Molecular Motifs in Vascular Morphogenesis: Vascular Endothelial Growth Factor A (VEGFA) as the Leading Promoter of Angiogenesis
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodivala-Dilke, K. Alphavbeta3 integrin and angiogenesis: A moody integrin in a changing environment. Curr. Opin. Cell Biol. 2008, 20, 514–519. [Google Scholar] [CrossRef]
- Hynes, R.O. Cell-matrix adhesion in vascular development. J. Thromb. Haemost. 2007, 5 (Suppl. 1), 32–40. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/Pericyte Interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef] [Green Version]
- George, E.L.; Baldwin, H.S.; Hynes, R.O. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood 1997, 90, 3073–3308. [Google Scholar] [CrossRef] [PubMed]
- George, E.L.; Georges-Labouesse, E.N.; Patel-King, R.S.; Rayburn, H.; Hynes, R.O. Defects in mesoderm, neural tube, and vascular development in mouse embryos lacking fibronectin. Development 1993, 119, 1079–1091. [Google Scholar] [CrossRef]
- Georges-Labouesse, E.N.; George, E.L.; Rayburn, H.; Hynes, R.O. Mesodermal development in mouse embryos mutant for fibronectin. Dev. Dyn. 1996, 207, 145–156. [Google Scholar] [CrossRef]
- Francis, S.E.; Goh, K.L.; Hodivala-Dilke, K.; Bader, B.L.; Stark, M.; Davidson, D.; Hynes, R.O. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 927–933. [Google Scholar] [CrossRef] [Green Version]
- Lucitti, J.L.; Jones, E.A.; Huang, C.; Chen, J.; Fraser, S.E.; Dickinson, M.E. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 2007, 134, 3317–3326. [Google Scholar] [CrossRef] [Green Version]
- Trinh, L.A.; Stainier, D.Y. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev. Cell 2004, 6, 371–382. [Google Scholar] [CrossRef] [Green Version]
- Koshida, S.; Kishimoto, Y.; Utsumi, H.; Shimizu, T.; Furutani-Seiki, M.; Kondoh, H.; Takada, S. Integrinalpha5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev. Cell 2005, 8, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Carlson, T.R.; Hu, H.; Braren, R.; Kim, Y.H.; Wang, R.A. Cell-autonomous requirement for beta1 integrin in endothelial cell adhesion, migration, and survival during angiogenesis in mice. Development 2008, 135, 2193–2202. [Google Scholar] [CrossRef] [Green Version]
- Wierzbicka-Patynowski, I.; Schwarzbauer, J.E. The ins and outs of fibronectin matrix assembly. J. Cell Sci. 2003, 116, 3269–3276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astrof, S.; Crowley, D.; Hynes, R.O. Multiple cardiovascular defects are caused by the absence of alternatively spliced segments of fibronectin. Dev. Biol. 2007, 311, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, D.Z.; Wang, Z.; Richardson, J.A.; Olson, E.N. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc. Natl. Acad. Sci. USA 2003, 100, 9366–9370. [Google Scholar] [CrossRef]
- Bergers, G.; Song, S.; Meyer-Morse, N.; Bergsland, E.; Hanahan, D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Investig. 2003, 111, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, A.M.; Onken, M.D.; Rosen, G.D.; Noakes, P.G.; Dean, D.C. Expanding roles for alpha four integrin and its ligands in development. Cell Adhes. Commun. 1994, 2, 27–43. [Google Scholar] [CrossRef]
- Vlahakis, N.E.; Young, B.A.; Atakilit, A.; Sheppard, D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J. Biol. Chem. 2005, 280, 4544–4552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchner, D.A.; Su, F.; Yamaoka, J.S.; Kamei, M.; Shavit, J.A.; Barthel, L.K.; McGee, B.; Amigo, J.D.; Kim, S.; Hanoch, A.W.; et al. pak2a mutations cause cerebral hemorrhage in redhead zebrafish. Proc. Natl. Acad. Sci. USA 2007, 104, 13996–14001. [Google Scholar] [CrossRef]
- Kamei, M.; Saunders, W.B.; Bayless, K.J.; Dye, L.; Davis, G.E.; Weinstein, B.M. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 2006, 442, 453–456. [Google Scholar] [CrossRef]
- Sennino, B.; Falcon, B.L.; McCauley, D.; Le, T.; McCauley, T.; Kurz, J.C.; Haskell, A.; Epstein, D.M.; McDonald, D.M. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res. 2007, 67, 7358–7367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coultas, L.; Chawengsaksophak, K.; Rossant, J. Endothelial cells and VEGF in vascular development. Nature 2005, 438, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J. Biochem. Mol. Biol. 2006, 39, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Noguera-Troise, I.; Daly, C.; Papadopoulos, N.J.; Coetzee, S.; Boland, P.; Gale, N.W.; Chieh Lin, H.; Yancopoulos, G.D.; Thurston, G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 2006, 444, 1032–1103. [Google Scholar] [CrossRef]
- Funahashi, Y.; Shawber, C.J.; Vorontchikhina, M.; Sharma, A.; Outtz, H.H.; Kitajewski, J. Notch regulates the angiogenic response via induction of VEGFR-1. J. Angiogenes Res. 2010, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Zovein, A.C.; Luque, A.; Turlo, K.A.; Hofmann, J.J.; Yee, K.M.; Becker, M.S.; Fassler, R.; Mellman, I.; Lane, T.F.; Iruela-Arispe, M.L. β1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev. Cell 2010, 18, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Tessier-Lavigne, M.; Goodman, C.S. The molecular biology of axon guidance. Science 1996, 274, 1123–1133. [Google Scholar] [CrossRef] [Green Version]
- Bedell, V.M.; Yeo, S.Y.; Park, K.W.; Chung, J.; Seth, P.; Shivalingappa, V.; Zhao, J.; Obara, T.; Sukhatme, V.P.; Drummond, I.A.; et al. Roundabout4 is essential for angiogenesis in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 6373–6378. [Google Scholar] [CrossRef]
- Koch, A.W.; Mathivet, T.; Larrivée, B.; Tong, R.K.; Kowalski, J.; Pibouin-Fragner, L.; Bouvrée, K.; Stawicki, S.; Nicholes, K.; Rathore, N.; et al. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev. Cell 2011, 20, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Neufeld, G.; Cohen, T.; Shraga, N.; Lange, T.; Kessler, O.; Herzog, Y. The neuropilins: Multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc. Med. 2002, 12, 13–19. [Google Scholar] [CrossRef]
- Pan, Q.; Chanthery, Y.; Liang, W.-C.; Stawicki, S.; Mak, J.; Rathore, N.; Tong, R.K.; Kowalski, J.; Yee, S.F.; Pacheco, G.; et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 2007, 11, 53–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo, C.-F.; Fontaine, R.H.; Soueid, J.; Tammela, T.; Makinen, T.; Alfaro-Cervello, C.; Bonnaud, F.; Miguez, A.; Benhaim, L.; Xu, Y.; et al. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev. 2011, 25, 831–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhnert, F.; Mancuso, M.R.; Hampton, J.; Stankunas, K.; Asano, T.; Chen, C.-Z.; Kuo, C.J. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development 2008, 135, 3989–3993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batchu, S.N.; Korshunov, V.A. Novel tyrosine kinase signaling pathways: Implications in vascular remodeling. Curr. Opin. Nephrol. Hypertens. 2012, 2, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, T.; Zhang, J.; Mao, Q.; Li, S.; Xiong, W.; Qiu, Y.; Xie, Q.; Ge, J. Notch1 promotes glioma cell migration and invasion by stimulating β-catenin and NF-κB signaling via AKT activation. Cancer Sci. 2012, 103, 181–190. [Google Scholar] [CrossRef]
- Muller, Y.A.; Chen, Y.; Christinger, H.W.; Li, B.; Cunningham, B.C.; Lowman, H.B.; de Vos, A.M. VEGF and the Fab fragment of a humanized neutralizing antibody: Crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface. Structure 1998, 6, 1153–1167. [Google Scholar] [CrossRef] [Green Version]
- Holmes, K.; Roberts, O.L.; Thomas, A.M.; Cross, M.J. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cell. Signal. 2007, 19, 2003–2012. [Google Scholar] [CrossRef]
- Boström, J.; Norrby, P.O.; Liljefors, T. Conformational energy penalties of protein-bound ligands. J. Comput. Aided Mol. Des. 1998, 12, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory—Design and description. J. Comput. Aid. Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef] [PubMed]
- CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1994.
- Hall, L.H.; Kier, L.B. The Molecular Connectivity Chi Indices and Kappa Shape Indices in Structure-Property Modeling. Rev. Comput. Chem. 1991, 2, 367–422. [Google Scholar]
- Balaban, A.T. Five New Topological Indices for the Branching of Tree-Like Graphs. Theor. Chim. Acta 1979, 53, 355–375. [Google Scholar] [CrossRef]
- Balaban, A.T. Highly Discriminating Distance-Based Topological Index. Chem. Phys. Lett. 1982, 89, 399–404. [Google Scholar] [CrossRef]
- Brillouin, L. Science and Information Theory; Academic Press: New York, NY, USA, 1956; ISBN 978-0-486-43918-1. [Google Scholar]
- Georgescu-Roegen, N. The Entropy Law and the Economic Process; Harvard University Press: Cambridge, MA, USA, 1971; ISBN 978-0-674-25781-8. [Google Scholar]
- Chen, J. The Physical Foundation of Economics—An Analytical Thermodynamic Theory; World Scientific: Singapore, 2005; ISBN 978-981-256-323-1. [Google Scholar]
- Kalinin, M.I.; Kononogov, S.A. Boltzmann’s constant. Meas. Tech. 2005, 48, 632–636. [Google Scholar] [CrossRef]
- Ben-Naim, A. Entropy Demystified the Second Law Reduced to Plain Common Sense, Expanded ed.; World Scientific: Singapore, 2008; ISBN 9789812832269. [Google Scholar]
- Vallino, J.J.; Algar, C.K.; González, N.F.; Huber, J.A. Use of Receding Horizon Optimal Control to Solve MaxEP-Based (max entropy production) Biogeochemistry Problems. In Production & Non-Equilibrium Systems. Living Systems as Catalysts; Springer: Berlin/Heidelberg, Germany, 2013; p. 340. ISBN 978-3642401534. [Google Scholar]
- Schneider, T. Twenty Years of Delila and Molecular Information Theory. Biol Theory. 2006, 1, 250–260. [Google Scholar] [CrossRef]
- Stuttfeld, E.; Ballmer-Hofer, K. Structure and function of VEGF receptors. IUBMB Life 2009, 61, 915–922. [Google Scholar] [CrossRef]
- Fujita, N.; Imai, J.-I.; Suzuki, T.; Yamada, M.; Ninomiya, K.; Miyamoto, K.; Iwasaki, R.; Morioka, H.; Matsumoto, M.; Chiba, K.; et al. Vascular endothelial growth factor-A is a survival factor for nucleus pulposus cells in the intervertebral disc. Biochem. Biophys. Res. Commun. 2008, 372, 367–372. [Google Scholar] [CrossRef]
- Zygmunt, T.; Gay, C.M.; Blondelle, J.; Singh, M.K.; Flaherty, K.M.; Means, P.C.; Herwig, L.; Krudewig, A.; Belting, H.G.; Affolter, M.; et al. Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Dev. Cell 2011, 21, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzog, B.; Pellet-Many, C.; Britton, G.; Hartzoulakis, B.; Zachary, I.C. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol. Biol. Cell 2011, 22, 2766–2776. [Google Scholar] [CrossRef] [PubMed]
- Mecollari, V.; Nieuwenhuis, B.; Verhaagen, J. A perspective on the role of class III semaphorin signaling in central nervous system trauma. Front. Cell. Neurosci. 2014, 8, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Lu, S.; Li, T.; Yu, L.; Zhang, Y.; Zeng, H.; Qian, X.; Bi, J.; Lin, Y. ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. J. Exp. Clin. Cancer Res. 2019, 38, 173. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Zhang, J.-Y.; Gong, L.-P.; Feng, Z.-Y.; Wang, D.; Pan, Y.-H.; Sun, L.-P.; Wen, J.-Y.; Chen, G.-F.; Liang, J.; et al. Hypoxia-induced EBV-circLMP2A promotes angiogenesis in EBV-associated gastric carcinoma through the KHSRP/VHL/HIF1α/VEGFA pathway. Cancer Lett. 2022, 526, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Gua, J.-H.; Cao, Z.-Y.; Guan, B.; Wei, L.-H.; Peng, J.; Chen, Y.-Q.; Sferra, T.J.; Sankararaman, S.; Zhan, Z.-X.; Lin, J.-M. Effect of Babao Dan on angiogenesis of gastric cancer in vitro by regulating VEGFA/VEGFR2 signaling pathway. Trans. Cancer Res. 2021, 10, 953–965. [Google Scholar]
- Luque, A.; Carpizo, D.R.; Iruela-Arispe, M.L. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J. Biol. Chem. 2003, 278, 23656–23665. [Google Scholar] [CrossRef] [Green Version]
- Obika, M.; Ogawa, H.; Takahashi, K.; Li, J.; Hatipoglu, O.F.; Cilek, M.Z.; Miyoshi, T.; Inagaki, J.; Ohtsuki, T.; Kusachi, S.; et al. Tumor growth inhibitory effect of ADAMTS1 is accompanied by the inhibition of tumor angiogenesis. Cancer Sci. 2012, 103, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhi, Y.; Chang, X.; Zhang, S.; Dai, D. Expression of ADAMTS1 and its correlation with angiogenesis in primary gastric cancer and lymph node metastasis. Dig. Dis. Sci. 2013, 58, 405–413. [Google Scholar] [CrossRef]
- Iruela-Arispe, M.L.; Carpizo, D.; Luque, A. ADAMTS1: A matrix metalloprotease with angioinhibitory properties. Ann. N. Y. Acad. Sci. 2003, 995, 183–190. [Google Scholar] [CrossRef]
- Lambert, J.; Makin, K.; Akbareian, S.; Johnson, R.; Alghamdi, A.A.A.; Robinson, S.D.; Edwards, D.R. ADAMTS-1 and syndecan-4 intersect in the regulation of cell migration and angiogenesis. J. Cell Sci. 2020, 133, jcs235762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prydz, K. Determinants of Glycosaminoglycan (GAG.) Structure. Biomolecules 2015, 5, 2003–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratta, M.A.; Yao, W.; Decicco, C.; Tortorella, M.D.; Liu, R.-Q.; Copeland, R.A.; Magolda, R.; Newton, R.C.; Trzaskos, J.M.; Arner, E.C. Aggrecan protects cartilage collagen from proteolytic cleavage. J. Biol. Chem. 2003, 278, 45539–45545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, H.M.; Robker, R.L.; Russell, D.L. Development and hormonal regulation of the ovarian lymphatic vasculature. Endocrinology 2010, 151, 5446–5455. [Google Scholar] [CrossRef]
- Lee, N.V.; Sato, M.; Annis, D.S.; Loo, J.A.; Wu, L.; Mosher, D.F.; Iruela-Arispe, M.L. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J. 2006, 25, 5270–5283. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.-C.; Lin, Y.-W.; Chu, C.-Y.; Yang, Y.-C.; Yang, S.-F.; Liu, Y.-F.; Hsiao, M.; Lee, W.-J.; Chien, M.-H. Melatonin-triggered post-transcriptional and post-translational modifications of ADAMTS1 coordinately retard tumorigenesis and metastasis of renal cell carcinoma. J. Pienal. Res. 2020, 69, e12668. [Google Scholar] [CrossRef]
- Vo, N.V.; Hartman, R.A.; Yurube, T.; Jacobs, L.J.; Sowa, G.A.; Kang, J.D. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013, 13, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Diamantis, I.; Lüthi, M.; Hösli, M.; Reichen, J. Cloning of the rat ADAMTS-1 gene and its down regulation in endothelial cells in cirrhotic rats. Liver 2000, 20, 165–172. [Google Scholar] [CrossRef]
- Voros, G.; Maquoi, E.; Collen, D.; Lijnen, H.R. Differential expression of plasminogen activator inhibitor-1, tumor necrosis factor-alpha, TNF-alpha converting enzyme and ADAMTS family members in murine fat territories. Biochim. Biophys. Acta 2003, 1625, 36–42. [Google Scholar] [CrossRef]
- Mitlöhner, J.; Kaushik, R.; Niekisch, H.; Blondiaux, A.; Gee, C.E.; Happel, M.F.K.; Gundelfinger, E.; Dityatev, A.; Frischknecht, R.; Seidenbecher, C. Dopamine Receptor Activation Modulates the Integrity of the Perisynaptic Extracellular Matrix at Excitatory Synapses. Cells 2020, 9, 260. [Google Scholar] [CrossRef] [Green Version]
- Arner, E.C. Aggrecanase-mediated cartilage degradation. Curr. Opin. Pharmacol. 2002, 2, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Okada, Y. ADAMs in cancer cell proliferation and progression. Cancer Sci. 2007, 98, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signaling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Wiesmann, C.; Fuh, G.; Christinger, H.W.; Eigenbrot, C.; Wells, J.A.; de Vos, A.M. Crystal structure at 1.7 A resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell 1997, 91, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Cheung, M.; Hunter, R.N.; Brown, M.L.; Veal, J.M.; Nolte, R.T.; Wang, L.; Liu, W.; Crosby, R.M.; Johnson, J.H.; et al. Discovery and evaluation of 2-anilino-5-aryloxazoles as a novel class of VEGFR2 kinase inhibitors. J. Med. Chem. 2005, 48, 1610–1619. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Leppanen, V.-M.; Tvorogov, D.; Kisko, K.; Prota, A.E.; Jeltsch, M.; Anisimov, A.; Markovic-Mueller, S.; Stuttfeld, E.; Goldie, K.N.; Ballmer-Hofer, K.; et al. Structural and Mechanistic Insights into Vegfr-3 Ligand Binding and Activation. Proc. Natl. Acad. Sci. USA 2013, 110, 12960. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, S.; Hassall, G.; Hawtin, P.; McCall, E.; Flavell, L.; Minshull, C.; Hargreaves, D.; Ting, A.; Pauptit, R.A.; Parker, A.E.; et al. Crystal structures of human ADAMTS-1 reveal a conserved catalytic domain and a disintegrin-like domain with a fold homologous to cysteine-rich domains. J. Mol. Biol. 2007, 373, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Nishida, T.; Shimo, T.; Kobayashi, K.; Kubo, T.; Tamatani, T.; Tezuka, K.; Takigawa, M. Effects of CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene, on the proliferation and differentiation of chondrocytes in culture. Endocrinology 2000, 141, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Kreusch, A.; McMullan, D.; Ng, K.; Spraggon, G. Crystal Structure of the Human Neuropilin-1 b1 Domain. Structure 2003, 11, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Pérez, A.; Marchán, I.; Svozil, D.; Sponer, J.; Cheatham, T.E.; Laughton, C.A.; Orozco, M. Refinement of the AMBER force field for nucleic acids: Improving the description of alpha/gamma conformers. Biophys. J. 2007, 92, 3817–3829. [Google Scholar] [CrossRef] [Green Version]
- Homeyer, N.; Horn, A.H.C.; Lanig, H.; Sticht, H. AMBER force-field parameters for phosphorylated amino acids in different protonation states: Phosphoserine, phosphothreonine, phosphotyrosine, and phosphohistidine. J. Mol. Model. 2006, 12, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Jung, E.; Bairoch, A. SWISS-PROT: Connecting Biomolecular Knowledge Via a Protein Database. Curr. Issues Mol. Biol. 2001, 3, 47–55. [Google Scholar] [PubMed]
- Bhachoo, J.; Beuming, T. Investigating Protein-Peptide Interactions Using the Schrödinger Computational Suite. Methods Mol. Biol. 2017, 1561, 235–254. [Google Scholar] [PubMed]
- Roy, U.; Luck, L.A. Molecular modeling of estrogen receptor using molecular operating environment. Biochem. Mol. Biol. Educ. 2007, 35, 238–243. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Q.; Han, W.; Laster, K.; Hu, Y.; Ma, F.; Chen, H.; Tian, X.; Qiao, Y.; Liu, H.; et al. Targeting integrin αvβ3 with indomethacin inhibits patient-derived xenograft tumour growth and recurrence in oesophageal squamous cell carcinoma. Clin. Transl. Med. 2021, 11, e548. [Google Scholar] [CrossRef]
- Wang, D.D.; Chan, M.-T.; Yan, H. Structure-based protein-ligand interaction fingerprints for binding affinity prediction. Comput. Struct. Biotechnol. J. 2021, 25, 6291–6300. [Google Scholar] [CrossRef]
- Brewerton, S.C. The use of protein-ligand interaction fingerprints in docking. Curr. Opin. Drug Discov. Dev. 2008, 11, 356–364. [Google Scholar]
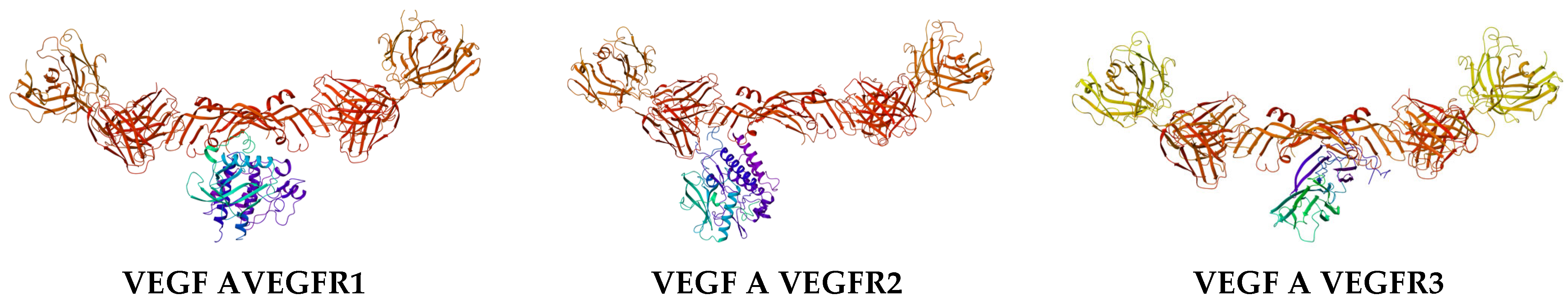
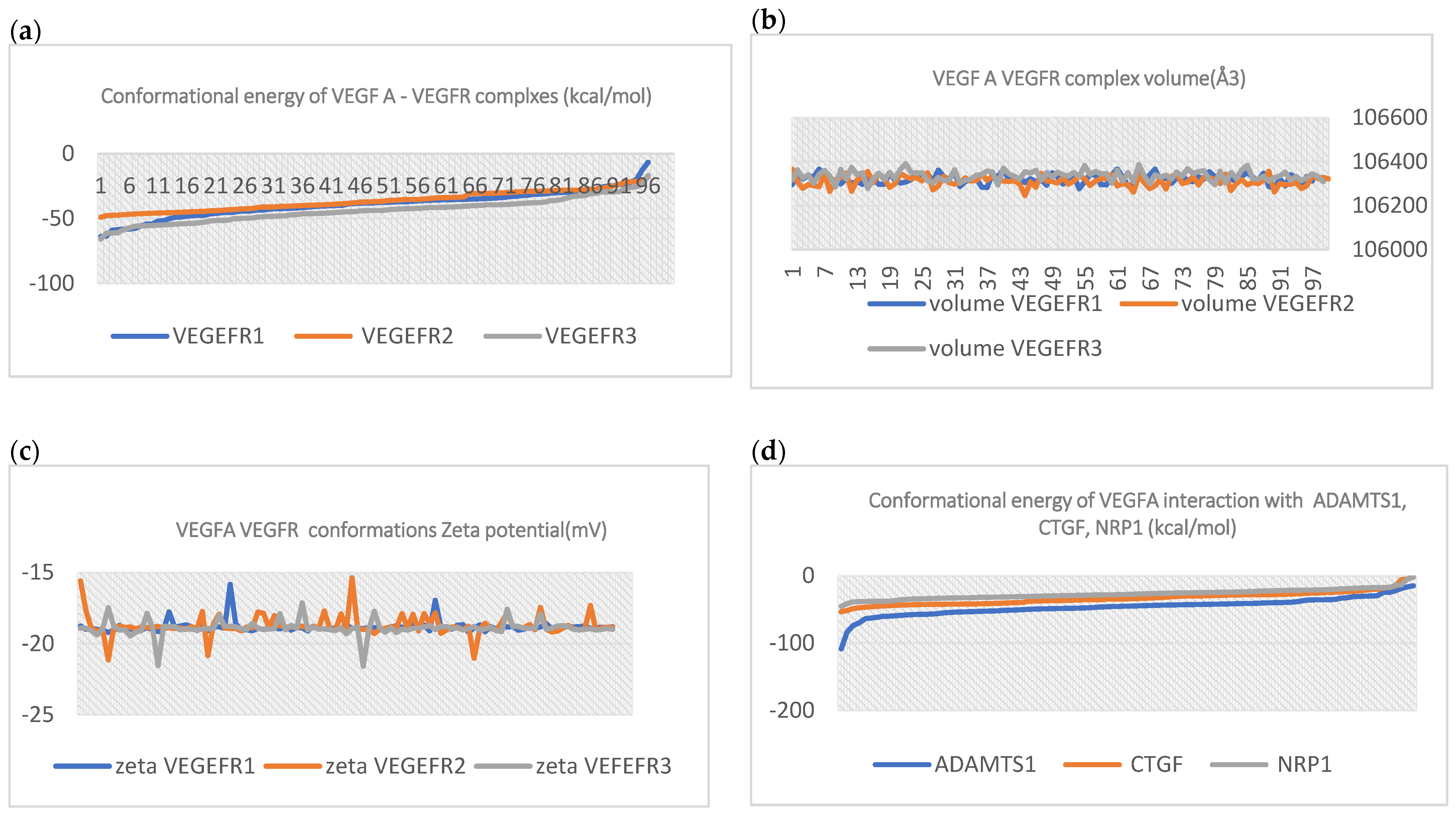
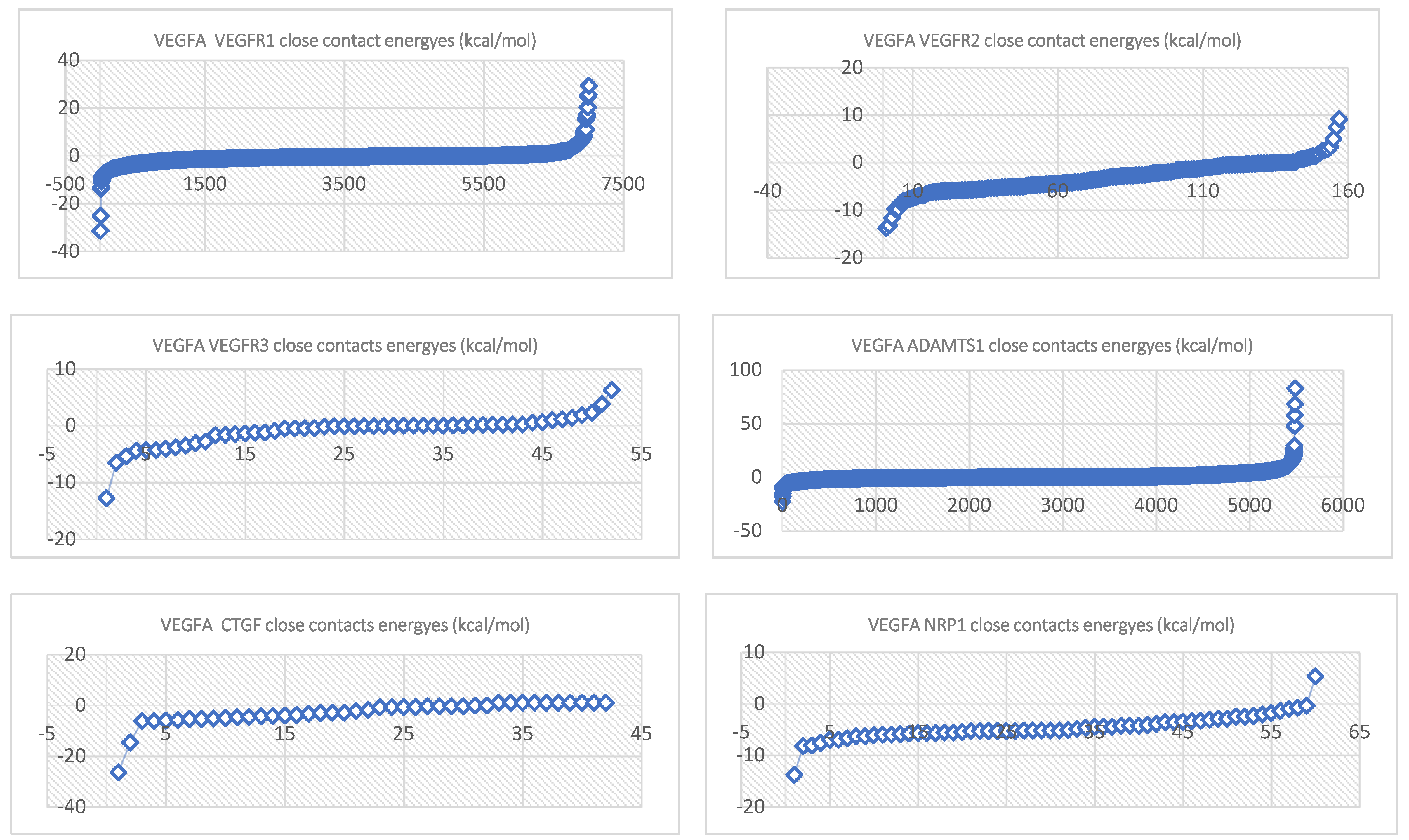
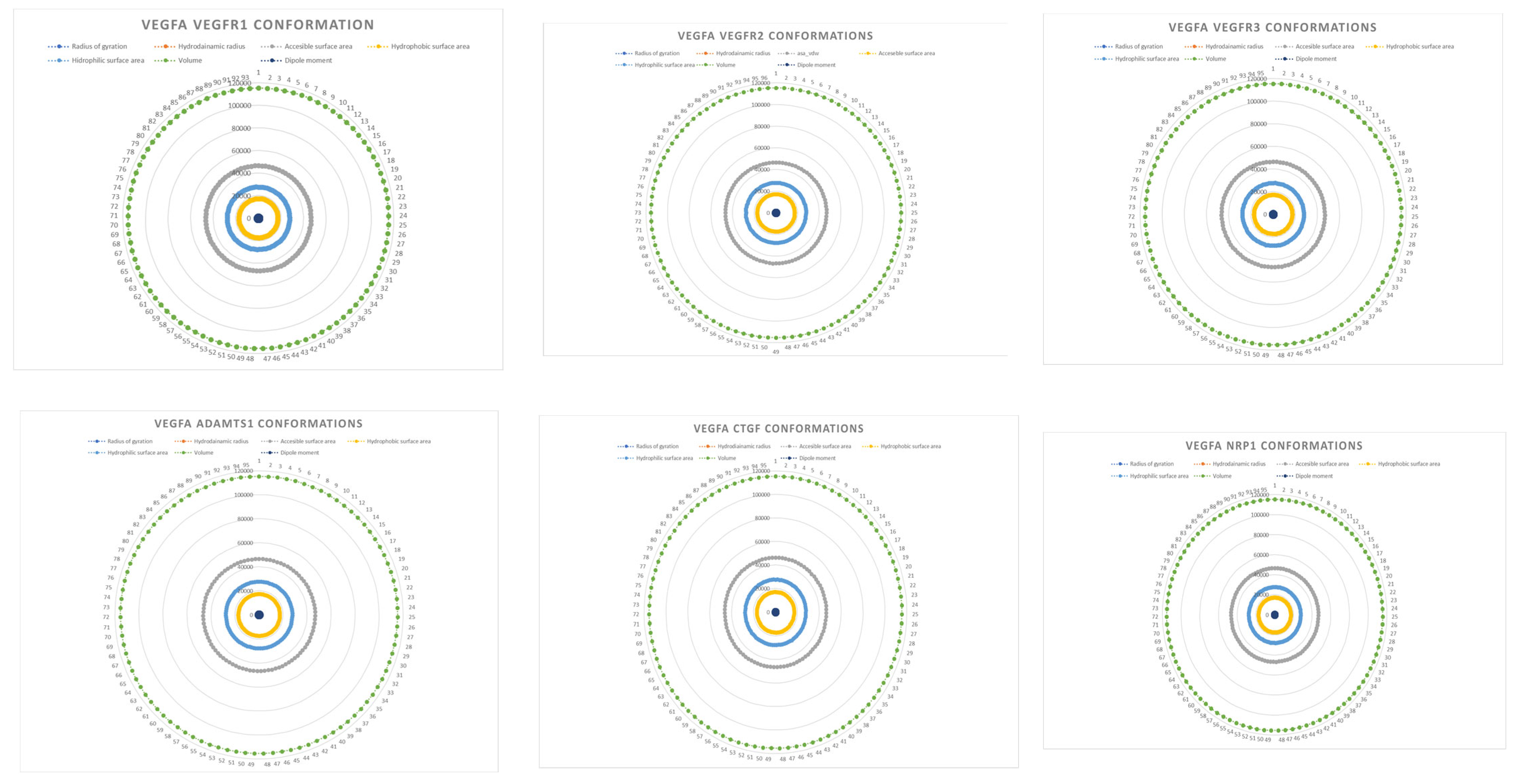
| Complex | Equation |
|---|---|
| VEGFA/VEGFR1 | y = 21x6 − 16x5 − 13x4 − 10x3 −0.6x2 + 0.0104x − 8.2713 |
| VEGFA/VEGFR2 | y = −11x6 − 10x5 − 0.6x4 + 0.0003x3 − 0.0201x2 + 0.6414x − 12.746 |
| VEGFA/VEGFR3 | y = −0.8x6 + −0.6x5 − 0.0004x4 + 0.0138x3 − 0.2601x2 + 2.5731x − 12.569 |
| VEGFA/ADAMTS1 | y = −19x6 − 15x5 − 11x4 − 0.8x3 – 0.5x2 − 0.0186x |
| VEGFA/CTGF | y = −0.7x6 – 0.5x5 − 0.0052x4 + 0.1408x3 − 1.9297x2 + 12.493x − 34.148 |
| VEGFA/NRP1 | y = −0.9x6 − 0.7x5 − 0.5x4 + 0.0043x3 − 0.1242x2 + 1.5741x − 12.772 |
| Nr | Riemann Surface Schematics | Riemann Surface 3D |
|---|---|---|
| 1 |  |  |
| 2 |  |  |
| 3 |  |  |
| 4 |  |  |
| 5 |  |  |
| 6 |  |  |
| Complex | Total Information Content | Mean Information Content | Vertex Adjacency Information Equal | Vertex Adjacency Information Mag |
|---|---|---|---|---|
| VEGFA–VEGFR1 | 30,187.6602 | 1.6931 | 0.0030 | 14.0463 |
| VEGFA–VEGFR2 | 30,196.4746 | 1.6927 | 0.0030 | 14.0463 |
| VEGFA–VEGFR3 | 30,190.5966 | 1.6930 | 0.0030 | 14.0463 |
| VEGFA–ADAMTS1 | 30,196.4746 | 1.6927 | 0.0030 | 14.0463 |
| VEGFA–CTGF | 30,192.5586 | 1.6929 | 0.0030 | 14.0463 |
| VEGFA–NRP1 | 30,184.7227 | 1.6932 | 0.0030 | 14.0463 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, C.N.; Mehedinti, M.C. Molecular Motifs in Vascular Morphogenesis: Vascular Endothelial Growth Factor A (VEGFA) as the Leading Promoter of Angiogenesis. Int. J. Mol. Sci. 2023, 24, 12169. https://doi.org/10.3390/ijms241512169
Lungu CN, Mehedinti MC. Molecular Motifs in Vascular Morphogenesis: Vascular Endothelial Growth Factor A (VEGFA) as the Leading Promoter of Angiogenesis. International Journal of Molecular Sciences. 2023; 24(15):12169. https://doi.org/10.3390/ijms241512169
Chicago/Turabian StyleLungu, Claudiu N., and Mihaela C. Mehedinti. 2023. "Molecular Motifs in Vascular Morphogenesis: Vascular Endothelial Growth Factor A (VEGFA) as the Leading Promoter of Angiogenesis" International Journal of Molecular Sciences 24, no. 15: 12169. https://doi.org/10.3390/ijms241512169





