Lipopolysaccharide Tolerance in Human Primary Monocytes and Polarized Macrophages
Abstract
:1. Introduction
2. Results
2.1. TLR1/2 Ligands but Not TNF-α Partly Tolerize against Subsequent TLR4 Stimulation
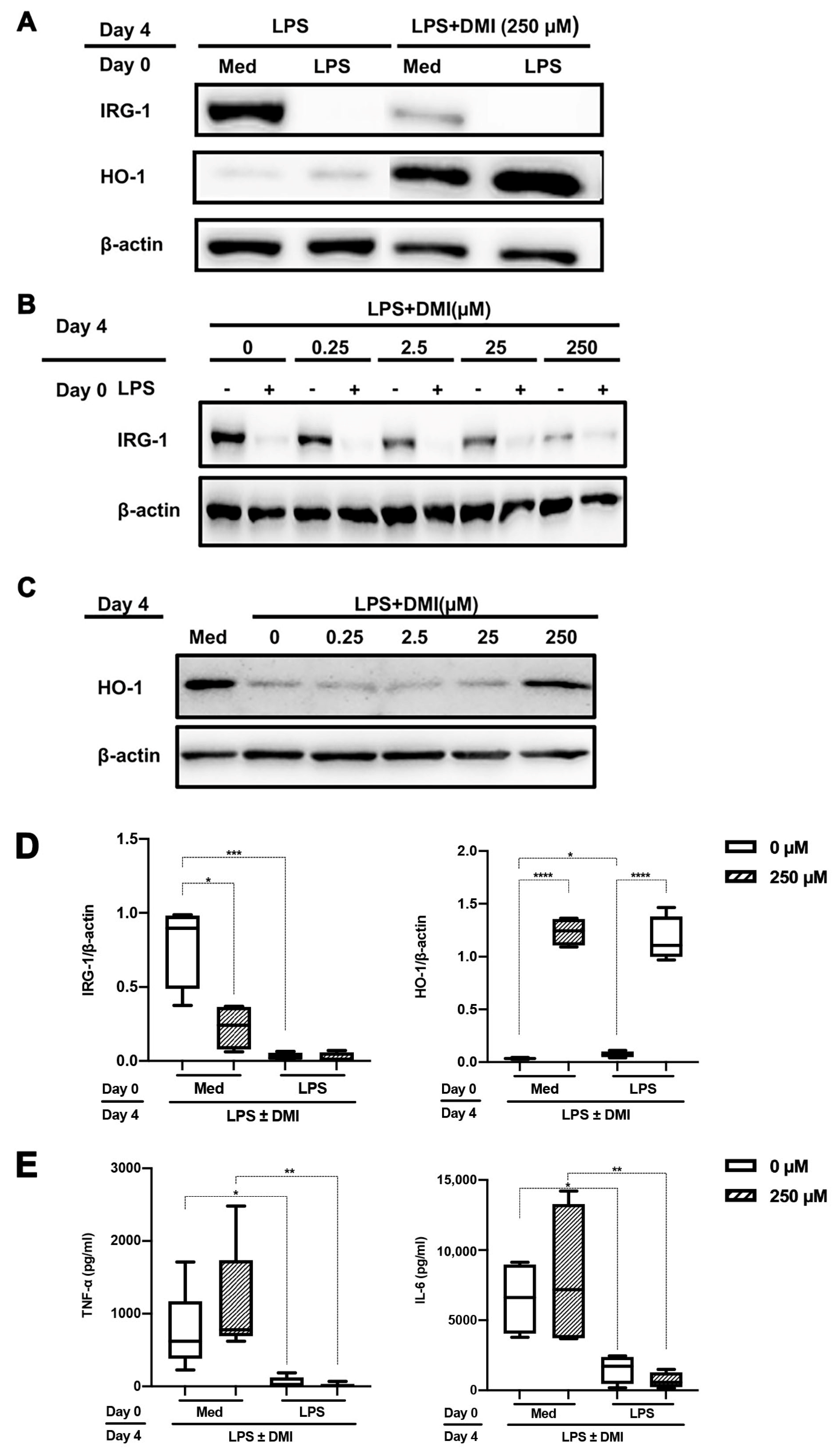
2.2. DMI Dose Dependently Abrogates LPS-mediated IRG-1 Induction
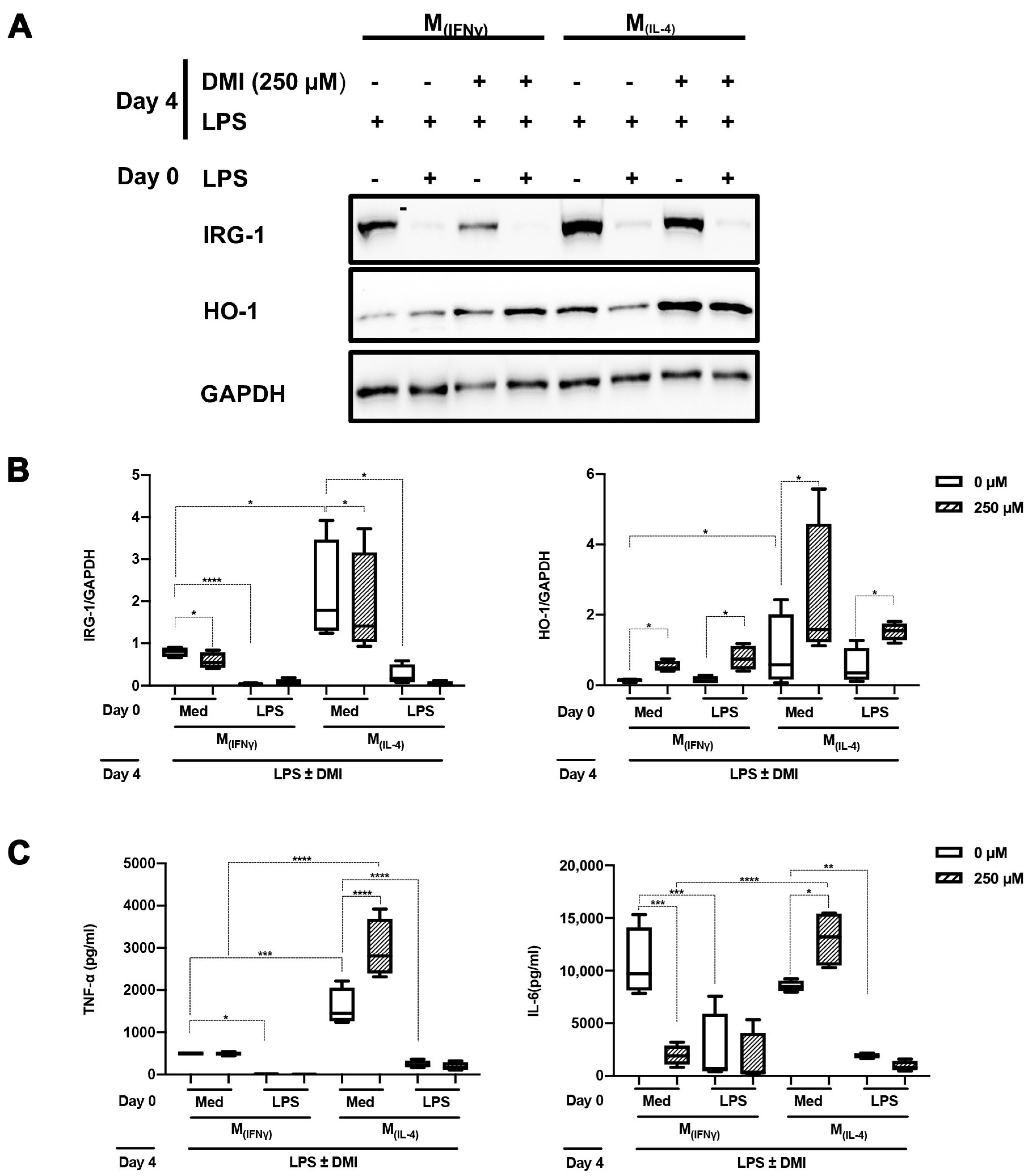
2.3. DMI Does Not Affect LPS Tolerance in M(IFNγ) and M(IL-4) Polarized Macrophages
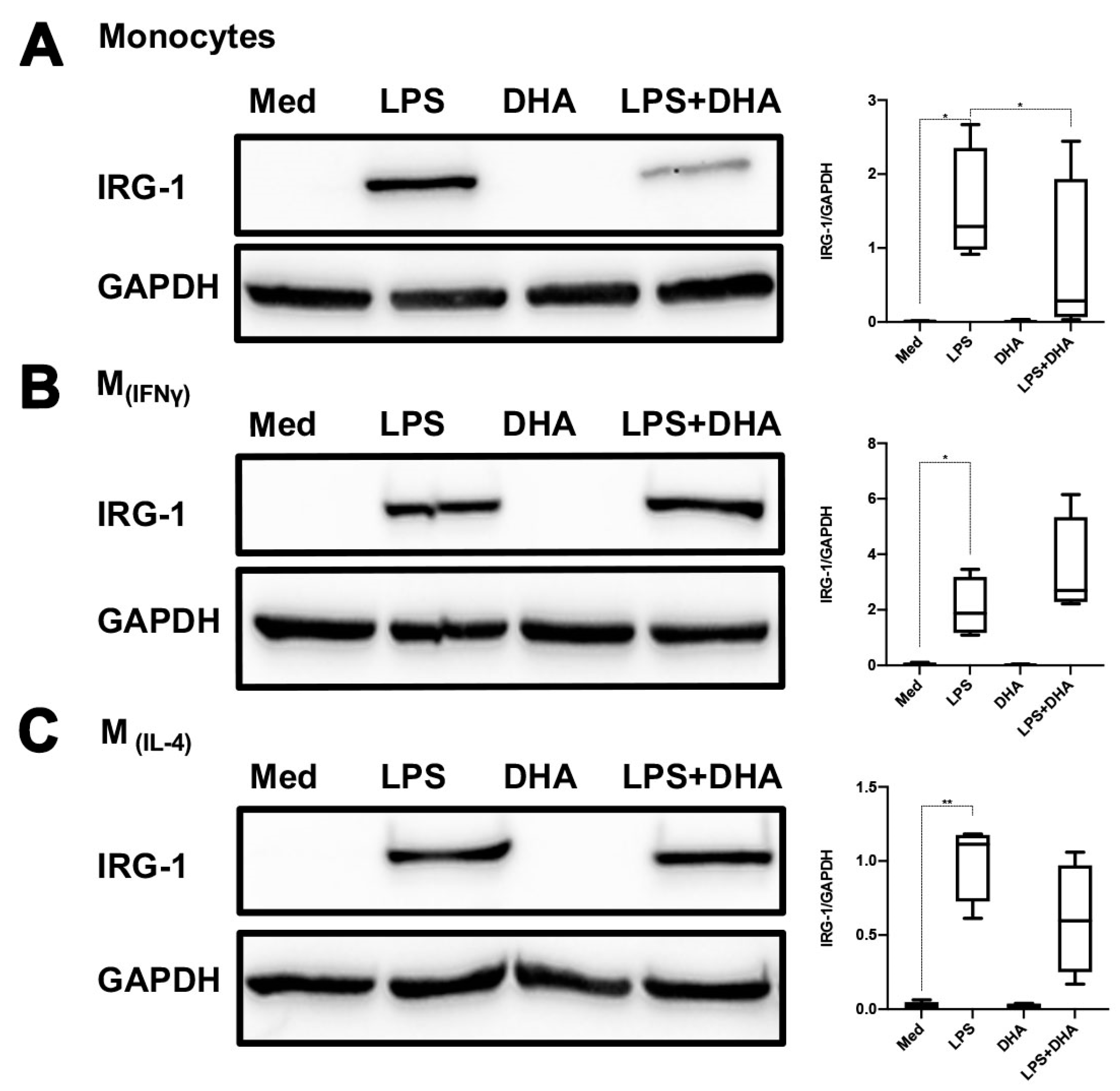
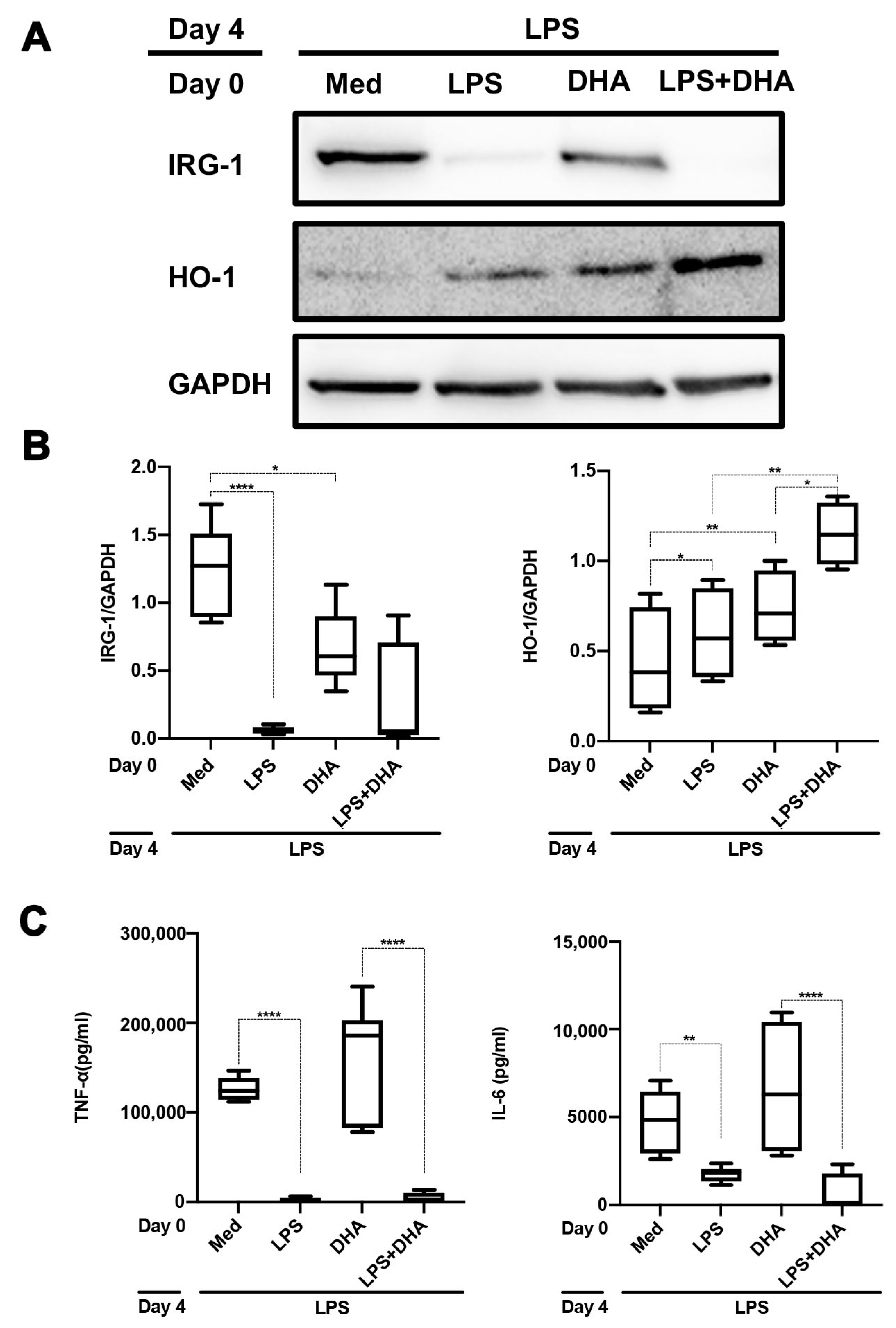
2.4. DHA Does Not Affect LPS Tolerance
3. Discussion
Limitations of This Study
4. Materials and Methods
4.1. Monocyte Isolation and Culture
4.2. Macrophage Polarization
4.3. Innate Immune Memory Assay
4.4. Western Blot Analysis
4.5. Cytokine Measurements
4.6. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATF3 | Activating transcriptional factor 3 |
| BCG | Bacille Calmette-Guérin |
| DHA | Docosahexaenoic acid |
| DMI | Dimethyl itaconate |
| HO-1 | Heme oxygenase 1 |
| IFNγ | Interferon-gamma |
| IL-6 | Interleukin 6 |
| IRG-1 | Immune-responsive gene 1 |
| IκBζ | Inhibitor of Kappa B zeta |
| LPS | Lipopolysaccharide |
| LTA | Lipoteichoic acid |
| M-CSF | Monocyte-colony stimulating factors |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PBMCs | Peripheral blood mononuclear cells |
| SDH | Succinate dehydrogenase |
| TCA | Tricarboxylic acid |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
References
- Ferreira, A.V.; Domiguéz-Andrés, J.; Netea, M.G. The Role of Cell Metabolism in Innate Immune Memory. J. Innate Immun. 2020, 14, 42–50. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’neill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Ozato, K. Innate Immune Memory in Hematopoietic Stem/Progenitor Cells: Myeloid-Biased Differentiation and the Role of Interferon. Front. Immunol. 2021, 12, 621333. [Google Scholar] [CrossRef]
- Zhang, B.; Moorlag, S.J.; Dominguez-Andres, J.; Bulut, Ö.; Kilic, G.; Liu, Z.; van Crevel, R.; Xu, C.-J.; Joosten, L.A.; Netea, M.G.; et al. Single-cell RNA sequencing reveals induction of distinct trained-immunity programs in human monocytes. J. Clin. Investig. 2022, 132, e147719. [Google Scholar] [CrossRef]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat. Immunol. 2020, 22, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, B.; Habibi, E.; Wang, S.-Y.; Arts, R.J.; Davar, R.; Megchelenbrink, W.; Kim, B.; Kuznetsova, T.; Kox, M.; Zwaag, J.; et al. β-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. Cell 2016, 167, 1354–1368.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stothers, C.L.; Burelbach, K.R.; Owen, A.M.; Patil, N.K.; McBride, M.A.; Bohannon, J.K.; Luan, L.; Hernandez, A.; Patil, T.K.; Williams, D.L.; et al. β-Glucan Induces Distinct and Protective Innate Immune Memory in Differentiated Macrophages. J. Immunol. 2021, 207, 2785–2798. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.G.; O’Neill, L.A.J. Krebs cycle rewired for macrophage and dendritic cell effector functions. FEBS Lett. 2017, 591, 2992–3006. [Google Scholar] [CrossRef] [Green Version]
- Bambouskova, M.; Gorvel, L.; Lampropoulou, V.; Sergushichev, A.; Loginicheva, E.; Johnson, K.; Korenfeld, D.; Mathyer, M.E.; Kim, H.; Huang, L.-H.; et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ–ATF3 inflammatory axis. Nature 2018, 556, 501–504. [Google Scholar] [CrossRef]
- Cordes, T.; Wallace, M.; Michelucci, A.; Divakaruni, A.S.; Sapcariu, S.C.; Sousa, C.; Koseki, H.; Cabrales, P.; Murphy, A.N.; Hiller, K.; et al. Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. J. Biol. Chem. 2016, 291, 14274–14284. [Google Scholar] [CrossRef] [Green Version]
- Michelucci, A.; Cordes, T.; Ghelfi, J.; Pailot, A.; Reiling, N.; Goldmann, O.; Binz, T.; Wegner, A.; Tallam, A.; Rausell, A.; et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. USA 2013, 110, 7820–7825. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiser, J.; Krämer, L.; Sapcariu, S.C.; Battello, N.; Ghelfi, J.; D’Herouel, A.F.; Skupin, A.; Hiller, K. Pro-inflammatory Macrophages Sustain Pyruvate Oxidation through Pyruvate Dehydrogenase for the Synthesis of Itaconate and to Enable Cytokine Expression. J. Biol. Chem. 2016, 291, 3932–3946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tretter, L.; Patocs, A.; Chinopoulos, C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta Bioenergy 2016, 1857, 1086–1101. [Google Scholar] [CrossRef] [PubMed]
- O’neill, L.A.J.; Artyomov, M.N. Itaconate: The poster child of metabolic reprogramming in macrophage function. Nat. Rev. Immunol. 2019, 19, 273–281. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and Protectins in Inflammation Resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Rizzolo, D.; Serra, A.; Colungo, C.; Sala-Vila, A.; Sisó-Almirall, A.; Gomis, R. Type 2 diabetes preventive effects with a 12-months sardine-enriched diet in elderly population with prediabetes: An interventional, randomized and controlled trial. Clin. Nutr. 2021, 40, 2587–2598. [Google Scholar] [CrossRef]
- Hernandez, P.; Passi, N.; Modarressi, T.; Kulkarni, V.; Soni, M.; Burke, F.; Bajaj, A.; Soffer, D. Clinical Management of Hypertriglyceridemia in the Prevention of Cardiovascular Disease and Pancreatitis. Curr. Atheroscler. Rep. 2021, 23, 72. [Google Scholar] [CrossRef]
- So, J.; Wu, D.; Lichtenstein, A.H.; Tai, A.K.; Matthan, N.R.; Maddipati, K.R.; Lamon-Fava, S. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: A randomized, double-blind, crossover study. Atherosclerosis 2020, 316, 90–98. [Google Scholar] [CrossRef]
- Vors, C.; Allaire, J.; Marin, J.; Lépine, M.-C.; Charest, A.; Tchernof, A.; Couture, P.; Lamarche, B. Inflammatory gene expression in whole blood cells after EPA vs. DHA supplementation: Results from the ComparED study. Atherosclerosis 2017, 257, 116–122. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Nomura, F.; Kawai, T.; Takeuchi, O.; Mühlradt, P.F.; Takeda, K.; Akira, S. Synergy and Cross-Tolerance Between Toll-Like Receptor (TLR) 2- and TLR4-Mediated Signaling Pathways. J. Immunol. 2000, 165, 7096–7101. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Michaeli, J.; Pallavi, P.; Breedijk, A.; Xu, X.; Dietrich, N.; Sigl, M.; Keese, M.; Nitschke, K.; Jarczyk, J.; et al. Concurrent stimulation of monocytes with CSF1 and polarizing cytokines reveals phenotypic and functional differences with classical polarized macrophages. J. Leukoc. Biol. 2022, 112, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Rod-In, W.; Monmai, C.; Jang, A.-Y.; Choi, J.; Park, W.-J. In Vitro Immune-Enhancement and Anti-Inflammatory Effects of Fatty Acids Extracted from the Halocynthia aurantium Gonad on RAW264.7 Macrophages. Nutrients 2022, 14, 4510. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.; Loscher, C.E.; Roche, H.M. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J. Nutr. Biochem. 2010, 21, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Kopydlowski, K.M.; Vogel, S.N. Inhibition of Lipopolysaccharide-Induced Signal Transduction in Endotoxin-Tolerized Mouse Macrophages: Dysregulation of Cytokine, Chemokine, and Toll-Like Receptor 2 and 4 Gene Expression. J. Immunol. 2000, 164, 5564–5574. [Google Scholar] [CrossRef]
- Lehner, M.D.; Morath, S.; Michelsen, K.S.; Schumann, R.R.; Hartung, T. Induction of Cross-Tolerance by Lipopolysaccharide and Highly Purified Lipoteichoic Acid Via Different Toll-Like Receptors Independent of Paracrine Mediators. J. Immunol. 2001, 166, 5161–5167. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Wang, G.; Liu, X.; Zhou, L.; Jiang, M.; Yang, L. A20 Is Critical for the Induction of Pam3CSK4-Tolerance in Monocytic THP-1 Cells. PLoS ONE 2014, 9, e87528. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ouyang, Y.; Guner, Y.; Ford, H.R.; Grishin, A.V. Ubiquitin-Editing Enzyme A20 Promotes Tolerance to Lipopolysaccharide in Enterocytes. J. Immunol. 2009, 183, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Qiu, F.; Piao, W.; Song, C.; Wahl, L.M.; Medvedev, A.E. Endotoxin Tolerance Impairs IL-1 Receptor-Associated Kinase (IRAK) 4 and TGF-β-activated Kinase 1 Activation, K63-linked Polyubiquitination and Assembly of IRAK1, TNF Receptor-associated Factor 6, and IκB Kinase γ and Increases A20 Expression. J. Biol. Chem. 2011, 286, 7905–7916. [Google Scholar] [CrossRef] [Green Version]
- Nahid, M.A.; Yao, B.; Dominguez-Gutierrez, P.R.; Kesavalu, L.; Satoh, M.; Chan, E.K.L. Regulation of TLR2-Mediated Tolerance and Cross-Tolerance through IRAK4 Modulation by miR-132 and miR-212. J. Immunol. 2013, 190, 1250–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF Receptor Superfamilies: Integrating Mammalian Biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takada, H.; Chen, N.-J.; Mirtsos, C.; Suzuki, S.; Suzuki, N.; Wakeham, A.; Mak, T.W.; Yeh, W.-C. Role of SODD in Regulation of Tumor Necrosis Factor Responses. Mol. Cell. Biol. 2003, 23, 4026–4033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devin, A.; Cook, A.; Lin, Y.; Rodriguez, Y.; Kelliher, M.; Liu, Z.-G. The Distinct Roles of TRAF2 and RIP in IKK Activation by TNF-R1: TRAF2 Recruits IKK to TNF-R1 while RIP Mediates IKK Activation. Immunity 2000, 12, 419–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.-C.; Griss, T.; et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef] [Green Version]
- Hooftman, A.; Angiari, S.; Hester, S.; Corcoran, S.E.; Runtsch, M.C.; Ling, C.; Ruzek, M.C.; Slivka, P.F.; McGettrick, A.F.; Banahan, K.; et al. The Immunomodulatory Metabolite Itaconate Modifies NLRP3 and Inhibits Inflammasome Activation. Cell Metab. 2020, 32, 468–478.e7. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M.S. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef]
- ElAzzouny, M.; Tom, C.T.M.B.; Evans, C.R.; Olson, L.L.; Tanga, M.J.; Gallagher, K.A.; Martin, B.R.; Burant, C.F. Dimethyl Itaconate Is Not Metabolized into Itaconate Intracellularly. J. Biol. Chem. 2017, 292, 4766–4769. [Google Scholar] [CrossRef] [Green Version]
- Nakkala, J.R.; Yao, Y.; Zhai, Z.; Duan, Y.; Zhang, D.; Mao, Z.; Lu, L.; Gao, C. Dimethyl Itaconate-Loaded Nanofibers Rewrite Macrophage Polarization, Reduce Inflammation, and Enhance Repair of Myocardic Infarction. Small 2021, 17, 2006992. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from Omega-3 Fatty Acids via Cyclooxygenase 2–Nonsteroidal Antiinflammatory Drugs and Transcellular Processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Controlling the Resolution of Acute Inflammation: A New Genus of Dual Anti-Inflammatory and Proresolving Mediators. J. Periodontol. 2008, 79, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Gonzalez, F.; Rueda, F.; Petriz, J.; Domingo, P.; Villarroya, F.; Diaz-Delfin, J.; A de Madariaga, M.; Domingo, J.C. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPARγ:RXR heterodimers: Comparison with other polyunsaturated fatty acids. J. Leukoc. Biol. 2008, 84, 1172–1182. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.W.; Kwon, M.-J.; Choi, A.M.; Kim, H.-P.; Nakahira, K.; Hwang, D.H. Fatty Acids Modulate Toll-like Receptor 4 Activation through Regulation of Receptor Dimerization and Recruitment into Lipid Rafts in a Reactive Oxygen Species-dependent Manner. J. Biol. Chem. 2009, 284, 27384–27392. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Stulnig, G.; Frisch, M.-T.; Crnkovic, S.; Stiegler, P.; Sereinigg, M.; Stacher, E.; Olschewski, H.; Olschewski, A.; Frank, S. Docosahexaenoic acid (DHA)-induced heme oxygenase-1 attenuates cytotoxic effects of DHA in vascular smooth muscle cells. Atherosclerosis 2013, 230, 406–413. [Google Scholar] [CrossRef]
- Kusunoki, C.; Yang, L.; Yoshizaki, T.; Nakagawa, F.; Ishikado, A.; Kondo, M.; Morino, K.; Sekine, O.; Ugi, S.; Nishio, Y.; et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2013, 430, 225–230. [Google Scholar] [CrossRef]
- Wang, S.; Hannafon, B.N.; Wolf, R.F.; Zhou, J.; Avery, J.E.; Wu, J.; Lind, S.E.; Ding, W.-Q. Characterization of docosahexaenoic acid (DHA)-induced heme oxygenase-1 (HO-1) expression in human cancer cells: The importance of enhanced BTB and CNC homology 1 (Bach1) degradation. J. Nutr. Biochem. 2014, 25, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Dorresteijn, M.J.; Paine, A.; Zilian, E.; Fenten, M.; Frenzel, E.; Janciauskiene, S.; Figueiredo, C.; Eiz-Vesper, B.; Blasczyk, R.; Dekker, D.; et al. Cell-type-specific downregulation of heme oxygenase-1 by lipopolysaccharide via Bach1 in primary human mononuclear cells. Free. Radic. Biol. Med. 2015, 78, 224–232. [Google Scholar] [CrossRef]
- Solanki, P.; Aminoshariae, A.; Jin, G.; A Montagnese, T.; Mickel, A. The effect of docosahexaenoic acid (DHA) on expression of IL-1ß, IL-6, IL-8, and TNF-α in normal and lipopolysaccharide (LPS)-stimulated macrophages. Quintessence Int. 2013, 44, 393. [Google Scholar] [CrossRef]
- Espiritu, M.M.; Lin, H.; Foley, E.; Tsang, V.; Rhee, E.; Perlman, J.; Cunningham-Rundles, S. Omega-3 fatty acids modulate neonatal cytokine response to endotoxin. J. Périnat. Med. 2016, 44, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Sekhon-Loodu, S.; Ziaullah; Rupasinghe, H.V. Docosahexaenoic acid ester of phloridzin inhibit lipopolysaccharide-induced inflammation in THP-1 differentiated macrophages. Int. Immunopharmacol. 2015, 25, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.A.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [Green Version]
- Arts, R.J.W.; Carvalho, A.; La Rocca, C.; Palma, C.; Rodrigues, F.; Silvestre, R.; Kleinnijenhuis, J.; Lachmandas, E.; Gonçalves, L.G.; Belinha, A.; et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 2016, 17, 2562–2571. [Google Scholar] [CrossRef] [Green Version]
- Kar, U.K.; Joosten, L.A.B. Training the trainable cells of the immune system and beyond. Nat. Immunol. 2020, 21, 115–119. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Breedijk, A.; Dietrich, N.; Nitschke, K.; Jarczyk, J.; Nuhn, P.; Krämer, B.K.; Yard, B.A.; Leipe, J.; Hauske, S. Lipopolysaccharide Tolerance in Human Primary Monocytes and Polarized Macrophages. Int. J. Mol. Sci. 2023, 24, 12196. https://doi.org/10.3390/ijms241512196
Li H, Breedijk A, Dietrich N, Nitschke K, Jarczyk J, Nuhn P, Krämer BK, Yard BA, Leipe J, Hauske S. Lipopolysaccharide Tolerance in Human Primary Monocytes and Polarized Macrophages. International Journal of Molecular Sciences. 2023; 24(15):12196. https://doi.org/10.3390/ijms241512196
Chicago/Turabian StyleLi, Hui, Annette Breedijk, Nadine Dietrich, Katja Nitschke, Jonas Jarczyk, Philipp Nuhn, Bernhard K. Krämer, Benito A. Yard, Jan Leipe, and Sibylle Hauske. 2023. "Lipopolysaccharide Tolerance in Human Primary Monocytes and Polarized Macrophages" International Journal of Molecular Sciences 24, no. 15: 12196. https://doi.org/10.3390/ijms241512196
APA StyleLi, H., Breedijk, A., Dietrich, N., Nitschke, K., Jarczyk, J., Nuhn, P., Krämer, B. K., Yard, B. A., Leipe, J., & Hauske, S. (2023). Lipopolysaccharide Tolerance in Human Primary Monocytes and Polarized Macrophages. International Journal of Molecular Sciences, 24(15), 12196. https://doi.org/10.3390/ijms241512196






