Oxidative Stress and Antioxidant Defense in the Brain of Bat Species with Different Feeding Habits
Abstract
1. Introduction
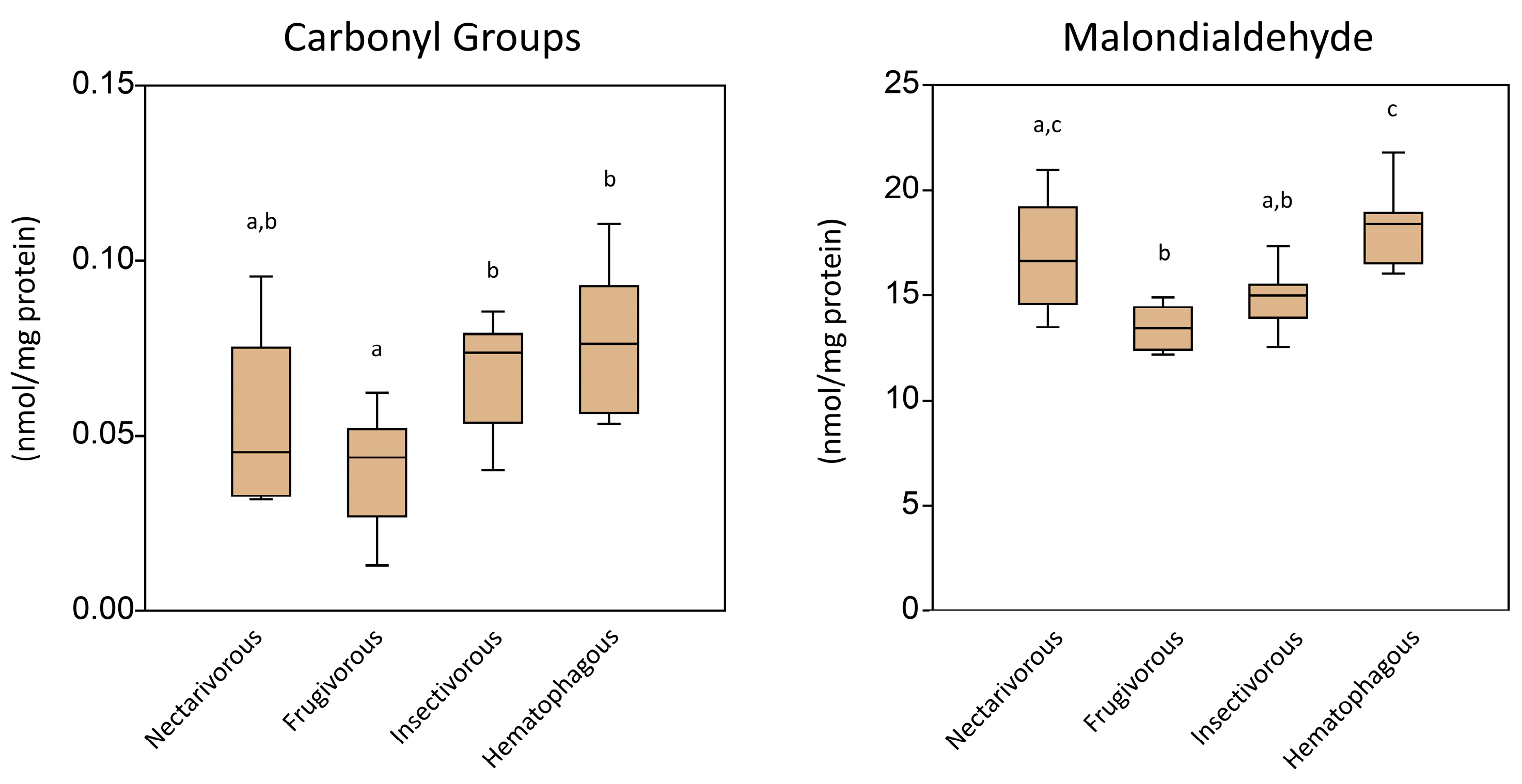
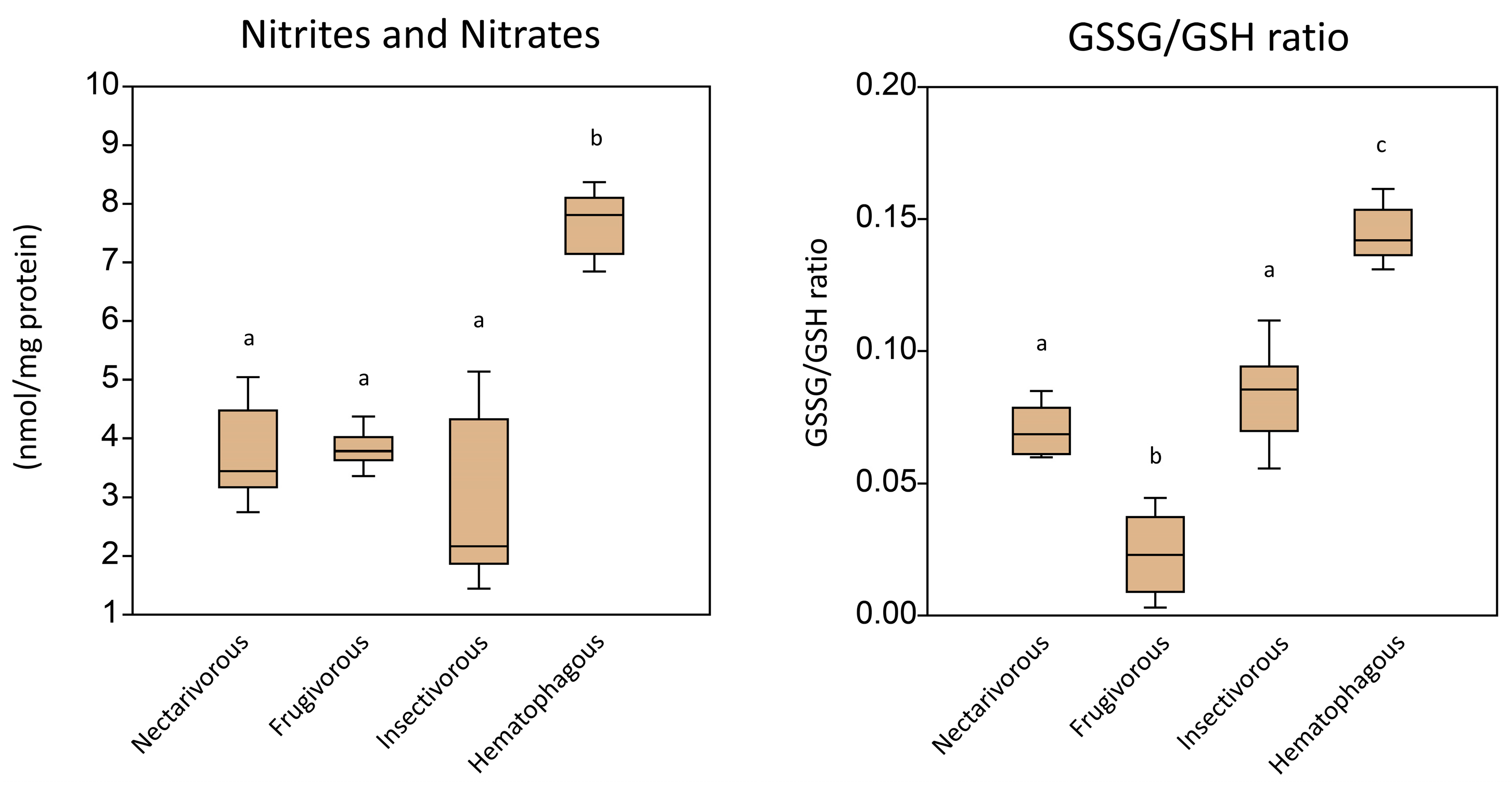
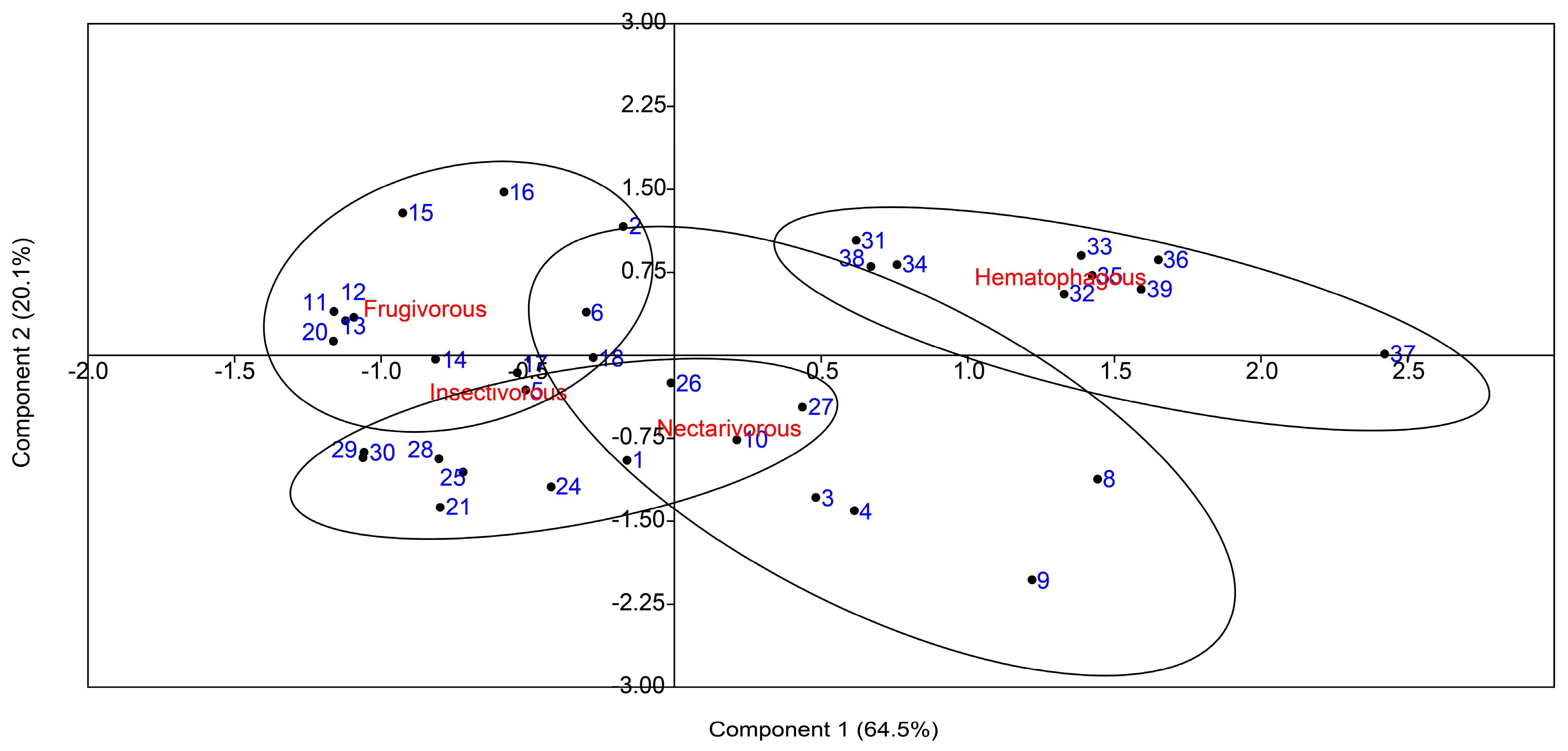
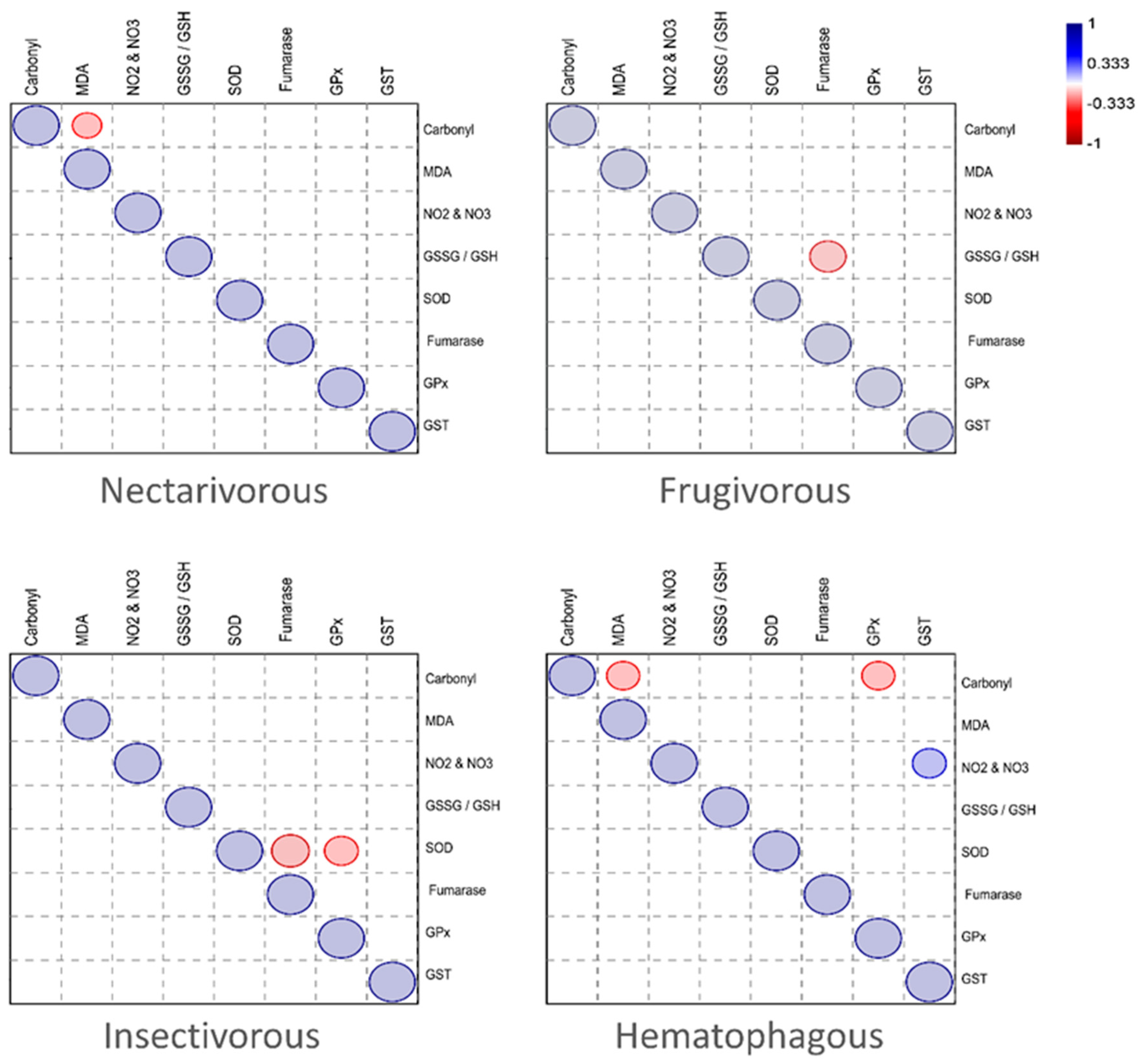
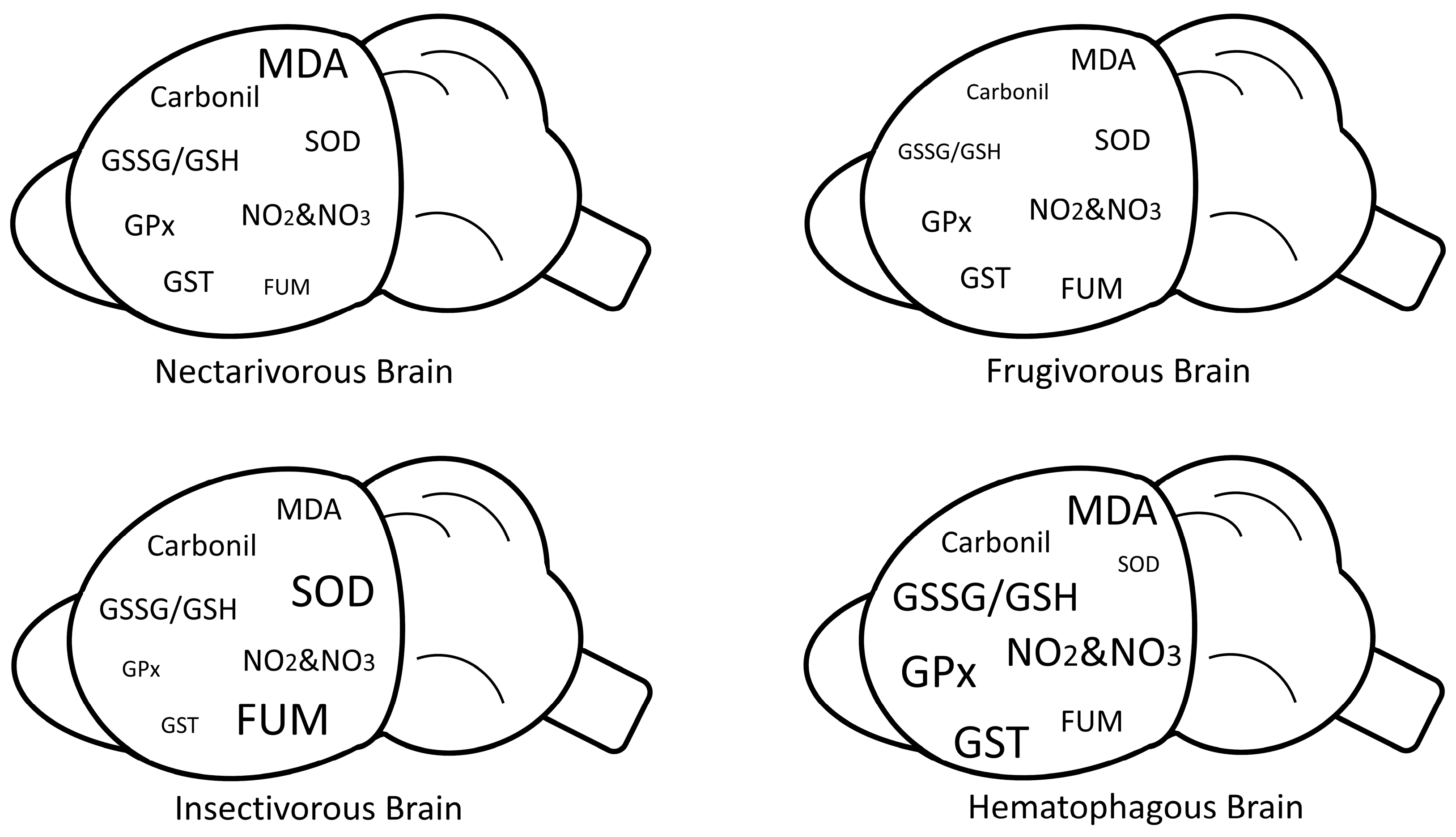
2. Results
3. Discussion
4. Materials and Methods
4.1. Ethical Aspects
4.2. Animal and Sample Collection
4.3. Organ Processing
4.4. Oxidative Damage
4.5. Enzymatic Activity
4.6. Non-Enzymatic Antioxidants
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maciejczyk, M.; Żebrowska, E.; Zalewska, A.; Chabowski, A. Redox balance, antioxidant defense, and oxidative damage in the hypothalamus and cerebral cortex of rats with high fat diet-induced insulin resistance. Oxid. Med. Cell. Longev. 2018, 2018, 6940515. [Google Scholar] [CrossRef]
- Leutner, S.; Eckert, A.; Müller, W.E. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J. Neural Transm. 2001, 108, 955–967. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Beneficial effects of walnuts on cognition and brain health. Nutrients 2020, 12, 550. [Google Scholar] [CrossRef]
- Potter, J.H.T.; Davies, K.T.J.; Yohe, L.R.; Sanchez, M.K.R.; Rengifo, E.M.; Struebig, M.; Warren, K.; Tsagkogeorga, G.; Lim, B.K.; dos Reis, M.; et al. Dietary diversification and specialization in neotropical bats facilitated by early molecular evolution. Mol. Biol. Evol. 2021, 38, 3864–3883. [Google Scholar] [CrossRef]
- Rojas, D.; Mancina, C.A.; Flores-Martínez, J.J.; Navarro, L. Phylogenetic signal, feeding behaviour and brain volume in Neotropical bats. J. Evol. Biol. 2013, 26, 1925–1933. [Google Scholar] [CrossRef]
- Freitas, R.M.P.; Oliveira, J.M.; Castro, D.L.J.; Sarandy, M.; Gonçalves, R.V.; Freitas, M.B. The antioxidant status of three neotropical bat species with different feeding habits. Acta Chiropterol. 2020, 21, 395–402. [Google Scholar] [CrossRef]
- Camiletti-Moirón, D.; Aparicio, V.A.; Nebot, E.; Medina, G.; Martínez, R.; Kapravelou, G.; Andrade, A.; Porres, J.M.; López-Jurado, M.; Aranda, P. High-protein diet induces oxidative stress in rat brain: Protective action of high-intensity exercise against lipid peroxidation. Nutr. Hosp. 2014, 31, 866–874. [Google Scholar] [CrossRef]
- Żebrowska, E.; Maciejczyk, M.; Żendzian-Piotrowska, M.; Zalewska, A.; Chabowski, A. High protein diet induces oxidative stress in rat cerebral cortex and hypothalamus. Int. J. Mol. Sci. 2019, 20, 1547. [Google Scholar] [CrossRef]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011, 711, 193–201. [Google Scholar] [CrossRef]
- Leshets, M.; Silas, Y.B.H.; Lehming, N.; Pines, O. Fumarase: From the TCA cycle to DNA damage response and tumor suppression. Front. Mol. Biosci. 2018, 5, 68. [Google Scholar] [CrossRef]
- Khan, A.I.; Liu, J.; Dutta, P. Iron transport kinetics through blood-brain barrier endothelial cells. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1168–1179. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Oliveira, M.P.; Paes, M.C.; Oliveira, M.F. Modulation of mitochondrial metabolism as a biochemical trait in blood feeding organisms: The redox vampire hypothesis redux. Cell Biol. Int. 2018, 42, 683–700. [Google Scholar] [CrossRef]
- Figueroa-Méndez, R.; Rivas-Arancibia, S. Vitamin C in health and disease: Its role in the metabolism of cells and redox state in the brain. Front. Physiol. 2015, 6, 397. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, A.Y.; Kim, H.C.; Ryu, D.; Jo, S.A.; Jung, Y.S. Effects of natural polyphenols on oxidative stress-mediated blood-brain barrier dysfunction. Antioxidants 2022, 11, 197. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Ma, L.; Hu, L.; Feng, X.; Wang, S. Nitrate and nitrite in health and disease. Aging Dis. 2018, 9, 938–945. [Google Scholar] [CrossRef]
- Chachlaki, K.; Prevot, V. Nitric oxide signaling in the brain and its control of bodily functions. Br. J. Pharmacol. 2020, 177, 5437–5458. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Picón-Pagès, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective effect of antioxidants in the brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.Y.; Kim, H.S. Oxidative stress and the antioxidant enzyme system in the developing brain. Korean J. Pediatr. 2013, 56, 107–111. [Google Scholar] [CrossRef]
- Lees-Miller, S.P. Fumarate in DNA repair. Nat. Cell Biol. 2015, 17, 1096–1097. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Duszenko, M.; Gospodaryov, D.V.; Garaschuk, O. Oxidative stress and energy metabolism in the brain: Midlife as a turning point. Antioxidants 2021, 10, 1715. [Google Scholar] [CrossRef] [PubMed]
- Chada, S.; Whitney, C.; Newburger, P.E. Post-transcriptional regulation of glutathione peroxidase gene expression by selenium in the HL-60 human myeloid cell line. Blood 1989, 74, 2535–2541. [Google Scholar]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Garbarino, V.R.; Orr, M.E.; Rodriguez, K.A.; Buffenstein, R. Mechanisms of oxidative stress resistance in the brain: Lessons learned from hypoxia tolerant extremophilic vertebrates. Arch. Biochem. Biophys. 2015, 576, 8–16. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. In Oxygen Radicals and Biological Systems Part B; Academic Press: New York, NY, USA, 1990; pp. 464–478. [Google Scholar]
- Karatepe, M. Simultaneous determination of ascorbic acid and free malondialdehyde in human serum by HPLC-UV. Lc-Gc N. Am. 2004, 22, 362–365. [Google Scholar]
- Mescam, M.; Vinnakota, K.C.; Beard, D.A. Identification of the catalytic Mechanism and estimation of kinetic parameters for Fumarase. J. Biol. Chem. 2011, 286, 21100–21109. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.W.; Schindler, M.S.Z.; Cora, D.H.; Thiel, N.; Siebel, A.M.; Galiano, D. Oxidative state of the frugivorous bat Sturnira lilium (Chiroptera: Phyllostomidae) in agricultural and urban areas of Southern Brazil. Environ. Sci. Pollut. Res. 2020, 27, 30868–30874. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. Rapid kinetic microassay for catalase activity. J. Biomol. Tech. 2007, 18, 185–187. [Google Scholar]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R. Quantitation of nitrate and nitrite in extracellular fluids. In Nitric Oxide Part A Sources and Detection of NO; NO Synthase; Academic Press: New York, NY, USA, 1996; pp. 237–246. [Google Scholar]


| Nectarivorous | Frugivorous | Insectivorous | Hematophagous | |
|---|---|---|---|---|
| Nectarivorous | 0.0036 * | 0.0018 * | 0.0006 * | |
| Frugivorous | 0.0036 * | 0.0054 * | 0.0006 * | |
| Insectivorous | 0.0018 * | 0.0054 * | 0.0006 * | |
| Hematophagous | 0.0006 * | 0.0006 * | 0.0006 * |
| Bat Species | Food Group | n | Location (City–State) | Coordinates |
|---|---|---|---|---|
| G. soricina | Nectarivorous | 10 | Dom Pedro Alcântara–RS | 29°24′22.35″ S 49°51′4.56″ W |
| S. lilium | Frugivorous | 10 | Dom Pedro Alcântara–RS | 29°24′22.35″ S 49°51′4.56″ |
| M. molossus | Insectivorous | 10 | Treviso–SC | 28°30′47.52″ S 49°27′26.6″ |
| D. rotundus | Hematophagous | 9 | Criciúma–SC | 28°41′27.7″ S 49°25′50.6″ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rampelotto, P.H.; Giannakos, N.R.O.; Mena Canata, D.A.; Pereira, F.D.; Hackenhaar, F.S.; Pereira, M.J.R.; Benfato, M.S. Oxidative Stress and Antioxidant Defense in the Brain of Bat Species with Different Feeding Habits. Int. J. Mol. Sci. 2023, 24, 12162. https://doi.org/10.3390/ijms241512162
Rampelotto PH, Giannakos NRO, Mena Canata DA, Pereira FD, Hackenhaar FS, Pereira MJR, Benfato MS. Oxidative Stress and Antioxidant Defense in the Brain of Bat Species with Different Feeding Habits. International Journal of Molecular Sciences. 2023; 24(15):12162. https://doi.org/10.3390/ijms241512162
Chicago/Turabian StyleRampelotto, Pabulo Henrique, Nikolas Raphael Oliveira Giannakos, Diego Antonio Mena Canata, Francielly Dias Pereira, Fernanda Schäfer Hackenhaar, María João Ramos Pereira, and Mara Silveira Benfato. 2023. "Oxidative Stress and Antioxidant Defense in the Brain of Bat Species with Different Feeding Habits" International Journal of Molecular Sciences 24, no. 15: 12162. https://doi.org/10.3390/ijms241512162
APA StyleRampelotto, P. H., Giannakos, N. R. O., Mena Canata, D. A., Pereira, F. D., Hackenhaar, F. S., Pereira, M. J. R., & Benfato, M. S. (2023). Oxidative Stress and Antioxidant Defense in the Brain of Bat Species with Different Feeding Habits. International Journal of Molecular Sciences, 24(15), 12162. https://doi.org/10.3390/ijms241512162








