Actions of a Novel Bacterial Topoisomerase Inhibitor against Neisseria gonorrhoeae Gyrase and Topoisomerase IV: Enhancement of Double-Stranded DNA Breaks
Abstract
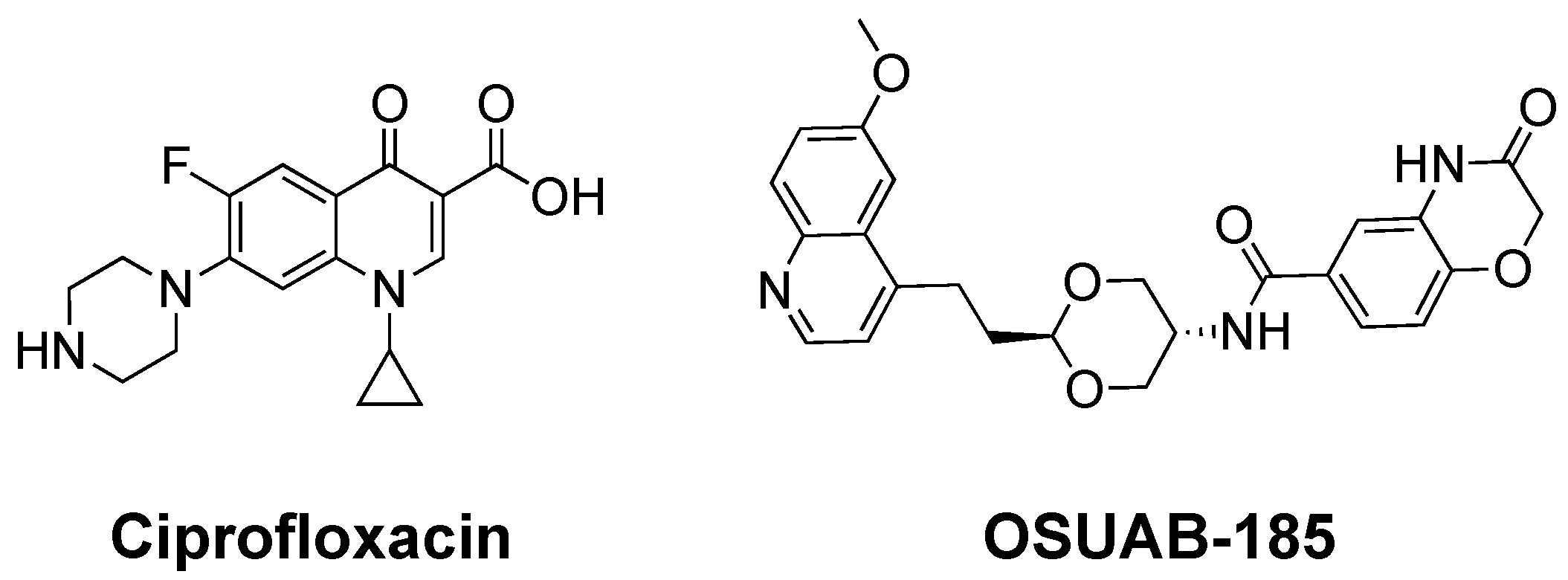
1. Introduction
2. Results
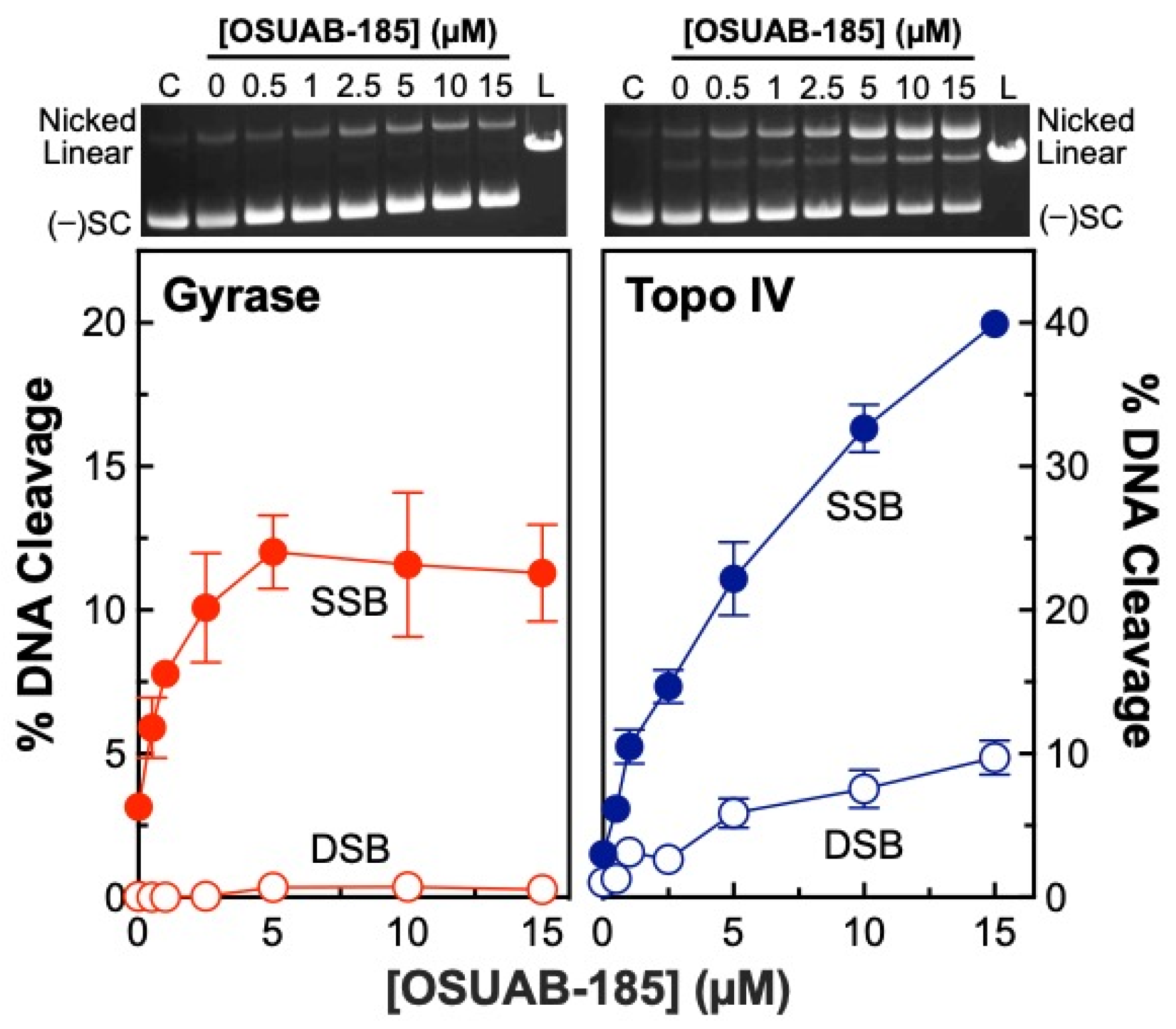
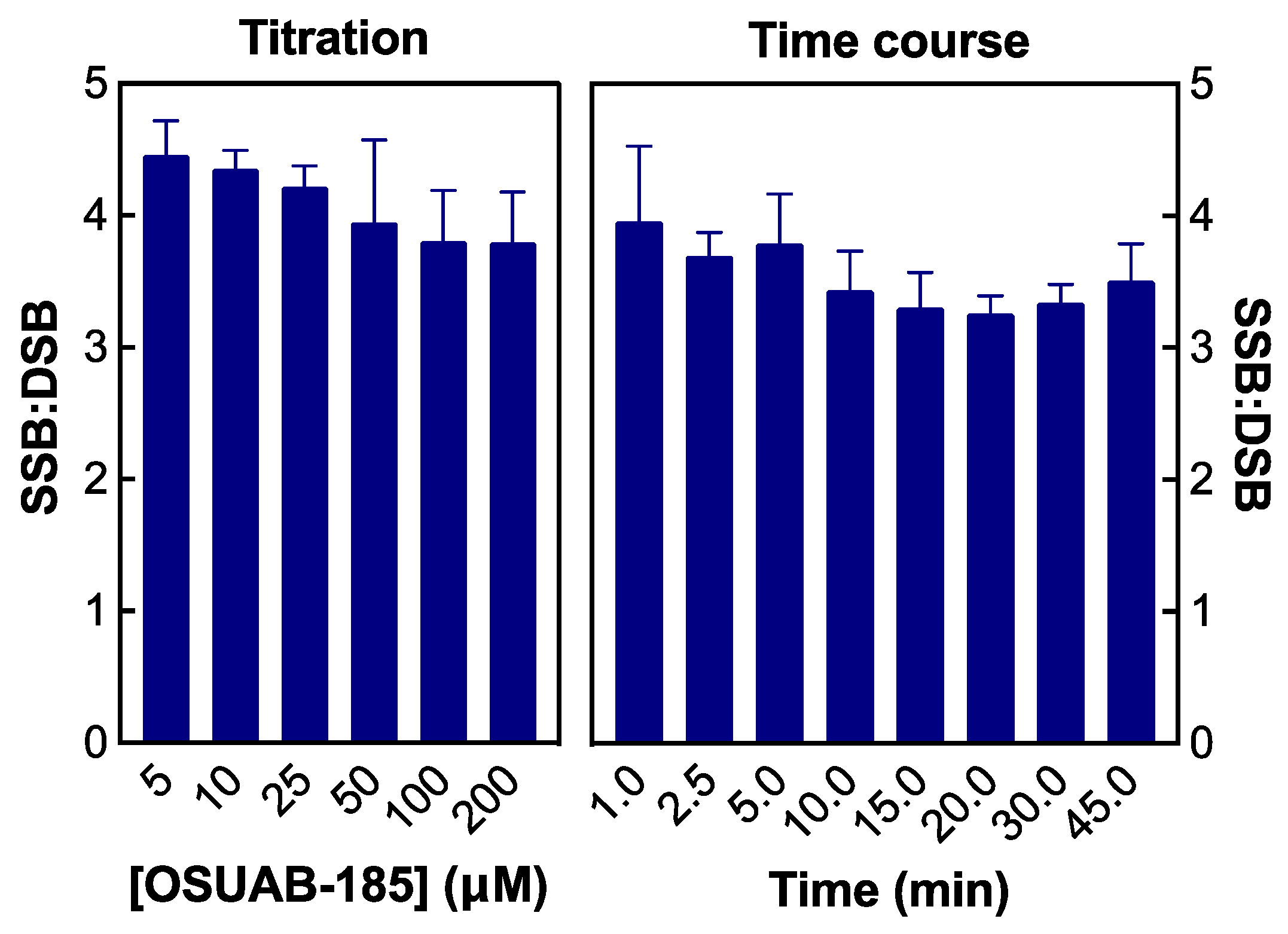
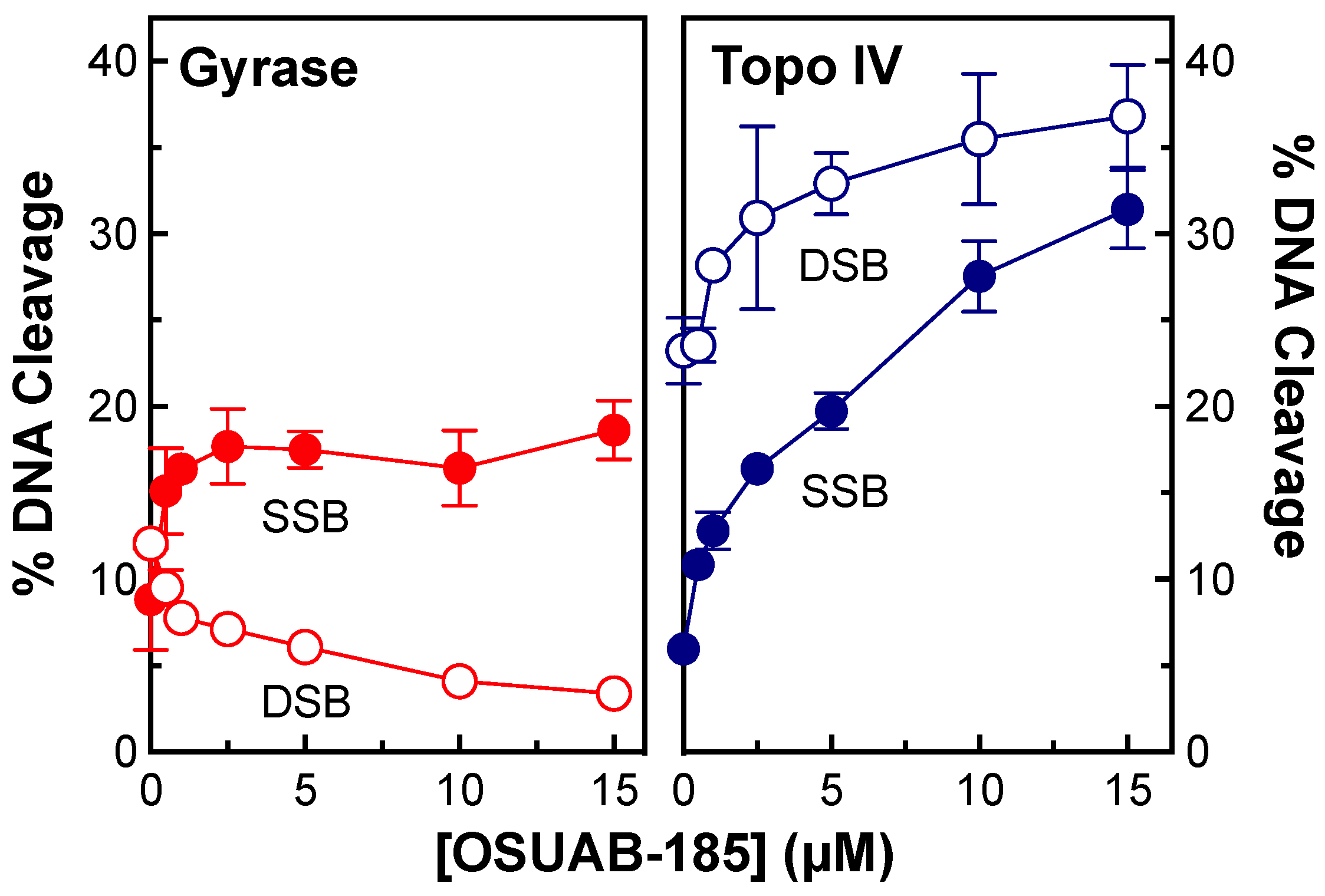
2.1. OSUAB-185 Induces Double-Stranded DNA Cleavage Mediated by N. gonorrhoeae Topoisomerase IV
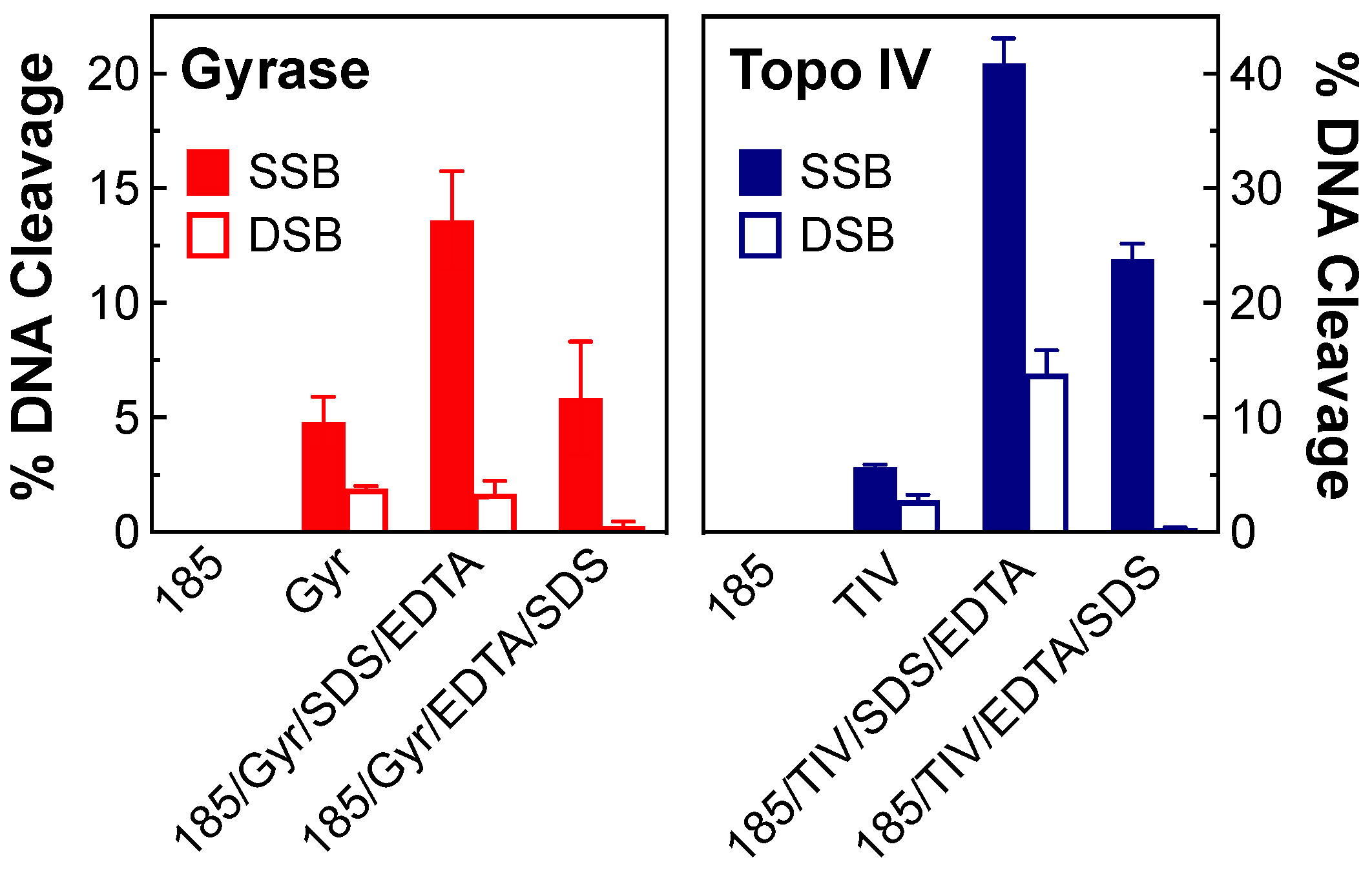
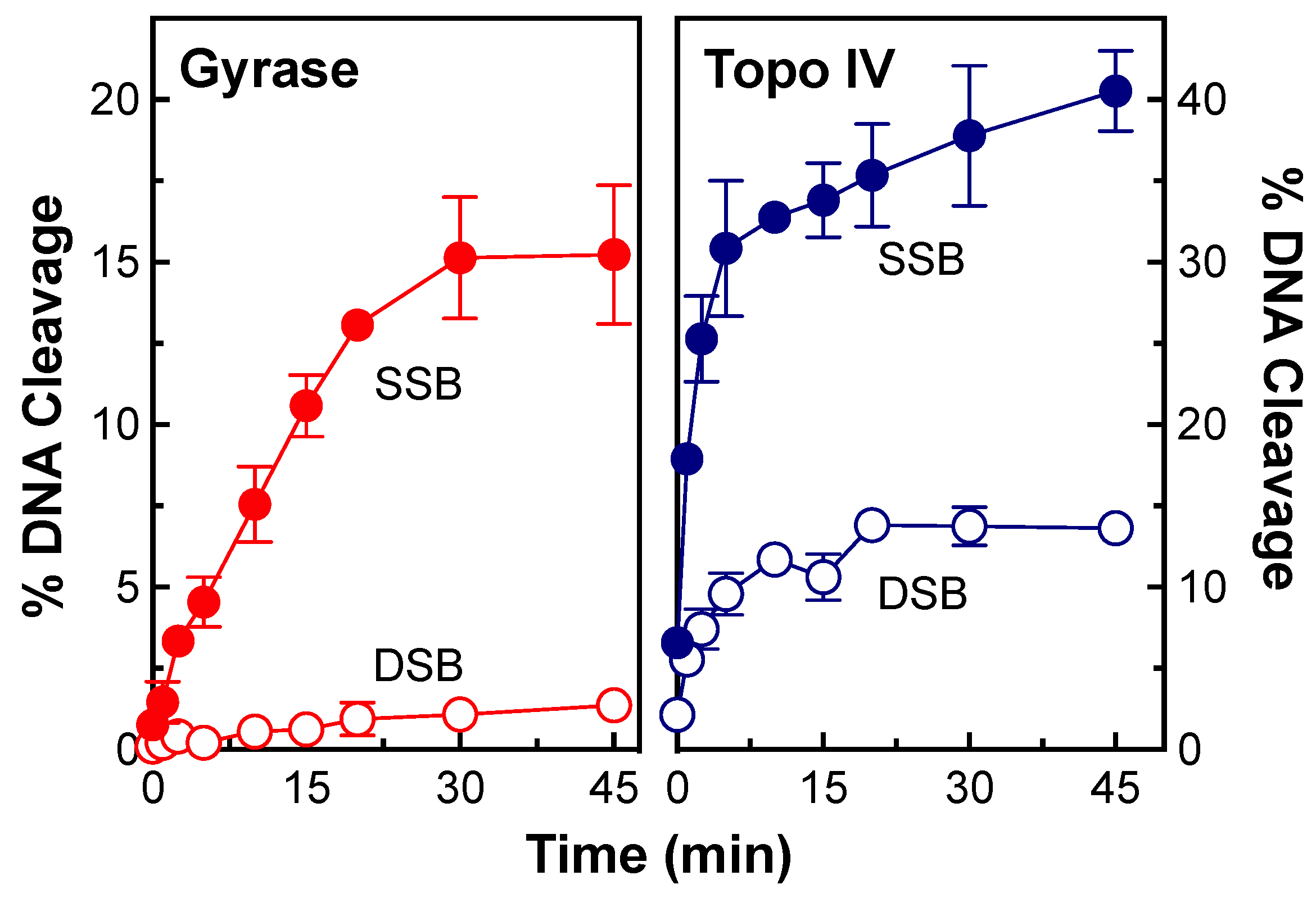
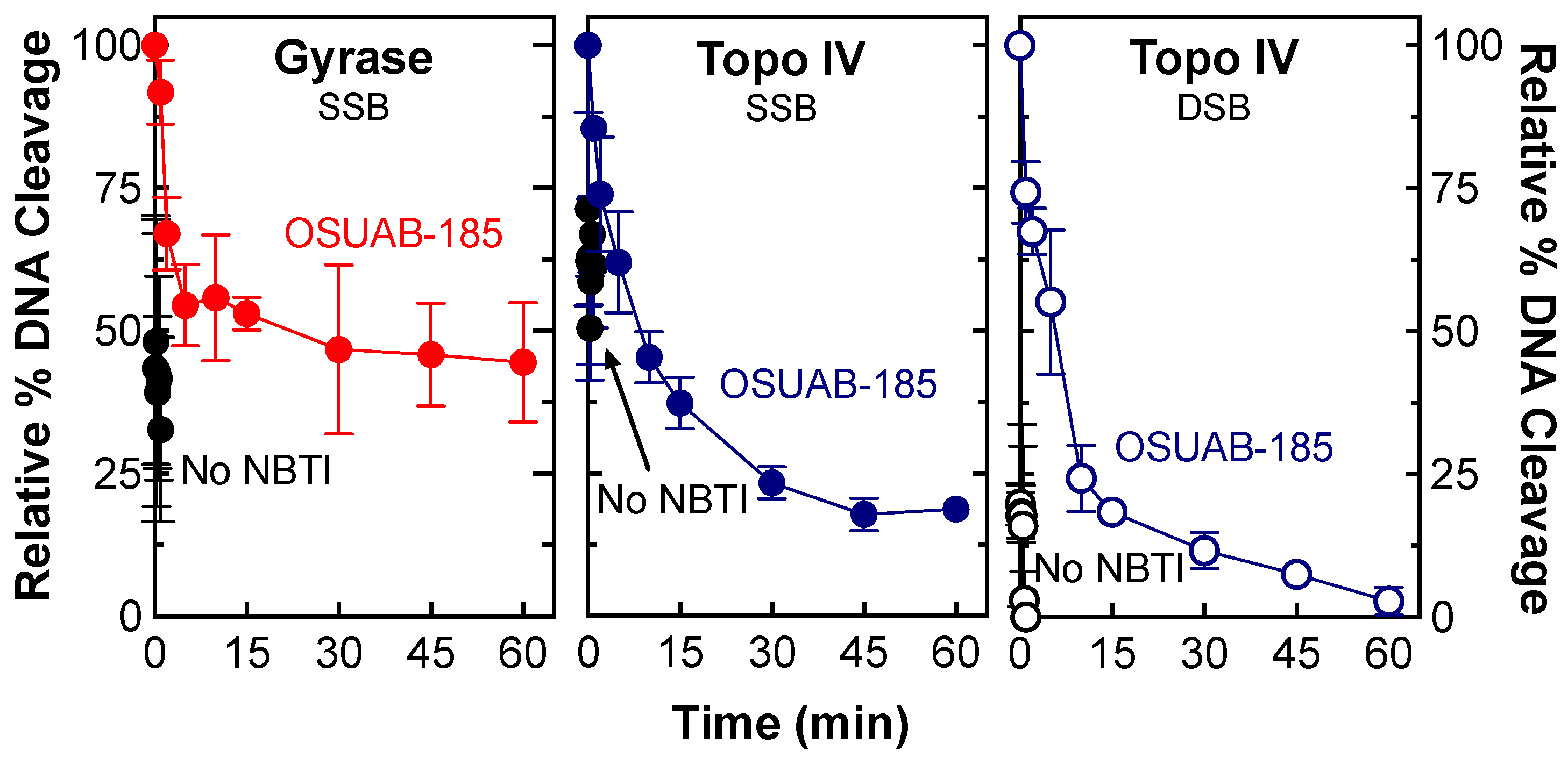
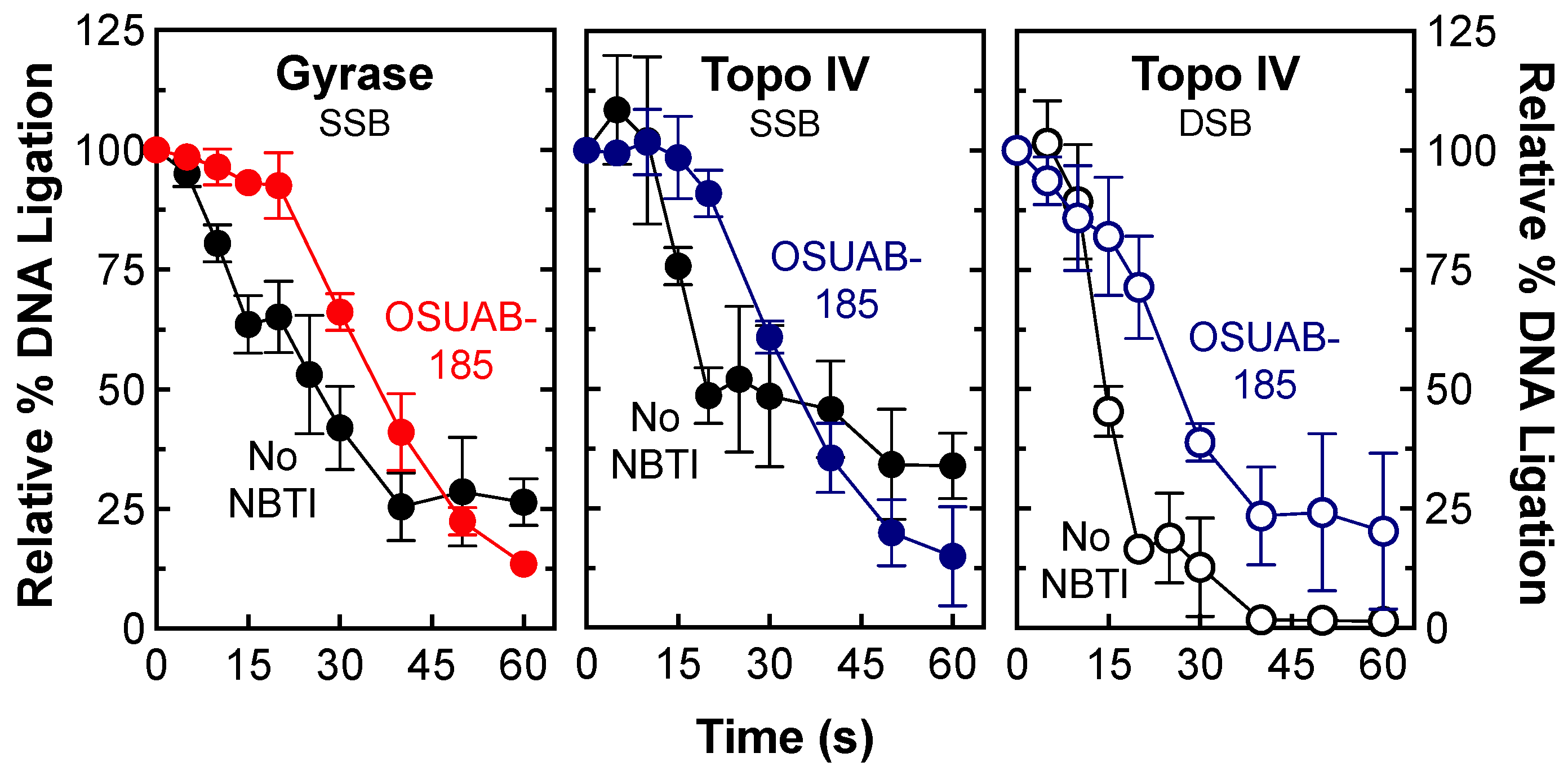
2.2. Stability of Single- and Double-Stranded DNA Breaks Induced by OSUAB-185
2.3. Effects of OSUAB-185 on Fluoroquinolone-Resistant N. gonorrhoeae Gyrase and Topoisomerase IV
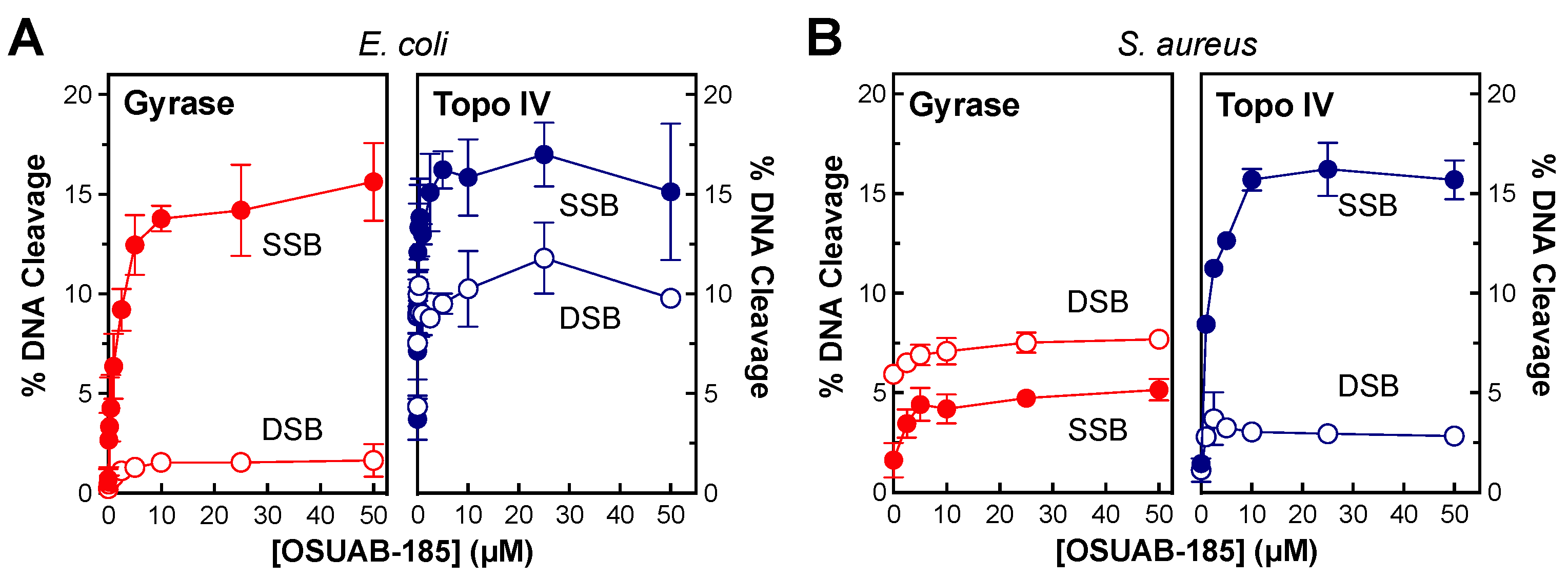
2.4. Effects of OSUAB-185 on Gyrase and Topoisomerase IV from E. coli and S. aureus
2.5. Effects of OSUAB-185 on Human Topoisomerase IIα
3. Discussion
4. Materials and Methods
4.1. Enzymes and Materials
4.2. DNA Cleavage
4.3. Persistence of Cleavage Complexes
4.4. DNA Ligation
4.5. Accession Codes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Outpatient Antibiotic Prescriptions—United States, 2017; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017.
- Basarab, G.S. Four ways to skin a cat: Inhibition of bacterial topoisomerases leading to the clinic. In Antibacterials: Volume I; Fisher, J.F., Mobashery, S., Miller, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 165–188. [Google Scholar]
- World Health Organization. Critically Important Antimicrobials for Human Medicine; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [PubMed]
- Gibson, E.G.; Ashley, R.E.; Kerns, R.J.; Osheroff, N. Fluoroquinolone interactions with bacterial type II topoisomerases and target-mediated drug resistance. In Antimicrobial Resistance and Implications for the 21st Century; Drlica, K., Shlaes, D., Fong, I.W., Eds.; Springer: New York, NY, USA, 2018; pp. 507–529. [Google Scholar]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, lethality and their contributions to antibiotic resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Unemo, M.; Del Rio, C.; Shafer, W.M. Antimicrobial resistance expressed by Neisseria gonorrhoeae: A major global public health problem in the 21st century. Microbiol. Spectr. 2016, 4, 213–237. [Google Scholar]
- Kirkcaldy, R.D.; Harvey, A.; Papp, J.R.; Del Rio, C.; Soge, O.O.; Holmes, K.K.; Hook, E.W., 3rd; Kubin, G.; Riedel, S.; Zenilman, J.; et al. Neisseria gonorrhoeae antimicrobial susceptibility surveillance—The gonococcal isolate surveillance project, 27 sites, United States, 2014. MMWR Surveill. Summ. 2016, 65, 1–19. [Google Scholar] [CrossRef]
- CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb. Mortal. Wkly. Rep. 2007, 56, 332–336. [Google Scholar]
- Sexually Transmitted Disease Surveillance 2021; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021.
- CDC. Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019.
- Galvin, S.R.; Cohen, M.S. The role of sexually transmitted diseases in HIV transmission. Nat. Rev. Microbiol. 2004, 2, 33–42. [Google Scholar] [CrossRef]
- Morgan, M.K.; Decker, C.F. Gonorrhea. Dis. Mon. 2016, 62, 260–268. [Google Scholar] [CrossRef]
- Koebler, J. World Health Organization Warns Gonorrhea Could Join HIV as ‘Uncurable’. U.S. News and World Report. 2012. Available online: https://www.usnews.com/news/articles/2012/06/06/world-health-organization-warns-gonorrhea-could-join-hiv-as-uncurable- (accessed on 20 June 2023).
- World Health Organization. Multi-Drug Resistant Gonorrhoea; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Liu, Z.; Deibler, R.W.; Chan, H.S.; Zechiedrich, L. The why and how of DNA unlinking. Nucleic Acids Res. 2009, 37, 661–671. [Google Scholar] [CrossRef]
- Vos, S.M.; Tretter, E.M.; Schmidt, B.H.; Berger, J.M. All tangled up: How cells direct, manage and exploit topoisomerase function. Nat. Rev. Mol. Cell Biol. 2011, 12, 827–841. [Google Scholar]
- Chen, S.H.; Chan, N.L.; Hsieh, T.S. New mechanistic and functional insights into DNA topoisomerases. Annu. Rev. Biochem. 2013, 82, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.G.; Evans-Roberts, K.; Maxwell, A. DNA topoisomerases. EcoSal Plus 2015, 6, 1–34. [Google Scholar] [CrossRef]
- Dalvie, E.D.; Osheroff, N. DNA topoisomerases: Type II. In The Encyclopedia of Biological Chemistry, 3rd ed.; Allewell, N.M., Ed.; Academic Press: Waltham, MA, USA, 2021; Volume 3, pp. 479–486. [Google Scholar]
- McKie, S.J.; Neuman, K.C.; Maxwell, A. DNA topoisomerases: Advances in understanding of cellular roles and multi-protein complexes via structure-function analysis. Bioessays 2021, 43, 2000286. [Google Scholar] [CrossRef] [PubMed]
- Ashley, R.E.; Osheroff, N. Regulation of DNA by topoisomerases: Mathematics at the molecular level. In Knots, Low-Dimensional Topology and Applications; Adams, C., Gordon, C.M., Jones, V., Kauffman, L., Lambropoulou, S., Millet, K., Przytycki, J., Ricca, R., Sazdanovic, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Dong, K.C.; Berger, J.M. Structural basis for gate-DNA recognition and bending by type IIA topoisomerases. Nature 2007, 450, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Deweese, J.E.; Osheroff, N. The DNA cleavage reaction of topoisomerase II: Wolf in sheep’s clothing. Nucleic Acids Res. 2009, 37, 738–749. [Google Scholar] [CrossRef]
- Laponogov, I.; Pan, X.S.; Veselkov, D.A.; McAuley, K.E.; Fisher, L.M.; Sanderson, M.R. Structural basis of gate-DNA breakage and resealing by type II topoisomerases. PLoS ONE 2010, 5, e11338. [Google Scholar] [CrossRef]
- Wohlkonig, A.; Chan, P.F.; Fosberry, A.P.; Homes, P.; Huang, J.; Kranz, M.; Leydon, V.R.; Miles, T.J.; Pearson, N.D.; Perera, R.L.; et al. Structural basis of quinolone inhibition of type IIA topoisomerases and target-mediated resistance. Nat. Struct. Mol. Biol. 2010, 17, 1152–1153. [Google Scholar] [CrossRef] [PubMed]
- Vann, K.R.; Oviatt, A.A.; Osheroff, N. Topoisomerase II poisons: Converting essential enzymes into molecular scissors. Biochemistry 2021, 60, 1630–1641. [Google Scholar] [CrossRef]
- Michel, B. After 30 years of study, the bacterial SOS response still surprises us. PLoS Biol. 2005, 3, e255. [Google Scholar] [CrossRef]
- Drlica, K.; Malik, M.; Kerns, R.J.; Zhao, X. Quinolone-mediated bacterial death. Antimicrob. Agents Chemother. 2008, 52, 385–392. [Google Scholar] [CrossRef]
- Schroder, W.; Goerke, C.; Wolz, C. Opposing effects of aminocoumarins and fluoroquinolones on the SOS response and adaptability in Staphylococcus aureus. J. Antimicrob. Chemother. 2013, 68, 529–538. [Google Scholar] [CrossRef]
- Heisig, P.; Schedletzky, H.; Falkensteinpaul, H. Mutations in the gyrA gene of a highly fluoroquinolone-resistant clinical isolate of Escherichia coli. Antimicrob. Agents Chemother. 1993, 37, 696–701. [Google Scholar] [CrossRef]
- Aldred, K.J.; McPherson, S.A.; Wang, P.; Kerns, R.J.; Graves, D.E.; Turnbough, C.L.; Osheroff, N. Drug interactions with Bacillus anthracis topoisomerase IV: Biochemical basis for quinolone action and resistance. Biochemistry 2012, 51, 370–381. [Google Scholar]
- Aldred, K.J.; McPherson, S.A.; Turnbough, C.L.; Kerns, R.J.; Osheroff, N. Topoisomerase IV-quinolone interactions are mediated through a water-metal ion bridge: Mechanistic basis of quinolone resistance. Nucleic Acids Res. 2013, 41, 4628–4639. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.D.; Chan, P.F.; Eggleston, D.S.; Fosberry, A.; Gentry, D.R.; Gorrec, F.; Giordano, I.; Hann, M.M.; Hennessy, A.; Hibbs, M.; et al. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 2010, 466, 935–940. [Google Scholar] [CrossRef]
- Basarab, G.S.; Kern, G.H.; McNulty, J.; Mueller, J.P.; Lawrence, K.; Vishwanathan, K.; Alm, R.A.; Barvian, K.; Doig, P.; Galullo, V.; et al. Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial type II topoisomerases. Sci. Rep. 2015, 5, 11827. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.G.; Bax, B.; Chan, P.F.; Osheroff, N. Mechanistic and structural basis for the actions of the antibacterial gepotidacin against Staphylococcus aureus gyrase. ACS Infect. Dis. 2019, 5, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.D.; Murshudov, G.; Maxwell, A.; Germe, T. DNA topoisomerase inhibitors: Trapping a DNA-cleaving machine in motion. J. Mol. Biol. 2019, 431, 3427–3449. [Google Scholar] [CrossRef] [PubMed]
- GlaxoSmithKline. A Phase III, Randomized, Multicenter, Parallel-Group, Double-Blind, Double-Dummy Study in Adolescent and Adult Female Participants Comparing the Efficacy and Safety of Gepotidacin to Nitrofurantoin in the Treatment of Uncomplicated Urinary Tract Infection (Acute Cystitis); National Library of Medicine: Bethesda, MD, USA, 2021.
- GlaxoSmithKline. A Phase III, Randomized, Multicenter, Open-Label Study in Adolescent and Adult Participants Comparing the Efficacy and Safety of Gepotidacin to Ceftriaxone Plus Azithromycin in the Treatment of Uncomplicated Urogenital Gonorrhea Caused by Neisseria gonorrhoeae; National Library of Medicine: Bethesda, MD, USA, 2022.
- Perry, C.; Hossain, M.; Powell, M.; Raychaudhuri, A.; Scangarella-Oman, N.; Tiffany, C.; Xu, S.; Dumont, E.; Janmohamed, S. Design of two phase III, randomized, multicenter studies comparing gepotidacin with nitrofurantoin for the treatment of uncomplicated urinary tract infection in female participants. Infect. Dis. Ther. 2022, 11, 2297–2310. [Google Scholar] [CrossRef]
- Scangarella-Oman, N.E.; Hossain, M.; Perry, C.R.; Tiffany, C.; Powell, M.; Swift, B.; Dumont, E.F. Dose selection for a phase III study evaluating gepotidacin (GSK2140944) in the treatment of uncomplicated urogenital gonorrhoea. Sex. Transm. Infect. 2023, 99, 64–69. [Google Scholar] [CrossRef]
- Watkins, R.R.; Thapaliya, D.; Lemonovich, T.L.; Bonomo, R.A. Gepotidacin: A novel, oral, ‘first-in-class’ triazaacenaphthylene antibiotic for the treatment of uncomplicated urinary tract infections and urogenital gonorrhoea. J. Antimicrob. Chemother. 2023, 78, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- GlaxoSmithKline. EAGLE-2 and EAGLE-3 Phase III Trials for Gepotidacin Stopped Early for Efficacy Following Pre-Planned Interim Analysis by Independent Data Monitoring Committee; GlaxoSmithKline: Brentford, UK, 2022. [Google Scholar]
- Vanden Broeck, A.; Lotz, C.; Ortiz, J.; Lamour, V. Cryo-EM structure of the complete E. coli DNA gyrase nucleoprotein complex. Nat. Commun. 2019, 10, 4935. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.F.; Srikannathasan, V.; Huang, J.; Cui, H.; Fosberry, A.P.; Gu, M.; Hann, M.M.; Hibbs, M.; Homes, P.; Ingraham, K.; et al. Structural basis of DNA gyrase inhibition by antibacterial QPT-1, anticancer drug etoposide and moxifloxacin. Nat. Commun. 2015, 6, 10048. [Google Scholar] [CrossRef] [PubMed]
- Blower, T.R.; Williamson, B.H.; Kerns, R.J.; Berger, J.M. Crystal structure and stability of gyrase-fluoroquinolone cleaved complexes from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2016, 113, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.G.; Blower, T.R.; Cacho, M.; Bax, B.; Berger, J.M.; Osheroff, N. Mechanism of action of Mycobacterium tuberculosis gyrase inhibitors: A novel class of gyrase poisons. ACS Infect. Dis. 2018, 4, 1211–1222. [Google Scholar] [CrossRef]
- Gibson, E.G.; Oviatt, A.A.; Cacho, M.; Neuman, K.C.; Chan, P.F.; Osheroff, N. Bimodal actions of a naphthyridone/aminopiperidine-based antibacterial that targets gyrase and topoisomerase IV. Biochemistry 2019, 58, 4447–4455. [Google Scholar] [CrossRef]
- Lu, Y.; Papa, J.L.; Nolan, S.; English, A.; Seffernick, J.T.; Shkolnikov, N.; Powell, J.; Lindert, S.; Wozniak, D.J.; Yalowich, J.; et al. Dioxane-linked amide derivatives as novel bacterial topoisomerase inhibitors against gram-positive Staphylococcus aureus. ACS Med. Chem. Lett. 2020, 11, 2446–2454. [Google Scholar] [CrossRef]
- Osheroff, N. Role of the divalent cation in topoisomerase II mediated reactions. Biochemistry 1987, 26, 6402–6406. [Google Scholar] [CrossRef]
- Osheroff, N.; Zechiedrich, E.L. Calcium-promoted DNA cleavage by eukaryotic topoisomerase II: Trapping the covalent enzyme-DNA complex in an active form. Biochemistry 1987, 26, 4303–4309. [Google Scholar] [CrossRef]
- Bandele, O.J.; Osheroff, N. The efficacy of topoisomerase II-targeted anticancer agents reflects the persistence of drug-induced cleavage complexes in cells. Biochemistry 2008, 47, 11900–11908. [Google Scholar] [CrossRef]
- Anderson, V.E.; Zaniewski, R.P.; Kaczmarek, F.S.; Gootz, T.D.; Osheroff, N. Quinolones inhibit DNA religation mediated by Staphylococcus aureus topoisomerase IV: Changes in drug mechanism across evolutionary boundaries. J. Biol. Chem. 1999, 274, 35927–35932. [Google Scholar] [PubMed]
- Hall, C.L.; Harrison, M.A.; Pond, M.J.; Chow, C.; Harding-Esch, E.M.; Sadiq, S.T. Genotypic determinants of fluoroquinolone and macrolide resistance in Neisseria gonorrhoeae. Sex. Health 2019, 16, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Dan, M. The use of fluoroquinolones in gonorrhoea: The increasing problem of resistance. Expert Opin. Pharmacother. 2004, 5, 829–854. [Google Scholar] [CrossRef] [PubMed]
- Mitton-Fry, M.J.; Brickner, S.J.; Hamel, J.C.; Brennan, L.; Casavant, J.M.; Chen, M.; Chen, T.; Ding, X.Y.; Driscoll, J.; Hardink, J.; et al. Novel quinoline derivatives as inhibitors of bacterial DNA gyrase and topoisomerase IV. Bioorg. Med. Chem. Lett. 2013, 23, 2955–2961. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Okumu, A.; Dellos-Nolan, S.; Li, Z.; Karmahapatra, S.; English, A.; Yalowich, J.C.; Wozniak, D.J.; Mitton-Fry, M.J. Synthesis and anti-staphylococcal activity of novel bacterial topoisomerase inhibitors with a 5-amino-1,3-dioxane linker moiety. Bioorg. Med. Chem. Lett. 2018, 28, 2477–2480. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Okumu, A.A.; Nolan, S.; English, A.; Vibhute, S.; Lu, Y.; Hervert-Thomas, K.; Seffernick, J.T.; Azap, L.; Cole, S.L.; et al. 1,3-dioxane-linked bacterial topoisomerase inhibitors with enhanced antibacterial activity and reduced hERG inhibition. ACS Infect. Dis. 2019, 5, 1115–1128. [Google Scholar] [CrossRef]
- Felix, C.A.; Kolaris, C.P.; Osheroff, N. Topoisomerase II and the etiology of chromosomal translocations. DNA Repair 2006, 5, 1093–1108. [Google Scholar] [CrossRef]
- Joannides, M.; Grimwade, D. Molecular biology of therapy-related leukaemias. Clin. Transl. Oncol. 2010, 12, 8–14. [Google Scholar] [CrossRef]
- Cowell, I.G.; Austin, C.A. Mechanism of generation of therapy related leukemia in response to anti-topoisomerase II agents. Int. J. Environ. Res. Public Health 2012, 9, 2075–2091. [Google Scholar] [CrossRef]
- Pendleton, M.; Lindsey, R.H., Jr.; Felix, C.A.; Grimwade, D.; Osheroff, N. Topoisomerase II and leukemia. Ann. N. Y. Acad. Sci. 2014, 1310, 98–110. [Google Scholar] [CrossRef]
- Ashley, R.E.; Dittmore, A.; McPherson, S.A.; Turnbough, C.L., Jr.; Neuman, K.C.; Osheroff, N. Activities of gyrase and topoisomerase IV on positively supercoiled DNA. Nucleic Acids Res. 2017, 45, 9611–9624. [Google Scholar] [CrossRef]
- Peng, H.; Marians, K.J. Escherichia coli topoisomerase IV. Purification, characterization, subunit structure, and subunit interactions. J. Biol. Chem. 1993, 268, 24481–24490. [Google Scholar] [CrossRef] [PubMed]
- Worland, S.T.; Wang, J.C. Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1989, 264, 4412–4416. [Google Scholar] [CrossRef] [PubMed]
- Kingma, P.S.; Greider, C.A.; Osheroff, N. Spontaneous DNA lesions poison human topoisomerase IIα and stimulate cleavage proximal to leukemic 11q23 chromosomal breakpoints. Biochemistry 1997, 36, 5934–5939. [Google Scholar] [CrossRef] [PubMed]
- Fortune, J.M.; Osheroff, N. Merbarone inhibits the catalytic activity of human topoisomerase IIα by blocking DNA cleavage. J. Biol. Chem. 1998, 273, 17643–17650. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauda, S.E.; Collins, J.A.; Byl, J.A.W.; Lu, Y.; Yalowich, J.C.; Mitton-Fry, M.J.; Osheroff, N. Actions of a Novel Bacterial Topoisomerase Inhibitor against Neisseria gonorrhoeae Gyrase and Topoisomerase IV: Enhancement of Double-Stranded DNA Breaks. Int. J. Mol. Sci. 2023, 24, 12107. https://doi.org/10.3390/ijms241512107
Dauda SE, Collins JA, Byl JAW, Lu Y, Yalowich JC, Mitton-Fry MJ, Osheroff N. Actions of a Novel Bacterial Topoisomerase Inhibitor against Neisseria gonorrhoeae Gyrase and Topoisomerase IV: Enhancement of Double-Stranded DNA Breaks. International Journal of Molecular Sciences. 2023; 24(15):12107. https://doi.org/10.3390/ijms241512107
Chicago/Turabian StyleDauda, Soziema E., Jessica A. Collins, Jo Ann W. Byl, Yanran Lu, Jack C. Yalowich, Mark J. Mitton-Fry, and Neil Osheroff. 2023. "Actions of a Novel Bacterial Topoisomerase Inhibitor against Neisseria gonorrhoeae Gyrase and Topoisomerase IV: Enhancement of Double-Stranded DNA Breaks" International Journal of Molecular Sciences 24, no. 15: 12107. https://doi.org/10.3390/ijms241512107
APA StyleDauda, S. E., Collins, J. A., Byl, J. A. W., Lu, Y., Yalowich, J. C., Mitton-Fry, M. J., & Osheroff, N. (2023). Actions of a Novel Bacterial Topoisomerase Inhibitor against Neisseria gonorrhoeae Gyrase and Topoisomerase IV: Enhancement of Double-Stranded DNA Breaks. International Journal of Molecular Sciences, 24(15), 12107. https://doi.org/10.3390/ijms241512107






