CXCL5 Promotes Acetaminophen-Induced Hepatotoxicity by Activating Kupffer Cells
Abstract
:1. Introduction
2. Results
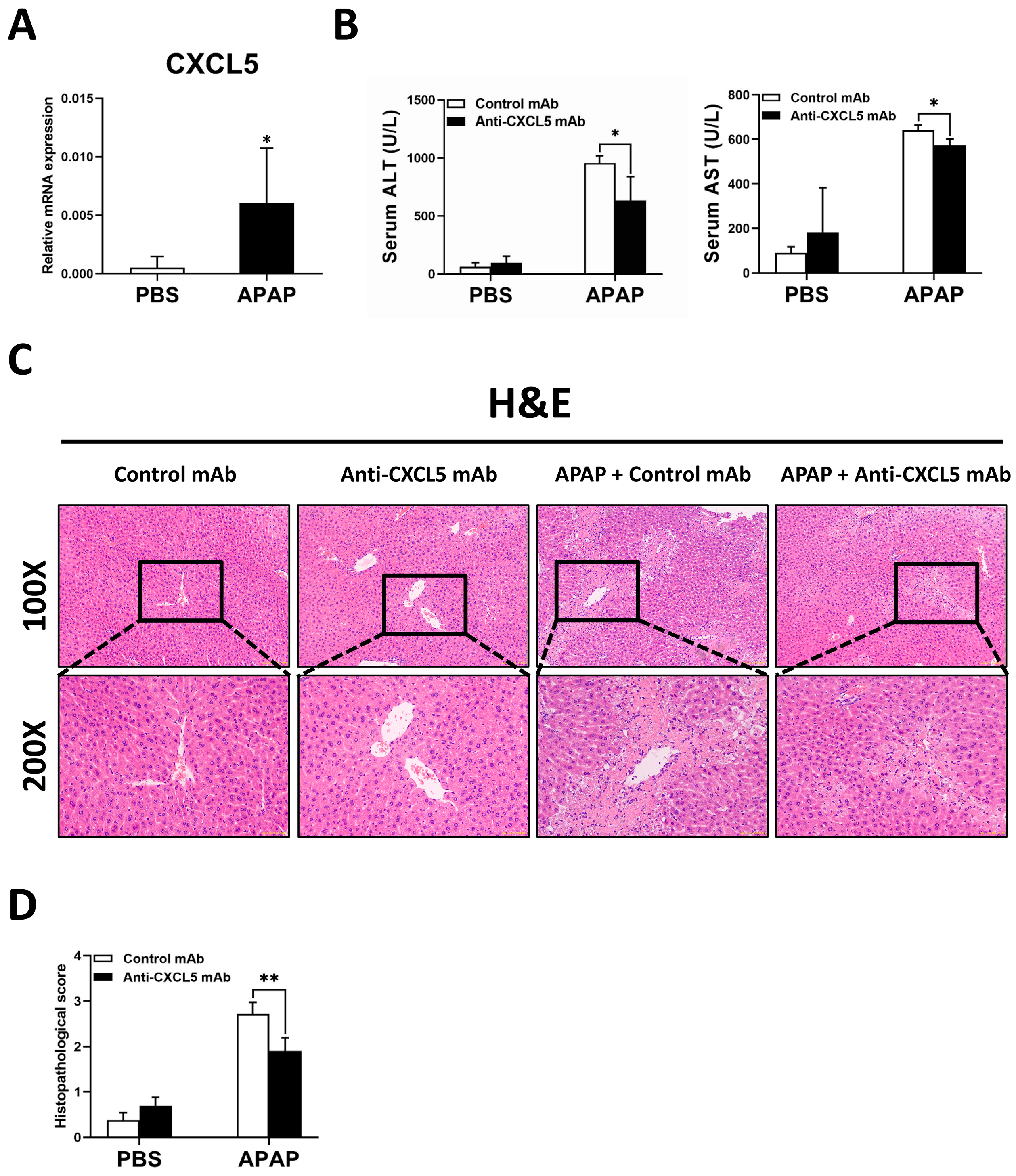
2.1. Anti-CXCL5 mAb Treatment Mitigates the Degree of APAP-Induced ALI in Mice
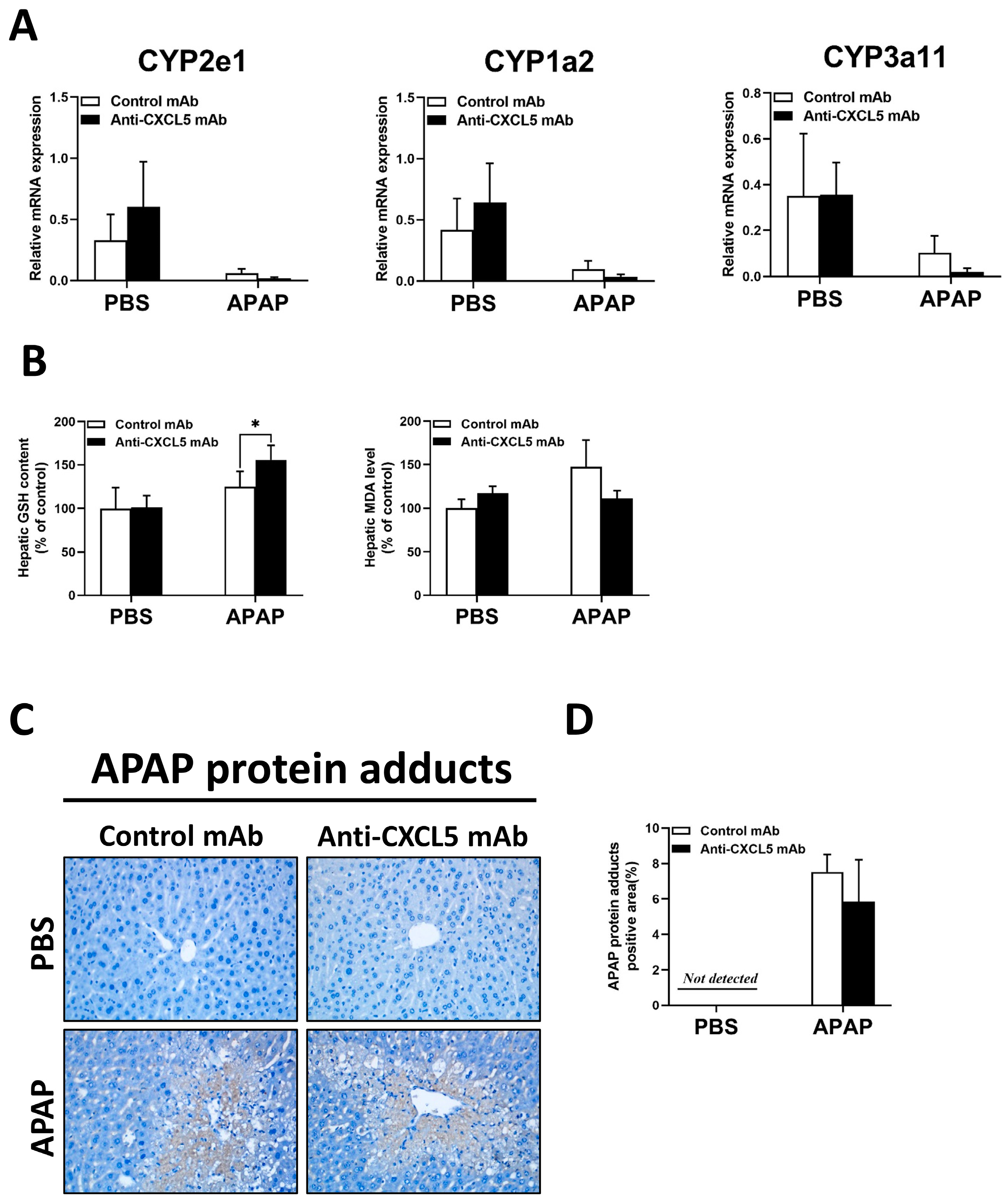
2.2. Anti-CXCL5 mAb Treatment Has No Significant Effect on Liver Metabolism in ALI Induced by APAP
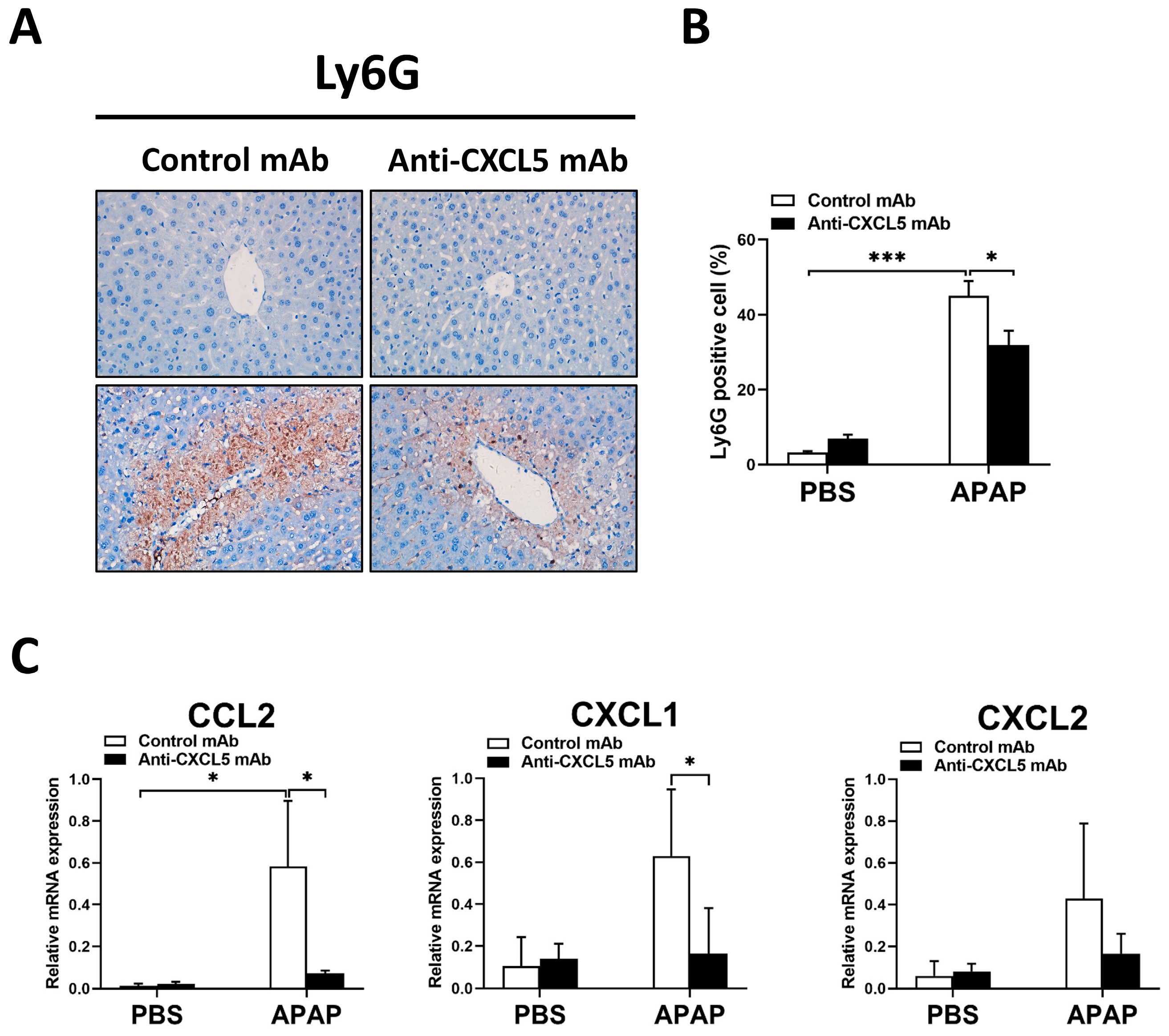
2.3. Anti-CXCL5 mAb Exhibits Anti-Inflammatory Effects in APAP-Injected Mice
2.4. Treatment with Anti-CXCL5 mAb Ameliorates Hepatocellular Death of APAP-Evoked ALI in Mice
2.5. rmCXCL5 Stimulates KCs to Produce Inflammatory Mediators In Vitro
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Animal Experiments
4.3. Histopathologic Examination
4.4. Biochemical Measurements
4.5. Immunohistochemistry (IHC) Staining
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Cell Cytotoxicity Assays (LDH)
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Cell Isolation and Culture
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, W.M. Drug-induced Acute Liver Failure. Clin. Liver Dis. 2013, 17, 575–586. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Huo, Y.; Yin, S.; Hu, H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018, 17, 274–283. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Jaeschke, H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J. Hepatol. 2017, 66, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Jaeschke, H.; Ramachandran, A. Mechanisms and pathophysiological significance of sterile inflammation during acetaminophen hepatotoxicity. Food Chem. Toxicol. 2020, 138, 111240. [Google Scholar] [CrossRef] [PubMed]
- Ferner, R.E.; Dear, J.W.; Bateman, D.N. Management of paracetamol poisoning. BMJ 2011, 342, d2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, P.S.; Campana, L.; Aleksieva, N.; Cartwright, J.A.; Mackinnon, A.; O’Duibhir, E.; Kendall, T.; Vermeren, M.; Thomson, A.; Gadd, V.; et al. Alternatively activated macrophages promote resolution of necrosis following acute liver injury. J. Hepatol. 2020, 73, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Tao, Y.; Wu, Y.; Zhao, X.; Ye, W.; Zhao, D.; Fu, L.; Tian, C.; Yang, J.; He, F.; et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 2019, 10, 1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Liu, M.; Sehrawat, T.S.; Shah, V.H. Regulation and functional roles of chemokines in liver diseases. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 630–647. [Google Scholar] [CrossRef]
- Liang, Y.; Li, H.; Li, J.; Yang, Z.N.; Li, J.L.; Zheng, H.W.; Chen, Y.L.; Shi, H.J.; Guo, L.; Liu, L.D. Role of neutrophil chemoattractant CXCL5 in SARS-CoV-2 infection-induced lung inflammatory innate immune response in an in vivo hACE2 transfection mouse model. Zool. Res. 2020, 41, 621–631. [Google Scholar] [CrossRef]
- Zhou, S.-L.; Dai, Z.; Zhou, Z.-J.; Wang, X.-Y.; Yang, G.-H.; Wang, Z.; Huang, X.-W.; Fan, J.; Zhou, J. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology 2012, 56, 2242–2254. [Google Scholar] [CrossRef]
- Chavey, C.; Lazennec, G.; Lagarrigue, S.; Clapé, C.; Iankova, I.; Teyssier, J.; Annicotte, J.-S.; Schmidt, J.; Mataki, C.; Yamamoto, H.; et al. CXC Ligand 5 Is an Adipose-Tissue Derived Factor that Links Obesity to Insulin Resistance. Cell Metab. 2009, 9, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Pickens, S.R.; Chamberlain, N.D.; Volin, M.V.; Gonzalez, M.; Pope, R.M.; Mandelin, A.M.; Kolls, J.K.; Shahrara, S. Anti-CXCL5 therapy ameliorates IL-17-induced arthritis by decreasing joint vascularization. Angiogenesis 2011, 14, 443–455. [Google Scholar] [CrossRef] [Green Version]
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of Acetaminophen-Induced Liver Necrosis. In Adverse Drug Reactions; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 196, pp. 369–405. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.T.; Akakpo, J.Y.; Weemhoff, J.L.; Ramachandran, A.; Ding, W.-X.; Jaeschke, H. Impaired protein adduct removal following repeat administration of subtoxic doses of acetaminophen enhances liver injury in fed mice. Arch. Toxicol. 2021, 95, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Shang, M.; Zhou, C.L.; Feng, L.Z.; Zhou, Q.S.; Hu, K. Mechanism of Cxc Chemokine Ligand 5 (CXCL5)/Cxc Chemokine Receptor 2 (CXCR2) Bio-Axis in Mice with Acute Respiratory Distress Syndrome. Med. Sci. Monit. 2019, 25, 5299–5305. [Google Scholar] [CrossRef]
- Patterson, A.M.; Schmutz, C.; Davis, S.; Gardner, L.; Ashton, B.A.; Middleton, J. Differential binding of chemokines to macrophages and neutrophils in the human inflamed synovium. Thromb. Haemost. 2002, 4, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Liang, J.J.; Ng, T.K.; Hu, Z.; Xu, C.; Chen, S.; Chen, S.L.; Xu, Y.; Zhuang, X.; Huang, S.; et al. CXCL5/CXCR2 modulates inflammation-mediated neural repair after optic nerve injury. Exp. Neurol. 2021, 341, 113711. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, J.; Shi, Y.; Li, W.; Shi, H.; Ji, R.; Mao, F.; Qian, H.; Xu, W.; Zhang, X. CXCL5 promotes gastric cancer metastasis by inducing epithelial-mesenchymal transition and activating neutrophils. Oncogenesis 2020, 9, 63. [Google Scholar] [CrossRef]
- Mossanen, J.C.; Krenkel, O.; Ergen, C.; Govaere, O.; Liepelt, A.; Puengel, T.; Heymann, F.; Kalthoff, S.; Lefebvre, E.; Eulberg, D.; et al. Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury. Hepatology 2016, 64, 1667–1682. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-X.; Govindarajan, S.; Kaplowitz, N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology 2004, 127, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Woolbright, B.L.; Jaeschke, H. Mechanisms of Inflammatory Liver Injury and Drug-Induced Hepatotoxicity. Curr. Pharmacol. Rep. 2018, 4, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Zhou, Z.; Yun, H.; Park, S.; Choi, S.-J.; Lee, S.-H.; Lim, C.W.; Lee, K.; Kim, B. Cigarette smoking differentially regulates inflammatory responses in a mouse model of nonalcoholic steatohepatitis depending on exposure time point. Food Chem. Toxicol. 2020, 135, 110930. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Zhao, J.; Xia, L.; Li, J.; Xu, M.; Wang, B.; Guo, H.; Yu, C.; Gao, Y.; et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell. Mol. Immunol. 2020, 17, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Aleksunes, L.M.; Manautou, J.E. Emerging Role of Nrf2 in Protecting Against Hepatic and Gastrointestinal Disease. Toxicol. Pathol. 2007, 35, 459–473. [Google Scholar] [CrossRef]
- Goldring, C.E.P.; Kitteringham, N.R.; Elsby, R.; Randle, L.E.; Clement, Y.N.; Williams, D.P.; McMahon, M.; Hayes, J.D.; Itoh, K.; Yamamoto, M.; et al. Activation of hepatic Nrf2in vivo by acetaminophen in CD-1 mice. Hepatology 2004, 39, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Williams, C.D.; McGill, M.R.; Jaeschke, H. Lower susceptibility of female mice to acetaminophen hepatotoxicity: Role of mitochondrial glutathione, oxidant stress and c-jun N-terminal kinase. Toxicol. Appl. Pharmacol. 2014, 281, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Wang, L.; Huang, J.; Lv, H.; Deng, X.; Ci, X. Pterostilbene Reduces Acetaminophen-Induced Liver Injury by Activating the Nrf2 Antioxidative Defense System via the AMPK/Akt/GSK3β Pathway. Cell. Physiol. Biochem. 2018, 49, 1943–1958. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Wei, J.-G.; Tu, M.-J.; Gu, J.-G.; Zhang, W. Fucoidan Alleviates Acetaminophen-Induced Hepatotoxicity via Oxidative Stress Inhibition and Nrf2 Translocation. Int. J. Mol. Sci. 2018, 19, 4050. [Google Scholar] [CrossRef] [Green Version]
- Van der Horst, D.; Carter-Timofte, M.; van Grevenynghe, J.; Laguette, N.; Dinkova-Kostova, A.; Olagnier, D. Regulation of innate immunity by Nrf2. Curr. Opin. Immunol. 2022, 78, 102247. [Google Scholar] [CrossRef]
- Patel, S.J.; Luther, J.; Bohr, S.; Iracheta-Vellve, A.; Li, M.; King, K.R.; Chung, R.T.; Yarmush, M.L. A Novel Resolvin-Based Strategy for Limiting Acetaminophen Hepatotoxicity. Clin. Transl. Gastroenterol. 2016, 7, e153. [Google Scholar] [CrossRef] [PubMed]



| Gene | Gene Accession Number | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| CYP2e1 | NM_021282 | AAGCGCTTCGGGCCAG | TAGCCATGCAGGACCACGA |
| CYP1a2 | NM_009993.3 | GGTCAGAAAGCCGTGGTTG | GACATGGCCTAACGTGCAG |
| CYP3a11 | NM_007818.3 | CGCCTCTCCTTGCTGTCACA | CTTTGCCTTCTGCCTCAAGT |
| TNFα | NM_013693.3 | GTCTACTCCCAGGTTTCTCTTCAAGG | GCAAATCGGCTGACGGTGTG |
| IL-1β | NM_008361.4 | CTCGCAGCAGCACATCAACA | CCACGGGAAAGACACAGGTA |
| IL-6 | NM_001314054.1 | CAACGATGATGCACTTGCAGA | CTCCAGGTAGCTATGGTACTCCAGA |
| CXCL1 | NM_008176.3 | TGCACCCAAACCGAAGTC | GTCAGAAGCCAGCGTTCACC |
| CXCL2 | NM_009140.2 | GCCAAGGGTTGACTTCAAGAACA | AGGCTCCTCCTTTCCAGGTCA |
| CCL2 | NM_011333.3 | AGCAGCAGGTGTCCCAAAGA | GTGCTGAAGACCTTAGGGCAGA |
| CD68 | NM_009853 | ACCGCCATGTAGTCCAGGTA | ATCCCCACCTGTCTCTCTCA |
| CD14 | NM_009841 | GGCCGCGCGGATTCCTAGTC | ATCGGGTCCGGTGGCTTCCA |
| Bax | NM_007527 | AAGCGCTTCGGGCCAG | TAGCCATGCAGGACCACGA |
| Bcl2 | NM_177410 | GGTCAGAAAGCCGTGGTTG | GACATGGCCTAACGTGCAG |
| NQO-1 | NM_008706 | CAGCCAATCAGCGTTCGGTA | CTTCATGGCGTAGTTGAATGATGTC |
| HO-1 | NM_010442 | TGCAGGTGATGCTGACAGAGG | GGGATGAGCTAGTGCTGATCTGG |
| GCLC | NM_010295 | AATGACTGTTGCCAGGTGGATG | GGTTGCACTTCCAAATGAGGCTA |
| GCLM | NM_008129 | AGTTGGAGCAGCTGTATCAGTGG | TTTAGCAAAGGCAGTCAAATCTGG |
| GAPDH | NM_001411840.1 | ACGGCAAATTCAACGGCACAG | GAAGACTCCACGACATACTCAGCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, K.; Pan, Y.; Huang, W.; Li, M.; Yan, X.; Zhou, Z.; Qi, J. CXCL5 Promotes Acetaminophen-Induced Hepatotoxicity by Activating Kupffer Cells. Int. J. Mol. Sci. 2023, 24, 12180. https://doi.org/10.3390/ijms241512180
Qiu K, Pan Y, Huang W, Li M, Yan X, Zhou Z, Qi J. CXCL5 Promotes Acetaminophen-Induced Hepatotoxicity by Activating Kupffer Cells. International Journal of Molecular Sciences. 2023; 24(15):12180. https://doi.org/10.3390/ijms241512180
Chicago/Turabian StyleQiu, Kexin, Yan Pan, Weizhi Huang, Mengyuan Li, Xueqing Yan, Zixiong Zhou, and Jing Qi. 2023. "CXCL5 Promotes Acetaminophen-Induced Hepatotoxicity by Activating Kupffer Cells" International Journal of Molecular Sciences 24, no. 15: 12180. https://doi.org/10.3390/ijms241512180





