HIV-1–Host Interaction in Gut-Associated Lymphoid Tissue (GALT): Effects on Local Environment and Comorbidities
Abstract
:1. Introduction
2. The Gastrointestinal Tract as a Primary Site of HIV Early Replication and Reservoir
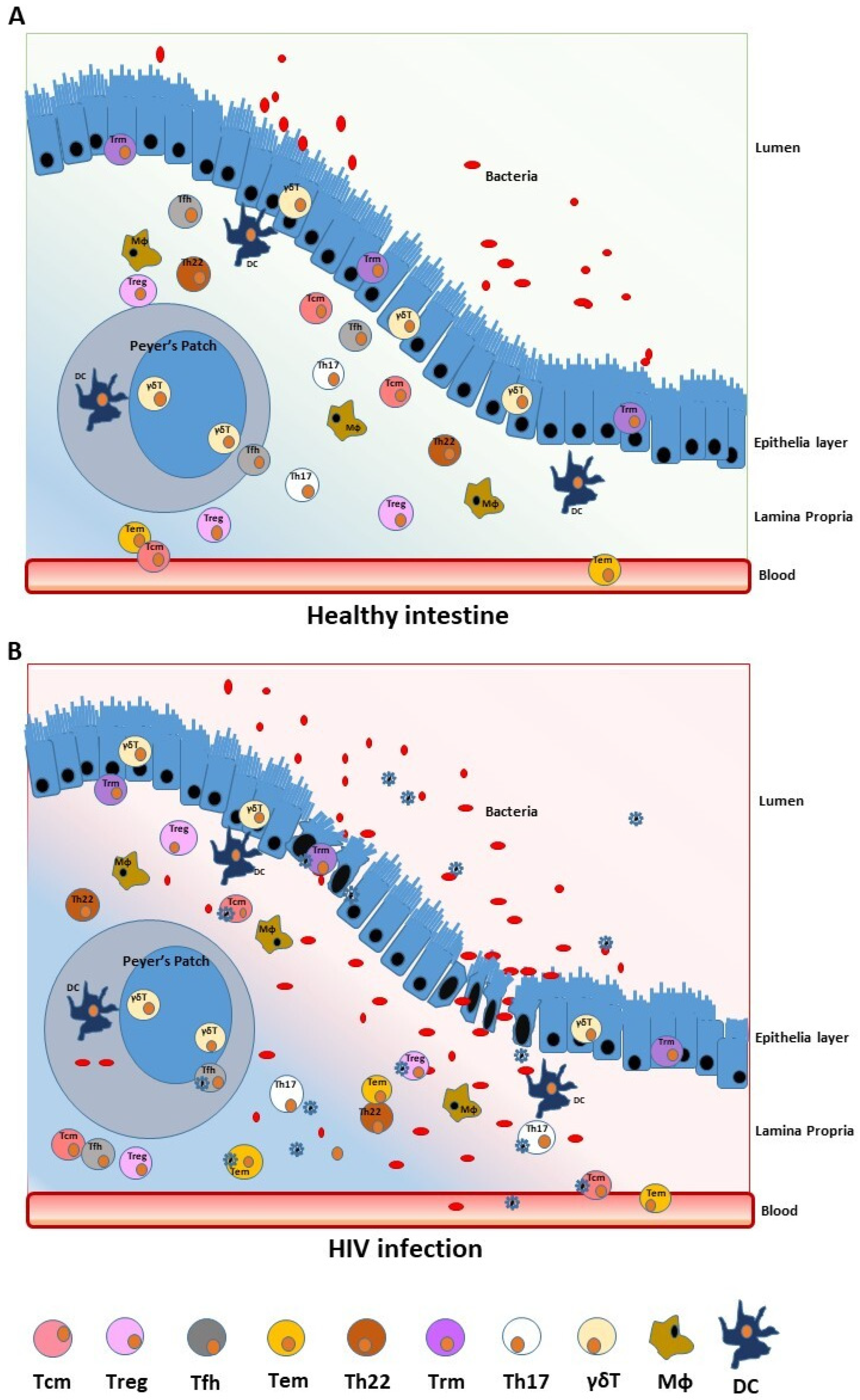
2.1. The Gut Barrier Function
2.2. The Role of Microbiota and GALT
2.3. T-Cell Subsets as Viral Reservoir in the Gut Mucosa
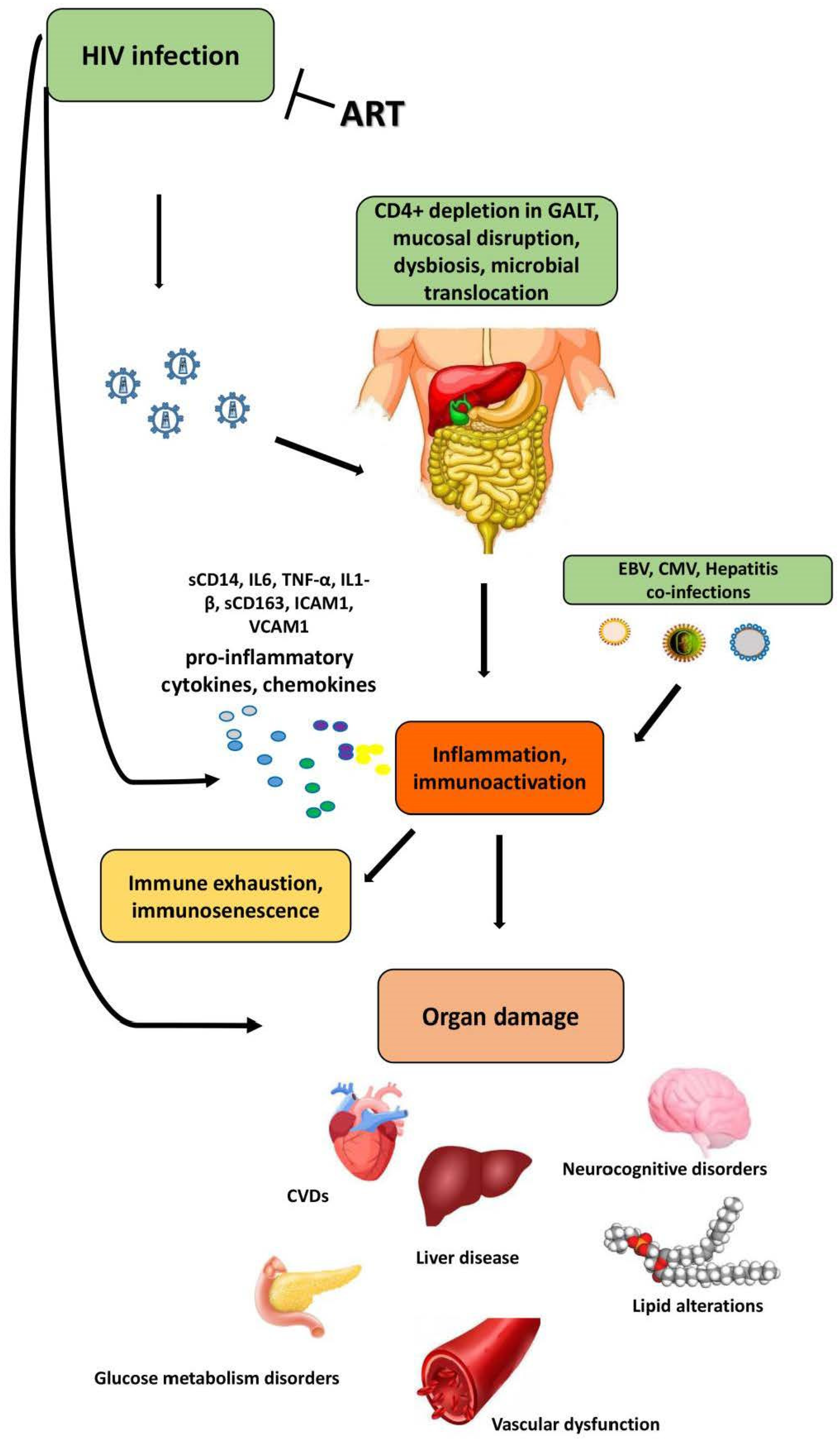
3. Effects of HIV Infection on Gut Microbiota and Systemic Immune Activation
3.1. The Healthy Microbiota
3.2. The Effects of HIV-1 Infection on Gut Microbiota
4. Role of HIV-Related Gut Dysbiosis in Non-AIDS Comorbidities
4.1. The Gut–Liver Axis and Liver Disease
4.2. The Cardiovascular and Metabolic Diseases
4.3. The Brain–Gut Axis and Neurocognitive Disorders
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, D.Y.; Wu, H.Y.; Yarla, N.S.; Xu, B.; Ding, J.; Lu, T.R. HAART in HIV/AIDS Treatments: Future Trends. Infect. Disord. Drug Targets 2018, 18, 15–22. [Google Scholar] [CrossRef]
- Buzon, M.J.; Martin-Gayo, E.; Pereyra, F.; Ouyang, Z.; Sun, H.; Li, J.Z.; Piovoso, M.; Shaw, A.; Dalmau, J.; Zangger, N.; et al. Long-term antiretroviral treatment initiated at primary HIV-1 infection affects the size, composition, and decay kinetics of the reservoir of HIV-1-infected CD4 T cells. J. Virol. 2014, 88, 10056–10065. [Google Scholar] [CrossRef] [Green Version]
- Besson, G.J.; Lalama, C.M.; Bosch, R.J.; Gandhi, R.T.; Bedison, M.A.; Aga, E.; Riddler, S.A.; McMahon, D.K.; Hong, F.; Mellors, J.W. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin. Infect. Dis. 2014, 59, 1312–1321. [Google Scholar] [CrossRef] [Green Version]
- Palermo, E.; Acchioni, C.; Di Carlo, D.; Zevini, A.; Muscolini, M.; Ferrari, M.; Castiello, L.; Virtuoso, S.; Borsetti, A.; Antonelli, G.; et al. Activation of Latent HIV-1 T Cell Reservoirs with a Combination of Innate Immune and Epigenetic Regulators. J. Virol. 2019, 93, e01194-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruner, K.M.; Wang, Z.; Simonetti, F.R.; Bender, A.M.; Kwon, K.J.; Sengupta, S.; Fray, E.J.; Beg, S.A.; Antar, A.A.R.; Jenike, K.M.; et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019, 566, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Siliciano, R.F. Targeting the Latent Reservoir for HIV-1. Immunity 2018, 48, 872–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohn, L.B.; Chomont, N.; Deeks, S.G. The Biology of the HIV-1 Latent Reservoir and Implications for Cure Strategies. Cell Host Microbe 2020, 27, 519–530. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Buzón, M.J.; Benito, J.M.; Rallón, N. Peering into the HIV reservoir. Rev. Med. Virol. 2018, 28, e1981. [Google Scholar] [CrossRef]
- Wong, M.E.; Jaworowski, A.; Hearps, A.C. The HIV Reservoir in Monocytes and Macrophages. Front. Immunol. 2019, 10, 1435. [Google Scholar] [CrossRef] [Green Version]
- Moretti, S.; Virtuoso, S.; Sernicola, L.; Farcomeni, S.; Maggiorella, M.T.; Borsetti, A. Advances in SIV/SHIV Non-Human Primate Models of NeuroAIDS. Pathogens 2021, 10, 1018. [Google Scholar] [CrossRef]
- Busman-Sahay, K.; Starke, C.E.; Nekorchuk, M.D.; Estes, J.D. Eliminating HIV reservoirs for a cure: The issue is in the tissue. Curr. Opin. HIV AIDS 2021, 16, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.; Nolan, D.J.; Maidji, E.; Stoddart, C.A.; Singer, E.J.; Lamers, S.L.; McGrath, M.S. Eradication of HIV from Tissue Reservoirs: Challenges for the Cure. AIDS Res. Hum. Retroviruses 2018, 34, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Capone, A.; Lo Presti, A.; Sernicola, L.; Farcomeni, S.; Ferrantelli, F.; Maggiorella, M.T.; Mee, E.T.; Rose, N.J.; Cella, E.; Ciccozzi, M.; et al. Genetic diversity in the env V1-V2 region of proviral quasispecies from long-term controller MHC-typed cynomolgus macaques infected with SHIVSF162P4cy. J. Gen. Virol. 2018, 99, 1717–1728. [Google Scholar] [CrossRef]
- Mattapallil, J.J.; Douek, D.C.; Hill, B.; Nishimura, Y.; Martin, M.; Roederer, M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005, 434, 1093–1097. [Google Scholar] [CrossRef]
- Sereti, I.; Krebs, S.J.; Phanuphak, N.; Fletcher, J.L.; Slike, B.; Pinyakorn, S.; O’Connell, R.J.; Rupert, A.; Chomont, N.; Valcour, V.; et al. Persistent, Albeit Reduced, Chronic Inflammation in Persons Starting Antiretroviral Therapy in Acute HIV Infection. Clin. Infect. Dis. 2017, 64, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, T.W.; Nickle, D.C.; Justement, J.S.; Meyers, J.H.; Roby, G.; Hallahan, C.W.; Kottilil, S.; Moir, S.; Mican, J.M.; Mullins, J.I.; et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J. Infect. Dis. 2008, 197, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Alzahrani, J.; Hussain, T.; Simar, D.; Palchaudhuri, R.; Abdel-Mohsen, M.; Crowe, S.M.; Mbogo, G.W.; Palmer, C.S. Inflammatory and immunometabolic consequences of gut dysfunction in HIV: Parallels with IBD and implications for reservoir persistence and non-AIDS comorbidities. EBioMedicine 2019, 46, 522–531. [Google Scholar] [CrossRef] [Green Version]
- Zevin, A.S.; McKinnon, L.; Burgener, A.; Klatt, N.R. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr. Opin. HIV AIDS 2016, 11, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Farcomeni, S.; Moretti, S.; Fimiani, C.; Sulekova, L.F.; Vescio, F.; Sernicola, L.; Maggiorella, M.T.; Remoli, A.L.; Picconi, O.; Mosca, L.; et al. Short- and Long-Term Immunological Responses in Chronic HCV/HIV Co-Infected Compared to HCV Mono-Infected Patients after DAA Therapy. Pathogens 2021, 10, 1488. [Google Scholar] [CrossRef]
- Nishio, J.; Honda, K. Immunoregulation by the gut microbiota. Cell Mol. Life Sci. 2012, 69, 3635–3650. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S.; Gordon, J.I.; Glimcher, L.H. Homeostasis and inflammation in the intestine. Cell 2010, 140, 859–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Sakuragi, N.; Takakuwa, A.; Ayabe, T. Paneth cell-defensins and enteric microbiota in health and disease. Biosci. Microbiota Food Health 2016, 35, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Kucharzik, T.; Lugering, N.; Rautenberg, K.; Lugering, A.; Schmidt, M.A.; Stoll, R.; Domschke, W. Role of M-cells in intestinal barrier function. Ann. N. Y. Acad. Sci. 2000, 915, 171–183. [Google Scholar] [CrossRef]
- Zeuthen, L.H.; Fink, L.N.; Frokiaer, H. Epithelial cells prime the immune response to an array of gut- derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor. Immunology 2008, 123, 197–208. [Google Scholar] [CrossRef]
- Baba, N.; Samson, S.; Bourdet-Sicard, R.; Rubio, M.; Sarfati, M. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T-cells. J. Leukoc. Biol. 2008, 84, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogier, R.; Koenders, M.I.; Abdollahi-Roodsaz, S. Toll-like receptor mediated modulation of T-cell response by commensal intestinal microbiota as a trigger for autoimmune. Arthritis 2015, 2015, 527696. [Google Scholar] [CrossRef]
- Abreu, M.T. Toll-like receptor signalling in the intestinal epithelium: How bacterial recognition shake intestinal function. Nat. Rev. Immunol. 2010, 11, 215. [Google Scholar] [CrossRef]
- Cario, E.; Gerken, G.; Podolsky, D. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenerology 2004, 127, 224–238. [Google Scholar] [CrossRef]
- Melmed, G.; Thomas, L.S.; Lee, N.; Tesfay, S.Y.; Lukasek, K.; Michelsen, K.S.; Hou, Y.; Hu, B.; Arditi, M.; Abreu, M.T. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: Implications for host-microbial interactions in the gut. J. Immunol. 2003, 170, 1406–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashida, H.; Ogawa, M.; Kim, M.; Mimuro, H.; Sasakawa, C. Bacteria and host interactions in the gut epithelial barrier. Nat. Chem. Biol. 2012, 8, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.L.; Huffnagle, G.B.; Noverr, M.C.; Kao, J.Y. Overview of gut immunology. Adv. Exp. Med. Biol. 2008, 635, 1–14. [Google Scholar] [PubMed]
- Mowat, A.M.; Viney, J.L. The anatomical basis of intestinal immunity. Immunol. Rev. 1997, 156, 145–166. [Google Scholar] [CrossRef]
- Kräutler, N.J.; Suan, D.; Butt, D.; Bourne, K.; Hermes, J.R.; Chan, T.D.; Sundling, C.; Kaplan, W.; Schofield, P.; Jackson, J.; et al. Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J. Exp. Med. 2017, 214, 1259–1267. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Mayassi, T.; Jabri, B. Human intraepithelial lymphocytes. Mucosal Immunol. 2018, 11, 1281–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogunovic, M.; Ginhoux, F.; Helft, J.; Shang, L.; Hashimoto, D.; Greter, M.; Liu, K.; Jakubzick, C.; Ingersoll, M.A.; Leboeuf, M.; et al. Origin of the lamina propria dendritic cell network. Immunity 2009, 31, 513–525. [Google Scholar] [CrossRef] [Green Version]
- Varol, C.; Zigmond, E.; Jung, S. Securing the immune tightrope: Mononuclear phagocytes in the intestinal lamina propria. Nat. Rev. Immunol. 2010, 10, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Tuganbaev, T.; Skelly, A.N.; Honda, K. T Cell Responses to the Microbiota. Annu. Rev. Immunol. 2022, 40, 559–587. [Google Scholar] [CrossRef]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef]
- Dillon, S.; Frank, D.N.; Wilson, C.C. The gut microbiome and HIV-1 pathogenesis: A two-way street. AIDS 2016, 30, 2737–2751. [Google Scholar] [CrossRef] [Green Version]
- Allers, K.; Fehr, M.; Conrad, K.; Epple, H.J.; Schürmann, D.; Geelhaar-Karsch, A.; Schinnerling, K.; Moos, V.; Schneider, T. Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. J. Infect. Dis. 2014, 209, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Grainger, J.R.; Konkel, J.E.; Zangerle-Murray, T.; Shaw, T.N. Macrophages in gastrointestinal homeostasis and inflammation. Pflug. Arch. Eur. J. Physiol. 2017, 469, 527–539. [Google Scholar] [CrossRef] [Green Version]
- Dubourg, G.; Surenaud, M.; Lévy, Y.; Hüe, S.; Raoult, D. Microbiome of HIV-infected people. Microb. Pathog. 2017, 106, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.M.; Lee, E.J.; Kotter, C.V.; Austin, G.L.; Gianella, S.; Siewe, B.; Smith, D.M.; Landay, A.L.; McManus, M.C.; Robertson, C.E.; et al. Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection. Mucosal Immunol. 2016, 9, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Favre, D.; Mold, J.; Hunt, P.W.; Kanwar, B.; Loke, P.; Seu, L.; Barbour, J.D.; Lowe, M.M.; Jayawardene, A.; Aweeka, F.; et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci. Transl. Med. 2010, 2, 32–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shacklett, B.L. Mucosal Immunity in HIV/SIV Infection: T Cells, B Cells and Beyond. Curr. Immunol. Rev. 2019, 15, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Baban, B.; Chandler, P.R.; Sharma, M.D.; Pihkala, J.; Koni, P.A.; Munn, D.H.; Mellor, A.L. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J. Immunol. 2009, 183, 2475–2483. [Google Scholar] [CrossRef] [Green Version]
- D’Ettorre, G.; Paiardini, M.; Zaffiri, L.; Andreotti, M.; Ceccarelli, G.; Rizza, C.; Indinnimeo, M.; Vella, S.; Mastroianni, C.M.; Silvestri, G.; et al. HIV persistence in the gut mucosa of HIV-infected subjects undergoing antiretroviral therapy correlates with immune activation and increased levels of LPS. Curr. HIV Res. 2011, 9, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Zaunders, J.; Danta, M.; Bailey, M.; Mak, G.; Marks, K.; Seddiki, N.; Xu, Y.; Templeton, D.J.; Cooper, D.A.; Boyd, M.A.; et al. CD4+ T follicular helper and IgA+ B cell numbers in gut biopsies from HIV-infected subjects on antiretroviral therapy are similar to HIV-uninfected individuals. Front. Immunol. 2016, 7, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Rasmussen, T.; Pahar, B.; Poonia, B.; Alvarez, X.; Lackner, A.A.; Veaze, R.S. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood 2007, 109, 1174–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yukl, S.A.; Gianella, S.; Sinclair, E.; Epling, L.; Li, Q.; Duan, L.; Choi, A.L.M.; Girling, V.; Ho, T.; Li, P.; et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J. Infect. Dis. 2010, 202, 1553–1561. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.K.; Yukl, S.A. Tissue reservoirs of HIV. Curr. Opin. HIV AIDS 2016, 11, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Martín-Moreno, A.; Muñoz-Fernández, M.A. Dendritic Cells, the Double Agent in the War against HIV-1. Front. Immunol. 2019, 10, 2485. [Google Scholar] [CrossRef] [Green Version]
- Thome, J.J.; Yudanin, N.; Ohmura, Y.; Kubota, M.; Grinshpun, B.; Sathaliyawala, T.; Kato, T.; Lerner, H.; Shen, Y.; Farber, D.L. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 2014, 159, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Kondo, T.; Matsuki, F.; Takata, H.; Takiguchi, M. Phenotypic classification of human CD4+ T cell subsets and their differentiation. Int. Immunol. 2008, 20, 1189–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beura, L.K.; Masopust, D. SnapShot: Resident memory T cells. Cell 2014, 157, 1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.T.; Ong, D.E.; Lim, F.S.; Teng, K.W.; McGovern, N.; Narayanan, S.; Ho, W.Q.; Cerny, D.; Tan, H.K.; Anicete, R.; et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity 2016, 45, 442–456. [Google Scholar] [CrossRef] [Green Version]
- Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014, 41, 529–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telwatte, S.; Lee, S.; Somsouk, M.; Hatano, H.; Baker, C.; Kaiser, P.; Kim, P.; Chen, T.H.; Milush, J.; Hunt, P.W.; et al. Gut and blood differ in constitutive blocks to HIV transcription, suggesting tissue-specific differences in the mechanisms that govern HIV latency. PLoS Pathog. 2018, 14, e1007357. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Telwatte, S.; Trapecar, M.; Yukl, S.; Sanjabi, S. Differentiating Immune Cell Targets in Gut-Associated Lymphoid Tissue for HIV Cure. AIDS Res. Hum. Retroviruses 2017, 33, S40–S58. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, S.; Graf, E.H.; Dahl, V.; Strain, M.C.; Yukl, S.A.; Lysenko, E.S.; Bosch, R.J.; Lai, J.; Chioma, S.; Emad, F.; et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013, 9, e1003174. [Google Scholar] [CrossRef] [PubMed]
- Sandquist, I.; Kolls, J. Update on regulation and effector functions of Th17 cells. F1000Research 2018, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kim, D.; Li, X.; Kiselinova, M.; Ouyang, Z.; Vandekerckhove, L.; Shang, H.; Rosenberg, E.S.; Yu, X.G.; Lichterfeld, M. Th1/17 Polarization of CD4 T Cells Supports HIV-1 Persistence during Antiretroviral Therapy. J. Virol. 2015, 89, 11284–11293. [Google Scholar] [CrossRef] [Green Version]
- Gosselin, A.; Monteiro, P.; Chomont, N.; Diaz-Griffero, F.; Said, E.A.; Fonseca, S.; Wacleche, V.; El-Far, M.; Boulassel, M.R.; Routy, J.P.; et al. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J. Immunol. 2010, 184, 1604–1616. [Google Scholar] [CrossRef] [Green Version]
- Prendergast, A.; Prado, J.G.; Kang, Y.H.; Chen, F.; Riddell, L.A.; Luzzi, G.; Goulder, P.; Klenerman, P. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS 2010, 24, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Chege, D.; Sheth, P.M.; Kain, T.; Kim, C.J.; Kovacs, C.; Loutfy, M.; Halpenny, R.; Kandel, G.; Chun, T.W.; Ostrowski, M.; et al. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS 2011, 25, 741–749. [Google Scholar] [CrossRef]
- Planas, D.; Routy, J.P.; Ancuta, P. New Th17-specific therapeutic strategies for HIV remission. Curr. Opin. HIV AIDS 2019, 14, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Fert, A.; Raymond Marchand, L.; Wiche Salinas, T.R.; Ancuta, P. Targeting Th17 cells in HIV-1 remission/cure interventions. Trends Immunol. 2022, 43, 580–594. [Google Scholar] [CrossRef]
- Muranski, P.; Borman, Z.A.; Kerkar, S.P.; Klebanoff, C.A.; Ji, Y.; Sanchez-Perez, L.; Sukumar, M.; Reger, R.N.; Yu, Z.; Kern, S.J.; et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 2011, 35, 972–985. [Google Scholar] [CrossRef] [Green Version]
- d’Ettorre, G.; Ceccarelli, G.; Andreotti, M.; Selvaggi, C.; Giustini, N.; Serafino, S.; Schietroma, I.; Nunnari, G.; Antonelli, G.; Vullo, V.; et al. Analysis of Th17 and Tc17 Frequencies and Antiviral Defenses in Gut-Associated Lymphoid Tissue of Chronic HIV-1 Positive Patients. Mediat. Inflamm. 2015, 2015, 395484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevalier, M.F.; Petitjean, G.; Dunyach-Rémy, C.; Didier, C.; Girard, P.M.; Manea, M.E.; Campa, P.; Meyer, L.; Rouzioux, C.; Lavigne, J.P.; et al. The Th17/Treg ratio, IL-1RA and sCD14 levels in primary HIV infection predict the T-cell activation set point in the absence of systemic microbial translocation. PLoS Pathog. 2013, 9, e1003453. [Google Scholar] [CrossRef] [Green Version]
- Wacleche, V.S.; Landay, A.; Routy, J.P.; Ancuta, P. The Th17 Lineage: From Barrier Surfaces Homeostasis to Autoimmunity, Cancer, and HIV-1 Pathogenesis. Viruses 2017, 9, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renault, C.; Veyrenche, N.; Mennechet, F.; Bedin, A.S.; Routy, J.P.; Van de Perre, P.; Reynes, J.; Tuaillon, E. Th17 CD4+ T-Cell as a Preferential Target for HIV Reservoirs. Front. Immunol. 2022, 13, 822576. [Google Scholar] [CrossRef]
- Gong, J.; Zhan, H.; Liang, Y.; He, Q.; Cui, D. Role of Th22 Cells in Human Viral Diseases. Front. Med. 2021, 8, 708140. [Google Scholar] [CrossRef] [PubMed]
- Doulabi, H.; Masoumi, E.; Rastin, M.; Foolady Azarnaminy, A.; Esmaeili, S.A.; Mahmoudi, M. The role of Th22 cells, from tissue repair to cancer progression. Cytokine 2022, 149, 155749. [Google Scholar] [CrossRef]
- Nayrac, M.; Requena, M.; Loiseau, C.; Cazabat, M.; Suc, B.; Carrere, N.; Barange, K.; Alric, L.; Martin-Blondel, G.; Izopet, J.; et al. Th22 cells are efficiently recruited in the gut by CCL28 as an alternative to CCL20 but do not compensate for the loss of Th17 cells in treated HIV-1-infected individuals. Mucosal Immunol. 2021, 14, 219–228. [Google Scholar] [CrossRef]
- Wan, Z.; Zhou, Z.; Liu, Y.; Lai, Y.; Luo, Y.; Peng, X.; Zou, W. Regulatory T cells and T helper 17 cells in viral infection. Scand. J. Immunol. 2020, 91, e12873. [Google Scholar] [CrossRef] [Green Version]
- López-Abente, J.; Correa-Rocha, R.; Pion, M. Functional Mechanisms of Treg in the Context of HIV Infection and the Janus Face of Immune Suppression. Front. Immunol. 2016, 7, 192. [Google Scholar] [CrossRef] [Green Version]
- Holmes, D.; Jiang, Q.; Zhang, L.; Su, L. Foxp3 and Treg cells in HIV-1 infection and immuno-pathogenesis. Immunol. Res. 2008, 41, 248–266. [Google Scholar] [CrossRef] [Green Version]
- Veazey, R.S.; DeMaria, M.; Chalifoux, L.V.; Shvetz, D.E.; Pauley, D.R.; Knight, H.L.; Rosenzweig, M.; Johnson, R.P.; Desrosiers, R.C.; Lackner, A.A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998, 280, 427–431. [Google Scholar] [CrossRef]
- Veazey, R.S. Intestinal CD4 Depletion in HIV / SIV Infection. Curr. Immunol. Rev. 2019, 15, 76–91. [Google Scholar] [CrossRef]
- Deenick, E.K.; Ma, C.S. The regulation and role of T follicular helper cells in immunity. Immunology 2011, 134, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Perreau, M.; Savoye, A.L.; De Crignis, E.; Corpataux, J.M.; Cubas, R.; Haddad, E.K.; De Leval, L.; Graziosi, C.; Pantaleo, G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 2013, 210, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Banga, R.; Procopio, F.A.; Noto, A.; Pollakis, G.; Cavassini, M.; Ohmiti, K.; Corpataux, J.M.; de Leval, L.; Pantaleo, G.; Perreau, M. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat. Med. 2016, 22, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Rahmberg, A.R.; Rajakumar, P.A.; Billingsley, J.M.; Johnson, R.P. Dynamic Modulation of Expression of Lentiviral Restriction Factors in Primary CD4+ T Cells following Simian Immunodeficiency Virus Infection. J. Virol. 2017, 91, e02189-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allam, A.; Majji, S.; Peachman, K.; Jagodzinski, L.; Kim, J.; Ratto-Kim, S.; Wijayalath, W.; Merbah, M.; Kim, J.H.; Michael, N.L.; et al. TFH cells accumulate in mucosal tissues of humanized-DRAG mice and are highly permissive to HIV-1. Sci. Rep. 2015, 5, 10443. [Google Scholar] [CrossRef] [Green Version]
- Aid, M.; Dupuy, F.P.; Moysi, E.; Moir, S.; Haddad, E.K.; Estes, J.D.; Sekaly, R.P.; Petrovas, C.; Ribeiro, S.P. Follicular CD4 T Helper Cells As a Major HIV Reservoir Compartment: A Molecular Perspective. Front. Immunol. 2018, 9, 895. [Google Scholar] [CrossRef]
- Rampoldi, F.; Prinz, I. Three Layers of Intestinal γδ T Cells Talk Different Languages With the Microbiota. Front. Immunol. 2022, 13, 849954. [Google Scholar] [CrossRef]
- Poccia, F.; Agrati, C.; Martini, F.; Capobianchi, M.R.; Wallace, M.; Malkovsky, M. Antiviral reactivities of gammadelta T cells. Microbes Infect. 2005, 7, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, M.L.; Strbo, N.; Romero, L.; Jones, D.L.; Rodriguez, V.J.; Arheart, K.; Martinez, O.; Bolivar, H.; Podack, E.R.; Fischl, M.A. Bacterial Vaginosis Is Associated with Loss of Gamma Delta T Cells in the Female Reproductive Tract in Women in the Miami Women Interagency HIV Study (WIHS): A Cross Sectional Study. PLoS ONE 2016, 11, e0153045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano-Sarabia, N.; Archin, N.M.; Bateson, R.; Dahl, N.P.; Crooks, A.M.; Kuruc, J.D.; Garrido, C.; Margolis, D.M. Peripheral Vγ9Vδ2 T Cells Are a Novel Reservoir of Latent HIV Infection. PLoS Pathog. 2015, 11, e1005201. [Google Scholar] [CrossRef]
- Farber, D.L.; Yudanin, N.A.; Restifo, N.P. Human memory T cells: Generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014, 14, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Fritsch, R.D.; Shen, X.; Sims, G.P.; Hathcock, K.S.; Hodes, R.J.; Lipsky, P.E. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J. Immunol. 2005, 175, 6489–6497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senda, T.; Dogra, P.; Granot, T.; Furuhashi, K.; Snyder, M.E.; Carpenter, D.J.; Szabo, P.A.; Thapa, P.; Miron, M.; Farber, D.L. Microanatomical dissection of human intestinal T-cell immunity reveals site-specific changes in gut-associated lymphoid tissues over life. Mucosal Immunol. 2019, 12, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Gantner, P.; Buranapraditkun, S.; Pagliuzza, A.; Dufour, C.; Pardons, M.; Mitchell, J.L.; Kroon, E.; Sacdalan, C.; Tulmethakaan, N.; Pinyakorn, S.; et al. HIV rapidly targets a diverse pool of CD4+ T cells to establish productive and latent infections. Immunity 2023, 56, 653–668.e5. [Google Scholar] [CrossRef]
- Yukl, S.A.; Shergill, A.K.; Ho, T.; Killian, M.; Girling, V.; Epling, L.; Li, P.; Wong, L.K.; Crouch, P.; Deeks, S.G.; et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: Implications for viral persistence. J. Infect. Dis. 2013, 208, 1212–1220. [Google Scholar] [CrossRef] [Green Version]
- Casey, K.A.; Fraser, K.A.; Schenkel, J.M.; Moran, A.; Abt, M.C.; Beura, L.K.; Lucas, P.J.; Artis, D.; Wherry, E.J.; Hogquist, K.; et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012, 188, 4866–4875. [Google Scholar] [CrossRef] [Green Version]
- Yukl, S.A.; Khan, S.; Chen, T.H.; Trapecar, M.; Wu, F.; Xie, G.; Telwatte, S.; Fulop, D.; Pico, A.R.; Laird, G.M.; et al. Shared Mechanisms Govern HIV Transcriptional Suppression in Circulating CD103+ and Gut CD4+ T Cells. J. Virol. 2020, 95, e01331-20. [Google Scholar] [CrossRef]
- Gattinoni, L.; Lugli, E.; Ji, Y.; Pos, Z.; Paulos, C.M.; Quigley, M.F.; Almeida, J.R.; Gostick, E.; Yu, Z.; Carpenito, C.; et al. Price, D.A.; June, C.H.; Marincola, F.M.; Roederer, M.; Restifo, N.P. A human memory T cell subset with stem cell-like properties. Nat. Med. 2011, 17, 1290–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugli, E.; Dominguez, M.H.; Gattinoni, L.; Chattopadhyay, P.K.; Bolton, D.L.; Song, K.; Klatt, N.R.; Brenchley, J.M.; Vaccari, M.; Gostick, E.; et al. Superior T memory stem cell persistence supports long-lived T cell memory. J. Clin. Investig. 2013, 123, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chachage, M. The gut-microbiome contribution to HIV-associated cardiovascular disease and metabolic disorders. Curr. Opin. Endocr. Metab. Res. 2021, 21, 100287. [Google Scholar] [CrossRef]
- D’Angelo, C.; Reale, M.; Costantini, E. Microbiota and Probiotics in Health and HIV Infection. Nutrients 2017, 9, 615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Troy, E.B.; Kasper, D.L. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front. Biosci. 2010, 15, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.A.; Ryu, J.H.; Jo, Y.; Hong, C. The role of gut microbiota in T cell immunity and immune mediated disorders. Int. J. Biol. Sci. 2023, 19, 1178–1191. [Google Scholar] [CrossRef]
- Geng, S.T.; Zhang, Z.Y.; Wang, Y.X.; Lu, D.; Yu, J.; Zhang, J.B.; Kuang, Y.Q.; Wang, K.H. Regulation of Gut Microbiota on Immune Reconstitution in Patients With Acquired Immunodeficiency Syndrome. Front. Microbiol. 2020, 11, 594820. [Google Scholar] [CrossRef]
- Deusch, S.; Serrano-Villar, S.; Rojo, D.; Martinez-Martinez, M.; Bargiela, R.; Vazquez-Castellanos, J.F.; Sainz, T.; Barbas, C.; Moya, A.; Moreno, S.; et al. Effects of HIV, antiretroviral therapy and prebiotics on the active fraction of the gut microbiota. AIDS 2018, 32, 1229–1237. [Google Scholar] [CrossRef]
- Boasso, A.; Hardy, A.W.; Anderson, S.A.; Dolan, M.J.; Shearer, G.M. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS ONE 2008, 13, e2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Hoel, H.; Hove-Skovsgaard, M.; Hov, J.R.; Gaardbo, J.C.; Holm, K.; Kummen, M.; Rudi, K.; Nwosu, F.; Valeur, J.; Gelpi, M.; et al. Impact of HIV and Type 2 diabetes on Gut Microbiota Diversity, Tryptophan Catabolism and Endothelial Dysfunction. Sci. Rep. 2018, 8, 6725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vujkovic-Cvijin, I.; Somsouk, M. HIV and the Gut Microbiota: Composition, Consequences, and Avenues for Amelioration. Curr. HIV/AIDS Rep. 2019, 16, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Paquin-Proulx, D.; Ching, C.; Vujkovic-Cvijin, I.; Fadrosh, D.; Loh, L.; Huang, Y.; Somsouk, M.; Lynch, S.V.; Hunt, P.W.; Nixon, D.F.; et al. Bacteroides are associated with GALT iNKT cell function and reduction of microbial translocation in HIV-1 infection. Mucosal Immunol. 2017, 10, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Suchard, M.S.; Mayne, E.; Green, V.A.; Shalekoff, S.; Donninger, S.L.; Stevens, W.S.; Gray, C.M.; Tiemessen, C.T. FOXP3 expression is upregulated in CD4T cells in progressive HIV-1 infection and is a marker of disease severity. PLoS ONE 2010, 5, e11762. [Google Scholar]
- Ferrari, B.; Da Silva, A.C.; Liu, K.H.; Saidakova, E.V.; Korolevskaya, L.B.; Shmagel, K.V.; Shive, C.; Pacheco Sanchez, G.; Retuerto, M.; Sharma, A.A.; et al. Gut-derived bacterial toxins impair memory CD4+ T cell mitochondrial function in HIV-1 infection. J. Clin. Investig. 2022, 132, e149571. [Google Scholar] [CrossRef]
- Monaco, C.L.; Gootenberg, D.B.; Zhao, G.; Handley, S.A.; Ghebremichael, M.S.; Lim, E.S.; Lankowski, A.; Baldridge, M.T.; Wilen, C.B.; Flagg, M.; et al. Altered Virome and Bacterial Microbiome in Human Immunodeficiency Virus-Associated Acquired Immunodeficiency Syndrome. Cell Host Microbe 2016, 19, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, G.; Bellistrì, G.M.; Borghi, E.; Tincati, C.; Ferramosca, S.; La Francesca, M.; Morace, G.; Gori, A.; Monforte, A.D. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS 2008, 22, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Tracy, R.; Douek, D.C. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013, 39, 633–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estes, J.; Baker, J.V.; Brenchley, J.M.; Khoruts, A.; Barthold, J.L.; Bantle, A.; Reilly, C.S.; Beilman, G.J.; George, M.E.; Douek, D.C.; et al. Collagen deposition limits immune reconstitution in the gut. J. Infect. Dis. 2008, 198, 456–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levesque, M.C.; Moody, M.A.; Hwang, K.K.; Marshall, D.J.; Whitesides, J.F.; Amos, J.D.; Gurley, T.C.; Allgood, S.; Haynes, B.B.; Vandergrift, N.A.; et al. Haynes, B.F. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009, 6, e1000107. [Google Scholar] [CrossRef]
- Kaspar, M.B.; Sterling, R.K. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol. 2017, 4, e000166. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S.N.; Cervasi, B.; Odorizzi, P.; Silverman, R.; Aberra, F.; Ginsberg, G.; Estes, J.D.; Paiardini, M.; Frank, I.; Silvestri, G. Disruption of intestinal CD4+ T cell homeostasis is a key marker of systemic CD4+ T cell activation in HIV-infected individuals. J. Immunol. 2010, 185, 5169–5179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakaroun, R.M.; Massier, L.; Kovacs, P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients 2020, 12, 1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crakes, K.R.; Jiang, G. Gut Microbiome Alterations During HIV/SIV Infection: Implications for HIV Cure. Front. Microbiol. 2019, 10, 1104. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, G.; Tincati, C.; Silvestri, G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin. Microbiol. Rev. 2013, 26, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Martin, H.R.; Sales Martinez, S.; Stebliankin, V.; Tamargo, J.A.; Campa, A.; Narasimhan, G.; Hernandez, J.; Rodriguez, J.A.B.; Teeman, C.; Johnson, A.; et al. Diet Quality and Liver Health in People Living with HIV in the MASH Cohort: A Multi-Omic Analysis of the Fecal Microbiome and Metabolome. Metabolites 2023, 13, 271. [Google Scholar] [CrossRef]
- Sim, J.H.; Mukerji, S.S.; Russo, S.C.; Lo, J. Gastrointestinal Dysfunction and HIV Comorbidities. Curr. HIV/AIDS Rep. 2021, 18, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, P.; Cima, S.; Corbella, M.; Comolli, G.; Chiesa, A.; Baldanti, F.; Klersy, C.; Novati, S.; Mulatto, P.; Mariconti, M.; et al. Liver fibrosis, microbial translocation and immune activation markers in HIV and HCV infections and in HIV/HCV co-infection. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2015, 47, 218–225. [Google Scholar] [CrossRef]
- Mastroianni, C.M.; Lichtner, M.; Mascia, C.; Zuccalà, P.; Vullo, V. Molecular mechanisms of liver fibrosis in HIV/HCV coinfection. Int. J. Mol. Sci. 2014, 15, 9184–9208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zilberman-Schapira, G.; Zmora, N.; Itav, S.; Bashiardes, S.; Elinav, H.; Elinav, E. The gut microbiome in human immunodeficiency virus infection. BMC Med. 2016, 14, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulos, P.D.; Tsigalou, C.; Valsamaki, P.N.; Konstantinidis, T.G.; Voidarou, C.; Bezirtzoglou, E. The Emerging Role of the Gut Microbiome in Cardiovascular Disease: Current Knowledge and Perspectives. Biomedicines 2022, 10, 948. [Google Scholar] [CrossRef]
- Wang, Z.; Peters, B.A.; Usyk, M.; Xing, J.; Hanna, D.B.; Wang, T.; Post, W.S.; Landay, A.L.; Hodis, H.N.; Weber, K.; et al. Gut Microbiota, Plasma Metabolomic Profiles, and Carotid Artery Atherosclerosis in HIV Infection. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1081–1093. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef] [Green Version]
- Srinivasa, S.; Fitch, K.V.; Lo, J.; Kadar, H.; Knight, R.; Wong, K.; Abbara, S.; Gauguier, D.; Capeau, J.; Boccara, F.; et al. Plaque burden in HIV-infected patients is associated with serum intestinal microbiota-generated trimethylamine. AIDS 2015, 29, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Haissman, J.M.; Knudsen, A.; Hoel, H.; Kjær, A.; Kristoffersen, U.S.; Berge, R.K.; Katzenstein, T.L.; Svardal, A.; Ueland, T.; Aukrust, P.; et al. Microbiota-Dependent Marker TMAO Is Elevated in Silent Ischemia but Is Not Associated With First-Time Myocardial Infarction in HIV Infection. J. Acquir. Immune Defic. Syndr. 2016, 71, 130–136. [Google Scholar] [CrossRef]
- Kehrmann, J.; Menzel, J.; Saeedghalati, M.; Obeid, R.; Schulze, C.; Holzendorf, V.; Farahpour, F.; Reinsch, N.; Klein-Hitpass, L.; Streeck, H.; et al. Gut Microbiota in Human Immunodeficiency Virus-Infected Individuals Linked to Coronary Heart Disease. J. Infect. Dis. 2019, 219, 497–508. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef] [Green Version]
- Haissman, J.M.; Haugaard, A.K.; Ostrowski, S.R.; Berge, R.K.; Hov, J.R.; Trøseid, M.; Nielsen, S.D. Microbiota-dependent metabolite and cardiovascular disease marker trimethylamine-N-oxide (TMAO) is associated with monocyte activation but not platelet function in untreated HIV infection. BMC Infect. Dis. 2017, 17, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siedner, M.J.; Kim, J.H.; Nakku, R.S.; Bibangambah, P.; Hemphill, L.; Triant, V.A.; Haberer, J.E.; Martin, J.N.; Mocello, A.R.; Boum, Y.; et al. Persistent Immune Activation and Carotid Atherosclerosis in HIV-Infected Ugandans Receiving Antiretroviral Therapy. J. Infect. Dis. 2016, 213, 370–378. [Google Scholar] [CrossRef] [Green Version]
- Kelesidis, T.; Kendall, M.A.; Yang, O.O.; Hodis, H.N.; Currier, J.S. Biomarkers of microbial translocation and macrophage activation: Association with progression of subclinical atherosclerosis in HIV-1 infection. J. Infect. Dis. 2012, 206, 1558–1567. [Google Scholar] [CrossRef]
- Longenecker, C.T.; Jiang, Y.; Orringer, C.E.; Gilkeson, R.C.; Debanne, S.; Funderburg, N.T.; Lederman, M.M.; Storer, N.; Labbato, D.E.; McComsey, G.A. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014, 28, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic-Cvijin, I.; Dunham, R.M.; Iwai, S.; Maher, M.C.; Albright, R.G.; Broadhurst, M.J.; Hernandez, R.D.; Lederman, M.M.; Huang, Y.; Somsouk, M.; et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci. Transl. Med. 2013, 5, 193ra91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byakwaga, H.; Boum, Y.; Huang, Y.; Muzoora, C.; Kembabazi, A.; Weiser, S.D.; Bennett, J.; Cao, H.; Haberer, J.E.; Deeks, S.G.; et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J. Infect. Dis. 2014, 210, 383–391. [Google Scholar] [CrossRef]
- Hanna, D.B.; Post, W.S.; Deal, J.A.; Hodis, H.N.; Jacobson, L.P.; Mack, W.J.; Anastos, K.; Gange, S.J.; Landay, A.L.; Lazar, J.M.; et al. HIV Infection Is Associated With Progression of Subclinical Carotid Atherosclerosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 61, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Qi, Q.; Hua, S.; Clish, C.B.; Scott, J.M.; Hanna, D.B.; Wang, T.; Haberlen, S.A.; Shah, S.J.; Glesby, M.J.; Lazar, J.M.; et al. Plasma Tryptophan-Kynurenine Metabolites Are Altered in Human Immunodeficiency Virus Infection and Associated With Progression of Carotid Artery Atherosclerosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 67, 235–242. [Google Scholar] [CrossRef]
- Dillon, S.M.; Kibbie, J.; Lee, E.J.; Guo, K.; Santiago, M.L.; Austin, G.L.; Gianella, S.; Landay, A.L.; Donovan, A.M.; Frank, D.N.; et al. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS 2017, 31, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Villar, S.; Vázquez-Castellanos, J.F.; Vallejo, A.; Latorre, A.; Sainz, T.; Ferrando-Martínez, S.; Rojo, D.; Martínez-Botas, J.; Del Romero, J.; Madrid, N.; et al. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol. 2017, 10, 1279–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.Y.; Zolnik, C.P.; Wang, Z.; Qiu, Y.; Usyk, M.; Wang, T.; Kizer, J.R.; Landay, A.L.; Kurland, I.J.; Anastos, K.; et al. Gut microbiota and plasma metabolites associated with diabetes in women with, or at high risk for, HIV infection. EBioMedicine 2018, 37, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Villanueva-Millán, M.J.; Pérez-Matute, P.; Recio-Fernández, E.; Lezana Rosales, J.M.; Oteo, J.A. Characterization of gut microbiota composition in HIV-infected patients with metabolic syndrome. J. Physiol. Biochem. 2019, 75, 299–309. [Google Scholar] [CrossRef]
- Pedro, M.N.; Rocha, G.Z.; Guadagnini, D.; Santos, A.; Magro, D.O.; Assalin, H.B.; Oliveira, A.G.; Pedro, R.J.; Saad, M.J.A. Insulin Resistance in HIV-Patients: Causes and Consequences. Front. Endocrinol. 2018, 9, 514. [Google Scholar] [CrossRef]
- Bourgi, K.; Wanjalla, C.; Koethe, J.R. Inflammation and Metabolic Complications in HIV. Curr. HIV/AIDS Rep. 2018, 15, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Fahme, S.A.; Bloomfield, G.S.; Peck, R. Hypertension in HIV-Infected Adults: Novel Pathophysiologic Mechanisms. Hypertension 2018, 72, 44–55. [Google Scholar] [CrossRef]
- Timmons, T.; Shen, C.; Aldrovandi, G.; Rollie, A.; Gupta, S.K.; Stein, J.H.; Dubé, M.P. Microbial translocation and metabolic and body composition measures in treated and untreated HIV infection. AIDS Res. Hum. Retroviruses 2014, 30, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Trautmann, L.; Janbazian, L.; Chomont, N.; Said, E.A.; Gimmig, S.; Bessette, B.; Boulassel, M.R.; Delwart, E.; Sepulveda, H.; Balderas, R.S.; et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006, 12, 1198–1202. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Rich, S.; Klann, E.; Bryant, V.; Richards, V.; Wijayabahu, A.; Bryant, K.; Mai, V.; Cook, R. A review of potential microbiome-gut-brain axis mediated neurocognitive conditions in persons living with HIV. Brain Behav. Immun. Health 2020, 9, 100168. [Google Scholar] [CrossRef]
- Ancuta, P.; Kamat, A.; Kunstman, K.J.; Kim, E.Y.; Autissier, P.; Wurcel, A.; Zaman, T.; Stone, D.; Mefford, M.; Morgello, S.; et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE 2008, 3, e2516. [Google Scholar] [CrossRef] [Green Version]
- Lyons, J.; Venna, N.; Cho, T.A. Atypical nervous system manifestations of HIV. Semin. Neurol. 2011, 31, 254–265. [Google Scholar] [CrossRef]
- Sharma, L.; Riva, A. Intestinal Barrier Function in Health and Disease-Any role of SARS-CoV-2. Microorganisms 2020, 8, 1744. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Santiago, J.; De Oliveira, M.F.; Var, S.R.; Day, T.R.C.; Woods, S.P.; Gianella, S.; Mehta, S.R. Increased cell-free mitochondrial DNA is a marker of ongoing inflammation and better neurocognitive function in virologically suppressed HIV-infected individuals. J. Neurovirol. 2017, 23, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson-Papp, J.; Nmashie, A.; Pedowitz, E.; Benn, E.K.T.; George, M.C.; Sharma, S.; Murray, J.; Machac, J.; Heiba, S.; Mehandru, S.; et al. Vagal dysfunction and small intestinal bacterial overgrowth: Novel pathways to chronic inflammation in HIV. AIDS 2018, 32, 1147–1156. [Google Scholar] [CrossRef]
- Galland, L. The gut microbiome and the brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef] [Green Version]
- McGettrick, P.; Barco, E.A.; Mallon, P.W.G. Ageing with HIV. Healthcare 2018, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Carrico, A.W.; Cherenack, E.M.; Rubin, L.H.; McIntosh, R.; Ghanooni, D.; Chavez, J.V.; Klatt, N.R.; Paul, R.H. Through the Looking-Glass: Psychoneuroimmunology and the Microbiome-Gut-Brain Axis in the Modern Antiretroviral Therapy Era. Psychosom. Med. 2022, 84, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kesarla, R.; Omri, A. Approaches for CNS delivery of drugs—Nose to brain targeting of antiretroviral agents as a potential attempt for complete elimination of major reservoir site of HIV to aid AIDS treatment. Expert Opin. Drug Deliv. 2019, 16, 287–300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, S.; Schietroma, I.; Sberna, G.; Maggiorella, M.T.; Sernicola, L.; Farcomeni, S.; Giovanetti, M.; Ciccozzi, M.; Borsetti, A. HIV-1–Host Interaction in Gut-Associated Lymphoid Tissue (GALT): Effects on Local Environment and Comorbidities. Int. J. Mol. Sci. 2023, 24, 12193. https://doi.org/10.3390/ijms241512193
Moretti S, Schietroma I, Sberna G, Maggiorella MT, Sernicola L, Farcomeni S, Giovanetti M, Ciccozzi M, Borsetti A. HIV-1–Host Interaction in Gut-Associated Lymphoid Tissue (GALT): Effects on Local Environment and Comorbidities. International Journal of Molecular Sciences. 2023; 24(15):12193. https://doi.org/10.3390/ijms241512193
Chicago/Turabian StyleMoretti, Sonia, Ivan Schietroma, Giuseppe Sberna, Maria Teresa Maggiorella, Leonardo Sernicola, Stefania Farcomeni, Marta Giovanetti, Massimo Ciccozzi, and Alessandra Borsetti. 2023. "HIV-1–Host Interaction in Gut-Associated Lymphoid Tissue (GALT): Effects on Local Environment and Comorbidities" International Journal of Molecular Sciences 24, no. 15: 12193. https://doi.org/10.3390/ijms241512193
APA StyleMoretti, S., Schietroma, I., Sberna, G., Maggiorella, M. T., Sernicola, L., Farcomeni, S., Giovanetti, M., Ciccozzi, M., & Borsetti, A. (2023). HIV-1–Host Interaction in Gut-Associated Lymphoid Tissue (GALT): Effects on Local Environment and Comorbidities. International Journal of Molecular Sciences, 24(15), 12193. https://doi.org/10.3390/ijms241512193








