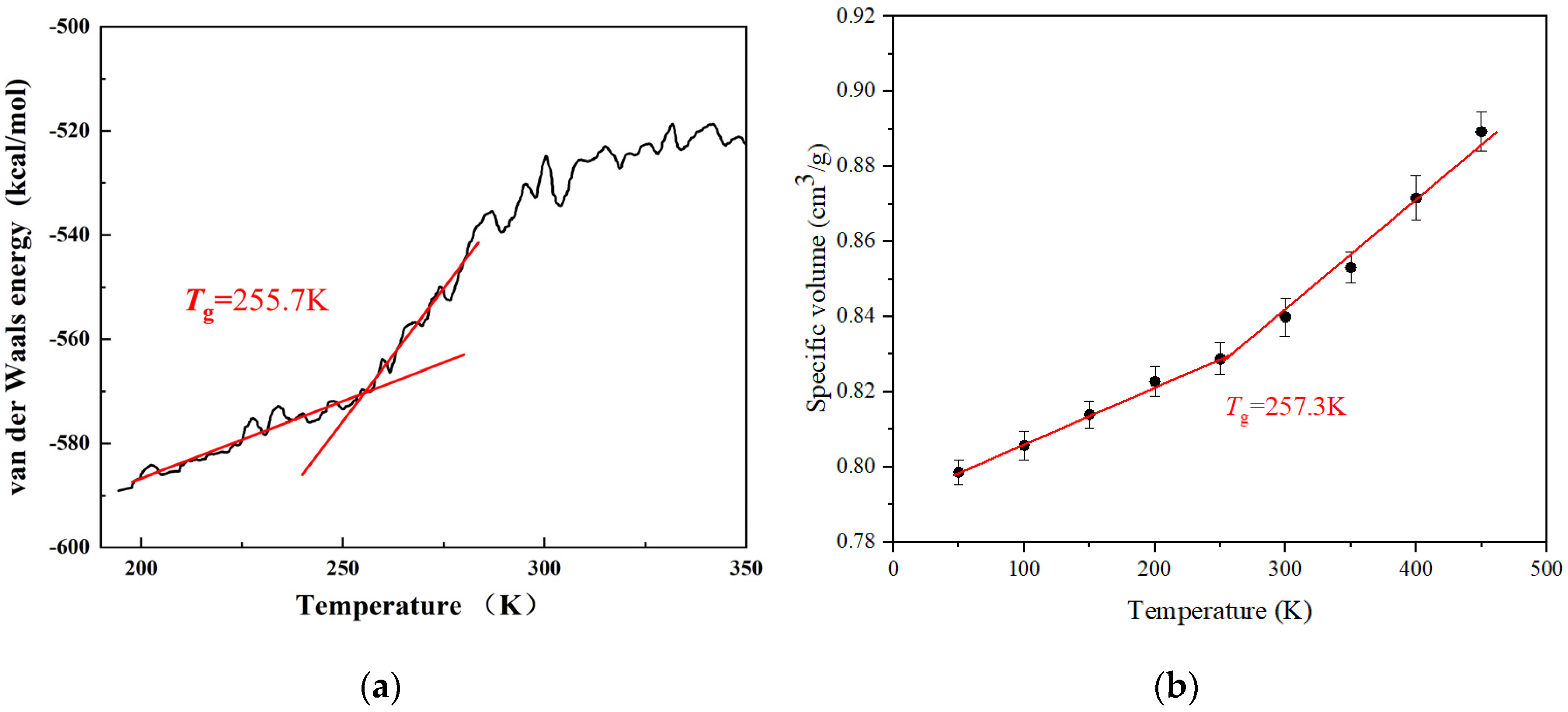
2.1. Glass Transition Temperature and Force Field Validation
2.2. Interface Interaction
2.3. Pair Correlation Function
2.4. N-NO2 Trigger Bond Length
2.5. Thermal Conductivity
3. Modelling and Computational Methods
3.1. Amorphous Polymer Chain
3.2. Models of CL-20/TNT and PBXs
3.3. Models and Methods for Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability:
References
- Xiao, H.M.; Xu, X.J. Theoretical Design of High Energy Density Materials; Science Press: Beijing, China, 2008; p. 135. [Google Scholar]
- Sun, T.; Xiao, J.J.; Liu, Q.; Zhao, F.; Xiao, H.M. Comparative study on structure, energetic and mechanical properties of a ε-CL-20/HMX cocrystal and its composite with molecular dynamics simulation. J. Mater. Chem. A 2014, 2, 13898. [Google Scholar] [CrossRef]
- Urbelis, J.H.; Swift, J.A. Solvent Effects on the Growth Morphology and Phase Purity of CL-20. Cryst. Growth Des. 2014, 14, 1642–1649. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.H.; Park, Y.C.; Hwang, J.H.; Kim, W.S. Control of crystal density of ε-hexanitrohexaazaisowurzitane in evaporation crystallization. Ind. Eng. Chem. Res. 2007, 46, 1500–1504. [Google Scholar] [CrossRef]
- Bumpus, J.A. A theoretical investigation of the ring strain energy, destabilization energy, and heat of formation of CL-20. Adv. Phys. Chem. 2012, 175146, 1–7. [Google Scholar] [CrossRef]
- Cao, Q.; Xiao, J.J.; Gao, P.; Li, S.S.; Zhao, F.; Wang, Y.A.; Xiao, H.M. Molecular dynamics simulations for CL-20/TNT co-crystal based polymer-bonded explosives. J. Theor. Comput. Chem. 2017, 16, 1750072. [Google Scholar] [CrossRef]
- Bolton, O.; Simke, L.R.; Pagoria, P.F.; Matzger, A.J. High power explosive with good sensitivity: A 2:1 cocrystal of CL-20:HMX. Cryst. Growth Des. 2012, 12, 4311–4314. [Google Scholar] [CrossRef]
- Li, H.; Shu, Y.; Gao, S.; Chen, L.; Ma, Q.; Ju, X. Easy methods to study the smart energetic TNT/CL-20 co-crystal. J. Mol. Model. 2013, 19, 4909–4917. [Google Scholar] [CrossRef]
- Hu, Y.; Yuan, S.; Li, X.; Liu, M.; Sun, F.; Yang, Y.; Hao, G.; Jiang, W. Preparation and characterization of nano-CL-20/TNT cocrystal explosives by mechanical ball-milling method. ACS Omega 2020, 5, 17761–17766. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xiao, J.J.; Zhang, J.; Zhao, F.; He, Z.H.; Xiao, H.M. Molecular dynamics simulation on CL-20/TNT cocrystal explosive. Chem. J. Chin. Univ. Chin. 2016, 37, 559–566. [Google Scholar]
- Bao, L.X.; Lv, P.H.; Fei, T.; Liu, Y.J.; Sun, C.H.; Pang, S. Crystal structure and explosive performance of a new CL-20/benzaldehyde cocrystal. J. Mol. Struct. 2020, 1215, 128267. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Lane, P.; Sjoberg, P.; Adolph, H.G. Shock-sensitivity relationships for nitramines and nitroaliphatics. Chem. Phys. Lett. 1991, 181, 78–82. [Google Scholar] [CrossRef]
- Xiao, J.J.; Wang, W.R.; Chen, J.; Ji, G.F.; Zhu, W.; Xiao, H.M. Study on structure, sensitivity and mechanical properties of HMX and HMX-based PBXs with molecular dynamics simulation. Comput. Theor. Chem. 2012, 999, 21–27. [Google Scholar] [CrossRef]
- Xiao, J.J.; Li, S.Y.; Chen, J.; Ji, G.F.; Zhu, W.; Zhao, F.; Wu, Q.; Xiao, H.M. Molecular dynamics study on the correlation between structure and sensitivity for defective RDX crystals and their PBXs. J. Mol. Model. 2013, 19, 803–809. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C. Review of the molecular and crystal correlations on sensitivities of energetic materials. J. Hazard. Mater. 2020, 398, 122910. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, B.; Chen, Y.; Li, Y.; Tan, X.; Wang, B.; Ye, B. Computational analysis the relationships of energy and mechanical properties with sensitivity for FOX-7 based PBXs via MD simulation. R. Soc. Open Sci. 2021, 8, 200345. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, A.; Koslowski, M. Effect of particle proximity and surface properties on the response of PBX under vibration. Comput. Mater. Sci. 2021, 192, 110334. [Google Scholar] [CrossRef]
- Mao, J.S.; Wang, B.G.; Chen, Y.F.; Fu, J.B.; Tian, X.; Ye, B.Y. Molecular dynamics simulation of CL20/DNDAP cocrystal-based PBXs. J. Mol. Model. 2023, 29, 199. [Google Scholar] [CrossRef]
- Mang, J.T.; Hjelm, R.P. Preferred void orientation in uniaxially pressed PBX 9502. Propellants Explos. Pyrotech. 2020, 46, 67–77. [Google Scholar] [CrossRef]
- Li, S.; Xiao, J. Molecular dynamics simulations for effects of fluoropolymer binder content in CL-20/TNT based polymer-bonded explosives. Molecules 2021, 26, 4876. [Google Scholar] [CrossRef]
- Peiris, S.M.; Piermarini, G.J. Static Compression of Energetic Materials; Springer: Berlin/Heidelberg, Germany, 2008; pp. 132–134. [Google Scholar]
- He, G.; Yang, Z.; Zhou, X.; Zhang, J.; Pan, L.; Liu, S. Polymer bonded explosives (PBXs) with reduced thermal stress and sensitivity by thermal conductivity enhancement with graphene nanoplatelets. Compos. Sci. Technol. 2016, 131, 22–31. [Google Scholar] [CrossRef]
- He, G.; Wang, P.; Dai, Y.; Sun, Y.; Zhang, J.; Yang, Z. Carbon nanofillers repair strategy for high-efficiency thermal conductivity enhancement of PBX composites at ultralow mass fraction. Compos. Part A Appl. Sci. Manuf. 2021, 148, 106492. [Google Scholar] [CrossRef]
- Li, Y.X.; Wu, P.; Hua, C.; Wang, J.; Huang, B.; Chen, J.; Qiao, Z.Q.; Yang, G.C. Determination of the mechanical and thermal properties, and Impact sensitivity of pressed hmx-based PBX. Cent. Eur. J. Energetic Mater. 2019, 16, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Mares, J.O.; Miller, J.K.; Sharp, N.D.; Moore, D.S.; Adams, D.E.; Groven, L.J.; Rhoads, J.F.; Son, S.F. Thermal and mechanical response of PBX 9501 under contact excitation. J. Appl. Phys. 2013, 113, 084904. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, H.; Li, J.; Zhang, H.; Zhu, W.; Xiao, H. Computation of interface interactions and mechanical properties of HMX-based PBX with Estane 5703 from atomic simulation. J. Mater. Sci. 2008, 43, 5685–5691. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Wang, X.; Fu, Y.; Huang, Z.; Xie, Q. Molecular dynamics studies of the mechanical behaviors and thermal conductivity of the DGEBA/MTHPA/CNB composites. Compos. Part B Eng. 2019, 164, 659–666. [Google Scholar] [CrossRef]
- Hossain, D.; Tschopp, M.A.; Ward, D.K.; Bouvard, J.L.; Wang, P.; Horstemeyer, M.F. Molecular dynamics simulations of deformation mechanisms of amorphous polyethylene. Polymer 2010, 51, 6071–6083. [Google Scholar] [CrossRef]
- Chen, T.K.; Chui, J.Y.; Shieh, T.S. Glass transition behaviors of a polyurethane hard segment based on 4,4′-diisocyanatodiphenylmethane and 1,4-butanediol and the calculation of microdomain composition. Macromolecules 1997, 30, 5068–5074. [Google Scholar] [CrossRef]
- Kazakevičiūtė-Makovska, R.; Özlem Özarmut, A.; Steeb, H. Characterization of shape memory polymer estane by means of dynamic mechanical thermal analysis technique. Smart Mater. Res. 2014, 250258, 1–9. [Google Scholar] [CrossRef]
- Tian, Q.; Yan, G.; Bai, L.; Li, X.; Zou, L.; Rosta, L.; Wacha, A.; Li, Q.; Krakovský, I.; Yan, M.; et al. Phase mixing and separation in polyester polyurethane studied by small-angle scattering: A polydisperse hard sphere model analysis. Polymer 2018, 147, 1–7. [Google Scholar] [CrossRef]
- Fernández-d’Arlas, B.; Baumann, R.P.; Pöselt, E.; Müller, A.J. Influence of composition on the isothermal crystallisation of segmented thermoplastic polyurethanes. CrystEngComm 2017, 19, 4720–4733. [Google Scholar] [CrossRef]
- Mark, J.E. Polymer Data Handbook; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Mark, J.E. Physical Properties of Polymers Handbook, 2nd ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Zhang, J.; Gao, P.; Xiao, J.J.; Zhao, F.; Xiao, H.M. CL-20/DNB co-crystal based PBX with PEG: Molecular dynamics simulation. Model. Simul. Mater. Sci. Eng. 2016, 24, 085008. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Q.; Chen, X.; Zhao, W.; Zhang, J.; Zhang, H.; Zeng, R.; Xu, S. A molecular simulation study of a series of cyclohexanone formaldehyde resins: Properties and applications in plastic printing. Polymer 2007, 48, 887–893. [Google Scholar] [CrossRef]
- Xiao, J.J.; Zhu, W.; Xiao, H.M. Molecular Dynamics of Energetic Materials; Science press: Beijing, China, 2013; p. 234. [Google Scholar]
- Andersen, H.C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Leiderman, K. Computation of the singular and regularized image systems for doubly-periodic Stokes flow in the presence of a wall. J. Comput. Phys. 2015, 297, 442–461. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applicationssoverview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Sha, Y.; Zhang, X. Impact sensitivity and moisture adsorption on the surface of CL-20/TNT cocrystal by molecular dynamics simulation. Appl. Surf. Sci. 2019, 483, 91–97. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, J.; Huang, H.; Li, J.; Xiao, H. Molecular dynamic simulations on the structures and properties of ε-CL-20 (0 0 1)/F2314 PBX. J. Hazard. Mater. 2010, 175, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Xiao, J.J.; Huang, H.; Li, J.S.; Xiao, H.M. Molecular dynamics simulations on the structures and properties of ε-CL-20-based PBXs. Sci. China Ser. B Chem. 2007, 50, 737–745. [Google Scholar] [CrossRef]
- Xiao, J.; Zhao, L.; Zhu, W.; Chen, J.; Ji, G.; Zhao, F.; Wu, Q.; Xiao, H. Molecular dynamics study on the relationships of modeling, structural and energy properties with sensitivity for RDX-based PBXs. Sci. China Chem. 2012, 55, 2587–2594. [Google Scholar] [CrossRef]
- Müller-Plathe, F. A simple non equilibrium molecular dynamics method for calculating the thermal conductivity. J. Chem. Phys. 1997, 106, 6082. [Google Scholar] [CrossRef]
- Mashhadzadeh, A.H.; Farzadian, O.; Dehaghani, M.Z.; Molaei, F.; Spitas, C.; Nouranian, S. Intrinsic thermal conductivities of BC3-C3N superlattice nanoribbons: A molecular dynamics study. Mater. Today Commun. 2022, 33, 104526. [Google Scholar] [CrossRef]
- Wei, N.; Chen, Y.; Cai, K.; Zhao, J.; Wang, H.-Q.; Zheng, J.-C. Thermal conductivity of graphene kirigami: Ultralow and strain robustness. Carbon 2016, 104, 203–213. [Google Scholar] [CrossRef]
- Nosé, S. Constant temperature molecular dynamics methods. Prog. Theor. Phys. 1991, 103, 1–46. [Google Scholar] [CrossRef]
- Hoover, W. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]







| Polymer | Density (g·cm−3) | Thermal Conductivity (W·K−1·m−1) | Tg (K) | ||||
|---|---|---|---|---|---|---|---|
| MD | Experiment | MD | Experiment | MD 1 | MD 2 | Experiment | |
| Estane5703 | 1.19 | 1.21 [33] | 0.20 | 0.21 [34] | 255.7 | 257.3 | ~250 [29,32] |
| Model | Ebind (kcal·mol−1) | Etotal (kcal·mol−1) | EEstane5703 (kcal·mol−1) | ECL-20TNT (kcal·mol−1) | Ebind’ (kcal·mol−1·nm−2) |
|---|---|---|---|---|---|
| PBX001 | 159.22 | −8558.08 | −72.81 | −8326.05 | 43.09 |
| (7.13) | (30.64) | (9.07) | (31.27) | (1.92) | |
| PBX010 | 178.16 | −8496.69 | −68.04 | −8250.49 | 44.69 |
| (4.47) | (30.82) | (9.16) | (28.65) | (1.10) | |
| PBX100 | 193.97 | −8505.28 | −61.96 | −8249.35 | 50.07 |
| (6.75) | (29.99) | (7.82) | (31.93) | (1.73) |
| Model | Distance (Å) | O1–H2 | H1–O2 | N1–H2 |
|---|---|---|---|---|
| PBX001 | 2.0–3.1 | 1.25 | 1.34 | 0.20 |
| 3.1–5.0 | 3.12 | 2.44 | 2.70 | |
| PBX010 | 2.0–3.1 | 1.77 | 1.20 | 0.41 |
| 3.1–5.0 | 3.92 | 4.20 | 3.18 | |
| PBX100 | 2.0–3.1 | 1.54 | 1.35 | 0.31 |
| 3.1–5.0 | 3.66 | 3.04 | 3.01 |
| Model | Lave | Lmax | |
|---|---|---|---|
| PBX001 | interfacial | 1.4115 (0.0325) −0.56 | 1.5423 (0.0012) −0.59 |
| internal | 1.4096 (0.0320) −1.91 | 1.5475 (0.0009) −0.26 | |
| PBX010 | interfacial | 1.4089 (0.0324) −2.41 | 1.5363 (0.0016) −0.98 |
| internal | 1.4102 (0.0319) −1.48 | 1.5433 (0.0017) −0.53 | |
| PBX100 | interfacial | 1.4095 (0.0316) −1.98 | 1.5283 (0.0011) −1.49 |
| internal | 1.4107 (0.0320) −1.13 | 1.5473 (0.0012) −0.27 | |
| CL-20/TNT | 1.4123 (0.0319) | 1.5515 (0.0012) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, Q.; Xiao, J. Interaction, Insensitivity and Thermal Conductivity of CL-20/TNT-Based Polymer-Bonded Explosives through Molecular Dynamics Simulation. Int. J. Mol. Sci. 2023, 24, 12067. https://doi.org/10.3390/ijms241512067
Li S, Li Q, Xiao J. Interaction, Insensitivity and Thermal Conductivity of CL-20/TNT-Based Polymer-Bonded Explosives through Molecular Dynamics Simulation. International Journal of Molecular Sciences. 2023; 24(15):12067. https://doi.org/10.3390/ijms241512067
Chicago/Turabian StyleLi, Shenshen, Qiaoli Li, and Jijun Xiao. 2023. "Interaction, Insensitivity and Thermal Conductivity of CL-20/TNT-Based Polymer-Bonded Explosives through Molecular Dynamics Simulation" International Journal of Molecular Sciences 24, no. 15: 12067. https://doi.org/10.3390/ijms241512067




