Neurologic and Psychiatric Manifestations of Bradykinin-Mediated Angioedema: Old and New Challenges
Abstract
:1. Introduction
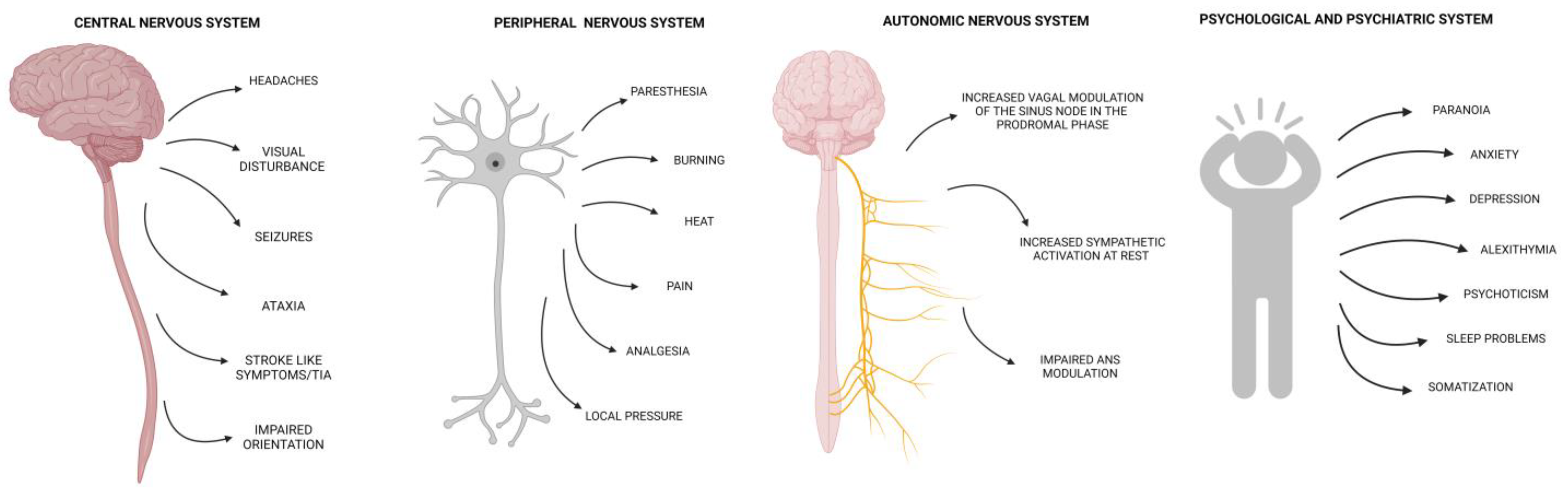
2. Central and Peripheral Nervous Systems Involvement
2.1. Physiopathological Aspects
2.2. Central Nervous System Involvement
2.2.1. Central Nervous System Involvement in Hereditary Angioedema
2.2.2. Iatrogenic Forms: Tissue Plasminogen Activator-Induced Angioedema
2.3. Peripheral Nervous System Involvement
2.4. Autonomic Nervous System Dysfunction
3. Psychological and Psychiatric Manifestations
4. Materials and Methods
5. Unmet Needs in Clinical Practice and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cicardi, M.; Zuraw, B.L. Angioedema Due to Bradykinin Dysregulation. J. Allergy Clin. Immunol. Pract. 2018, 6, 1132–1141. [Google Scholar] [CrossRef]
- Mormile, I.; Cocchiaro, A.; Bova, M.; Loffredo, S.; de Paulis, A.; Spadaro, G.; Petraroli, A. Gastrointestinal manifestations of angioedema: A potential area of misdiagnosis. Eur. J. Gastroenterol. Hepatol. 2021, 33, 787–793. [Google Scholar] [CrossRef]
- Bork, K.; Hardt, J.; Witzke, G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1-INH deficiency. J. Allergy Clin. Immunol. 2012, 130, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Mormile, I.; Bova, M.; Cocchiaro, A.; Rossi, F.W.; Granata, F.; Spadaro, G.; de Paulis, A.; Petraroli, A. Clinical features and burden of genital attacks in hereditary angioedema. J. Allergy Clin. Immunol. Pract. 2022, 10, 643–644.e642. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, H.J.; Bork, K. Hereditary angioedema: An update on causes, manifestations and treatment. Br. J. Hosp. Med. 2019, 80, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, R.; D’Andrea, G.; Maffione, A.B.; Margaglione, M.; d’Apolito, M. The Genetics of Hereditary Angioedema: A Review. J. Clin. Med. 2021, 10, 2023. [Google Scholar] [CrossRef]
- Ariano, A.; D’Apolito, M.; Bova, M.; Bellanti, F.; Loffredo, S.; D’Andrea, G.; Intrieri, M.; Petraroli, A.; Maffione, A.B.; Spadaro, G.; et al. A myoferlin gain-of-function variant associates with a new type of hereditary angioedema. Allergy 2020, 75, 2989–2992. [Google Scholar] [CrossRef]
- Bork, K.; Wulff, K.; Mohl, B.S.; Steinmuller-Magin, L.; Witzke, G.; Hardt, J.; Meinke, P. Novel hereditary angioedema linked with a heparan sulfate 3-O-sulfotransferase 6 gene mutation. J. Allergy Clin. Immunol. 2021, 148, 1041–1048. [Google Scholar] [CrossRef]
- Bova, M.; De Feo, G.; Parente, R.; De Pasquale, T.; Gravante, C.; Pucci, S.; Nettis, E.; Triggiani, M. Hereditary and Acquired Angioedema: Heterogeneity of Pathogenesis and Clinical Phenotypes. Int. Arch. Allergy Immunol. 2018, 175, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Zotter, Z.; Csuka, D.; Szabo, E.; Czaller, I.; Nebenfuhrer, Z.; Temesszentandrasi, G.; Fust, G.; Varga, L.; Farkas, H. The influence of trigger factors on hereditary angioedema due to C1-inhibitor deficiency. Orphanet J. Rare Dis. 2014, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Maurer, M.; Magerl, M.; Betschel, S.; Aberer, W.; Ansotegui, I.J.; Aygoren-Pursun, E.; Banerji, A.; Bara, N.A.; Boccon-Gibod, I.; Bork, K.; et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2021 revision and update. Allergy 2022, 77, 1961–1990. [Google Scholar] [CrossRef] [PubMed]
- Pines, J.M.; Poarch, K.; Hughes, S. Recognition and Differential Diagnosis of Hereditary Angioedema in the Emergency Department. J. Emerg. Med. 2021, 60, 35–43. [Google Scholar] [CrossRef]
- Zafra, H. Hereditary Angioedema: A Review. WMJ 2022, 121, 48–53. [Google Scholar] [PubMed]
- Jindal, A.K.; Reshef, A.; Longhurst, H.; GEHM workgroup (Global Equity in HAE Management). Mitigating Disparity in Health-care Resources Between Countries for Management of Hereditary Angioedema. Clin. Rev. Allergy Immunol. 2021, 61, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A.; Davis, K.H.; Brown, T.M.; Hollis, K.; Hunter, S.M.; Long, J.; Jain, G.; Devercelli, G. Patient-reported burden of hereditary angioedema: Findings from a patient survey in the United States. Ann. Allergy Asthma Immunol. 2020, 124, 600–607. [Google Scholar] [CrossRef]
- Riedl, M.A.; Maurer, M.; Bernstein, J.A.; Banerji, A.; Longhurst, H.J.; Li, H.H.; Lu, P.; Hao, J.; Juethner, S.; Lumry, W.R.; et al. Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks. Allergy 2020, 75, 2879–2887. [Google Scholar] [CrossRef]
- Aygoren-Pursun, E.; Bygum, A.; Grivcheva-Panovska, V.; Magerl, M.; Graff, J.; Steiner, U.C.; Fain, O.; Huissoon, A.; Kinaciyan, T.; Farkas, H.; et al. Oral Plasma Kallikrein Inhibitor for Prophylaxis in Hereditary Angioedema. N. Engl. J. Med. 2018, 379, 352–362. [Google Scholar] [CrossRef]
- Bork, K.; Bygum, A.; Hardt, J. Benefits and risks of danazol in hereditary angioedema: A long-term survey of 118 patients. Ann. Allergy Asthma Immunol. 2008, 100, 153–161. [Google Scholar] [CrossRef]
- Cesoni Marcelli, A.; Loffredo, S.; Petraroli, A.; Carucci, L.; Mormile, I.; Ferrara, A.L.; Spadaro, G.; Genovese, A.; Bova, M. Nailfold Videocapillaroscopy Findings in Bradykinin-Mediated Angioedema. J. Investig. Allergol. Clin. Immunol. 2021, 31, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Gambardella, J.; Sorriento, D.; Bova, M.; Rusciano, M.; Loffredo, S.; Wang, X.; Petraroli, A.; Carucci, L.; Mormile, I.; Oliveti, M.; et al. Role of Endothelial G Protein-Coupled Receptor Kinase 2 in Angioedema. Hypertension 2020, 76, 1625–1636. [Google Scholar] [CrossRef]
- Molinari, F.; Meskanaite, V.; Munnich, A.; Sonderegger, P.; Colleaux, L. Extracellular proteases and their inhibitors in genetic diseases of the central nervous system. Hum. Mol. Genet. 2003, 12, R195–R200. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lu, F.; Qin, G.; Fernandes, S.M.; Li, J.; Davis, A.E., 3rd. C1 inhibitor-mediated protection from sepsis. J. Immunol. 2007, 179, 3966–3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Shiosaka, S. Plasticity-related serine proteases in the brain (review). Int. J. Mol. Med. 1999, 3, 405–409. [Google Scholar] [CrossRef]
- Davis, A.E., 3rd; Lu, F.; Mejia, P. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb. Haemost. 2010, 104, 886–893. [Google Scholar] [CrossRef]
- Chung, J.Y.; Kim, M. Migraine-like headache in a patient with complement 1 inhibitor deficient hereditary angioedema. J. Korean Med. Sci. 2012, 27, 104–106. [Google Scholar] [CrossRef] [Green Version]
- Markowitz, S.; Saito, K.; Moskowitz, M.A. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J. Neurosci. 1987, 7, 4129–4136. [Google Scholar] [CrossRef] [Green Version]
- Tradtrantip, L.; Asavapanumas, N.; Phuan, P.W.; Verkman, A.S. Potential therapeutic benefit of C1-esterase inhibitor in neuromyelitis optica evaluated in vitro and in an experimental rat model. PLoS ONE 2014, 9, e106824. [Google Scholar] [CrossRef]
- Testori, A.; Melamed, I. Neurologic manifestations of hereditary angioedema. Ann. Allergy Asthma Immunol. 2017, 118, 119–120. [Google Scholar] [CrossRef]
- Sunder, T.R.; Balsam, M.J.; Vengrow, M.I. Neurological manifestations of angioedema. Report of two cases and review of the literature. JAMA 1982, 247, 2005–2007. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, M.; Meta, D.; Kalo, T. Hereditary angioedema as a potential cause of cerebral edema. Otorhinolaryngol. Head Neck Surg. 2013, 51, 31–34. [Google Scholar]
- Krause, K.H.; Rentrop, U.; Mehregan, U. [Cerebral manifestations in angioneurotic edema (author’s transl)]. J. Neurol. Sci. 1979, 42, 429–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnaud, I.; Rouaud, V.; Guyot, M.; Debiais, S.; Saudeau, D.; de Toffol, B.; Farber, C.M. Exceptional stroke-like episodes in a patient with type I autosomal angioedema. Neurology 2012, 78, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Lasek-Bal, A.; Holecki, M.; Handzlik-Orlik, G.; Smertka, M.; Dulawa, J. Hereditary angioedema with dominant cerebral symptoms finally leading to chronic disability. Clin. Neurol. Neurosurg. 2015, 135, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Bork, K.; Meng, G.; Staubach, P.; Hardt, J. Hereditary angioedema: New findings concerning symptoms, affected organs, and course. Am. J. Med. 2006, 119, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz-Wojcik, K.; Porebski, G. Life-threatening laryngeal attacks in hereditary angioedema patients. Otolaryngol. Pol. 2020, 74, 42–46. [Google Scholar] [CrossRef]
- Bork, K.; Barnstedt, S.E. Laryngeal edema and death from asphyxiation after tooth extraction in four patients with hereditary angioedema. J. Am. Dent. Assoc. 2003, 134, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.; Boursiquot, J.N.; Chapdelaine, H.; Laramee, B.; Desjardins, M.; Gagnon, R.; Payette, N.; Lepeshkina, O.; Vincent, M. Bradykinin-induced angioedema in the emergency department. Int. J. Emerg. Med. 2022, 15, 15. [Google Scholar] [CrossRef]
- Neri, S.G.F.; Rizzotto, A. An Unusual Case of Cerebral Oedema. EJCRIM 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- Frohlich, K.; Macha, K.; Gerner, S.T.; Bobinger, T.; Schmidt, M.; Dorfler, A.; Hilz, M.J.; Schwab, S.; Seifert, F.; Kallmunzer, B.; et al. Angioedema in Stroke Patients With Thrombolysis. Stroke 2019, 50, 1682–1687. [Google Scholar] [CrossRef]
- Liang, B.A.; Lew, R.; Zivin, J.A. Review of tissue plasminogen activator, ischemic stroke, and potential legal issues. Arch. Neurol. 2008, 65, 1429–1433. [Google Scholar] [CrossRef] [Green Version]
- Menon, B.K.; Buck, B.H.; Singh, N.; Deschaintre, Y.; Almekhlafi, M.A.; Coutts, S.B.; Thirunavukkarasu, S.; Khosravani, H.; Appireddy, R.; Moreau, F.; et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): A pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet 2022, 400, 161–169. [Google Scholar] [CrossRef]
- Duangmee, K.; Boonmuang, P.; Santimaleeworagun, W.; Prasitdumrong, H. Urticaria, angioedema, and type I hypersensitivity reactions associated with fibrinolytic agents. Asian Pac. J. Allergy Immunol. 2022, 40, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Duymun, S.; Reddy, V.; Bentley, E.; Bose-Kolanu, A. Tissue Plasminogen Activator-Induced Angioedema Involving a Posterior Cerebral Artery Infarct: A Case Presentation. Am. J. Case Rep. 2021, 22, e927137. [Google Scholar] [CrossRef]
- Brown, E.; Campana, C.; Zimmerman, J.; Brooks, S. Icatibant for the treatment of orolingual angioedema following the administration of tissue plasminogen activator. Am. J. Emerg. Med. 2018, 36, 1125.e1–1125.e2. [Google Scholar] [CrossRef] [PubMed]
- Engelter, S.T.; Fluri, F.; Buitrago-Tellez, C.; Marsch, S.; Steck, A.J.; Ruegg, S.; Lyrer, P.A. Life-threatening orolingual angioedema during thrombolysis in acute ischemic stroke. J. Neurol. 2005, 252, 1167–1170. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Wang, G.N.; Zhang, X.M.; Zhang, J.S. Orolingual angioedema during thrombolysis in acute ischemic stroke: A case report. World J. Emerg. Med. 2022, 13, 71–73. [Google Scholar] [CrossRef]
- Hurford, R.; Rezvani, S.; Kreimei, M.; Herbert, A.; Vail, A.; Parry-Jones, A.R.; Douglass, C.; Molloy, J.; Alachkar, H.; Tyrrell, P.J.; et al. Incidence, predictors and clinical characteristics of orolingual angio-oedema complicating thrombolysis with tissue plasminogen activator for ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2015, 86, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Tang, S.C.; Tsai, L.K.; Yeh, S.J.; Hsiao, Y.J.; Chen, Y.W.; Chen, K.H.; Yip, B.S.; Shen, L.J.; Wu, F.L.; et al. Orolingual angioedema after alteplase therapy of acute ischaemic stroke: Incidence and risk of prior angiotensin-converting enzyme inhibitor use. Eur. J. Neurol. 2014, 21, 1285–1291. [Google Scholar] [CrossRef]
- Yakhkind, A.; Lang, A.E.; Montalvo, M.; Beland, M.D.; Cutting, S. Gastrointestinal Angioedema as a Side Effect of Alteplase for Acute Stroke. J. Vasc. Interv. Radiol. 2020, 31, 1921–1924. [Google Scholar] [CrossRef]
- Chaucer, B.; Whelan, D.; Veys, C.; Upadhyaya, M. Angioedema Secondary to IV Tissue Plasminogen Activator Administration for Treatment of Acute Ischemic Stroke. Case Rep. Crit. Care 2018, 2018, 3257215. [Google Scholar] [CrossRef] [Green Version]
- Bennett, W.R.; Yawn, D.H.; Migliore, P.J.; Young, J.B.; Pratt, C.M.; Raizner, A.E.; Roberts, R.; Bolli, R. Activation of the complement system by recombinant tissue plasminogen activator. J. Am. Coll. Cardiol. 1987, 10, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Busse, P.J.; Christiansen, S.C.; Riedl, M.A.; Banerji, A.; Bernstein, J.A.; Castaldo, A.J.; Craig, T.; Davis-Lorton, M.; Frank, M.M.; Li, H.H.; et al. US HAEA Medical Advisory Board 2020 Guidelines for the Management of Hereditary Angioedema. J. Allergy Clin. Immunol. Pract. 2021, 9, 132–150.e133. [Google Scholar] [CrossRef]
- Cheong, E.; Dodd, L.; Smith, W.; Kleinig, T. Icatibant as a Potential Treatment of Life-Threatening Alteplase-Induced Angioedema. J. Stroke Cerebrovasc. Dis. 2018, 27, e36–e37. [Google Scholar] [CrossRef]
- Foreman, A.; He, T.; Chan, Y.; Gilbert, R.; Gullane, P. Persistent, severe post-thrombolysis angioedema: Simple management of a difficult problem. Am. J. Otolaryngol. 2015, 36, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Leibovich-Nassi, I.; Reshef, A. The Enigma of Prodromes in Hereditary Angioedema (HAE). Clin. Rev. Allergy Immunol. 2021, 61, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.M.; Gelfand, J.A.; Atkinson, J.P. Hereditary angioedema: The clinical syndrome and its management. Ann. Intern. Med. 1976, 84, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Landerman, N.S. Hereditary angioneurotic edema. I. Case reports and review of the literature. J. Allergy 1962, 33, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Beck, P.; Willis, D.; Davies, G.T.; Lachmann, P.J.; Sussman, M. A family study of hereditary angioneurotic oedema. Q. J. Med. 1973, 42, 317–339. [Google Scholar]
- Osler, W. Landmark publication from The American Journal of the Medical Sciences: Hereditary angio-neurotic oedema. Am. J. Med. Sci. 2010, 339, 175–178. [Google Scholar] [CrossRef]
- Crowder, J.R.; Crowder, T.R. Five generations of angioneurotic edema. Arch. Intern. Med. 1917, 20, 840–852. [Google Scholar] [CrossRef] [Green Version]
- Prematta, M.J.; Kemp, J.G.; Gibbs, J.G.; Mende, C.; Rhoads, C.; Craig, T.J. Frequency, timing, and type of prodromal symptoms associated with hereditary angioedema attacks. Allergy Asthma Proc. 2009, 30, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Reshef, A.; Prematta, M.J.; Craig, T.J. Signs and symptoms preceding acute attacks of hereditary angioedema: Results of three recent surveys. Allergy Asthma Proc. 2013, 34, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.W.; Gran, J.T.; Straume, B.; Mellbye, O.J.; Johansen, H.T.; Mollnes, T.E. Hereditary angio-oedema: New clinical observations and autoimmune screening, complement and kallikrein-kinin analyses. J. Intern. Med. 1996, 239, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, L.; Liu, B.; Zhang, Y.; Chen, Q.; Li, C. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS ONE 2014, 9, e102342. [Google Scholar] [CrossRef]
- Perego, F.; De Maria, B.; Bova, M.; Petraroli, A.; Marcelli Cesoni, A.; De Grazia, V.; Zingale, L.C.; Porta, A.; Spadaro, G.; Dalla Vecchia, L.A. Analysis of Heart-Rate Variability during Angioedema Attacks in Patients with Hereditary C1-Inhibitor Deficiency. Int. J. Environ. Res. Public Health 2021, 18, 2900. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; McGuire, M.; Shuler, S.A.; Deliu, E.; Barr, J.L.; Abood, M.E.; Brailoiu, G.C. Modulation of cardiac vagal tone by bradykinin acting on nucleus ambiguus. Neuroscience 2017, 365, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.A.; Casella, F.; Perego, F.; Suffritti, C.; Afifi Afifi, N.; Tobaldini, E.; Zanichelli, A.; Cogliati, C.; Montano, N.; Cicardi, M. Hereditary angioedema: Assessing the hypothesis for underlying autonomic dysfunction. PLoS ONE 2017, 12, e0187110. [Google Scholar] [CrossRef] [Green Version]
- Savarese, L.; Mormile, I.; Bova, M.; Petraroli, A.; Maiello, A.; Spadaro, G.; Freda, M.F. Psychology and hereditary angioedema: A systematic review. Allergy Asthma Proc. 2021, 42, e1–e7. [Google Scholar] [CrossRef]
- Kuman Tuncel, O.; Gokmen, N.M.; Demir, E.; Gulbahar, O.; Pirildar, S. The impact of hereditary angioedema on quality of life and family planning decisions. Int. J. Psychiatry Med. 2019, 54, 377–394. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Xu, Y.; Xu, Q.; Zhi, Y. Risk factors for diagnostic delay in Chinese patients with hereditary angioedema. Allergy Asthma Proc. 2019, 40, 343–349. [Google Scholar] [CrossRef]
- Fouche, A.S.; Saunders, E.F.; Craig, T. Depression and anxiety in patients with hereditary angioedema. Ann. Allergy Asthma Immunol. 2014, 112, 371–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballero, T.; Sala-Cunill, A.; Cancian, M.; Craig, T.J.; Neri, S.; Keith, P.K.; Boccon-Gibod, I.; Bethune, C.; Bork, K. Current status of implementation of self-administration training in various regions of Europe, Canada and the USA in the management of hereditary angioedema. Int. Arch. Allergy Immunol. 2013, 161 (Suppl. 1), 10–16. [Google Scholar] [CrossRef] [PubMed]
- Savarese, L.; Bova, M.; De Falco, R.; Guarino, M.D.; De Luca Picione, R.; Petraroli, A.; Senter, R.; Traverso, C.; Zabotto, M.; Zanichelli, A.; et al. Emotional processes and stress in children affected by hereditary angioedema with C1-inhibitor deficiency: A multicenter, prospective study. Orphanet J. Rare Dis. 2018, 13, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, G.J.; Bagby, R.M. New trends in alexithymia research. Psychother. Psychosom. 2004, 73, 68–77. [Google Scholar] [CrossRef]
- Savarese, L.; Bova, M.; Maiello, A.; Petraroli, A.; Mormile, I.; Cancian, M.; Senter, R.; Zanichelli, A.; Spadaro, G.; Freda, M.F. Psychological processes in the experience of hereditary angioedema in adult patients: An observational study. Orphanet J. Rare Dis. 2021, 16, 23. [Google Scholar] [CrossRef]
- Mormile, I.; Gigliotti, M.C.; Petraroli, A.; Cocchiaro, A.; Furno, A.; Granata, F.; Rossi, F.W.; Portella, G.; de Paulis, A. Immunogenicity and Safety of Anti-SARS-CoV-2 mRNA Vaccines in a Cohort of Patients with Hereditary Angioedema. Vaccines 2023, 11, 215. [Google Scholar] [CrossRef]
- Cicardi, M.; Agostoni, A. Hereditary angioedema. N. Engl. J. Med. 1996, 334, 1666–1667. [Google Scholar] [CrossRef]
- Mormile, I.; Petraroli, A.; Loffredo, S.; Rossi, F.W.; Mormile, M.; Del Mastro, A.; Spadaro, G.; de Paulis, A.; Bova, M. Episodic Angioedema with Hypereosinophilia (Gleich’s Syndrome): A Case Report and Extensive Review of the Literature. J. Clin. Med. 2021, 10, 1442. [Google Scholar] [CrossRef]
- Zanichelli, A.; Longhurst, H.J.; Maurer, M.; Bouillet, L.; Aberer, W.; Fabien, V.; Andresen, I.; Caballero, T.; Group, I.O.S.S. Misdiagnosis trends in patients with hereditary angioedema from the real-world clinical setting. Ann. Allergy Asthma Immunol. 2016, 117, 394–398. [Google Scholar] [CrossRef] [Green Version]
- Bygum, A.; Aygoren-Pursun, E.; Beusterien, K.; Hautamaki, E.; Sisic, Z.; Wait, S.; Boysen, H.B.; Caballero, T. Burden of Illness in Hereditary Angioedema: A Conceptual Model. Acta Derm. Venereol. 2015, 95, 706–710. [Google Scholar] [CrossRef]
- Mendivil, J.; Murphy, R.; de la Cruz, M.; Janssen, E.; Boysen, H.B.; Jain, G.; Aygoren-Pursun, E.; Hirji, I.; Devercelli, G. Clinical characteristics and burden of illness in patients with hereditary angioedema: Findings from a multinational patient survey. Orphanet J. Rare Dis. 2021, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Granero-Molina, J.; Sanchez-Hernandez, F.; Fernandez-Sola, C.; Jimenez-Lasserrotte, M.D.M.; Antequera-Raynal, L.H.; Hernandez-Padilla, J.M. The Diagnosis of Hereditary Angioedema: Family Caregivers’ Experiences. Clin. Nurs. Res. 2020, 29, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A. The burden of illness in patients with hereditary angioedema. Ann. Allergy Asthma Immunol. 2013, 111, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Magerl, M. Long-term prophylaxis of hereditary angioedema with androgen derivates: A critical appraisal and potential alternatives. J. Dtsch. Dermatol. Ges. 2011, 9, 99–107. [Google Scholar] [CrossRef]
- Riedl, M.A.; Banerji, A.; Gower, R. Current medical management of hereditary angioedema: Follow-up survey of US physicians. Ann. Allergy Asthma Immunol. 2021, 126, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.C.; Davis, D.K.; Castaldo, A.J.; Zuraw, B.L. Pediatric Hereditary Angioedema: Onset, Diagnostic Delay, and Disease Severity. Clin. Pediatr. 2016, 55, 935–942. [Google Scholar] [CrossRef]
- Longhurst, H.J.; Dempster, J.; Lorenzo, L.; Buckland, M.; Grigoriadou, S.; Symons, C.; Bethune, C.; Fabien, V.; Bangs, C.; Garcez, T. Real-world outcomes in hereditary angioedema: First experience from the Icatibant Outcome Survey in the United Kingdom. Allergy Asthma Clin. Immunol. 2018, 14, 28. [Google Scholar] [CrossRef] [Green Version]
- Riedl, M.A.; Lumry, W.R.; Busse, P.; Levy, H.; Steele, T.; Dayno, J.; Li, H.H. Prevalence of hereditary angioedema in untested first-degree blood relatives of known subjects with hereditary angioedema. Allergy Asthma Proc. 2015, 36, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Pittock, S.J.; Lennon, V.A.; McKeon, A.; Mandrekar, J.; Weinshenker, B.G.; Lucchinetti, C.F.; O’Toole, O.; Wingerchuk, D.M. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: An open-label pilot study. Lancet Neurol. 2013, 12, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M.; Lennon, V.A.; Lucchinetti, C.F.; Pittock, S.J.; Weinshenker, B.G. The spectrum of neuromyelitis optica. Lancet Neurol. 2007, 6, 805–815. [Google Scholar] [CrossRef]
- Jacob, A.; McKeon, A.; Nakashima, I.; Sato, D.K.; Elsone, L.; Fujihara, K.; de Seze, J. Current concept of neuromyelitis optica (NMO) and NMO spectrum disorders. J. Neurol. Neurosurg. Psychiatry 2013, 84, 922–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhong, L.; Geng, J. Neuromyelitis optica spectrum disorder: Pathogenesis, treatment, and experimental models. Mult. Scler. Relat. Disord. 2019, 27, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Mealy, M.A. Purified human C1-esterase inhibitor is safe in acute relapses of neuromyelitis optica. Neurol. Neuroimmunol. Neuroinflammation 2014, 1, e5. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mormile, I.; Palestra, F.; Petraroli, A.; Loffredo, S.; Rossi, F.W.; Spadaro, G.; de Paulis, A.; Bova, M. Neurologic and Psychiatric Manifestations of Bradykinin-Mediated Angioedema: Old and New Challenges. Int. J. Mol. Sci. 2023, 24, 12184. https://doi.org/10.3390/ijms241512184
Mormile I, Palestra F, Petraroli A, Loffredo S, Rossi FW, Spadaro G, de Paulis A, Bova M. Neurologic and Psychiatric Manifestations of Bradykinin-Mediated Angioedema: Old and New Challenges. International Journal of Molecular Sciences. 2023; 24(15):12184. https://doi.org/10.3390/ijms241512184
Chicago/Turabian StyleMormile, Ilaria, Francesco Palestra, Angelica Petraroli, Stefania Loffredo, Francesca Wanda Rossi, Giuseppe Spadaro, Amato de Paulis, and Maria Bova. 2023. "Neurologic and Psychiatric Manifestations of Bradykinin-Mediated Angioedema: Old and New Challenges" International Journal of Molecular Sciences 24, no. 15: 12184. https://doi.org/10.3390/ijms241512184
APA StyleMormile, I., Palestra, F., Petraroli, A., Loffredo, S., Rossi, F. W., Spadaro, G., de Paulis, A., & Bova, M. (2023). Neurologic and Psychiatric Manifestations of Bradykinin-Mediated Angioedema: Old and New Challenges. International Journal of Molecular Sciences, 24(15), 12184. https://doi.org/10.3390/ijms241512184






