Surveillance of Human Rotaviruses in Wuhan, China (2019–2022): Whole-Genome Analysis of Emerging DS-1-like G8P[8] Rotavirus
Abstract
:1. Introduction
2. Results
2.1. Detection of Rotaviruses and VP7 and VP4 Genotyping
2.2. The Genotype Constellation of RVAs
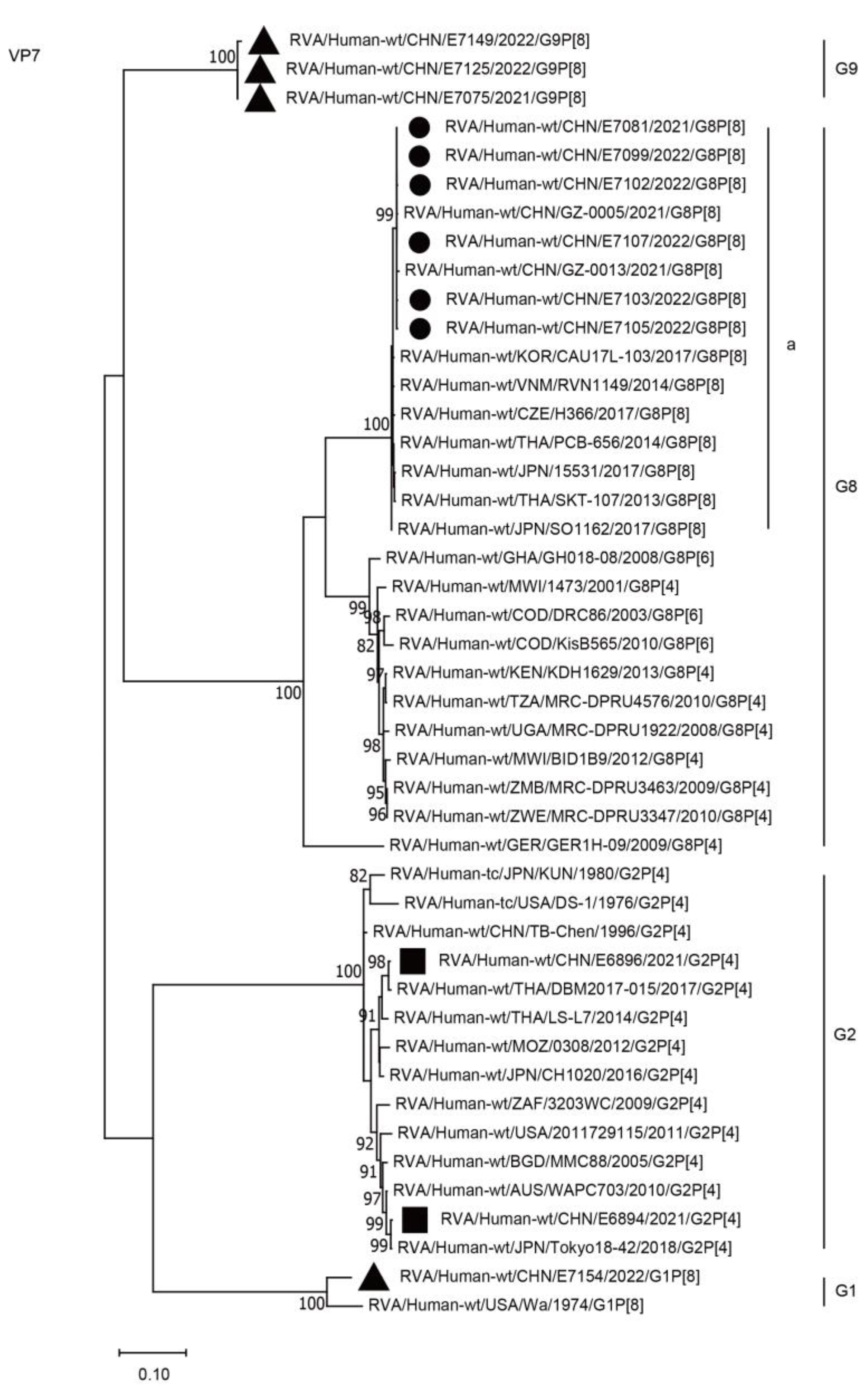
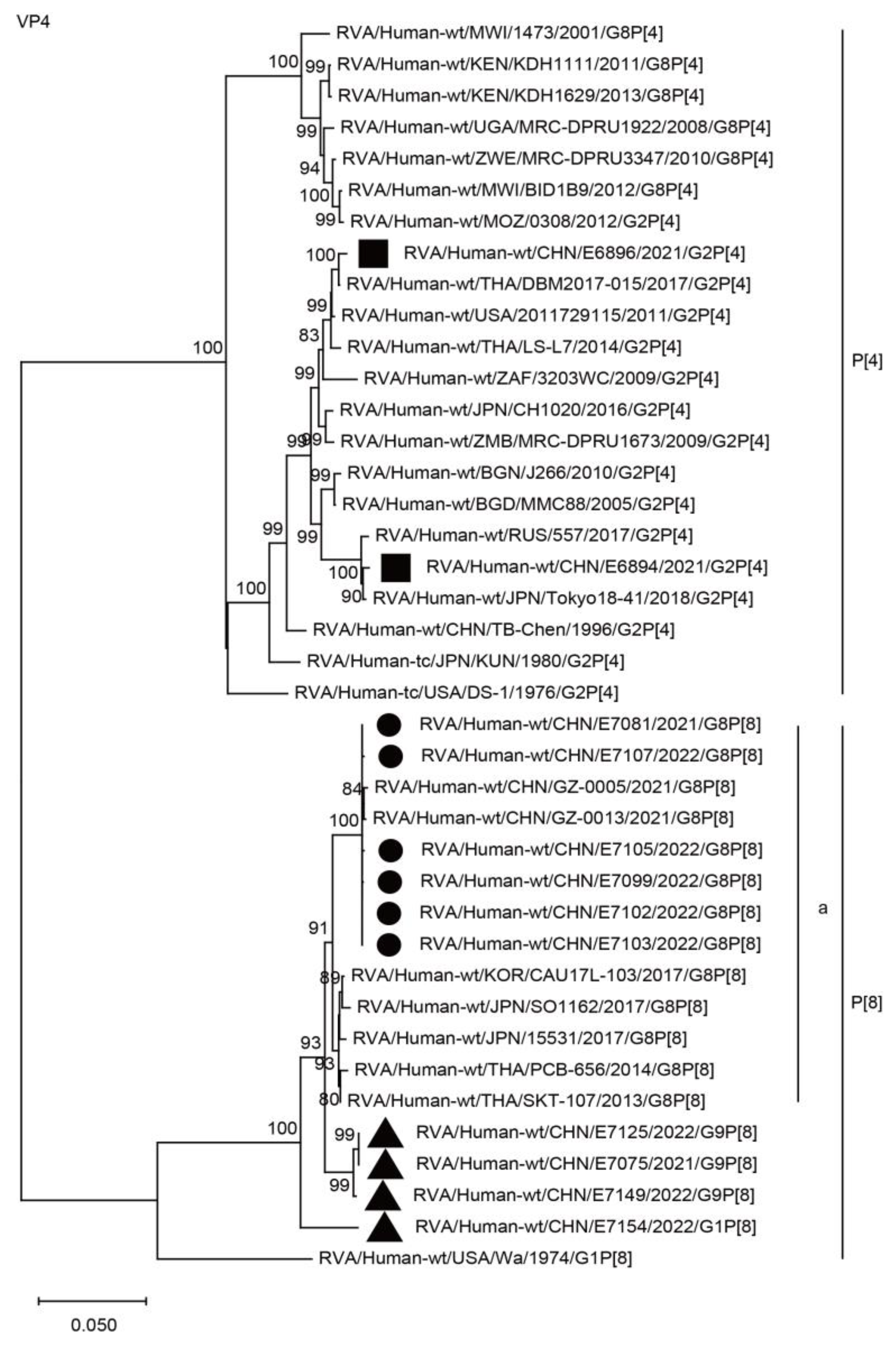
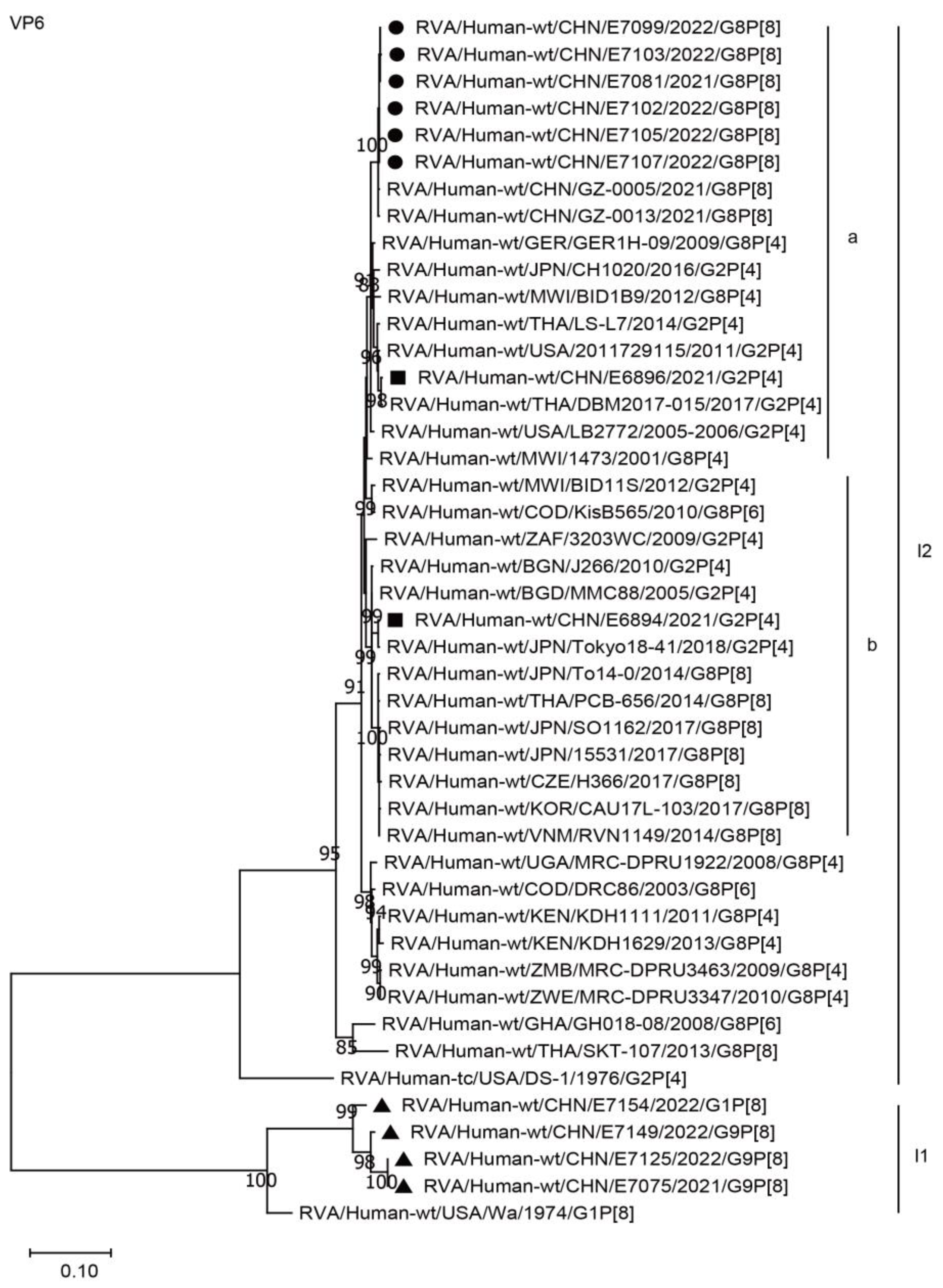
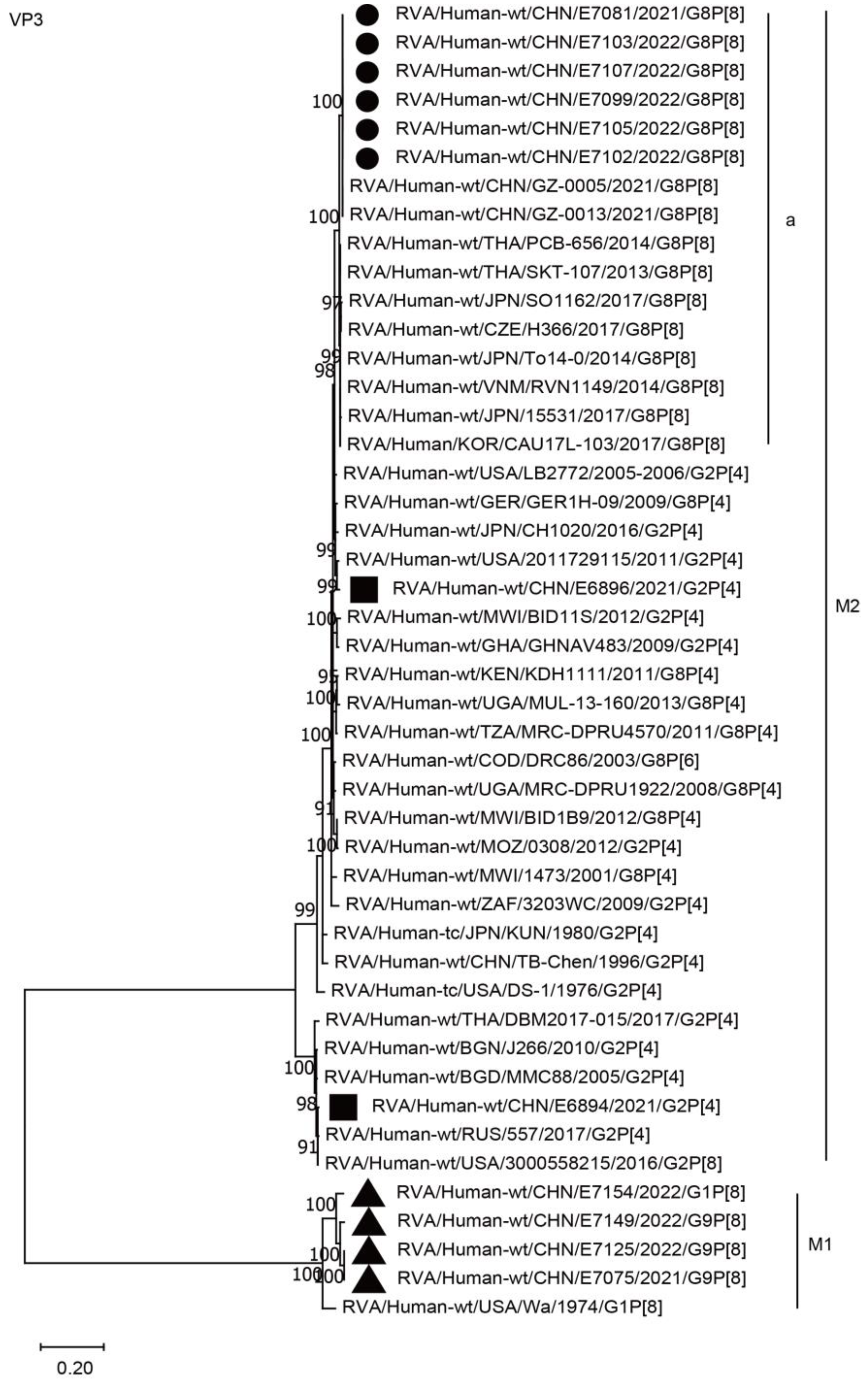
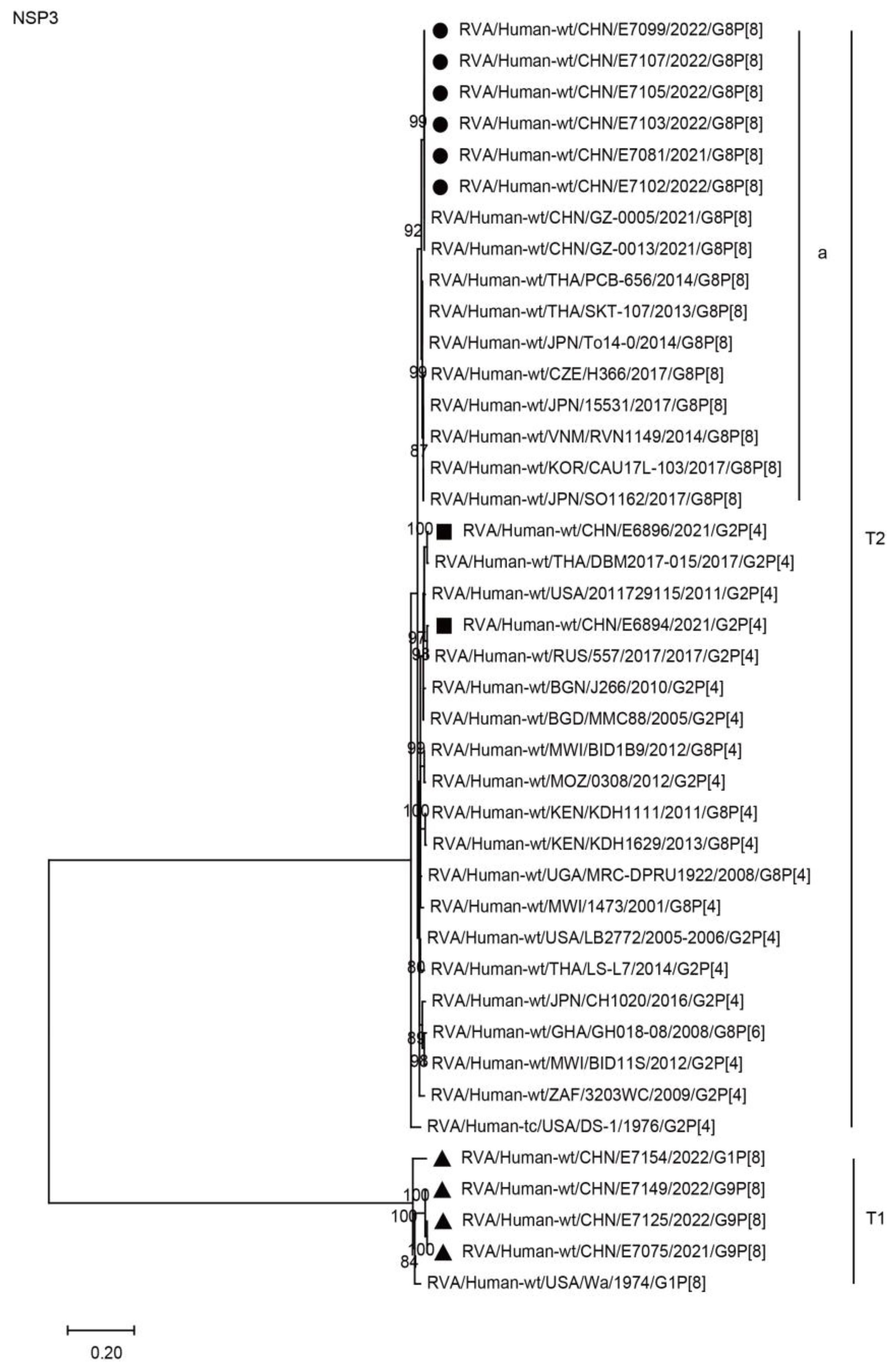
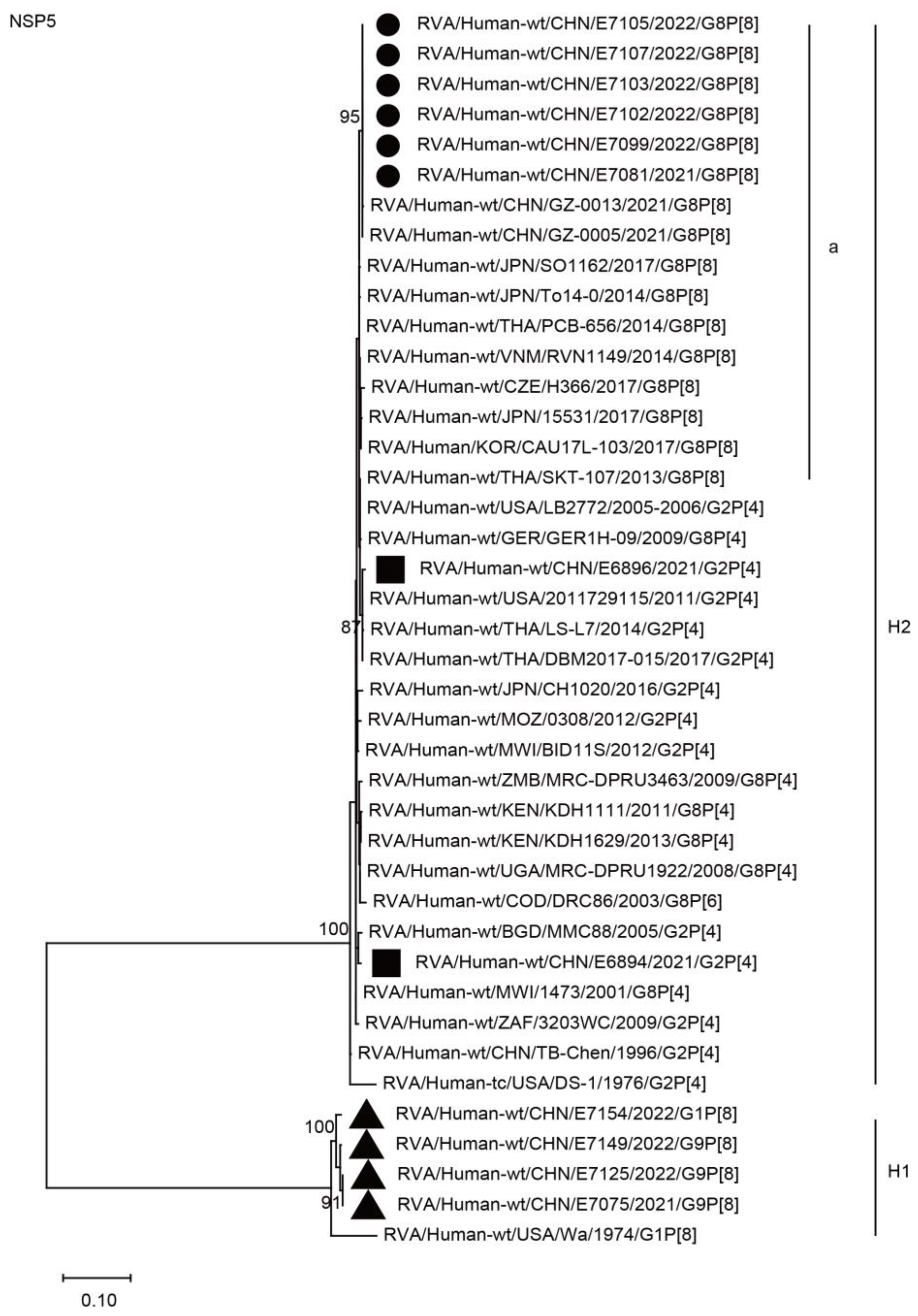
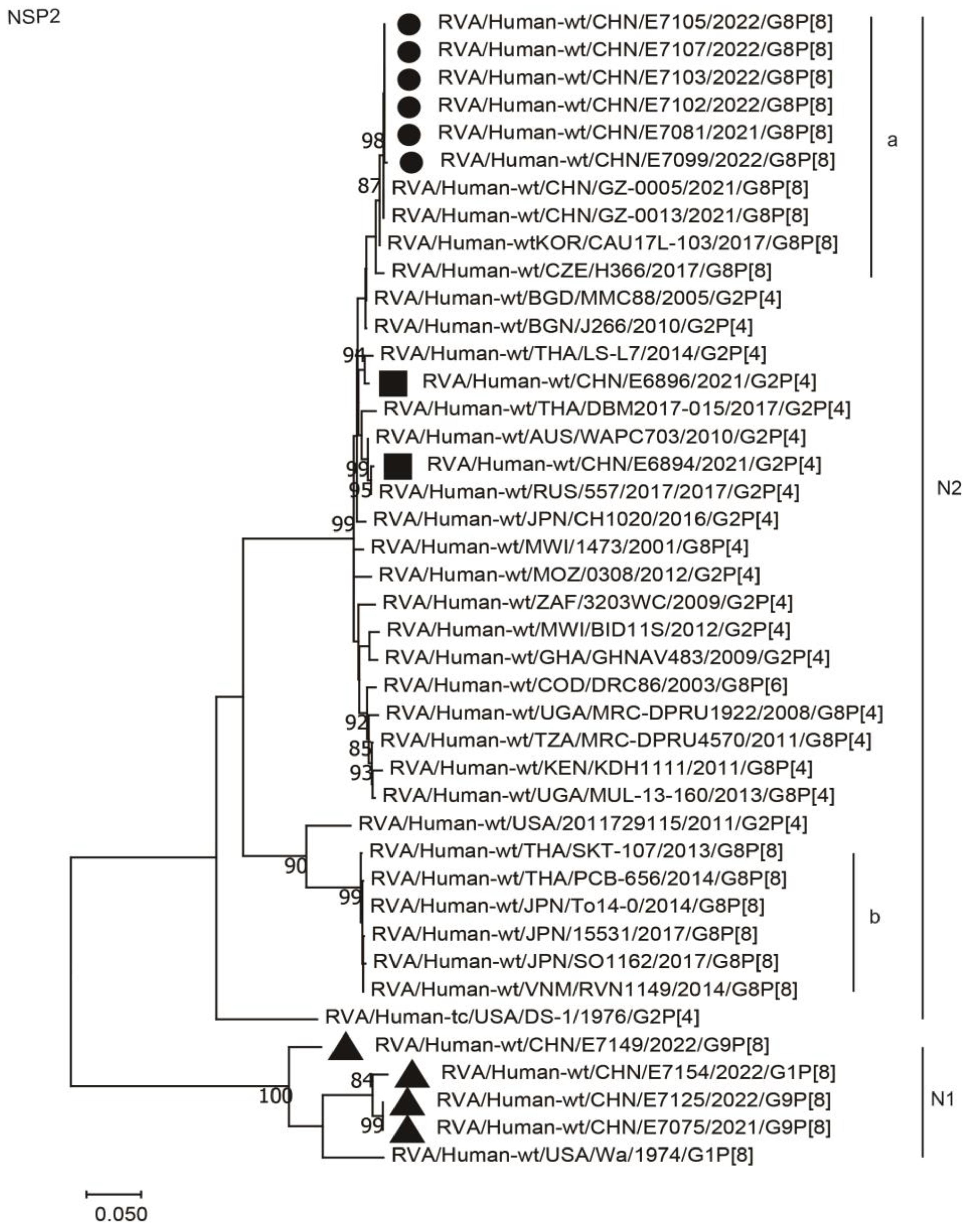
2.3. Phylogenetic Analysis
2.3.1. Structural Protein Genes
2.3.2. Nonstructural Protein Genes
3. Discussion
4. Materials and Methods
4.1. Specimens
4.2. Detection of Rotavirus
4.3. Genotyping of RVAs
4.4. Whole-Genome Sequencing
4.5. Phylogenetic Analysis
4.6. Statistical Analysis
4.7. Accession Numbers of Nucleotide Sequences in Genbank
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Y.; Chen, C.; Zhang, X.; Yan, D.; Jiang, D.; Liu, X.; Yang, M.; Ding, C.; Lan, L.; Hecht, R.; et al. Global burden and trends of rotavirus infection-associated deaths from 1990 to 2019: An observational trend study. Virol. J. 2022, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Mohammadreza Naghipour, T.N.; Osamu, N. Issues with reducing the rotavirus-associated mortality by vaccination in developing countries. Vaccine 2008, 26, 3236–3241. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.D.; Burton, A.; Lanata, C.; Boschi-Pinto, C.; Shibuya, K.; Steele, D.; Glass, R.I. Global Mortality Associated with Rotavirus Disease among Children in 2004. J. Infect. Dis. 2009, 200 (Suppl. 1), S9–S15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omatola, C.A.; Olaniran, A.O. Rotaviruses: From Pathogenesis to Disease Control-A Critical Review. Viruses 2022, 14, 875. [Google Scholar] [CrossRef]
- Estes, M.K.K.; Rotaviruses, A.Z. Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams &Wilkins: Philadelphia, PA, USA, 2007; Volume 5, pp. 1917–1974. [Google Scholar]
- RCWG. Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg (accessed on 22 April 2023).
- Liu, N.; Xu, Z.; Li, D.; Zhang, Q.; Wang, H.; Duan, Z.J. Update on the disease burden and circulating strains of rotavirus in China: A systematic review and meta-analysis. Vaccine 2014, 32, 4369–4375. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Wang, M.; Wang, J.; Ma, Y.; Liu, X.; Wang, M.; Sun, X.; Li, L.; Li, H.; Zhang, Q.; et al. Phylogenetic analysis of the viral proteins VP4/VP7 of circulating human rotavirus strains in China from 2016 to 2019 and comparison of their antigenic epitopes with those of vaccine strains. Front. Cell Infect. Microbiol. 2022, 12, 927490. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, L.N.; Wang, S.M.; Zhou, H.L.; Qiu, C.; Jiang, B.; Qiu, T.Y.; Chen, S.L.; von Seidlein, L.; Wang, X.Y. Genetic characterization of two G8P[8] rotavirus strains isolated in Guangzhou, China, in 2020/21: Evidence of genome reassortment. BMC Infect. Dis. 2022, 22, 579. [Google Scholar] [CrossRef]
- Lu, Y.; Xie, H.; Wang, D.; Lu, J. Nosocomial infection caused by a rare G8P[8] rotavirus subtype in a pediatric unit in Guangzhou, Southern China. Hum. Vaccin. Immunother. 2021, 17, 3619–3622. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; Rahman, M.; Attoui, H.; Banyai, K.; Estes, M.K.; Gentsch, J.R.; Iturriza-Gomara, M.; Kirkwood, C.D.; Martella, V.; et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 2008, 153, 1621–1629. [Google Scholar] [CrossRef] [Green Version]
- Matthijnssens, J.; Ciarlet, M.; Heiman, E.; Arijs, I.; Delbeke, T.; McDonald, S.M.; Palombo, E.A.; Iturriza-Gomara, M.; Maes, P.; Patton, J.T.; et al. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008, 82, 3204–3219. [Google Scholar] [CrossRef] [Green Version]
- Martella, V.; Bányai, K.; Matthijnssens, J.; Buonavoglia, C.; Ciarlet, M. Zoonotic aspects of rotaviruses. Vet. Microbiol. 2010, 140, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Geletu, U.S.; Usmael, M.A.; Bari, F.D. Rotavirus in Calves and Its Zoonotic Importance. Vet. Med. Int. 2021, 2021, 6639701. [Google Scholar] [CrossRef]
- Taniguchi, K.; Urasawa, T.; Urasawa, S. Independent segregation of the VP4 and the VP7 genes in bovine rotaviruses as confirmed by VP4 sequence analysis of G8 and G10 bovine rotavirus strains. J. Gen. Virol. 1993, 74, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Browning, G.F.; Nakagomi, O.; Kaga, E.; Sarasini, A.; Gerna, G. Human and bovine serotype G8 rotaviruses may be derived by reassortment. Arch. Virol. 1992, 125, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Heiman, E.M.; McDonald, S.M.; Barro, M.; Taraporewala, Z.F.; Bar-Magen, T.; Patton, J.T. Group A Human Rotavirus Genomics: Evidence that Gene Constellations Are Influenced by Viral Protein Interactions. J. Virol. 2008, 82, 11106–11116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yodmeeklin, A.; Khamrin, P.; Kumthip, K.; Malasao, R.; Ukarapol, N.; Ushijima, H.; Maneekarn, N. Increasing predominance of G8P[8] species A rotaviruses in children admitted to hospital with acute gastroenteritis in Thailand, 2010–2013. Arch. Virol. 2018, 163, 2165–2178. [Google Scholar] [CrossRef]
- Tacharoenmuang, R.; Komoto, S.; Guntapong, R.; Ide, T.; Sinchai, P.; Upachai, S.; Yoshikawa, T.; Tharmaphornpilas, P.; Sangkitporn, S.; Taniguchi, K. Full Genome Characterization of Novel DS-1-Like G8P[8] Rotavirus Strains that Have Emerged in Thailand: Reassortment of Bovine and Human Rotavirus Gene Segments in Emerging DS-1-Like Intergenogroup Reassortant Strains. PLoS ONE 2016, 11, e0165826. [Google Scholar] [CrossRef] [Green Version]
- Kondo, K.; Tsugawa, T.; Ono, M.; Ohara, T.; Fujibayashi, S.; Tahara, Y.; Kubo, N.; Nakata, S.; Higashidate, Y.; Fujii, Y.; et al. Clinical and Molecular Characteristics of Human Rotavirus G8P[8] Outbreak Strain, Japan, 2014. Emerg. Infect. Dis. 2017, 23, 968–972. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.; Kobayashi, M.; Nagasawa, K.; Hatazawa, R.; Thi Kim Pham, N.; Miyashita, H.; Komoto, S.; Tajima, T.; Baba, T.; Okitsu, S.; et al. Whole genome sequencing and evolutionary analysis of G8P [8] rotaviruses emerging in Japan. Virusdisease 2022, 33, 215–218. [Google Scholar] [CrossRef]
- Kamiya, H.; Tacharoenmuang, R.; Ide, T.; Negoro, M.; Tanaka, T.; Asada, K.; Nakamura, H.; Sugiura, K.; Umemoto, M.; Kuroki, H.; et al. Characterization of an Unusual DS-1-Like G8P[8] Rotavirus Strain from Japan in 2017: Evolution of Emerging DS-1-Like G8P[8] Strains through Reassortment. Jpn. J. Infect. Dis. 2019, 72, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Hoa-Tran, T.N.; Nakagomi, T.; Vu, H.M.; Do, L.P.; Gauchan, P.; Agbemabiese, C.A.; Nguyen, T.T.; Nakagomi, O.; Thanh, N.T. Abrupt emergence and predominance in Vietnam of rotavirus A strains possessing a bovine-like G8 on a DS-1-like background. Arch. Virol. 2016, 161, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Kobayashi, N.; Zhou, D.J.; Yang, Z.Q.; Zhou, X.; Peng, J.S.; Zhu, Z.R.; Zhao, D.F.; Liu, M.Q.; Gong, J. Molecular epidemiologic analysis of group A rotaviruses in adults and children with diarrhea in Wuhan city, China, 2000-2006. Arch. Virol. 2007, 152, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Kobayashi, N.; Zhou, X.; Nagashima, S.; Zhu, Z.R.; Peng, J.S.; Liu, M.Q.; Hu, Q.; Zhou, D.J.; Watanabe, S.; et al. Phylogenetic analysis of rotaviruses with predominant G3 and emerging G9 genotypes from adults and children in Wuhan, China. J. Med. Virol. 2009, 81, 382–389. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhou, X.; Ghosh, S.; Zhou, D.J.; Pang, B.B.; Peng, J.S.; Hu, Q.; Kobayashi, N. Prevalence of human rotavirus genotypes in Wuhan, China, during 2008–2011: Changing trend of predominant genotypes and emergence of strains with the P[8]b subtype of the VP4 gene. Arch. Virol. 2011, 156, 2221–2231. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.H.; Pang, B.B.; Chen, N.; Kobayashi, N. Surveillance of Human Rotavirus in Wuhan, China (2011-2019): Predominance of G9P[8] and Emergence of G12. Pathogens 2020, 9, 810. [Google Scholar] [CrossRef]
- Vetter, V.; Gardner, R.C.; Debrus, S.; Benninghoff, B.; Pereira, P. Established and new rotavirus vaccines: A comprehensive review for healthcare professionals. Hum. Vaccin. Immunother. 2022, 18, 1870395. [Google Scholar] [CrossRef]
- Nan, X.; Jinyuan, W.; Yan, Z.; Maosheng, S.; Hongjun, L. Epidemiological and clinical studies of rotavirus-induced diarrhea in China from 1994-2013. Hum. Vaccin. Immunother. 2014, 10, 3672–3680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp, H.; Laszlo, B.; Jakab, F.; Ganesh, B.; De Grazia, S.; Matthijnssens, J.; Ciarlet, M.; Martella, V.; Banyai, K. Review of group A rotavirus strains reported in swine and cattle. Vet. Microbiol. 2013, 165, 190–199. [Google Scholar] [CrossRef]
- Cunliffe, N.A.; Gondwe, J.S.; Broadhead, R.L.; Molyneux, M.E.; Woods, P.A.; Bresee, J.S.; Glass, R.I.; Gentsch, J.R.; Hart, C.A. Rotavirus G and P types in children with acute diarrhea in Blantyre, Malawi, from 1997 to 1998: Predominance of novel P[6]G8 strains. J. Med. Virol. 1999, 57, 308–312. [Google Scholar]
- Cunliffe, N.A.; Gentsch, J.R.; Kirkwood, C.D.; Gondwe, J.S.; Dove, W.; Nakagomi, O.; Nakagomi, T.; Hoshino, Y.; Bresee, J.S.; Glass, R.I.; et al. Molecular and serologic characterization of novel serotype G8 human rotavirus strains detected in Blantyre, Malawi. Virology 2000, 274, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Holmes, J.L.; Kirkwood, C.D.; Gerna, G.; Clemens, J.D.; Rao, M.R.; Naficy, A.B.; Abu-Elyazeed, R.; Savarino, S.J.; Glass, R.I.; Gentsch, J.R. Characterization of unusual G8 rotavirus strains isolated from Egyptian children. Arch. Virol. 1999, 144, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.D.; Parker, S.P.; Peenze, I.; Pager, C.T.; Taylor, M.B.; Cubitt, W.D. Comparative studies of human rotavirus serotype G8 strains recovered in South Africa and the United Kingdom. J. Gen. Virol. 1999, 80 Pt 11, 3029–3034. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Rahman, M.; Yang, X.; Delbeke, T.; Arijs, I.; Kabue, J.P.; Muyembe, J.J.; Van Ranst, M. G8 rotavirus strains isolated in the Democratic Republic of Congo belong to the DS-1-like genogroup. J. Clin. Microbiol. 2006, 44, 1801–1809. [Google Scholar] [CrossRef] [Green Version]
- Nakagomi, T.; Doan, Y.H.; Dove, W.; Ngwira, B.; Iturriza-Gomara, M.; Nakagomi, O.; Cunliffe, N.A. G8 rotaviruses with conserved genotype constellations detected in Malawi over 10 years (1997–2007) display frequent gene reassortment among strains co-circulating in humans. J. Gen. Virol. 2013, 94, 1273–1295. [Google Scholar] [CrossRef] [Green Version]
- Dennis, F.E.; Fujii, Y.; Haga, K.; Damanka, S.; Lartey, B.; Agbemabiese, C.A.; Ohta, N.; Armah, G.E.; Katayama, K. Identification of novel Ghanaian G8P[6] human-bovine reassortant rotavirus strain by next generation sequencing. PLoS ONE 2014, 9, e100699. [Google Scholar] [CrossRef]
- Moutelikova, R.; Sauer, P.; Dvorakova Heroldova, M.; Hola, V.; Prodelalova, J. Emergence of Rare Bovine-Human Reassortant DS-1-Like Rotavirus A Strains with G8P[8] Genotype in Human Patients in the Czech Republic. Viruses 2019, 11, 1015. [Google Scholar] [CrossRef] [Green Version]
- Diaz Alarcon, R.G.; Liotta, D.J.; Mino, S. Zoonotic RVA: State of the Art and Distribution in the Animal World. Viruses 2022, 14, 2554. [Google Scholar] [CrossRef]
- Strydom, A.; Donato, C.M.; Nyaga, M.M.; Boene, S.S.; Peenze, I.; Mogotsi, M.T.; Joao, E.D.; Munlela, B.; Potgieter, A.C.; Seheri, M.L.; et al. Genetic Characterisation of South African and Mozambican Bovine Rotaviruses Reveals a Typical Bovine-like Artiodactyl Constellation Derived through Multiple Reassortment Events. Pathogens 2021, 10, 1308. [Google Scholar] [CrossRef]
- Yan, N.; Li, R.; Wang, Y.; Zhang, B.; Yue, H.; Tang, C. High prevalence and genomic characteristics of G6P[1] Bovine Rotavirus A in yak in China. J. Gen. Virol. 2020, 101, 701–711. [Google Scholar] [CrossRef]
- Gentsch, G.R., Jr.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.K.; Bhan, M.K. Identificationof group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1365–1373. [Google Scholar] [CrossRef] [Green Version]
- Gouvea, V.; Glass, R.I.; Woods, P.; Taniguchi, K.; Clark, H.F.; Forrester, B.; Fang, Z.Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 1990, 28, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Iturriza-Gómara, M.; Kang, G.; Gray, J. Rotavirus genotyping: Keeping up with an evolving population of human rotaviruses. J. Clin. Virol. 2004, 31, 259–265. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.M.; Matthijnssens, J.; McAllen, J.K.; Hine, E.; Overton, L.; Wang, S.; Lemey, P.; Zeller, M.; Van Ranst, M.; Spiro, D.J.; et al. Evolutionary dynamics of human rotaviruses: Balancing reassortment with preferred genome constellations. PLoS Pathog. 2009, 5, e1000634. [Google Scholar] [CrossRef]
- Wang, Y.H.; Pang, B.B.; Ghosh, S.; Zhou, X.; Shintani, T.; Urushibara, N.; Song, Y.W.; He, M.Y.; Liu, M.Q.; Tang, W.F.; et al. Molecular epidemiology and genetic evolution of the whole genome of G3P[8] human rotavirus in Wuhan, China, from 2000 through 2013. PLoS ONE 2014, 9, e88850. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef] [Green Version]




| G-Type/P-Type | June 2019–May 2020 | June 2020–May 2021 | June 2021–May 2022 | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Child | Adult | Child | Adult | Child | Adult | Child | Adult | |
| G1/P[8] | 0 | 0 | 1 | 0 | 3 | 0 | 4 | 0 |
| G2/P[4] | 0 | 0 | 5 | 0 | 0 | 0 | 5 | 0 |
| G3/P[8] | 15 | 0 | 7 | 0 | 0 | 0 | 22 | 0 |
| G8/P[8] | 0 | 0 | 0 | 0 | 22 | 0 | 22 | 0 |
| G9/P[8] | 42 | 4 | 34 | 0 | 13 | 4 | 89 | 8 |
| Total | 57 | 4 | 47 | 0 | 38 | 4 | 142 | 8 |
| Strain | Genotypes of Viral Protein Genes and Nucleotide Sequence Identities (%) to E7081 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VP7 | VP[4] | VP6 | VP1 | VP2 | VP3 | NSP1 | NSP2 | NSP3 | NSP[4] | NSP5 | ||||||||||||
| RVA/Human-wt/CHN/E7081/2021/G8P[8] | G8 | — | P[8] | — | I2 | — | R2 | — | C2 | — | M2 | — | A2 | — | N2 | — | T2 | — | E2 | — | H2 | — |
| RVA/Human-wt/CHN/E7099/2022/G8P[8] | G8 | 100.0 | P[8] | 100.0 | I2 | 100.0 | R2 | 99.9 | C2 | 99.8 | M2 | 100.0 | A2 | 99.9 | N2 | 99.8 | T2 | 99.9 | E2 | 99.8 | H2 | 100.0 |
| RVA/Human-wt/CHN/E7102/2022/G8P[8] | G8 | 99.9 | P[8] | 100.0 | I2 | 99.9 | R2 | 99.9 | C2 | 99.9 | M2 | 99.9 | A2 | 99.8 | N2 | 100.0 | T2 | 99.9 | E2 | 99.8 | H2 | 100.0 |
| RVA/Human-wt/CHN/E7103/2022/G8P[8] | G8 | 99.9 | P[8] | 100.0 | I2 | 99.9 | R2 | 99.9 | C2 | 99.7 | M2 | 99.9 | A2 | 99.8 | N2 | 100.0 | T2 | 100.0 | E2 | 99.8 | H2 | 100.0 |
| RVA/Human-wt/CHN/E7105/2022/G8P[8] | G8 | 99.9 | P[8] | 99.8 | I2 | 99.9 | R2 | 99.9 | C2 | 99.8 | M2 | 100.0 | A2 | 99.9 | N2 | 100.0 | T2 | 100.0 | E2 | 99.8 | H2 | 100.0 |
| RVA/Human-wt/CHN/E7107/2022/G8P[8] | G8 | 100.0 | P[8] | 99.9 | I2 | 99.9 | R2 | 99.9 | C2 | 99.9 | M2 | 99.9 | A2 | 99.9 | N2 | 100.0 | T2 | 99.9 | E2 | 99.6 | H2 | 100.0 |
| RVA/Human-wt/CHN/E6894/2021/G2P[4] | G2 | 73.1 | P[4] | 87.0 | I2 | 96.4 | R2 | 97.0 | C2 | 96.7 | M2 | 87.1 | A2 | 96.5 | N2 | 96.5 | T2 | 96.4 | E2 | 97.5 | H2 | 98.4 |
| RVA/Human-wt/CHN/E6896/2021/G2P[4] | G2 | 73.3 | P[4] | 86.6 | I2 | 97.7 | R2 | 97.6 | C2 | 97.5 | M2 | 96.2 | A2 | 95.7 | N2 | 96.5 | T2 | 95.8 | E2 | 97.0 | H2 | 98.7 |
| RVA/Human-wt/CHN/E7154/2022/G1P[8] | G1 | 73.4 | P[8] | 95.7 | I1 | 79.5 | R1 | 79.3 | C1 | 79.1 | M1 | 77.4 | A1 | 75.0 | N1 | 83.4 | T1 | 79.6 | E1 | 80.7 | H1 | 83.4 |
| RVA/Human-wt/CHN/E7075/2021/G9P[8] | G9 | 76.2 | P[8] | 97.2 | I1 | 80.1 | R1 | 80.1 | C1 | 79.4 | M1 | 77.1 | A1 | 74.9 | N1 | 82.8 | T1 | 79.3 | E1 | 80.5 | H1 | 83.9 |
| RVA/Human-wt/CHN/E7125/2022/G9P[8] | G9 | 76.2 | P[8] | 97.2 | I1 | 80.1 | R1 | 80.1 | C1 | 79.4 | M1 | 77.1 | A1 | 75.1 | N1 | 82.8 | T1 | 79.3 | E1 | 80.5 | H1 | 83.9 |
| RVA/Human-wt/CHN/E7149/2022/G9P[8] | G9 | 76.1 | P[8] | 97.2 | I1 | 79.9 | R1 | 80.0 | C1 | 79.1 | M1 | 77.2 | A1 | 75.2 | N1 | 83.3 | T1 | 79.2 | E1 | 80.3 | H1 | 83.6 |
| RVA/Human-wt/CHN/GZ-0005/2021/G8P[8] | G8 | 99.9 | P[8] | 99.7 | I2 | 99.7 | R2 | 99.8 | C2 | 99.7 | M2 | 99.7 | A2 | 99.9 | N2 | 99.9 | T2 | 99.9 | E2 | 99.6 | H2 | 100.0 |
| RVA/Human-wt/CHN/GZ-0013/2021/G8P[8] | G8 | 99.7 | P[8] | 99.8 | I2 | 99.7 | R2 | 99.8 | C2 | 99.8 | M2 | 99.7 | A2 | 99.8 | N2 | 99.9 | T2 | 99.9 | E2 | 99.4 | H2 | 99.8 |
| RVA/Human-wt/KOR/CAU17L-103/2017/G8P[8] | G8 | 99.4 | P[8] | 98.3 | I2 | 96.4 | R2 | 86.1 | C2 | 98.7 | M2 | 98.3 | A2 | 98.6 | N2 | 99.5 | T2 | 98.4 | E2 | 93.4 | H2 | 98.2 |
| RVA/Human-wt/JPN/15531/2017/G8P[8] | G8 | 99.2 | P[8] | 98.2 | I2 | 96.4 | R2 | 86.1 | C2 | 98.4 | M2 | 98.2 | A2 | 98.6 | N2 | 87.3 | T2 | 98.6 | E2 | 93.2 | H2 | 99.0 |
| RVA/Human-wt/JPN/SO1162/2017/G8P[8] | G8 | 99.3 | P[8] | 98.2 | I2 | 96.5 | R2 | 93.8 | C2 | 98.5 | M2 | 98.4 | A2 | 98.6 | N2 | 87.0 | T2 | 98.4 | E2 | 93.8 | H2 | 99.3 |
| RVA/Human-wt/CZE/H366/2017/G8P[8] | G8 | 99.3 | P[8] | 98.1 | I2 | 96.3 | R2 | 86.1 | C2 | 98.3 | M2 | 98.4 | A2 | 98.6 | N2 | 98.3 | T2 | 98.3 | E2 | 93.2 | H2 | 98.7 |
| RVA/Human-wt/THA/PCB-656/2014/G8P[8] | G8 | 99.4 | P[8] | 98.2 | I2 | 96.6 | R2 | 86.0 | C2 | 97.4 | M2 | 98.6 | A2 | 98.7 | N2 | 87.2 | T2 | 98.8 | E2 | 93.2 | H2 | 99.5 |
| RVA/Human-wt/JPN/To14-0/2014/G8P[8] | G8 | 98.2 | P[8] | 98.3 | I2 | 96.6 | R2 | 85.9 | C2 | 99.0 | M2 | 98.6 | A2 | 98.8 | N2 | 87.3 | T2 | 98.7 | E2 | 93.4 | H2 | 99.3 |
| RVA/Human-wt/VNM/RVN1149/2014/G8P[8] | G8 | 99.4 | P[8] | 98.3 | I2 | 96.6 | R2 | 86.0 | C2 | 98.7 | M2 | 98.5 | A2 | 98.8 | N2 | 87.2 | T2 | 98.6 | E2 | 93.4 | H2 | 99.3 |
| RVA/Human-wt/THA/SKT-107/2013/G8P[8] | G8 | 99.2 | P[8] | 98.4 | I2 | 91.3 | R2 | 86.1 | C2 | 99.0 | M2 | 98.6 | A2 | 98.8 | N2 | 87.5 | T2 | 98.8 | E2 | 93.8 | H2 | 99.3 |
| RVA/Human-wt/COD/DRC86/2003/G8P[6] | G8 | 86.2 | P[6] | 75.3 | I2 | 96.2 | R2 | 94.3 | C2 | 97.0 | M2 | 96.5 | A2 | 95.9 | N2 | 95.6 | T2 | 96.9 | E2 | 89.2 | H2 | 97.9 |
| RVA/Human-wt/KEN/KDH1629/2013/G8P[4] | G8 | 86.1 | P[4] | 87.4 | I2 | 95.7 | R2 | 94.3 | C2 | 97.4 | M2 | 96.0 | A2 | 96.0 | N2 | 95.3 | T2 | 96.4 | E2 | 85.2 | H2 | 98.5 |
| RVA/Human-wt/MWI/1473/2001/G8P[4] | G8 | 86.9 | P[4] | 87.0 | I2 | 97.8 | R2 | 86.3 | C2 | 96.8 | M2 | 95.6 | A2 | 96.2 | N2 | 96.1 | T2 | 97.0 | E2 | 88.8 | H2 | 99.0 |
| RVA/Human-wt/THA/DBM2017-015/2017/G2P[4] | G2 | 73.2 | P[4] | 86.8 | I2 | 97.8 | R2 | 99.1 | C2 | 96.2 | M2 | 87.2 | A2 | 97.6 | N2 | 96.3 | T2 | 95.7 | E2 | 97.0 | H2 | 99.0 |
| RVA/Human-tc/USA/DS-1/1976/G2P[4] | G2 | 72.6 | P[4] | 86.9 | I2 | 87.4 | R2 | 90.2 | C2 | 93.7 | M2 | 92.6 | A2 | 92.6 | N2 | 86.1 | T2 | 94.5 | E2 | 89.8 | H2 | 95.7 |
| RVA/Human-wt/USA/Wa/1974/G1P[8] | G1 | 73.2 | P[8] | 90.1 | I1 | 79.6 | R1 | 79.7 | C1 | 80.0 | M1 | 77.0 | A1 | 75.4 | N1 | 82.0 | T1 | 79.6 | E1 | 80.7 | H1 | 83.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Wang, Y.; Chen, N.; Pang, B.; Liu, M.; Cai, K.; Kobayashi, N. Surveillance of Human Rotaviruses in Wuhan, China (2019–2022): Whole-Genome Analysis of Emerging DS-1-like G8P[8] Rotavirus. Int. J. Mol. Sci. 2023, 24, 12189. https://doi.org/10.3390/ijms241512189
Zhou X, Wang Y, Chen N, Pang B, Liu M, Cai K, Kobayashi N. Surveillance of Human Rotaviruses in Wuhan, China (2019–2022): Whole-Genome Analysis of Emerging DS-1-like G8P[8] Rotavirus. International Journal of Molecular Sciences. 2023; 24(15):12189. https://doi.org/10.3390/ijms241512189
Chicago/Turabian StyleZhou, Xuan, Yuanhong Wang, Nan Chen, Beibei Pang, Manqing Liu, Kun Cai, and Nobumichi Kobayashi. 2023. "Surveillance of Human Rotaviruses in Wuhan, China (2019–2022): Whole-Genome Analysis of Emerging DS-1-like G8P[8] Rotavirus" International Journal of Molecular Sciences 24, no. 15: 12189. https://doi.org/10.3390/ijms241512189





