Ocular Vascular Diseases: From Retinal Immune Privilege to Inflammation
Abstract
1. Introduction
2. Immune Privilege in the Healthy Eye
2.1. Physical Immune Barriers
2.2. The Immune Regulatory System in the Eye
3. Ocular Inflammation
3.1. Microglia in the Retina
3.1.1. Location and Morphology of Microglia in the Retina
3.1.2. Interaction between Microglia and Other Cells in the Retina
3.1.3. Roles of Microglia in Ocular Vascular Diseases
Retinopathy of Prematurity (ROP)
Age-Related Macular Degeneration (AMD)
Diabetic Retinopathy (DR)
3.2. Inflammatory Mediators and Modulators
3.2.1. Complement Systems
3.2.2. Cytokines
| Factor Types | Genes | Diseases | Pathological Function | References |
|---|---|---|---|---|
| Chemokines | CXCL1 | AMD, DR | Promotes neutrophil recruitment | [195,196,197] |
| CXCL10 | AMD | Antiangiogenic | [198] | |
| CXCL12 | AMD, DR | Chemoattractant | [199] | |
| CCL2 | AMD, DR | Knockout mice exhibit some features of AMD | [143,200,201,202] | |
| CCL5 | AMD, DR | Promotes Th1 cell recruitment | [203] | |
| CCL11 | AMD, DR | Proangiogenic | [204,205] | |
| CCL24 | AMD | N/A | [206] | |
| CX3CL1 | RP, AMD, DR, | Prolongs cone survival; involved in microglia activation and recruitment | [207,208,209] | |
| Colony stimulating factors | GCSF | ROP, AMD, DR | Attenuates oxidative-stress-induced apoptosis; Neuroprotective; anti-inflammatory | [210,211,212] |
| GMCSF | AMD, DR | N/A | [210,213] | |
| MCSF | ROP, AMD, DR | Proangiogenic | [214,215] | |
| Interferons | IFN-α | AMD | Anti-inflammatory | [216] |
| IFN-β | AMD | Immunoprotective | [217,218] | |
| IFN-γ | AMD | N/A | [219] | |
| Interleukins | IL-1α | AMD, DR | Proinflammatory | [220,221] |
| IL-1β | AMD, ROP, DR | Proinflammatory; induce rod degeneration in AMD model | [101,222,223] | |
| IL-1Ra | DR, ROP, | Antagonist for IL-1α, IL-1β | [157,221] | |
| IL-2 | DR | ND | [224] | |
| IL-4 | AMD, DR | Proangiogenic | [225,226] | |
| IL-6 | ROP, AMD, DR | Proinflammatory; promote choroidal neovascularization | [102,227,228,229] | |
| IL-8 | AMD, ROP, DR | Chemokine, proinflammatory | [168,228,230] | |
| IL-10 | AMD, ROP, DR | Proangiogenic in AMD and ROP | [231,232] | |
| IL-11 | DR | N/A | [233] | |
| IL-12 | AMD, ROP, DR | Antiangiogenic | [183,234,235] | |
| IL-13 | AMD, DR | Anti-inflammatory | [215,236,237] | |
| IL-17A | ROP, AMD, DR | Proinflammatory; proangiogenic | [238,239] | |
| IL-18 | AMD, DR | N/A | [193,240] | |
| IL-21 | AMD, DR | Promotes Th17-cell differentiation | [241,242] | |
| IL-22 | AMD, DR | Anti-inflammatory | [242] | |
| IL-23 | AMD, DR | Proinflammatory | [243,244] | |
| IL-26 | DR | N/A | [245] | |
| IL-27 | AMD, DR | Anti-inflammatory; antiangiogenic | [246,247] | |
| IL-31 | DR | N/A | [248] | |
| IL-33 | ROP, AMD | Pro-inflammatory in AMD; anti-inflammatory in RD | [249,250] | |
| IL-35 | DR | Anti-inflammatory | [63,68] | |
| IL-37 | ROP, DR, | Anti-inflammatory; proangiogenic | [251,252] | |
| IL-38 | ROP | Antiangiogenic | [253] | |
| Transforming growth factor | TGF-β | AMD, DR | Anti-inflammatory; antiangiogenic | [254,255] |
| Tumor necrosis factor | TNF-α | ROP, AMD, DR, | Alters RPE morphological changes and BRP breakdown; proinflammatory. | [256,257,258,259] |
3.2.3. Chemokines
3.2.4. Cyclooxygenase (COX) Enzyme
3.2.5. Other Mediators
3.3. Key Pathways during Retinal Inflammation
3.3.1. Janus Kinase/Signal Transducers and Activators of Transcription (JAK/STAT3/SOCS3) Pathway
3.3.2. c-Jun N-Terminal Kinases/Activator Protein 1 (AP-1) Pathway
3.3.3. Nuclear Factor Kappa B (NF-κB) Pathway
3.3.4. VEGF Pathway
3.3.5. Angiopoietin–Tie (Ang/Tie) Pathway
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Streilein, J.W. Ocular immune privilege: The eye takes a dim but practical view of immunity and inflammation. J. Leukoc. Biol. 2003, 74, 179–185. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826. [Google Scholar] [PubMed]
- Carson, M.J.; Thrash, J.C.; Walter, B. The cellular response in neuroinflammation: The role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin. Neurosci. Res. 2006, 6, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Wei, W.B.; Xu, L.; Wang, Y.X. Systemic inflammation and eye diseases. The Beijing Eye Study. PLoS ONE 2018, 13, e0204263. [Google Scholar]
- Hessen, M.; Akpek, E.K. Dry eye: An inflammatory ocular disease. J. Ophthalmic Vis. Res. 2014, 9, 240. [Google Scholar]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef]
- Chen, M.; Lechner, J.; Zhao, J.; Toth, L.; Hogg, R.; Silvestri, G.; Kissenpfennig, A.; Chakravarthy, U.; Xu, H. STAT3 Activation in Circulating Monocytes Contributes to Neovascular Age-Related Macular Degeneration. Curr. Mol. Med. 2016, 16, 412–423. [Google Scholar]
- Tolsma, K.W.; Allred, E.N.; Chen, M.L.; Duker, J.; Leviton, A.; Dammann, O. Neonatal bacteremia and retinopathy of prematurity: The ELGAN study. Arch. Ophthalmol. 2011, 129, 1555–1563. [Google Scholar] [CrossRef]
- Chen, M.; Citil, A.; McCabe, F.; Leicht, K.M.; Fiascone, J.; Dammann, C.E.; Dammann, O. Infection, oxygen, and immaturity: Interacting risk factors for retinopathy of prematurity. Neonatology 2011, 99, 125–132. [Google Scholar]
- Medawar, P.B. Immunity to homologous grafted skin. III. the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948, 29, 58–69. [Google Scholar]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. Creating immune privilege: Active local suppression that benefits friends, but protects foes. Nat. Rev. Immunol. 2008, 8, 74–80. [Google Scholar]
- Caspi, R.R. Ocular autoimmunity: The price of privilege? Immunol. Rev. 2006, 213, 23–35. [Google Scholar] [PubMed]
- Hori, J.; Joyce, N.; Streilein, J.W. Epithelium-deficient corneal allografts display immune privilege beneath the kidney capsule. Investig. Ophthalmol. Vis. Sci. 2000, 41, 443–452. [Google Scholar]
- Hori, J.; Joyce, N.C.; Streilein, J.W. Immune privilege and immunogenicity reside among different layers of the mouse cornea. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3032–3042. [Google Scholar]
- Wenkel, H.; Streilein, J.W. Evidence that retinal pigment epithelium functions as an immune-privileged tissue. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3467–3473. [Google Scholar]
- Ng, T.F.; Osawa, H.; Hori, J.; Young, M.J.; Streilein, J.W. Allogeneic neonatal neuronal retina grafts display partial immune privilege in the subcapsular space of the kidney. J. Immunol. 2002, 169, 5601–5606. [Google Scholar]
- Streilein, J.W. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 2003, 3, 879–889. [Google Scholar] [PubMed]
- Niederkorn, J.Y.; Stein-Streilein, J. History and physiology of immune privilege. Ocul. Immunol. Inflamm. 2010, 18, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y. Corneal transplantation and immune privilege. Int. Rev. Immunol. 2013, 32, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Hori, J.; Yamaguchi, T.; Keino, H.; Hamrah, P.; Maruyama, K. Immune privilege in corneal transplantation. Prog. Retin. Eye Res. 2019, 72, 100758. [Google Scholar]
- Forrester, J.V.; Xu, H. Good news-bad news: The Yin and Yang of immune privilege in the eye. Front. Immunol. 2012, 3, 338. [Google Scholar]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune regulation in the aging retina. Prog. Retin. Eye Res. 2019, 69, 159–172. [Google Scholar] [CrossRef]
- Van Dooremaal, J. Die entwickelung der in fremden grund versetzten lebenden gewebe. Albrecht Von Graefes Arch. Für Ophthalmol. 1873, 19, 359–373. [Google Scholar] [CrossRef]
- Streilein, J.W.; Niederkorn, J.Y. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. Ocul. Immunol. Inflamm. 2007, 15, 187–194. [Google Scholar] [PubMed]
- Streilein, J.W.; Toews, G.B.; Bergstresser, P.R. Corneal allografts fail to express Ia antigens. Nature 1979, 282, 326–327. [Google Scholar] [PubMed]
- Kunishige, T.; Taniguchi, H.; Terada, M.; Akiba, H.; Yagita, H.; Abe, R.; Hori, J. Protective Role of ICOS and ICOS Ligand in Corneal Transplantation and in Maintenance of Immune Privilege. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6815–6823. [Google Scholar]
- Shimmura-Tomita, M.; Wang, M.; Taniguchi, H.; Akiba, H.; Yagita, H.; Hori, J. Galectin-9-mediated protection from allo-specific T cells as a mechanism of immune privilege of corneal allografts. PLoS ONE 2013, 8, e63620. [Google Scholar]
- Neelam, S.; Niederkorn, J.Y. Corneal Nerve Ablation Abolishes Ocular Immune Privilege by Downregulating CD103 on T Regulatory Cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 25. [Google Scholar]
- Hori, J.; Wang, M.; Miyashita, M.; Tanemoto, K.; Takahashi, H.; Takemori, T.; Okumura, K.; Yagita, H.; Azuma, M. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J. Immunol. 2006, 177, 5928–5935. [Google Scholar] [CrossRef] [PubMed]
- Stuart, P.M.; Griffith, T.S.; Usui, N.; Pepose, J.; Yu, X.; Ferguson, T.A. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J. Clin. Investig. 1997, 99, 396–402. [Google Scholar]
- Yamagami, S.; Kawashima, H.; Tsuru, T.; Yamagami, H.; Kayagaki, N.; Yagita, H.; Okumura, K.; Gregerson, D.S. Role of Fas-Fas ligand interactions in the immunorejection of allogeneic mouse corneal transplants. Transplantation 1997, 64, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Hori, J.; Taniguchi, H.; Wang, M.; Oshima, M.; Azuma, M. GITR ligand-mediated local expansion of regulatory T cells and immune privilege of corneal allografts. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6556–6565. [Google Scholar]
- Shakib, M.; Cunha-Vaz, J. Studies on the permeability of the blood-retinal barrier: IV. Junctional complexes of the retinal vessels and their role in the permeability of the blood-retinal barrier. Exp. Eye Res. 1966, 5, 229-IN16. [Google Scholar]
- Peyman, G.A.; Bok, D. Peroxidase diffusion in the normal and laser-coagulated primate retina. Investig. Ophthalmol. Vis. Sci. 1972, 11, 35–45. [Google Scholar]
- Sugita, S. Role of ocular pigment epithelial cells in immune privilege. Arch. Immunol. Ther. Exp. 2009, 57, 263–268. [Google Scholar] [CrossRef]
- Mochizuki, M.; Sugita, S.; Kamoi, K. Immunological homeostasis of the eye. Prog. Retin. Eye Res. 2013, 33, 10–27. [Google Scholar] [CrossRef]
- Mendes-Jorge, L.; Ramos, D.; Luppo, M.; Llombart, C.; Alexandre-Pires, G.; Nacher, V.; Melgarejo, V.; Correia, M.; Navarro, M.; Carretero, A.; et al. Scavenger function of resident autofluorescent perivascular macrophages and their contribution to the maintenance of the blood-retinal barrier. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5997–6005. [Google Scholar]
- Rajesh, A.; Droho, S.; Lavine, J.A. Macrophages in close proximity to the vitreoretinal interface are potential biomarkers of inflammation during retinal vascular disease. J. Neuroinflamm. 2022, 19, 203. [Google Scholar]
- Raviola, G. The structural basis of the blood-ocular barriers. Exp. Eye Res. 1977, 25 (Suppl. S1), 27–63. [Google Scholar] [PubMed]
- Cunha-Vaz, J. The blood-ocular barriers. Surv. Ophthalmol. 1979, 23, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Coca-Prados, M. The blood-aqueous barrier in health and disease. J. Glaucoma 2014, 23, S36–S38. [Google Scholar] [PubMed]
- Cunha-Vaz, J. The blood-retinal barrier in the management of retinal disease: EURETINA award lecture. Ophthalmologica 2017, 237, 1–10. [Google Scholar]
- Taylor, A.W.; Ng, T.F. Negative regulators that mediate ocular immune privilege. J. Leukoc. Biol. 2018, 103, 1179–1187. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52. [Google Scholar]
- Taylor, A.W. A review of the influence of aqueous humor on immunity. Ocul. Immunol. Inflamm. 2003, 11, 231–241. [Google Scholar] [CrossRef]
- Namba, K.; Kitaichi, N.; Nishida, T.; Taylor, A.W. Induction of regulatory T cells by the immunomodulating cytokines α-melanocyte-stimulating hormone and transforming growth factor-β2. J. Leukoc. Biol. 2002, 72, 946–952. [Google Scholar]
- Cousins, S.W.; Trattler, W.B.; Streilein, J.W. Immune privilege and suppression of immunogenic inflammation in the anterior chamber of the eye. Curr. Eye Res. 1991, 10, 287–297. [Google Scholar]
- Nishida, T.; Taylor, A.W. Specific aqueous humor factors induce activation of regulatory T cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2268–2274. [Google Scholar]
- Zamiri, P.; Masli, S.; Kitaichi, N.; Taylor, A.W.; Streilein, J.W. Thrombospondin plays a vital role in the immune privilege of the eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.H.; Taylor, A.W. The immune privileged retina mediates an alternative activation of J774A. 1 cells. Ocul. Immunol. Inflamm. 2009, 17, 380–389. [Google Scholar]
- Wilbanks, G.A.; Wayne Streilein, J. Fluids from immune privileged sites endow macrophages with the capacity to induce antigen-specific immune deviation via a mechanism involving transforming growth factor-β. Eur. J. Immunol. 1992, 22, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Vendomèle, J.; Khebizi, Q.; Fisson, S. Cellular and molecular mechanisms of Anterior Chamber-Associated Immune Deviation (ACAID): What we have learned from knockout mice. Front. Immunol. 2017, 8, 1686. [Google Scholar] [PubMed]
- Wenkel, H.; Chen, P.W.; Ksander, B.R.; Streilein, J.W. Immune privilege is extended, then withdrawn, from allogeneic tumor cell grafts placed in the subretinal space. Investig. Ophthalmol. Vis. Sci. 1999, 40, 3202–3208. [Google Scholar]
- Jiang, L.Q.; Jorquera, M.; Streilein, J.W. Subretinal space and vitreous cavity as immunologically privileged sites for retinal allografts. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3347–3354. [Google Scholar]
- Reyes, N.J.; O’Koren, E.G.; Saban, D.R. New insights into mononuclear phagocyte biology from the visual system. Nat. Rev. Immunol. 2017, 17, 322–332. [Google Scholar]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [PubMed]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [PubMed]
- Reichenbach, A.; Bringmann, A. Glia of the human retina. Glia 2020, 68, 768–796. [Google Scholar] [CrossRef] [PubMed]
- O’Koren, E.G.; Yu, C.; Klingeborn, M.; Wong, A.Y.W.; Prigge, C.L.; Mathew, R.; Kalnitsky, J.; Msallam, R.A.; Silvin, A.; Kay, J.N.; et al. Microglial Function Is Distinct in Different Anatomical Locations during Retinal Homeostasis and Degeneration. Immunity 2019, 50, 723–737.e7. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.; Li, H.; Xia, L. IL-35 alleviates inflammation progression in a rat model of diabetic neuropathic pain via inhibition of JNK signaling. J. Inflamm. 2019, 16, 19. [Google Scholar] [CrossRef]
- Santos, A.M.; Calvente, R.; Tassi, M.; Carrasco, M.C.; Martín-Oliva, D.; Marín-Teva, J.L.; Navascués, J.; Cuadros, M.A. Embryonic and postnatal development of microglial cells in the mouse retina. J. Comp. Neurol. 2008, 506, 224–239. [Google Scholar] [CrossRef]
- Li, F.; Jiang, D.; Samuel, M.A. Microglia in the developing retina. Neural Dev. 2019, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Araya, C.M.; Provis, J.M.; Penfold, P.L.; Billson, F.A. Development of microglial topography in human retina. J. Comp. Neurol. 1995, 363, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M.; Mayer, E.J.; Forrester, J.V.; Dick, A.D. Turnover of resident retinal microglia in the normal adult mouse. Glia 2007, 55, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; You, H.; Zhang, X. Levels of interleukin 27 and interleukin 35 in the serum and vitreous of patients with proliferative diabetic retinopathy. Ocul. Immunol. Inflamm. 2018, 26, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal microglia: Just bystander or target for therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef]
- Sun, T.H.; Zhao, X. CD200-CD200R Interaction: An important regulator after stroke. Front. Neurosci. 2019, 13, 840. [Google Scholar]
- Hernangómez, M.; J Carrillo-Salinas, F.; Mecha, M.; Correa, F.; Mestre, L.; Loría, F.; Feliú, A.; Docagne, F.; Guaza, C. Brain innate immunity in the regulation of neuroinflammation: Therapeutic strategies by modulating CD200-CD200R interaction involve the cannabinoid system. Curr. Pharm. Des. 2014, 20, 4707–4722. [Google Scholar] [CrossRef] [PubMed]
- Zujovic, V.; Benavides, J.; Vigé, X.; Carter, C.; Taupin, V. Fractalkine modulates TNF-α secretion and neurotoxicity induced by microglial activation. Glia 2000, 29, 305–315. [Google Scholar] [CrossRef]
- Zujovic, V.; Schussler, N.; Jourdain, D.; Duverger, D.; Taupin, V. In vivo neutralization of endogenous brain fractalkine increases hippocampal TNFα and 8-isoprostane production induced by intracerebroventricular injection of LPS. J. Neuroimmunol. 2001, 115, 135–143. [Google Scholar] [PubMed]
- Finneran, D.J.; Nash, K.R. Neuroinflammation and fractalkine signaling in Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Jobling, A.I.; Waugh, M.; Vessey, K.A.; Phipps, J.A.; Trogrlic, L.; Greferath, U.; Mills, S.A.; Tan, Z.L.; Ward, M.M.; Fletcher, E.L. The role of the microglial Cx3cr1 pathway in the postnatal maturation of retinal photoreceptors. J. Neurosci. 2018, 38, 4708–4723. [Google Scholar] [PubMed]
- Liang, K.J.; Lee, J.E.; Wang, Y.D.; Ma, W.; Fontainhas, A.M.; Fariss, R.N.; Wong, W.T. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4444–4451. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wong, W.T. Microglia-Müller cell interactions in the retina. In Retinal Degenerative Diseases; Springer: Berlin/Heidelberg, Germany, 2014; pp. 333–338. [Google Scholar]
- Wang, M.; Ma, W.; Zhao, L.; Fariss, R.N.; Wong, W.T. Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J. Neuroinflamm. 2011, 8, 173. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Z.; Bao, Z.; Yan, W.; You, G.; Wang, Y.; Hu, H.; Zhang, W.; Zhang, Q.; Jiang, T. SOCS3 promoter hypermethylation is a favorable prognosticator and a novel indicator for G-CIMP-positive GBM patients. PLoS ONE 2014, 9, e91829. [Google Scholar]
- Karlstetter, M.; Nothdurfter, C.; Aslanidis, A.; Moeller, K.; Horn, F.; Scholz, R.; Neumann, H.; Weber, B.H.; Rupprecht, R.; Langmann, T. Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. J. Neuroinflamm. 2014, 11, 3. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Russo, M.V.; McGavern, D.B. Immune surveillance of the CNS following infection and injury. Trends Immunol. 2015, 36, 637–650. [Google Scholar] [CrossRef]
- Ronning, K.E.; Karlen, S.J.; Burns, M.E. Structural and functional distinctions of co-resident microglia and monocyte-derived macrophages after retinal degeneration. J. Neuroinflamm. 2022, 19, 299. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, H.; Xu, J.; Yang, Q.; Ma, Q.; Mao, X.; Xu, Z.; Zhou, Y.; Da, Q.; Cai, Y.; et al. Single-cell transcriptome analyses reveal microglia types associated with proliferative retinopathy. JCI Insight 2022, 7, e160940. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Boneva, S.; Rosmus, D.D.; Agostini, H.; Schlunck, G.; Wieghofer, P.; Schlecht, A.; Lange, C. In-Depth Molecular Profiling Specifies Human Retinal Microglia Identity. Front. Immunol. 2022, 13, 863158. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional microglia–neuron communication in health and disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar]
- Rivera, J.C.; Holm, M.; Austeng, D.; Morken, T.S.; Zhou, T.E.; Beaudry-Richard, A.; Sierra, E.M.; Dammann, O.; Chemtob, S. Retinopathy of prematurity: Inflammation, choroidal degeneration, and novel promising therapeutic strategies. J. Neuroinflamm. 2017, 14, 165. [Google Scholar]
- Wu, W.-C.; Shih, C.-P.; Wang, N.-K.; Lien, R.; Chen, Y.-P.; Chao, A.-N.; Chen, K.-J.; Chen, T.-L.; Hwang, Y.-S.; Lai, C.-C. Choroidal thickness in patients with a history of retinopathy of prematurity. JAMA Ophthalmol. 2013, 131, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Dorfman, A.L.; Seshadri, S.; Djavari, M.; Kermorvant-Duchemin, E.; Sennlaub, F.; Blais, M.; Polosa, A.; Varma, D.R.; Joyal, J.-S.; et al. Choroidal involution is a key component of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6238–6248. [Google Scholar] [CrossRef]
- Kaur, C.; Sivakumar, V.; Foulds, W.S.; Luu, C.D.; Ling, E.-A. Cellular and vascular changes in the retina of neonatal rats after an acute exposure to hypoxia. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5364–5374. [Google Scholar] [CrossRef]
- Zin, A.; Gole, G.A. Retinopathy of prematurity-incidence today. Clin. Perinatol. 2013, 40, 185–200. [Google Scholar] [CrossRef]
- Raghuveer, T.S.; Bloom, B.T. A paradigm shift in the prevention of retinopathy of prematurity. Neonatology 2011, 100, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E.; Penn, J.S. Mechanisms and management of retinopathy of prematurity. N. Engl. J. Med. 2012, 367, 2515–2526. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D’amato, R.; Sullivan, R.; D’Amore, P.A. Oxygen-induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Mutlu, F.M.; Sarici, S.U. Treatment of retinopathy of prematurity: A review of conventional and promising new therapeutic options. Int. J. Ophthalmol. 2013, 6, 228. [Google Scholar]
- Hellström, A.; Smith, L.E.; Dammann, O. Retinopathy of prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef]
- Hartnett, M.E. Advances in understanding and management of retinopathy of prematurity. Surv. Ophthalmol. 2017, 62, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Dammann, O. Perinatal Infection, Inflammation, and Retinopathy of Prematurity. Semin. Fetal Neonatal Med. 2012, 17, 26–29. [Google Scholar] [CrossRef]
- Kim, C.Y.; Jung, E.; Kim, E.N.; Kim, C.J.; Lee, J.Y.; Hwang, J.H.; Song, W.S.; Lee, B.S.; Kim, E.A.-R.; Kim, K.-S. Chronic placental inflammation as a risk factor of severe retinopathy of prematurity. J. Pathol. Transl. Med. 2018, 52, 290. [Google Scholar] [CrossRef]
- Silveira, R.C.; Fortes Filho, J.B.; Procianoy, R.S. Assessment of the contribution of cytokine plasma levels to detect retinopathy of prematurity in very low birth weight infants. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Sood, B.G.; Madan, A.; Saha, S.; Schendel, D.; Thorsen, P.; Skogstrand, K.; Hougaard, D.; Shankaran, S.; Carlo, W. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr. Res. 2010, 67, 394–400. [Google Scholar] [CrossRef]
- Fischer, F.; Martin, G.; Agostini, H.T. Activation of retinal microglia rather than microglial cell density correlates with retinal neovascularization in the mouse model of oxygen-induced retinopathy. J. Neuroinflamm. 2011, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Checchin, D.; Sennlaub, F.; Levavasseur, E.; Leduc, M.; Chemtob, S. Potential role of microglia in retinal blood vessel formation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3595–3602. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.R.; Banin, E.; Moreno, S.K.; Aguilar, E.; Dorrell, M.I.; Friedlander, M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J. Clin. Investig. 2006, 116, 3266–3276. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tsang, J.K.W.; Fung, F.K.C.; Chung, S.K.; Fu, Z.; Lo, A.C.Y. Retinal microglia protect against vascular damage in a mouse model of retinopathy of prematurity. Front. Pharmacol. 2022, 13, 945130. [Google Scholar] [CrossRef]
- Smith, W.; Assink, J.; Klein, R.; Mitchell, P.; Klaver, C.C.; Klein, B.E.; Hofman, A.; Jensen, S.; Wang, J.J.; de Jong, P.T. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology 2001, 108, 697–704. [Google Scholar] [CrossRef]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef]
- Bowes Rickman, C.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry age-related macular degeneration: Mechanisms, therapeutic targets, and imaging. Investig. Opthalmol. Vis. Sci. 2013, 54, ORSF68–ORSF80. [Google Scholar] [CrossRef]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef]
- Chen, Y.; Bedell, M.; Zhang, K. Age-related macular degeneration: Genetic and environmental factors of disease. Mol. Interv. 2010, 10, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Reynolds, R.; Yu, Y.; Daly, M.J.; Rosner, B. Risk models for progression to advanced age-related macular degeneration using demographic, environmental, genetic, and ocular factors. Ophthalmology 2011, 118, 2203–2211. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Mele, M.C.; Merendino, N.; Cintoni, M.; Anselmi, G.; Caporossi, A.; Gasbarrini, A.; Minnella, A.M. The Role of Diet, Micronutrients and the Gut Microbiota in Age-Related Macular Degeneration: New Perspectives from the Gut-Retina Axis. Nutrients 2018, 10, 1677. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef]
- Behnke, V.; Wolf, A.; Langmann, T. The role of lymphocytes and phagocytes in age-related macular degeneration (AMD). Cell. Mol. Life Sci. 2020, 77, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, H. Parainflammation, chronic inflammation, and age-related macular degeneration. J. Leukoc. Biol. 2015, 98, 713–725. [Google Scholar] [CrossRef]
- Gupta, N.; Brown, K.E.; Milam, A.H. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp. Eye Res. 2003, 76, 463–471. [Google Scholar] [CrossRef]
- Silverman, S.M.; Wong, W.T. Microglia in the Retina: Roles in Development, Maturity, and Disease. Annu. Rev. Vis. Sci. 2018, 4, 45–77. [Google Scholar] [CrossRef]
- Dietrich, L.; Lucius, R.; Roider, J.; Klettner, A. Interaction of inflammatorily activated retinal pigment epithelium with retinal microglia and neuronal cells. Exp. Eye Res. 2020, 199, 108167. [Google Scholar] [CrossRef]
- Rathnasamy, G.; Foulds, W.S.; Ling, E.-A.; Kaur, C. Retinal microglia—A key player in healthy and diseased retina. Prog. Neurobiol. 2019, 173, 18–40. [Google Scholar] [CrossRef]
- Madeira, M.H.; Boia, R.; Santos, P.F.; Ambrósio, A.F.; Santiago, A.R. Contribution of Microglia-Mediated Neuroinflammation to Retinal Degenerative Diseases. Mediat. Inflamm. 2015, 2015, 673090. [Google Scholar] [CrossRef]
- Wang, S.K.; Xue, Y.; Cepko, C.L. Microglia modulation by TGF-β1 protects cones in mouse models of retinal degeneration. J. Clin. Investig. 2020, 130, 4360–4369. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, P.; Xie, X.; Tomita, Y.; Cho, S.; Tsirukis, D.; Lam, E.; Luo, H.R.; Sun, Y. Myeloid lineage contributes to pathological choroidal neovascularization formation via SOCS3. EBioMedicine 2021, 73, 103632. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, C.; Sonoda, K.H.; Egashira, K.; Qiao, H.; Hisatomi, T.; Nakao, S.; Ishibashi, M.; Charo, I.F.; Sakamoto, T.; Murata, T.; et al. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J. Leukoc. Biol. 2003, 74, 25–32. [Google Scholar] [CrossRef]
- Tan, X.; Fujiu, K.; Manabe, I.; Nishida, J.; Yamagishi, R.; Terashima, Y.; Matsushima, K.; Kaburaki, T.; Nagai, R.; Yanagi, Y. Choroidal Neovascularization Is Inhibited in Splenic-Denervated or Splenectomized Mice with a Concomitant Decrease in Intraocular Macrophage. PLoS ONE 2016, 11, e0160985. [Google Scholar] [CrossRef]
- Droho, S.; Thomson, B.R.; Makinde, H.M.; Cuda, C.M.; Perlman, H.; Lavine, J.A. Ocular macrophage origin and heterogeneity during steady state and experimental choroidal neovascularization. J. Neuroinflamm. 2020, 17, 341. [Google Scholar] [CrossRef] [PubMed]
- Droho, S.; Rajesh, A.; Cuda, C.M.; Perlman, H.; Lavine, J.A. CD11c+ macrophages are proangiogenic and necessary for experimental choroidal neovascularization. JCI Insight 2023, 8, e168142. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Cheung, G.C.M.; Wong, T.Y. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Federation, I.D. IDF Diabetes Atlas, 6th ed; Novo Nordisk: Bagsværd, Denmark, 2015. [Google Scholar]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.-P.; Simo, R. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Powell, E.U.; Field, R. Diabetic retinopathy and rheumatoid arthritis. Lancet 1964, 284, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of inflammation in diabetic retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Ruiz, R.; Montalvo-Martínez, L.; Fuentes-Mera, L.; Camacho, A. Microglia activation due to obesity programs metabolic failure leading to type two diabetes. Nutr. Diabetes 2017, 7, e254. [Google Scholar] [CrossRef]
- Hsieh, C.-F.; Liu, C.-K.; Lee, C.-T.; Yu, L.-E.; Wang, J.-Y. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci. Rep. 2019, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Z.; Li, Y.; Li, L.; Shah, K.H.; Bernstein, K.E.; Lyden, P.; Shi, P. Microglia participate in neurogenic regulation of hypertension. Hypertension 2015, 66, 309–316. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Green, W.R.; Tso, M.O. Microglial activation in human diabetic retinopathy. Arch. Ophthalmol. 2008, 126, 227–232. [Google Scholar] [CrossRef]
- Saadane, A.; Veenstra, A.A.; Minns, M.S.; Tang, J.; Du, Y.; Abubakr Elghazali, F.; Lessieur, E.M.; Pearlman, E.; Kern, T.S. CCR2-positive monocytes contribute to the pathogenesis of early diabetic retinopathy in mice. Diabetologia 2023, 66, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.; McGuire, P.G.; Franco Nitta, C.; Monickaraj, F.; Oruganti, S.R.; Das, A. Chemokine mediated monocyte trafficking into the retina: Role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS ONE 2014, 9, e108508. [Google Scholar] [CrossRef] [PubMed]
- Beli, E.; Dominguez, J.M., 2nd; Hu, P.; Thinschmidt, J.S.; Caballero, S.; Li Calzi, S.; Luo, D.; Shanmugam, S.; Salazar, T.E.; Duan, Y.; et al. CX3CR1 deficiency accelerates the development of retinopathy in a rodent model of type 1 diabetes. J. Mol. Med. 2016, 94, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Chen, M.; Xu, H. Complement gene expression and regulation in mouse retina and retinal pigment epithelium/choroid. Mol. Vis. 2011, 17, 1588. [Google Scholar]
- Schäfer, N.; Grosche, A.; Schmitt, S.I.; Braunger, B.M.; Pauly, D. Complement components showed a time-dependent local expression pattern in constant and acute white light-induced photoreceptor damage. Front. Mol. Neurosci. 2017, 10, 197. [Google Scholar] [CrossRef]
- Rutar, M.; Natoli, R.; Albarracin, R.; Valter, K.; Provis, J. 670-nm light treatment reduces complement propagation following retinal degeneration. J. Neuroinflamm. 2012, 9, 257. [Google Scholar] [CrossRef]
- Xu, H.; Chen, M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur. J. Pharmacol. 2016, 787, 94–104. [Google Scholar] [CrossRef]
- Chen, M.; Muckersie, E.; Forrester, J.V.; Xu, H. Immune activation in retinal aging: A gene expression study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5888–5896. [Google Scholar] [CrossRef]
- Chen, M.; Muckersie, E.; Robertson, M.; Forrester, J.V.; Xu, H. Up-regulation of complement factor B in retinal pigment epithelial cells is accompanied by complement activation in the aged retina. Exp. Eye Res. 2008, 87, 543–550. [Google Scholar] [CrossRef]
- Ma, W.; Cojocaru, R.; Gotoh, N.; Gieser, L.; Villasmil, R.; Cogliati, T.; Swaroop, A.; Wong, W.T. Gene expression changes in aging retinal microglia: Relationship to microglial support functions and regulation of activation. Neurobiol. Aging 2013, 34, 2310–2321. [Google Scholar] [CrossRef]
- Pauly, D.; Agarwal, D.; Dana, N.; Schäfer, N.; Biber, J.; Wunderlich, K.A.; Jabri, Y.; Straub, T.; Zhang, N.R.; Gautam, A.K.; et al. Cell-Type-Specific Complement Expression in the Healthy and Diseased Retina. Cell Rep. 2019, 29, 2835–2848.e4. [Google Scholar] [CrossRef] [PubMed]
- Chirco, K.R.; Tucker, B.A.; Stone, E.M.; Mullins, R.F. Selective accumulation of the complement membrane attack complex in aging choriocapillaris. Exp. Eye Res. 2016, 146, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.F.; Khanna, A.; Schoo, D.P.; Tucker, B.A.; Sohn, E.H.; Drack, A.V.; Stone, E.M. Is age-related macular degeneration a microvascular disease? Retin. Degener. Dis. 2014, 801, 283–289. [Google Scholar]
- Park, D.H.; Connor, K.M.; Lambris, J.D. The Challenges and Promise of Complement Therapeutics for Ocular Diseases. Front. Immunol. 2019, 10, 1007. [Google Scholar] [CrossRef]
- Rathi, S.; Jalali, S.; Patnaik, S.; Shahulhameed, S.; Musada, G.R.; Balakrishnan, D.; Rani, P.K.; Kekunnaya, R.; Chhablani, P.P.; Swain, S.; et al. Abnormal Complement Activation and Inflammation in the Pathogenesis of Retinopathy of Prematurity. Front. Immunol. 2017, 8, 1868. [Google Scholar] [CrossRef]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.-Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T. Complement factor H polymorphism in age-related macular degeneration. Science 2005, 308, 385–389. [Google Scholar] [CrossRef]
- Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; Hancox, L.S.; Taiber, A.J.; Hardisty, L.I.; Hageman, J.L.; Stockman, H.A.; Borchardt, J.D.; Gehrs, K.M. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 7227–7232. [Google Scholar] [CrossRef]
- Haines, J.L.; Hauser, M.A.; Schmidt, S.; Scott, W.K.; Olson, L.M.; Gallins, P.; Spencer, K.L.; Kwan, S.Y.; Noureddine, M.; Gilbert, J.R. Complement factor H variant increases the risk of age-related macular degeneration. Science 2005, 308, 419–421. [Google Scholar] [CrossRef]
- Gold, B.; Merriam, J.E.; Zernant, J.; Hancox, L.S.; Taiber, A.J.; Gehrs, K.; Cramer, K.; Neel, J.; Bergeron, J.; Barile, G.R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 2006, 38, 458–462. [Google Scholar] [CrossRef]
- Maller, J.; George, S.; Purcell, S.; Fagerness, J.; Altshuler, D.; Daly, M.J.; Seddon, J.M. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat. Genet. 2006, 38, 1055–1059. [Google Scholar] [CrossRef]
- Yates, J.R.; Sepp, T.; Matharu, B.K.; Khan, J.C.; Thurlby, D.A.; Shahid, H.; Clayton, D.G.; Hayward, C.; Morgan, J.; Wright, A.F. Complement C3 variant and the risk of age-related macular degeneration. N. Engl. J. Med. 2007, 357, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.L.; Olson, L.M.; Anderson, B.M.; Schnetz-Boutaud, N.; Scott, W.K.; Gallins, P.; Agarwal, A.; Postel, E.A.; Pericak-Vance, M.A.; Haines, J.L. C3 R102G polymorphism increases risk of age-related macular degeneration. Hum. Mol. Genet. 2008, 17, 1821–1824. [Google Scholar] [CrossRef]
- Baas, D.C.; Ho, L.; Ennis, S.; Merriam, J.E.; Tanck, M.W.; Uitterlinden, A.G.; de Jong, P.T.; Cree, A.J.; Griffiths, H.L.; Rivadeneira, F. The complement component 5 gene and age-related macular degeneration. Ophthalmology 2010, 117, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Despriet, D.D.; van Duijn, C.M.; Oostra, B.A.; Uitterlinden, A.G.; Hofman, A.; Wright, A.F.; Jacoline, B.; Bakker, A.; de Jong, P.T.; Vingerling, J.R. Complement component C3 and risk of age-related macular degeneration. Ophthalmology 2009, 116, 474–480.e2. [Google Scholar] [CrossRef] [PubMed]
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Jonas, J.B.; Tao, Y.; Neumaier, M.; Findeisen, P. Cytokine concentration in aqueous humour of eyes with exudative age-related macular degeneration. Acta Ophthalmol. 2012, 90, e381–e388. [Google Scholar] [CrossRef]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef]
- Shahulhameed, S.; Vishwakarma, S.; Chhablani, J.; Tyagi, M.; Pappuru, R.R.; Jakati, S.; Chakrabarti, S.; Kaur, I. A Systematic Investigation on Complement Pathway Activation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 154. [Google Scholar] [CrossRef]
- Smith, J.M.; Mandava, N.; Tirado-Gonzalez, V.; Garcia-Santisteban, R.; Geiger, M.D.; Patnaik, J.L.; Frazer-Abel, A.; Lynch, A.M.; Mandava, N.; Holers, V.M.; et al. Correlation of Complement Activation in Aqueous and Vitreous in Patients With Proliferative Diabetic Retinopathy. Transl. Vis. Sci. Technol. 2022, 11, 13. [Google Scholar] [CrossRef]
- Dinarello, C.A. Historical insights into cytokines. Eur. J. Immunol. 2007, 37 (Suppl. S1), S34–S45. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, Z.; Liu, C.-H.; Gong, Y.; Liegl, R.; Fredrick, T.W.; Meng, S.S.; Burnim, S.B.; Wang, Z.; Akula, J.D.; et al. Inflammatory signals from photoreceptor modulate pathological retinal angiogenesis via c-Fos. J. Exp. Med. 2017, 214, 1753–1767. [Google Scholar] [CrossRef]
- Hong, S.; Van Kaer, L. Immune privilege: Keeping an eye on natural killer T cells. J. Exp. Med. 1999, 190, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Wu, C.-C.; Roy, S.; Lee, S.-M.; Liu, J.-H. Increased interleukin-6 in aqueous humor of neovascular glaucoma. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2627–2632. [Google Scholar]
- Yi, Q.Y.; Wang, Y.Y.; Chen, L.S.; Li, W.D.; Shen, Y.; Jin, Y.; Yang, J.; Wang, Y.; Yuan, J.; Cheng, L. Implication of inflammatory cytokines in the aqueous humour for management of macular diseases. Acta Ophthalmol. 2020, 98, e309–e315. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.I.; Tombran-Tink, J.; Hykin, P.G.; Gregor, Z.J.; Cree, I.A. Vitreous and aqueous concentrations of proangiogenic, antiangiogenic factors and other cytokines in diabetic retinopathy patients with macular edema: Implications for structural differences in macular profiles. Exp. Eye Res. 2006, 82, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, S.; Xia, X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr. Eye Res. 2012, 37, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Eastlake, K.; Banerjee, P.; Angbohang, A.; Charteris, D.; Khaw, P.; Limb, G. Müller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathy. Glia 2016, 64, 495–506. [Google Scholar] [CrossRef]
- Xi, H.; Katschke Jr, K.J.; Li, Y.; Truong, T.; Lee, W.P.; Diehl, L.; Rangell, L.; Tao, J.; Arceo, R.; Eastham-Anderson, J. IL-33 amplifies an innate immune response in the degenerating retina. J. Exp. Med. 2016, 213, 189–207. [Google Scholar] [CrossRef]
- Mimura, T.; Funatsu, H.; Noma, H.; Shimura, M.; Kamei, Y.; Yoshida, M.; Kondo, A.; Watanabe, E.; Mizota, A. Aqueous Humor Levels of Cytokines in Patients with Age-Related Macular Degeneration. Ophthalmologica 2019, 241, 81–89. [Google Scholar] [CrossRef]
- Sakurada, Y.; Nakamura, Y.; Yoneyama, S.; Mabuchi, F.; Gotoh, T.; Tateno, Y.; Sugiyama, A.; Kubota, T.; Iijima, H. Aqueous humor cytokine levels in patients with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Ophthalmic Res. 2015, 53, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, X.; Yuan, M.; Chen, Y. Comparison of cytokine levels in the aqueous humor of polypoidal choroidal vasculopathy and neovascular age-related macular degeneration patients. BMC Ophthalmol. 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Balne, P.K.; Wei, X.; Bijin, V.A.; Lee, B.; Ghosh, A.; Narayanan, R.; Agrawal, M.; Connolly, J. Cytokine profiling in patients with exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 376–382. [Google Scholar] [CrossRef]
- Wei, X.; Balne, P.K.; Au, V.B.; Lee, B.; Khandelwal, N.; Connolly, J.; Narayanan, R.; Sethu, S.; Agrawal, R. Cytokine profiling in patients with exudative age-related macular degeneration due to choroidal neovascularization and polypoidal choroidal vasculopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 349. [Google Scholar]
- Sato, T.; Takeuchi, M.; Karasawa, Y.; Takayama, K.; Enoki, T. Comprehensive expression patterns of inflammatory cytokines in aqueous humor of patients with neovascular age-related macular degeneration. Sci. Rep. 2019, 9, 19447. [Google Scholar] [CrossRef] [PubMed]
- Subhi, Y.; Krogh Nielsen, M.; Molbech, C.R.; Oishi, A.; Singh, A.; Nissen, M.H.; Sørensen, T.L. Plasma markers of chronic low-grade inflammation in polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Acta Ophthalmol. 2019, 97, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Ko, A.; Partanen, M.; Pakzad-Vaezi, K.; Merkur, A.B.; Albiani, D.A.; Kirker, A.W.; Wang, A.; Cui, J.Z.; Forooghian, F.; et al. Relationship between systemic cytokines and complement factor H Y402H polymorphism in patients with dry age-related macular degeneration. Am. J. Ophthalmol. 2013, 156, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Krogh Nielsen, M.; Subhi, Y.; Molbech, C.R.; Falk, M.K.; Nissen, M.H.; Sørensen, T.L. Systemic Levels of Interleukin-6 Correlate With Progression Rate of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Izumi-Nagai, K.; Nagai, N.; Ozawa, Y.; Mihara, M.; Ohsugi, Y.; Kurihara, T.; Koto, T.; Satofuka, S.; Inoue, M.; Tsubota, K. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am. J. Pathol. 2007, 170, 2149–2158. [Google Scholar] [CrossRef]
- Doyle, S.L.; Ozaki, E.; Brennan, K.; Humphries, M.M.; Mulfaul, K.; Keaney, J.; Kenna, P.F.; Maminishkis, A.; Kiang, A.-S.; Saunders, S.P.; et al. IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration. Sci. Transl. Med. 2014, 6, 230ra44. [Google Scholar] [CrossRef]
- Touhami, S.; Beguier, F.; Augustin, S.; Charles-Messance, H.; Vignaud, L.; Nandrot, E.F.; Reichman, S.; Forster, V.; Mathis, T.; Sahel, J.-A. Chronic exposure to tumor necrosis factor alpha induces retinal pigment epithelium cell dedifferentiation. J. Neuroinflamm. 2018, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Parkunan, S.M.; Randall, C.B.; Astley, R.A.; Furtado, G.C.; Lira, S.A.; Callegan, M.C. CXCL1, but not IL-6, significantly impacts intraocular inflammation during infection. J. Leukoc. Biol. 2016, 100, 1125–1134. [Google Scholar] [CrossRef]
- Sakamoto, S.; Takahashi, H.; Tan, X.; Inoue, Y.; Nomura, Y.; Arai, Y.; Fujino, Y.; Kawashima, H.; Yanagi, Y. Changes in multiple cytokine concentrations in the aqueous humour of neovascular age-related macular degeneration after 2 months of ranibizumab therapy. Br. J. Ophthalmol. 2018, 102, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Monickaraj, F.; Acosta, G.; Cabrera, A.; Das, A. The Chemokine CXCL1 Contributes to Vascular Inflammation and Disruption of Tight-Junctions Associated with Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2021, 62, 3032. [Google Scholar]
- Bucher, F.; Aguilar, E.; Marra, K.V.; Rapp, J.; Arnold, J.; Diaz-Aguilar, S.; Lange, C.; Agostini, H.; Schlunck, G.; Stahl, A. CNTF Prevents Development of Outer Retinal Neovascularization Through Upregulation of CxCl10. Investig. Ophthalmol. Vis. Sci. 2020, 61, 20. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Jia, J.; Yao, J.; Xu, Z. Stromal cell-derived factor 1 (SDF-1) and its receptor CXCR4 improves diabetic retinopathy. Biosci. Biotechnol. Biochem. 2019, 83, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Tuo, J.; Bojanowski, C.M.; Zhou, M.; Shen, D.; Ross, R.J.; Rosenberg, K.I.; Cameron, D.J.; Yin, C.; Kowalak, J.A.; Zhuang, Z. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3827–3836. [Google Scholar] [CrossRef]
- Taghavi, Y.; Hassanshahi, G.; Kounis, N.G.; Koniari, I.; Khorramdelazad, H. Monocyte chemoattractant protein-1 (MCP-1/CCL2) in diabetic retinopathy: Latest evidence and clinical considerations. J. Cell Commun. Signal. 2019, 13, 451–462. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Subhi, Y.; Molbech, C.R.; Falk, M.K.; Nissen, M.H.; Sørensen, T.L. Chemokine profile and the alterations in CCR5-CCL5 axis in geographic atrophy secondary to age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 28. [Google Scholar] [CrossRef]
- Monickaraj, F.; Oruganti, S.R.; McGuire, P.; Das, A. A potential novel therapeutic target in diabetic retinopathy: A chemokine receptor (CCR2/CCR5) inhibitor reduces retinal vascular leakage in an animal model. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.K.; Singh, A.; Faber, C.; Nissen, M.H.; Hviid, T.; Sørensen, T.L. Blood expression levels of chemokine receptor CCR3 and chemokine CCL11 in age-related macular degeneration: A case–control study. BMC Ophthalmol. 2014, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.M.; Becerra, Y.G.; García, M. Blood expression of CCL11 in patients with active choroidal neovascularization associated with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5368. [Google Scholar]
- Sharma, N.K.; Prabhakar, S.; Gupta, A.; Singh, R.; Gupta, P.K.; Gupta, P.K.; Anand, A. New biomarker for neovascular age-related macular degeneration: Eotaxin-2. DNA Cell Biol. 2012, 31, 1618–1627. [Google Scholar] [CrossRef]
- Tuo, J.; Smith, B.C.; Bojanowski, C.M.; Meleth, A.D.; Gery, I.; Csaky, K.G.; Chew, E.Y.; Chan, C.-C. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004, 18, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D.; Kochanowski, J.; Pyzlak, M.; Szewczyk, G.; Stangret, A.; Mittal, T.K. Fractalkine (CX3CL1) and its receptor CX3CR1 may contribute to increased angiogenesis in diabetic placenta. Mediat. Inflamm. 2013, 2013, 437576. [Google Scholar] [CrossRef]
- Zabel, M.K.; Zhao, L.; Zhang, Y.; Gonzalez, S.R.; Ma, W.; Wang, X.; Fariss, R.N.; Wong, W.T. Microglial phagocytosis and activation underlying photoreceptor degeneration is regulated by CX3CL1-CX3CR1 signaling in a mouse model of retinitis pigmentosa. Glia 2016, 64, 1479–1491. [Google Scholar] [CrossRef]
- Hang, H.; Yuan, S.; Yang, Q.; Yuan, D.; Liu, Q. Multiplex bead array assay of plasma cytokines in type 2 diabetes mellitus with diabetic retinopathy. Mol. Vis. 2014, 20, 1137. [Google Scholar]
- Kojima, H.; Otani, A.; Oishi, A.; Makiyama, Y.; Nakagawa, S.; Yoshimura, N. Granulocyte colony-stimulating factor attenuates oxidative stress–induced apoptosis in vascular endothelial cells and exhibits functional and morphologic protective effect in oxygen-induced retinopathy. Blood J. Am. Soc. Hematol. 2011, 117, 1091–1100. [Google Scholar] [CrossRef]
- Sun, T.; Wei, Q.; Gao, P.; Zhang, Y.; Peng, Q. Cytokine and Chemokine Profile Changes in Patients with Neovascular Age-Related Macular Degeneration After Intravitreal Ranibizumab Injection for Choroidal Neovascularization. Drug Des. Dev. Ther. 2021, 15, 2457. [Google Scholar] [CrossRef]
- Litwińska, Z.; Sobuś, A.; Łuczkowska, K.; Grabowicz, A.; Mozolewska-Piotrowska, K.; Safranow, K.; Kawa, M.P.; Machaliński, B.; Machalińska, A. The interplay between systemic inflammatory factors and microRNAs in age-related macular degeneration. Front. Aging Neurosci. 2019, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Takubo, K.; Shimizu, T.; Ohno, H.; Kishi, K.; Shibuya, M.; Saya, H.; Suda, T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J. Exp. Med. 2009, 206, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kobayashi, Y.; Nakama, T.; Zhou, Y.; Ishikawa, K.; Arita, R.; Nakao, S.; Miyazaki, M.; Sassa, Y.; Oshima, Y. Increased expression of M-CSF and IL-13 in vitreous of patients with proliferative diabetic retinopathy: Implications for M2 macrophage-involving fibrovascular membrane formation. Br. J. Ophthalmol. 2015, 99, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Engler, C.B.; Sander, B.; Bendtzen, K. IFN-α Antibodies in Patients with Age-Related Macular Degeneration Treated with Recombinant Human IFN-α 2a. J. Interferon Cytokine Res. 2002, 22, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Lückoff, A.; Caramoy, A.; Scholz, R.; Prinz, M.; Kalinke, U.; Langmann, T. Interferon-beta signaling in retinal mononuclear phagocytes attenuates pathological neovascularization. EMBO Mol. Med. 2016, 8, 670–678. [Google Scholar] [CrossRef]
- Behnke, V.; Langmann, T. IFN-β signaling dampens microglia reactivity but does not prevent from light-induced retinal degeneration. Biochem. Biophys. Rep. 2020, 24, 100866. [Google Scholar] [CrossRef]
- Yu, Y.; Ren, X.R.; Wen, F.; Chen, H.; Su, S.B. T-helper-associated cytokines expression by peripheral blood mononuclear cells in patients with polypoidal choroidal vasculopathy and age-related macular degeneration. BMC Ophthalmol. 2016, 16, 80. [Google Scholar] [CrossRef]
- Nassar, K.; Grisanti, S.; Elfar, E.; Lüke, J.; Lüke, M.; Grisanti, S. Serum cytokines as biomarkers for age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, S.; D’Amico, A.G.; Federico, C.; Saccone, S.; Magro, G.; Bucolo, C.; Drago, F.; D’Agata, V. Different Retinal Expression Patterns of IL-1α, IL-1β, and Their Receptors in a Rat Model of Type 1 STZ-Induced Diabetes. J. Mol. Neurosci. 2015, 56, 431–439. [Google Scholar] [CrossRef]
- Zhao, M.; Bai, Y.; Xie, W.; Shi, X.; Li, F.; Yang, F.; Sun, Y.; Huang, L.; Li, X. Interleukin-1β level is increased in vitreous of patients with neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV). PLoS ONE 2015, 10, e0125150. [Google Scholar] [CrossRef]
- Mao, C.; Yan, H. Roles of elevated intravitreal IL-1β and IL-10 levels in proliferative diabetic retinopathy. Indian J. Ophthalmol. 2014, 62, 699. [Google Scholar]
- Suzuki, Y.; Nakazawa, M.; Suzuki, K.; Yamazaki, H.; Miyagawa, Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. JPN J. Ophthalmol. 2011, 55, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Miyazaki, D.; Inata, K.; Uotani, R.; Miyake, H.; Sasaki, S.-i.; Shimizu, Y.; Inoue, Y.; Nakamura, K. Role of IL-4 in bone marrow driven dysregulated angiogenesis and age-related macular degeneration. Elife 2020, 9, e54257. [Google Scholar] [CrossRef] [PubMed]
- Chernykh, V.; Varvarinsky, E.; Smirnov, E.; Chernykh, D.; Trunov, A.N. Proliferative and inflammatory factors in the vitreous of patients with proliferative diabetic retinopathy. Indian J. Ophthalmol. 2015, 63, 33. [Google Scholar]
- Seddon, J.M.; George, S.; Rosner, B.; Rifai, N. Progression of age-related macular degeneration: Prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch. Ophthalmol. 2005, 123, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Canataroglu, H.; Varinli, I.; Ozcan, A.A.; Canataroglu, A.; Doran, F.; Varinli, S. Interleukin (IL)-6, interleukin (IL)-8 levels and cellular composition of the vitreous humor in proliferative diabetic retinopathy, proliferative vitreoretinopathy, and traumatic proliferative vitreoretinopathy. Ocul. Immunol. Inflamm. 2005, 13, 375–381. [Google Scholar] [CrossRef]
- Mocan, M.C.; Kadayifcilar, S.; Eldem, B. Elevated intravitreal interleukin-6 levels in patients with proliferative diabetic retinopathy. Can. J. Ophthalmol. 2006, 41, 747–752. [Google Scholar] [CrossRef]
- Woo, S.J.; Park, K.H.; Lee, S.Y.; Ahn, S.J.; Ahn, J.; Park, K.H.; Oh, K.J.; Ryu, A. The relationship between cord blood cytokine levels and perinatal factors and retinopathy of prematurity: A gestational age-matched case-control study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3434–3439. [Google Scholar] [CrossRef]
- Dace, D.S.; Khan, A.A.; Kelly, J.; Apte, R.S. Interleukin-10 promotes pathological angiogenesis by regulating macrophage response to hypoxia during development. PLoS ONE 2008, 3, e3381. [Google Scholar] [CrossRef]
- Rivera, J.C.; Sapieha, P.; Joyal, J.-S.; Duhamel, F.; Shao, Z.; Sitaras, N.; Picard, E.; Zhou, E.; Lachapelle, P.; Chemtob, S. Understanding retinopathy of prematurity: Update on pathogenesis. Neonatology 2011, 100, 343–353. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Ahmad, A.; Allegaert, E.; Siddiquei, M.M.; Gikandi, P.W.; De Hertogh, G.; Opdenakker, G. Interleukin-11 Overexpression and M2 Macrophage Density are Associated with Angiogenic Activity in Proliferative Diabetic Retinopathy. Ocul. Immunol. Inflamm. 2020, 28, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yoshida, S.; Kubo, Y.; Kobayashi, Y.; Nakama, T.; Yamaguchi, M.; Ishikawa, K.; Nakao, S.; Ikeda, Y.; Ishibashi, T. Interleukin-12 inhibits pathological neovascularization in mouse model of oxygen-induced retinopathy. Sci. Rep. 2016, 6, 28140. [Google Scholar] [CrossRef]
- Antunica, A.G.; Karaman, K.; Znaor, L.; Sapunar, A.; Buško, V.; Puzović, V. IL-12 concentrations in the aqueous humor and serum of diabetic retinopathy patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Liu, Z.-L.; Zhang, H.; Gu, F. Interleukin-13 and age-related macular degeneration. Int. J. Ophthalmol. 2017, 10, 535. [Google Scholar]
- Roberge, F.G.; de Smet, M.D.; Benichou, J.; Kriete, M.F.; Raber, J.; Hakimi, J. Treatment of uveitis with recombinant human interleukin-13. Br. J. Ophthalmol. 1998, 82, 1195–1198. [Google Scholar] [PubMed]
- Qiu, A.-W.; Wang, J.-L.; Liu, Q.-H. Blocking IL-17A alleviates diabetic retinopathy in rodents. Cell. Physiol. Biochem. 2017, 41, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tan, W.; Demetriades, A.M.; Cai, Y.; Gao, Y.; Sui, A.; Lu, Q.; Shen, X.; Jiang, C.; Xie, B. Interleukin-17A neutralization alleviated ocular neovascularization by promoting M2 and mitigating M1 macrophage polarization. Immunology 2016, 147, 414–428. [Google Scholar] [CrossRef]
- Song, Z.; Sun, M.; Zhou, F.; Huang, F.; Qu, J.; Chen, D. Increased intravitreous interleukin-18 correlated to vascular endothelial growth factor in patients with active proliferative diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1229–1234. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, B.; Yuan, L.; Peng, Q.; Cheng, L.; Zhong, P.; Yang, X.; Yu, H. Dysregulations of follicular helper T cells through IL-21 pathway in age-related macular degeneration. Mol. Immunol. 2019, 114, 243–250. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sato, T.; Tanaka, A.; Muraoka, T.; Taguchi, M.; Sakurai, Y.; Karasawa, Y.; Ito, M. Elevated levels of cytokines associated with Th2 and Th17 cells in vitreous fluid of proliferative diabetic retinopathy patients. PLoS ONE 2015, 10, e0137358. [Google Scholar] [CrossRef]
- Sasaki, S.-i.; Miyazaki, D.; Miyake, K.-i.; Terasaka, Y.; Kaneda, S.; Ikeda, Y.; Funakoshi, T.; Baba, T.; Yamasaki, A.; Inoue, Y. Associations of IL-23 with polypoidal choroidal vasculopathy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3424–3430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, L.; Huang, R.; Wu, P.; He, L. Comparison of inflammatory cytokines levels in the aqueous humor with diabetic retinopathy. Int. Ophthalmol. 2020, 40, 2763–2769. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, W.-Y.; Zhang, X.-D. Increased interleukin-26 expression in proliferative diabetic retinopathy. Int. J. Ophthalmol. 2019, 12, 1688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; da Cunha, A.; Li, S.; Hao, Q.; Kainz, V.; Huang, Q.; Wu, H. IL-27 regulates HIF-1α-mediated VEGFA response in macrophages of diabetic retinopathy patients and healthy individuals. Cytokine 2019, 113, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, E.; Oshima, Y.; Takeda, A.; Saeki, K.; Yoshida, H.; Sonoda, K.H.; Ishibashi, T. IL-27 inhibits pathophysiological intraocular neovascularization due to laser burn. J. Leukoc. Biol. 2012, 91, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Sato, T.; Sakurai, Y.; Taguchi, M.; Harimoto, K.; Karasawa, Y.; Ito, M. Association between aqueous humor and vitreous fluid levels of Th17 cell-related cytokines in patients with proliferative diabetic retinopathy. PLoS ONE 2017, 12, e0178230. [Google Scholar] [CrossRef]
- Theodoropoulou, S.; Copland, D.A.; Liu, J.; Wu, J.; Gardner, P.J.; Ozaki, E.; Doyle, S.L.; Campbell, M.; Dick, A.D. Interleukin-33 regulates tissue remodelling and inhibits angiogenesis in the eye. J. Pathol. 2017, 241, 45–56. [Google Scholar] [CrossRef]
- Cakir, U.; Tayman, C.; Yucel, C.; Ozdemir, O. Can IL-33 and Endocan be New Markers for Retinopathy of Prematurity? Comb. Chem. High Throughput Screen. 2019, 22, 41–48. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Zhao, Y.; Dua, P.; Rogaev, E.I.; Lukiw, W.J. microRNA-34a-Mediated Down-Regulation of the Microglial-Enriched Triggering Receptor and Phagocytosis-Sensor TREM2 in Age-Related Macular Degeneration. PLoS ONE 2016, 11, e0150211. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lin, Q.; Zhao, M.; Hu, Y.; Yu, Y.; Jin, J.; Zhou, H.; Hu, X.; Wei, R.; Zhang, X. IL-37 is a novel proangiogenic factor of developmental and pathological angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2638–2646. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, R.; Chen, J.; Jin, J.; Yu, Y.; Tian, Y.; Li, W.; Wang, W.; Zhou, H.; Su, S.B. The effect of interleukin 38 on angiogenesis in a model of oxygen-induced retinopathy. Sci. Rep. 2017, 7, 2756. [Google Scholar] [CrossRef]
- Tosi, G.M.; Neri, G.; Caldi, E.; Fusco, F.; Bacci, T.; Tarantello, A.; Nuti, E.; Marigliani, D.; Baiocchi, S.; Traversi, C. TGF-β concentrations and activity are down-regulated in the aqueous humor of patients with neovascular age-related macular degeneration. Sci. Rep. 2018, 8, 8053. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, V.; Platania, C.B.M.; Lazzara, F.; Conti, F.; Pizzo, C.; Reibaldi, M.; Russo, A.; Fallico, M.; Ortisi, E.; Pignatelli, F. TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy. Int. J. Mol. Sci. 2020, 21, 9558. [Google Scholar] [CrossRef]
- Al-Gayyar, M.; Elsherbiny, N. Contribution of TNF-α to the development of retinal neurodegenerative disorders. Eur. Cytokine Netw. 2013, 24, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Doehmen, S.; Le, M.L.; Koizumi, K.; Radetzky, S.; Krohne, T.U.; Poulaki, V.; Semkova, I.; Kociok, N. TNF-α mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol. Vis. 2009, 15, 1418. [Google Scholar]
- Gustavsson, C.; Agardh, E.; Bengtsson, B.; Agardh, C.-D. TNF-α is an independent serum marker for proliferative retinopathy in type 1 diabetic patients. J. Diabetes Its Complicat. 2008, 22, 309–316. [Google Scholar] [CrossRef]
- Whitmore, H.A.; Amarnani, D.; O’Hare, M.; Delgado-Tirado, S.; Gonzalez-Buendia, L.; An, M.; Pedron, J.; Bushweller, J.H.; Arboleda-Velasquez, J.F.; Kim, L.A. TNF-α signaling regulates RUNX1 function in endothelial cells. FASEB J. 2021, 35, e21155. [Google Scholar] [CrossRef]
- Wu, H.; Hwang, D.K.; Song, X.; Tao, Y. Association between Aqueous Cytokines and Diabetic Retinopathy Stage. J. Ophthalmol. 2017, 2017, 9402198. [Google Scholar] [CrossRef]
- Tsai, T.; Kuehn, S.; Tsiampalis, N.; Vu, M.K.; Kakkassery, V.; Stute, G.; Dick, H.B.; Joachim, S.C. Anti-inflammatory cytokine and angiogenic factors levels in vitreous samples of diabetic retinopathy patients. PLoS ONE 2018, 13, e0194603. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yu, H.; Yu, Y.; Geng, Y.; Li, D.; Yang, C.; Lv, Q.; Lu, L.; Liu, T.; Li, G.; et al. Levels of Inflammatory Cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in Aqueous Humour of Patients with Diabetic Retinopathy. J. Diabetes Res. 2018, 2018, 8546423. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Da Mota, S.E.; Soto-Bahena, J.J.; Viveros-Sandoval, M.E.; Cardiel-Ríos, M. Pro-inflammatory serum cytokines in diabetic retinopathy. Cirugía Cir. 2015, 83, 100–106. [Google Scholar] [CrossRef]
- Cardoso, J.F.; Gomes, K.B.; Fernandes, A.P.; Domingueti, C.P. Evaluation of cytokines in type 1 diabetes patients with and without retinopathy. J. Bras. Patol. Med. Lab. 2017, 53, 31–37. [Google Scholar] [CrossRef]
- Jo, D.H.; Yun, J.H.; Cho, C.S.; Kim, J.H.; Kim, J.H.; Cho, C.H. Interaction between microglia and retinal pigment epithelial cells determines the integrity of outer blood-retinal barrier in diabetic retinopathy. Glia 2019, 67, 321–331. [Google Scholar] [CrossRef]
- Kowluru, R.; Odenbach, S. Role of interleukin-1β in the pathogenesis of diabetic retinopathy. Br. J. Ophthalmol. 2004, 88, 1343–1347. [Google Scholar] [CrossRef]
- Nagineni, C.N.; Kommineni, V.K.; Ganjbaksh, N.; Nagineni, K.K.; Hooks, J.J.; Detrick, B. Inflammatory cytokines induce expression of chemokines by human retinal cells: Role in chemokine receptor mediated age-related macular degeneration. Aging Dis. 2015, 6, 444. [Google Scholar] [CrossRef]
- Nakazawa, T.; Hisatomi, T.; Nakazawa, C.; Noda, K.; Maruyama, K.; She, H.; Matsubara, A.; Miyahara, S.; Nakao, S.; Yin, Y. Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proc. Natl. Acad. Sci. USA 2007, 104, 2425–2430. [Google Scholar] [CrossRef]
- Newman, A.M.; Gallo, N.B.; Hancox, L.S.; Miller, N.J.; Radeke, C.M.; Maloney, M.A.; Cooper, J.B.; Hageman, G.S.; Anderson, D.H.; Johnson, L.V. Systems-level analysis of age-related macular degeneration reveals global biomarkers and phenotype-specific functional networks. Genome Med. 2012, 4, 16. [Google Scholar] [CrossRef]
- Takeda, A.; Baffi, J.Z.; Kleinman, M.E.; Cho, W.G.; Nozaki, M.; Yamada, K.; Kaneko, H.; Albuquerque, R.J.; Dridi, S.; Saito, K. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature 2009, 460, 225–230. [Google Scholar] [CrossRef]
- Li, Y.; Huang, D.; Xia, X.; Wang, Z.; Luo, L.; Wen, R. CCR3 and choroidal neovascularization. PLoS ONE 2011, 6, e17106. [Google Scholar] [CrossRef][Green Version]
- Mason, A.B.; Hoh, J. CCR3: Shedding new light on a dark problem? J. Mol. Cell Biol. 2009, 1, 17–19. [Google Scholar] [CrossRef][Green Version]
- El-Asrar, A.M.A.; Struyf, S.; Kangave, D.; Geboes, K.; Van Damme, J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur. Cytokine Netw. 2006, 17, 155–165. [Google Scholar]
- Meleth, A.D.; Agrón, E.; Chan, C.-C.; Reed, G.F.; Arora, K.; Byrnes, G.; Csaky, K.G.; Ferris, F.L.; Chew, E.Y. Serum inflammatory markers in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4295–4301. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Mbonye, U.R.; DeLong, C.J.; Wada, M.; Smith, W.L. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog. Lipid Res. 2007, 46, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Aïd, S.; Bosetti, F. Targeting cyclooxygenases-1 and-2 in neuroinflammation: Therapeutic implications. Biochimie 2011, 93, 46–51. [Google Scholar] [CrossRef]
- Choi, S.-H.; Aid, S.; Bosetti, F. The distinct roles of cyclooxygenase-1 and-2 in neuroinflammation: Implications for translational research. Trends Pharmacol. Sci. 2009, 30, 174–181. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L.; Alousis, N.S.; Kelly, D.J.; Gilbert, R.E. COX-2 inhibition and retinal angiogenesis in a mouse model of retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 2003, 44, 974–979. [Google Scholar] [CrossRef]
- Chin, M.S.; Nagineni, C.N.; Hooper, L.C.; Detrick, B.; Hooks, J.J. Cyclooxygenase-2 gene expression and regulation in human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2338–2346. [Google Scholar]
- Du, Y.; Sarthy, V.P.; Kern, T.S. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R735–R741. [Google Scholar] [CrossRef]
- Surh, Y.-J.; Chun, K.-S.; Cha, H.-H.; Han, S.S.; Keum, Y.-S.; Park, K.-K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 480–481, 243–268. [Google Scholar] [CrossRef]
- Ayalasomayajula, S.P.; Amrite, A.C.; Kompella, U.B. Inhibition of cyclooxygenase-2, but not cyclooxygenase-1, reduces prostaglandin E2 secretion from diabetic rat retinas. Eur. J. Pharmacol. 2004, 498, 275–278. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Engerman, R.L.; Kern, T.S. Abnormalities of retinal metabolism in diabetes or experimental galactosemia VIII. Prevention by aminoguanidine. Curr. Eye Res. 2000, 21, 814–819. [Google Scholar] [CrossRef]
- Du, Y.; Smith, M.A.; Miller, C.M.; Kern, T.S. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J. Neurochem. 2002, 80, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Newman, E.A. Inhibition of inducible nitric oxide synthase reverses the loss of functional hyperemia in diabetic retinopathy. Glia 2010, 58, 1996–2004. [Google Scholar] [CrossRef]
- Heldin, C.-H.; Lu, B.; Evans, R.; Gutkind, J.S. Signals and receptors. Cold Spring Harb. Perspect. Biol. 2016, 8, a005900. [Google Scholar] [CrossRef]
- Newton, A.C.; Bootman, M.D.; Scott, J.D. Second messengers. Cold Spring Harb. Perspect. Biol. 2016, 8, a005926. [Google Scholar] [CrossRef]
- Levy, J.; Zhou, D.; Zippin, J. Cyclic adenosine monophosphate signaling in inflammatory skin disease. J. Clin. Exp. Dermatol. Res. 2016, 7, 1000326. [Google Scholar]
- Raker, V.K.; Becker, C.; Steinbrink, K. The cAMP pathway as therapeutic target in autoimmune and inflammatory diseases. Front. Immunol. 2016, 7, 123. [Google Scholar] [CrossRef]
- Dang, T.A.; Schunkert, H.; Kessler, T. cGMP signaling in cardiovascular diseases: Linking genotype and phenotype. J. Cardiovasc. Pharmacol. 2020, 75, 516–525. [Google Scholar] [CrossRef] [PubMed]
- van der Wijk, A.-E.; Vogels, I.M.; van Noorden, C.J.; Klaassen, I.; Schlingemann, R.O. TNFα-Induced Disruption of the Blood–Retinal Barrier In Vitro Is Regulated by Intracellular 3′, 5′-Cyclic Adenosine Monophosphate Levels. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3496–3505. [Google Scholar] [CrossRef]
- Vincent, S.R. Nitric oxide neurons and neurotransmission. Prog. Neurobiol. 2010, 90, 246–255. [Google Scholar]
- Pepke-Zaba, J.; Higenbottam, T.W.; Dinh-Xuan, A.T.; Stone, D.; Wallwork, J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet 1991, 338, 1173–1174. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar]
- Duarte, A.; Candeias, E.; Correia, S.C.; Santos, R.X.; Carvalho, C.; Cardoso, S.; PLacido, A.; Santos, M.S.; Oliveira, C.R.; Moreira, P.I. The Janus Face of Insulin in Brain. In Metabolic Syndrome and Neurological Disorders; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 85–113. [Google Scholar]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar]
- Anavi, S.; Tirosh, O. iNOS as a metabolic enzyme under stress conditions. Free Radic. Biol. Med. 2020, 146, 16–35. [Google Scholar] [CrossRef]
- Crane, T.; Mellor, D.H. There is no question of physicalism. Mind 1990, 99, 185–206. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar]
- Sennlaub, F.; Courtois, Y.; Goureau, O. Inducible nitric oxide synthase mediates retinal apoptosis in ischemic proliferative retinopathy. J. Neurosci. 2002, 22, 3987–3993. [Google Scholar]
- Sierra, A.; Navascués, J.; Cuadros, M.A.; Calvente, R.; Martín-Oliva, D.; Ferrer-Martín, R.M.; Martín-Estebané, M.; Carrasco, M.-C.; Marín-Teva, J.L. Expression of inducible nitric oxide synthase (iNOS) in microglia of the developing quail retina. PLoS ONE 2014, 9, e106048. [Google Scholar]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Rao, X.; Sigdel, K.R. Regulation of Inflammation in Autoimmune Disease. J. Immunol. Res. 2019, 2019, 7403796. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Gadina, M.; Schreiber, R.D. Cytokine signaling in 2002: New surprises in the Jak/Stat pathway. Cell 2002, 109, S121–S131. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Janus kinases in immune cell signaling. Immunol. Rev. 2009, 228, 273–287. [Google Scholar]
- Arboccó, F.C.V.; Persia, F.A.; Hapon, M.B.; Jahn, G.A. Hypothyroidism decreases JAK/STAT signaling pathway in lactating rat mammary gland. Mol. Cell. Endocrinol. 2017, 450, 14–23. [Google Scholar]
- Malemud, C.J.; Pearlman, E. Targeting JAK/STAT signaling pathway in inflammatory diseases. Curr. Signal Transduct. Ther. 2009, 4, 201–221. [Google Scholar] [CrossRef]
- Thomas, S.; Snowden, J.; Zeidler, M.; Danson, S. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365. [Google Scholar] [CrossRef]
- Wormald, S.; Hilton, D.J. Inhibitors of cytokine signal transduction. J. Biol. Chem. 2004, 279, 821–824. [Google Scholar]
- Lang, R.; Pauleau, A.-L.; Parganas, E.; Takahashi, Y.; Mages, J.; Ihle, J.N.; Rutschman, R.; Murray, P.J. SOCS3 regulates the plasticity of gp130 signaling. Nat. Immunol. 2003, 4, 546–550. [Google Scholar]
- Ozawa, Y.; Nakao, K.; Shimazaki, T.; Shimmura, S.; Kurihara, T.; Ishida, S.; Yoshimura, A.; Tsubota, K.; Okano, H. SOCS3 is required to temporally fine-tune photoreceptor cell differentiation. Dev. Biol. 2007, 303, 591–600. [Google Scholar]
- Ozawa, Y.; Nakao, K.; Kurihara, T.; Shimazaki, T.; Shimmura, S.; Ishida, S.; Yoshimura, A.; Tsubota, K.; Okano, H. Roles of STAT3/SOCS3 pathway in regulating the visual function and ubiquitin-proteasome-dependent degradation of rhodopsin during retinal inflammation. J. Biol. Chem. 2008, 283, 24561–24570. [Google Scholar] [CrossRef]
- Smith, P.D.; Sun, F.; Park, K.K.; Cai, B.; Wang, C.; Kuwako, K.; Martinez-Carrasco, I.; Connolly, L.; He, Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron 2009, 64, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Kurihara, T.; Tsubota, K.; Okano, H. Regulation of posttranscriptional modification as a possible therapeutic approach for retinal neuroprotection. J. Ophthalmol. 2010, 2011, 506137. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Obasanmi, G.; Armstrong, D.; Lavery, N.-J.; Kissenpfennig, A.; Lois, N.; Xu, H. STAT3 activation in circulating myeloid-derived cells contributes to retinal microvascular dysfunction in diabetes. J. Neuroinflamm. 2019, 16, 138. [Google Scholar] [CrossRef]
- Stahl, A.; Joyal, J.-S.; Chen, J.; Sapieha, P.; Juan, A.M.; Hatton, C.J.; Pei, D.T.; Hurst, C.G.; Seaward, M.R.; Krah, N.M. SOCS3 is an endogenous inhibitor of pathologic angiogenesis. Blood J. Am. Soc. Hematol. 2012, 120, 2925–2929. [Google Scholar] [CrossRef]
- Sun, Y.; Ju, M.; Lin, Z.; Fredrick, T.W.; Evans, L.P.; Tian, K.T.; Saba, N.J.; Morss, P.C.; Pu, W.T.; Chen, J. SOCS3 in retinal neurons and glial cells suppresses VEGF signaling to prevent pathological neovascular growth. Sci. Signal. 2015, 8, ra94. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, J.; Ali, I.H.; Marry, S.; Augustine, J.; Bhuckory, M.; Lynch, A.; Kissenpfennig, A.; Xu, H. Cytokine signaling protein 3 deficiency in myeloid cells promotes retinal degeneration and angiogenesis through arginase-1 up-regulation in experimental autoimmune uveoretinitis. Am. J. Pathol. 2018, 188, 1007–1020. [Google Scholar] [CrossRef]
- Wagner, E.F.; Nebreda, Á.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Huang, C.; Rajfur, Z.; Borchers, C.; Schaller, M.D.; Jacobson, K. JNK phosphorylates paxillin and regulates cell migration. Nature 2003, 424, 219–223. [Google Scholar] [CrossRef]
- Liu, G.; Rondinone, C.M. JNK: Bridging the insulin signaling and inflammatory pathway. Curr. Opin. Investig. Drugs 2005, 6, 979–987. [Google Scholar]
- Johnson, G.L.; Nakamura, K. The c-jun kinase/stress-activated pathway: Regulation, function and role in human disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 1341–1348. [Google Scholar] [CrossRef]
- Eferl, R.; Wagner, E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef]
- Pan, M.; Hu, H.; Wang, R.; Zhou, Y.; Zhang, L.; Wang, C.; Wang, Q. JNK1 Induces Notch1 Expression to Regulate Genes Governing Photoreceptor Production. Cells 2019, 8, 970. [Google Scholar] [CrossRef]
- Guma, M.; Rius, J.; Duong-Polk, K.X.; Haddad, G.G.; Lindsey, J.D.; Karin, M. Genetic and pharmacological inhibition of JNK ameliorates hypoxia-induced retinopathy through interference with VEGF expression. Proc. Natl. Acad. Sci. USA 2009, 106, 8760–8765. [Google Scholar] [CrossRef]
- Du, H.; Sun, X.; Guma, M.; Luo, J.; Ouyang, H.; Zhang, X.; Zeng, J.; Quach, J.; Nguyen, D.H.; Shaw, P.X. JNK inhibition reduces apoptosis and neovascularization in a murine model of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 2377–2382. [Google Scholar] [CrossRef]
- Tonade, D.; Kern, T.S. Photoreceptor cells and RPE contribute to the development of diabetic retinopathy. Prog. Retin. Eye Res. 2020, 83, 100919. [Google Scholar] [CrossRef]
- Tonade, D.; Liu, H.; Palczewski, K.; Kern, T.S. Photoreceptor cells produce inflammatory products that contribute to retinal vascular permeability in a mouse model of diabetes. Diabetologia 2017, 60, 2111–2120. [Google Scholar] [CrossRef]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef]
- Tonade, D.; Liu, H.; Kern, T.S. Photoreceptor cells produce inflammatory mediators that contribute to endothelial cell death in diabetes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4264–4271. [Google Scholar] [CrossRef]
- Rana, T.; Kotla, P.; Fullard, R.; Gorbatyuk, M. TNFa knockdown in the retina promotes cone survival in a mouse model of autosomal dominant retinitis pigmentosa. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 92–102. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Wong, E.T.; Tergaonkar, V. Roles of NF-κB in health and disease: Mechanisms and therapeutic potential. Clin. Sci. 2009, 116, 451–465. [Google Scholar] [CrossRef]
- Moynagh, P.N. The NF-κB pathway. J. Cell Sci. 2005, 118, 4589–4592. [Google Scholar] [CrossRef]
- Palazzo, I.; Deistler, K.; Hoang, T.V.; Blackshaw, S.; Fischer, A.J. NF-κB signaling regulates the formation of proliferating Müller glia-derived progenitor cells in the avian retina. Development 2020, 147, dev183418. [Google Scholar]
- Zeng, H.-y.; Tso, M.O.; Lai, S.; Lai, H. Activation of nuclear factor-κB during retinal degeneration in rd mice. Mol. Vis. 2008, 14, 1075. [Google Scholar]
- Wu, T.; Chen, Y.; Chiang, S.K.; Tso, M.O. NF-κB activation in light-induced retinal degeneration in a mouse model. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2834–2840. [Google Scholar]
- Sakamoto, K.; Okuwaki, T.; Ushikubo, H.; Mori, A.; Nakahara, T.; Ishii, K. Activation inhibitors of nuclear factor kappa B protect neurons against the NMDA-induced damage in the rat retina. J. Pharmacol. Sci. 2017, 135, 72–80. [Google Scholar]
- Yoshida, A.; Yoshida, S.; Hata, Y.; Khalil, A.K.; Ishibashi, T.; Inomata, H. The role of NF-κB in retinal neovascularization in the rat: Possible involvement of cytokine-induced neutrophil chemoattractant (CINC), a member of the interleukin-8 family. J. Histochem. Cytochem. 1998, 46, 429–436. [Google Scholar]
- Sui, A.; Chen, X.; Demetriades, A.M.; Shen, J.; Cai, Y.; Yao, Y.; Yao, Y.; Zhu, Y.; Shen, X.; Xie, B. Inhibiting NF-κB Signaling Activation Reduces Retinal Neovascularization by Promoting a Polarization Shift in Macrophages. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4. [Google Scholar] [CrossRef]
- Romeo, G.; Liu, W.-H.; Asnaghi, V.; Kern, T.S.; Lorenzi, M. Activation of nuclear factor-κB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes 2002, 51, 2241–2248. [Google Scholar] [PubMed]
- Rajamani, U.; Jialal, I. Hyperglycemia induces Toll-like receptor-2 and -4 expression and activity in human microvascular retinal endothelial cells: Implications for diabetic retinopathy. J. Diabetes Res. 2014, 2014, 790902. [Google Scholar] [PubMed]
- Miller, J.W.; Adamis, A.P.; Aiello, L.P. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes/Metab. Rev. 1997, 13, 37–50. [Google Scholar] [CrossRef]
- Stone, J.; Itin, A.; Alon, T.; Pe’Er, J.; Gnessin, H.; Chan-Ling, T.; Keshet, E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 1995, 15, 4738–4747. [Google Scholar] [CrossRef] [PubMed]
- Robbins, S.G.; Conaway, J.R.; Ford, B.L.; Roberto, K.A.; Penn, J.S. Detection of vascular endothelial growth factor (VEGF) protein in vascular and non-vascular cells of the normal and oxygen-injured rat retina. Growth Factors 1997, 14, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Northrup, J.M.; Keyt, B.A.; Takagi, H.; Iwamoto, M.A. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch. Ophthalmol. 1995, 113, 1538–1544. [Google Scholar]
- Ida, H.; Tobe, T.; Nambu, H.; Matsumura, M.; Uyama, M.; Campochiaro, P.A. RPE cells modulate subretinal neovascularization, but do not cause regression in mice with sustained expression of VEGF. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5430–5437. [Google Scholar]
- Krause, T.A.; Alex, A.F.; Engel, D.R.; Kurts, C.; Eter, N. VEGF-production by CCR2-dependent macrophages contributes to laser-induced choroidal neovascularization. PLoS ONE 2014, 9, e94313. [Google Scholar] [CrossRef] [PubMed]
- Rezzola, S.; Loda, A.; Corsini, M.; Semeraro, F.; Annese, T.; Presta, M.; Ribatti, D. Angiogenesis-Inflammation Cross Talk in Diabetic Retinopathy: Novel Insights From the Chick Embryo Chorioallantoic Membrane/Human Vitreous Platform. Front. Immunol. 2020, 11, 581288. [Google Scholar]
- Nguyen, Q.D.; Heier, J.S.; Do, D.V.; Mirando, A.C.; Pandey, N.B.; Sheng, H.; Heah, T. The Tie2 signaling pathway in retinal vascular diseases: A novel therapeutic target in the eye. Int. J. Retin. Vitr. 2020, 6, 48. [Google Scholar]
- Gragoudas, E.S.; Adamis, A.P.; Cunningham Jr, E.T.; Feinsod, M.; Guyer, D.R. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.A.; D’Amore, P.A. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am. J. Pathol. 2012, 181, 376–379. [Google Scholar] [CrossRef]
- Yorston, D. Anti-VEGF drugs in the prevention of blindness. Community Eye Health 2014, 27, 44–46. [Google Scholar]
- Fleckenstein, M.; Keenan, T.D.L. Age-related macular degeneration. Lancet 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kim, H.G.; So, J.N.; Kim, J.H.; Kwak, H.J.; Koh, G.Y. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ. Res. 2000, 86, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kontos, C.D.; Cha, E.H.; York, J.D.; Peters, K.G. The endothelial receptor tyrosine kinase Tie1 activates phosphatidylinositol 3-kinase and Akt to inhibit apoptosis. Mol. Cell. Biol. 2002, 22, 1704–1713. [Google Scholar] [CrossRef]
- Daly, C.; Wong, V.; Burova, E.; Wei, Y.; Zabski, S.; Griffiths, J.; Lai, K.M.; Lin, H.C.; Ioffe, E.; Yancopoulos, G.D.; et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1). Genes Dev. 2004, 18, 1060–1071. [Google Scholar] [CrossRef]
- Hughes, D.P.; Marron, M.B.; Brindle, N.P. The antiinflammatory endothelial tyrosine kinase Tie2 interacts with a novel nuclear factor-κB inhibitor ABIN-2. Circ. Res. 2003, 92, 630–636. [Google Scholar] [CrossRef]
- Ziegler, T.; Horstkotte, J.; Schwab, C.; Pfetsch, V.; Weinmann, K.; Dietzel, S.; Rohwedder, I.; Hinkel, R.; Gross, L.; Lee, S.; et al. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J. Clin. Investig. 2013, 123, 3436–3445. [Google Scholar] [CrossRef]
- Menden, H.; Welak, S.; Cossette, S.; Ramchandran, R.; Sampath, V. Lipopolysaccharide (LPS)-mediated angiopoietin-2-dependent autocrine angiogenesis is regulated by NADPH oxidase 2 (Nox2) in human pulmonary microvascular endothelial cells. J. Biol. Chem. 2015, 290, 5449–5461. [Google Scholar] [CrossRef]
- Reiss, Y.; Droste, J.; Heil, M.; Tribulova, S.; Schmidt, M.H.; Schaper, W.; Dumont, D.J.; Plate, K.H. Angiopoietin-2 impairs revascularization after limb ischemia. Circ. Res. 2007, 101, 88–96. [Google Scholar] [CrossRef]
- Kim, M.; Allen, B.; Korhonen, E.A.; Nitschké, M.; Yang, H.W.; Baluk, P.; Saharinen, P.; Alitalo, K.; Daly, C.; Thurston, G.; et al. Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. J. Clin. Investig. 2016, 126, 3511–3525. [Google Scholar] [CrossRef]
- Khalaf, N.; Helmy, H.; Labib, H.; Fahmy, I.; El Hamid, M.A.; Moemen, L. Role of Angiopoietins and Tie-2 in Diabetic Retinopathy. Electron. Physician 2017, 9, 5031–5035. [Google Scholar] [CrossRef] [PubMed]
- Lip, P.L.; Chatterjee, S.; Caine, G.J.; Hope-Ross, M.; Gibson, J.; Blann, A.D.; Lip, G.Y.H. Plasma vascular endothelial growth factor, angiopoietin-2, and soluble angiopoietin receptor tie-2 in diabetic retinopathy: Effects of laser photocoagulation and angiotensin receptor blockade. Br. J. Ophthalmol. 2004, 88, 1543. [Google Scholar] [CrossRef]
- Ng, D.S.; Yip, Y.W.; Bakthavatsalam, M.; Chen, L.J.; Ng, T.K.; Lai, T.Y.; Pang, C.P.; Brelén, M.E. Elevated angiopoietin 2 in aqueous of patients with neovascular age related macular degeneration correlates with disease severity at presentation. Sci. Rep. 2017, 7, 45081. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Shima, C.; Kusaka, S. Vitreous levels of angiopoietin-1 and angiopoietin-2 in eyes with retinopathy of prematurity. Am. J. Ophthalmol. 2011, 151, 353–357.e1. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Frye, M.; Lee, B.L.; Reinardy, J.L.; McClung, J.M.; Ding, K.; Kojima, M.; Xia, H.; Seidel, C.; Lima e Silva, R.; et al. Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J. Clin. Investig. 2014, 124, 4564–4576. [Google Scholar] [CrossRef]
- Regula, J.T.; Lundh von Leithner, P.; Foxton, R.; Barathi, V.A.; Cheung, C.M.; Bo Tun, S.B.; Wey, Y.S.; Iwata, D.; Dostalek, M.; Moelleken, J.; et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2016, 8, 1265–1288. [Google Scholar] [CrossRef]
- Uemura, A.; Fruttiger, M.; D’Amore, P.A.; De Falco, S.; Joussen, A.M.; Sennlaub, F.; Brunck, L.R.; Johnson, K.T.; Lambrou, G.N.; Rittenhouse, K.D.; et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog. Retin. Eye Res. 2021, 84, 100954. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Abreu, F.; Adamis, A.P.; Basu, K.; Eichenbaum, D.A.; Haskova, Z.; Lin, H.; Loewenstein, A.; Mohan, S.; Pearce, I.A.; et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): Two randomised, double-masked, phase 3 trials. Lancet 2022, 399, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.J.; Hsieh, C.M.; Nepali, K.; Liou, J.P. Ocular Disease Therapeutics: Design and Delivery of Drugs for Diseases of the Eye. J. Med. Chem. 2020, 63, 10533–10593. [Google Scholar] [CrossRef]
- Wang, S.K.; Cepko, C.L. Targeting Microglia to Treat Degenerative Eye Diseases. Front. Immunol. 2022, 13, 843558. [Google Scholar] [CrossRef]
- Nazari, H.; Zhang, L.; Zhu, D.; Chader, G.J.; Falabella, P.; Stefanini, F.; Rowland, T.; Clegg, D.O.; Kashani, A.H.; Hinton, D.R.; et al. Stem cell based therapies for age-related macular degeneration: The promises and the challenges. Prog. Retin. Eye Res. 2015, 48, 1–39. [Google Scholar] [CrossRef] [PubMed]
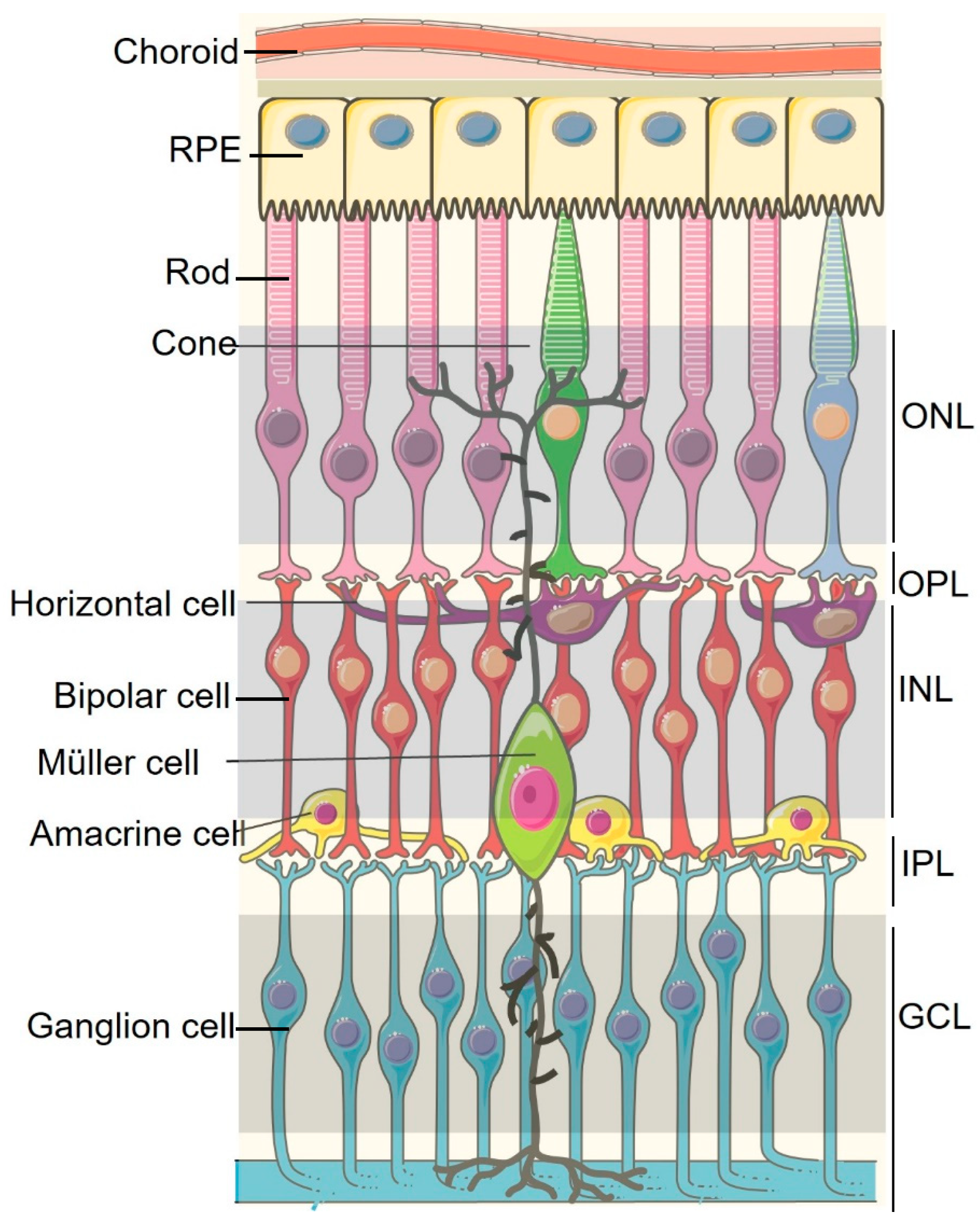
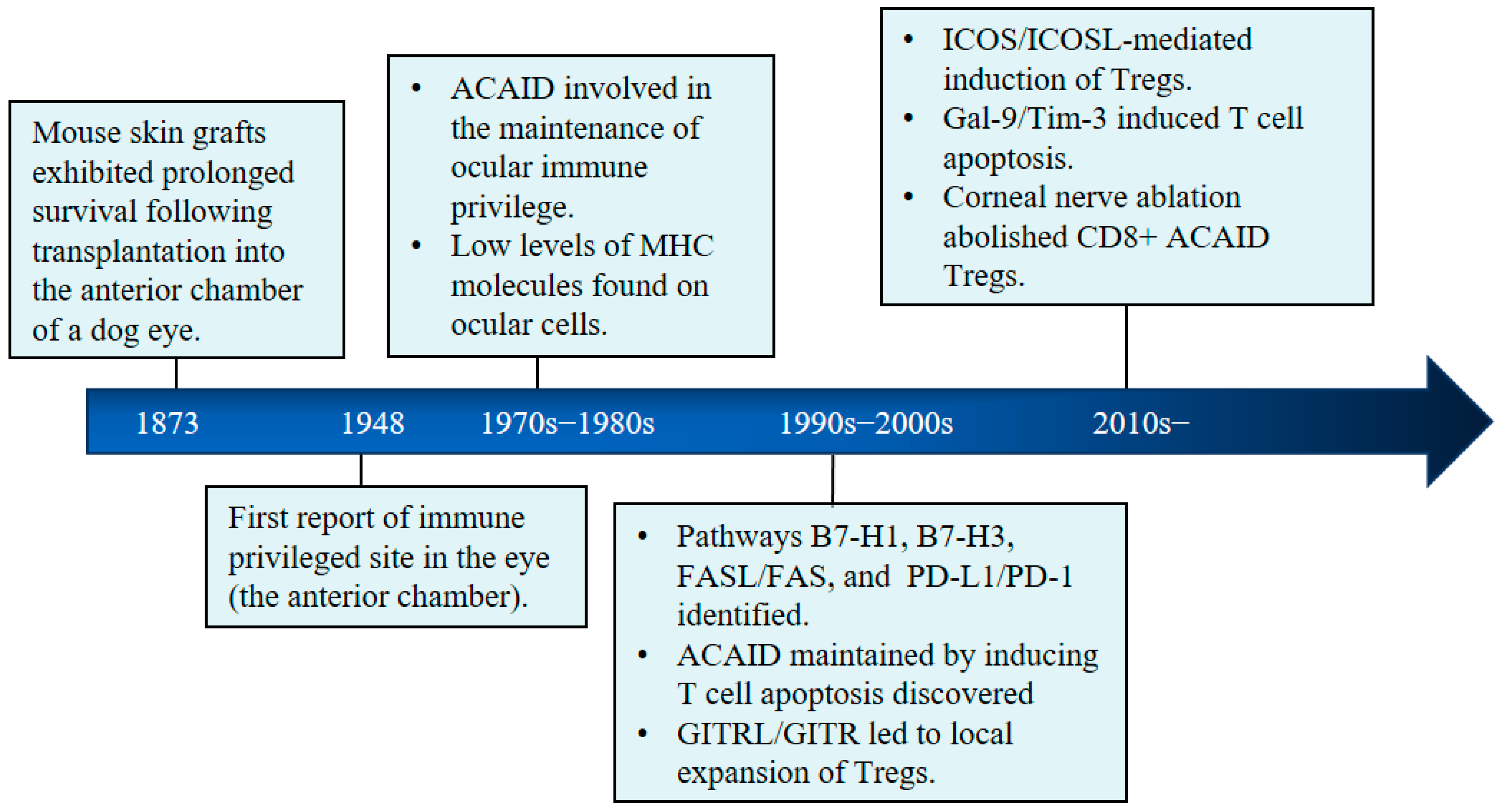
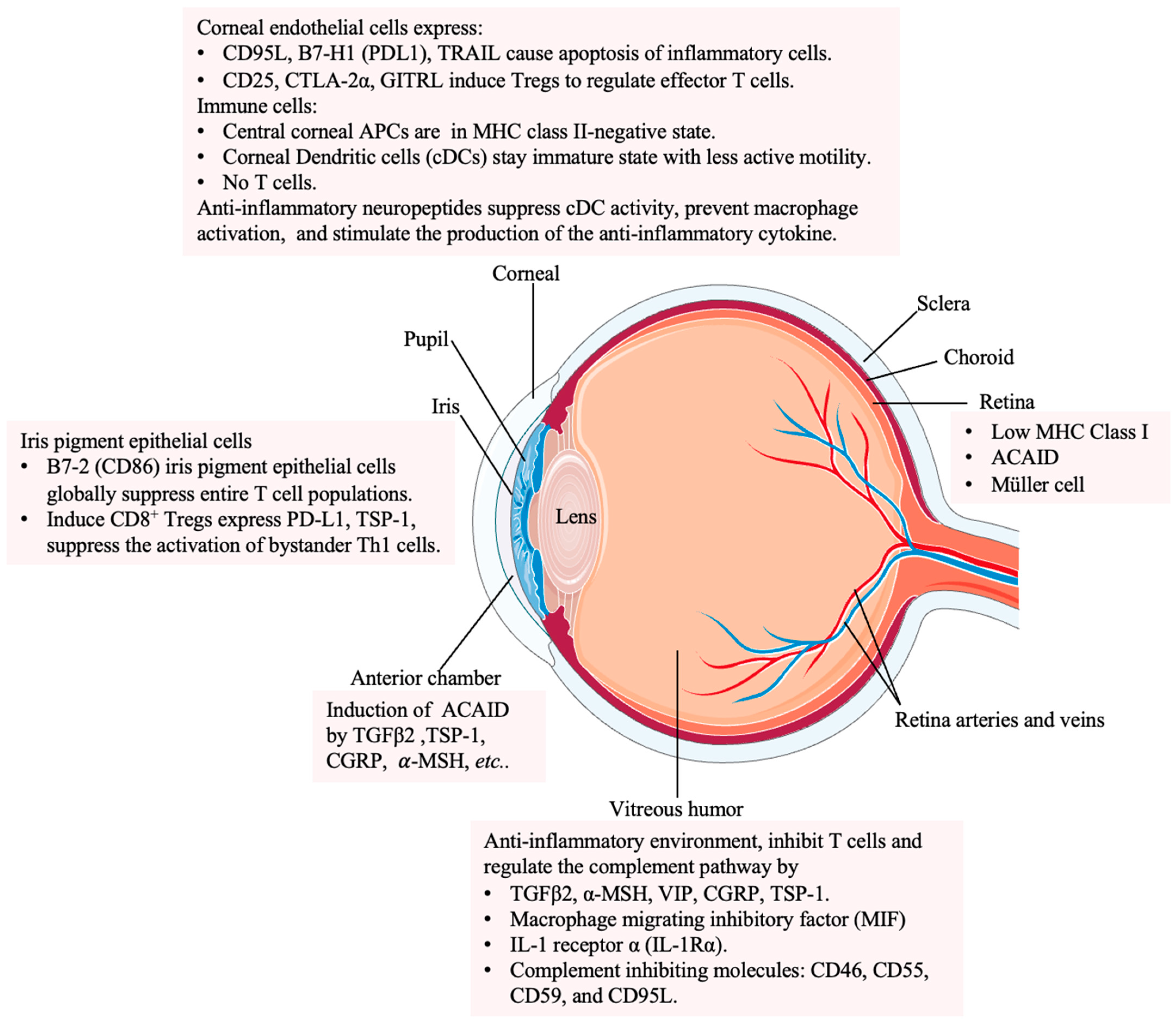
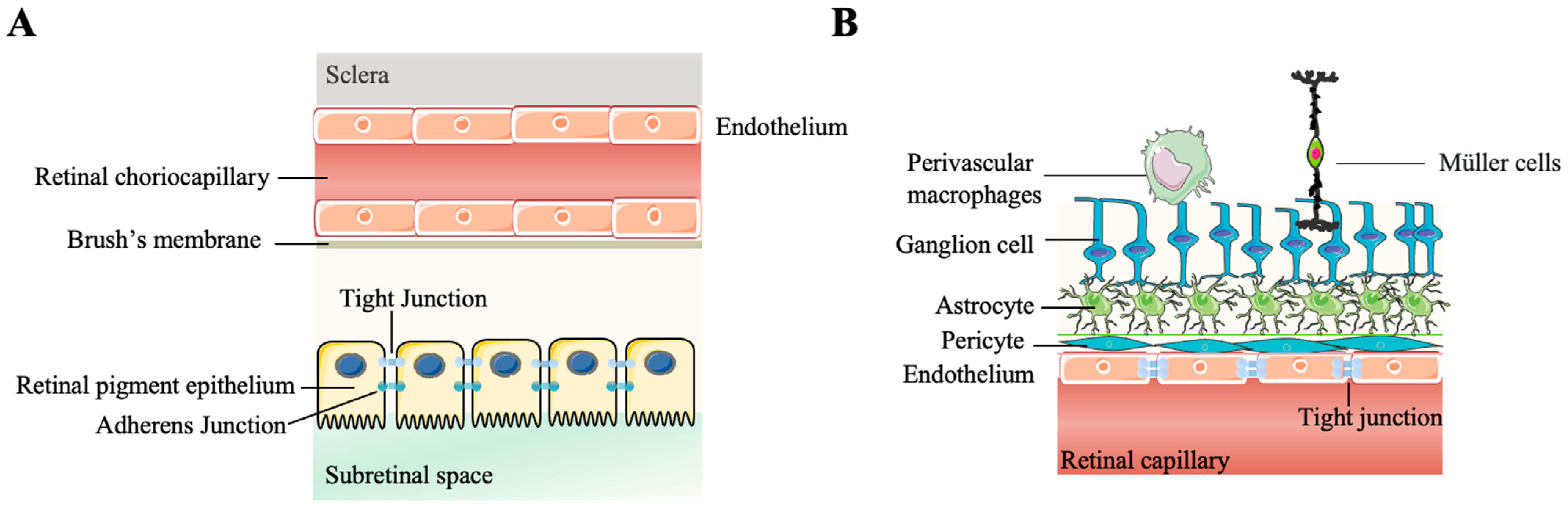
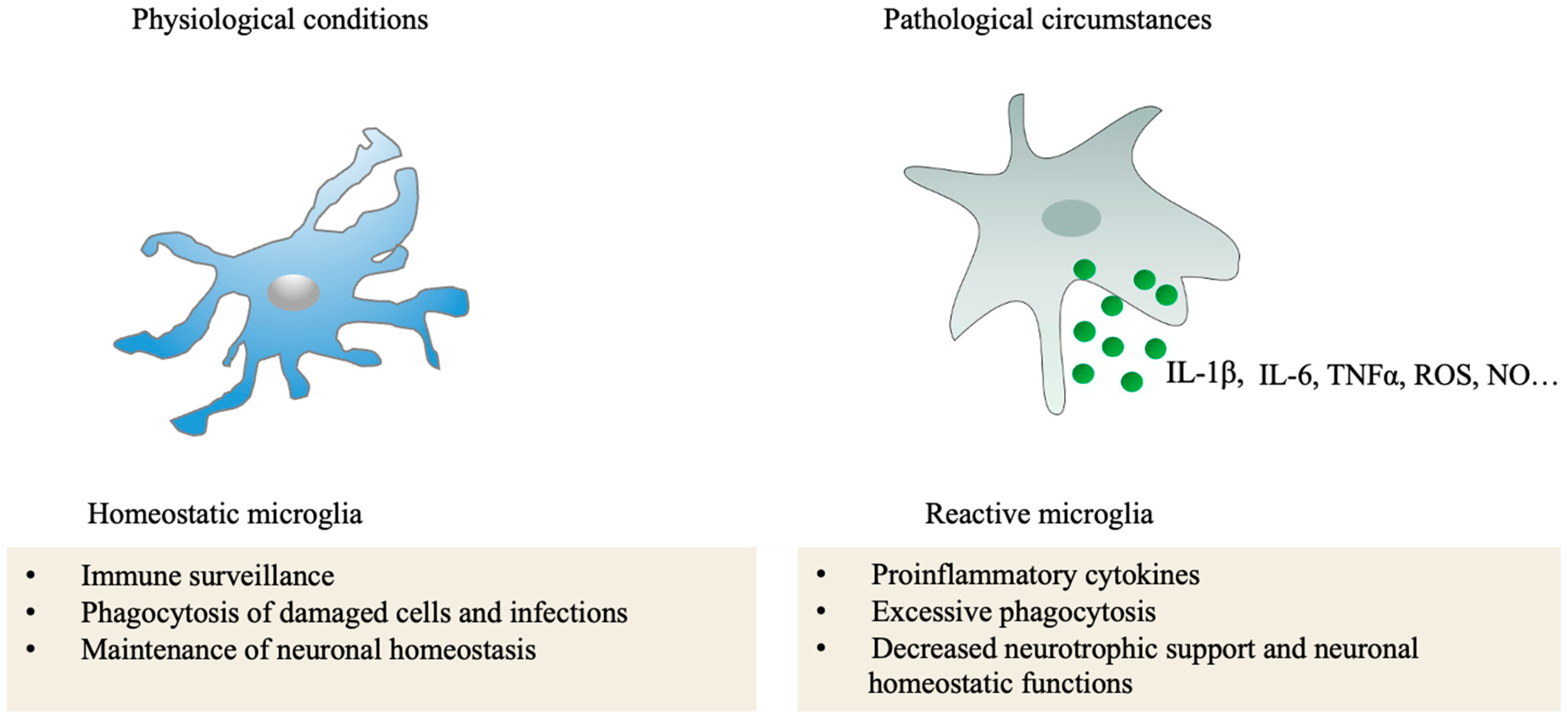
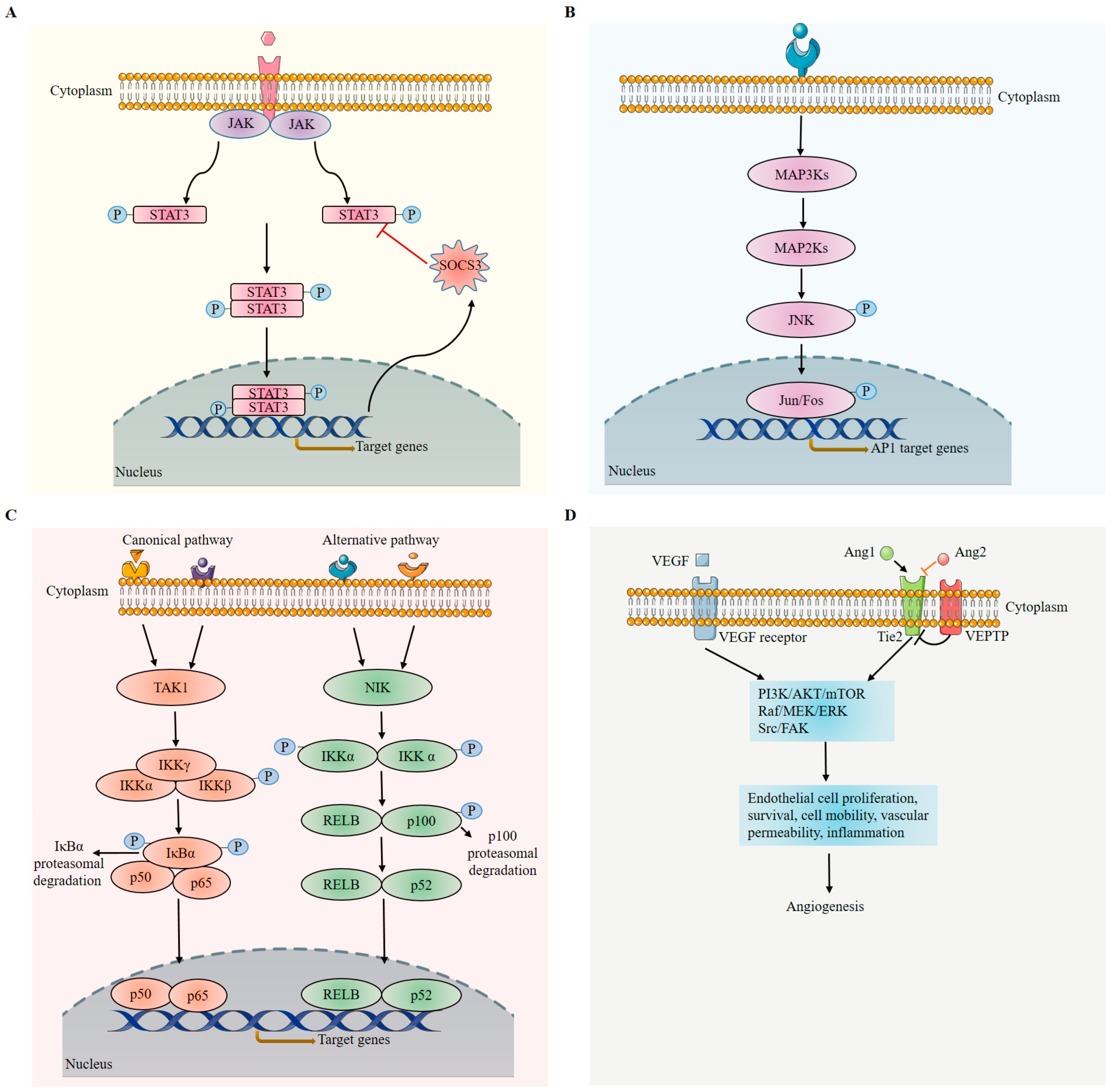
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, T.; Lam, E.; Alvarez, D.; Sun, Y. Ocular Vascular Diseases: From Retinal Immune Privilege to Inflammation. Int. J. Mol. Sci. 2023, 24, 12090. https://doi.org/10.3390/ijms241512090
Wang X, Wang T, Lam E, Alvarez D, Sun Y. Ocular Vascular Diseases: From Retinal Immune Privilege to Inflammation. International Journal of Molecular Sciences. 2023; 24(15):12090. https://doi.org/10.3390/ijms241512090
Chicago/Turabian StyleWang, Xudong, Tianxi Wang, Enton Lam, David Alvarez, and Ye Sun. 2023. "Ocular Vascular Diseases: From Retinal Immune Privilege to Inflammation" International Journal of Molecular Sciences 24, no. 15: 12090. https://doi.org/10.3390/ijms241512090
APA StyleWang, X., Wang, T., Lam, E., Alvarez, D., & Sun, Y. (2023). Ocular Vascular Diseases: From Retinal Immune Privilege to Inflammation. International Journal of Molecular Sciences, 24(15), 12090. https://doi.org/10.3390/ijms241512090






