Parkinson’s Disease and the Heart: Studying Cardiac Metabolism in the 6-Hydroxydopamine Model
Abstract
1. Introduction
2. Results
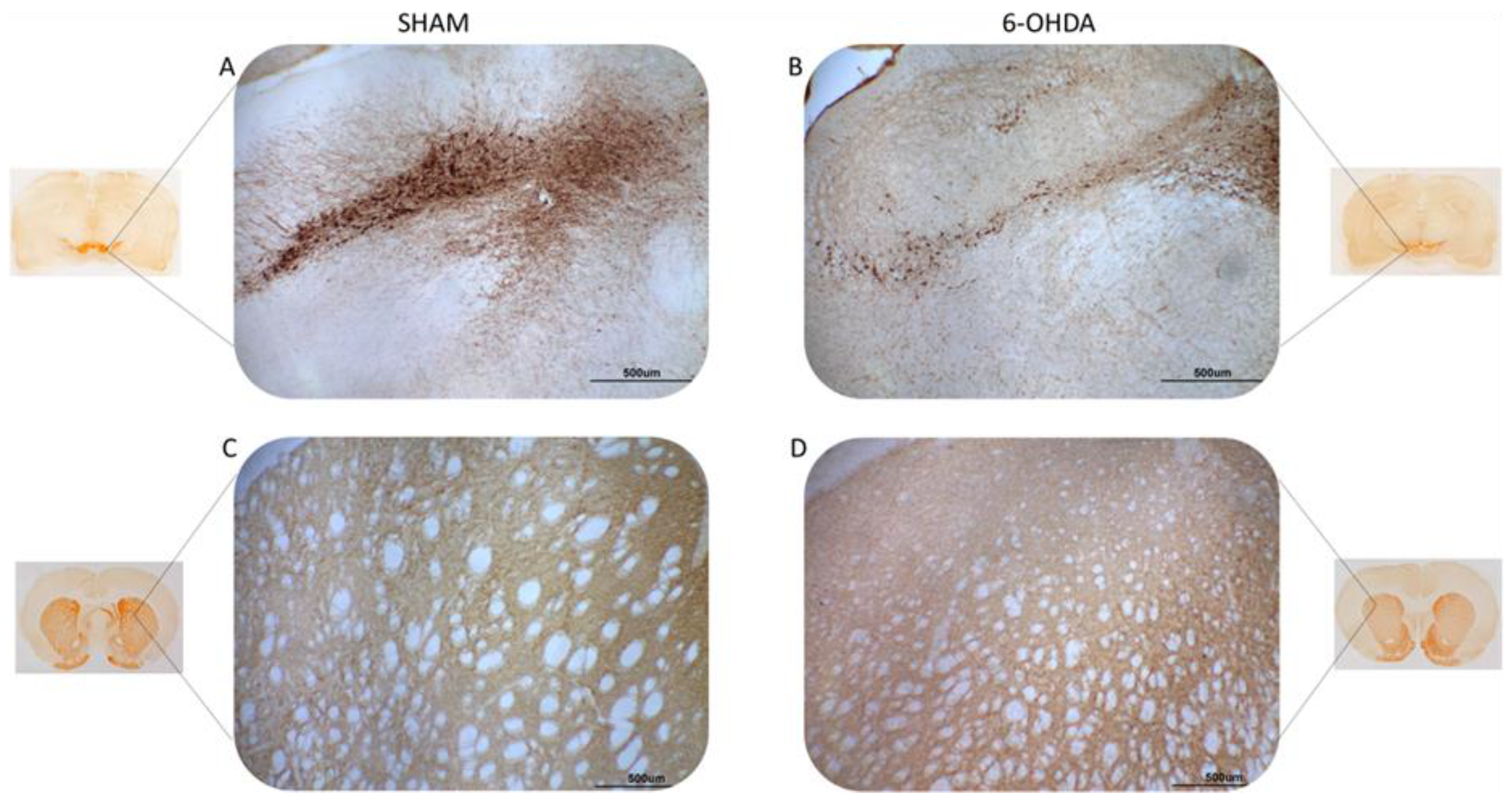
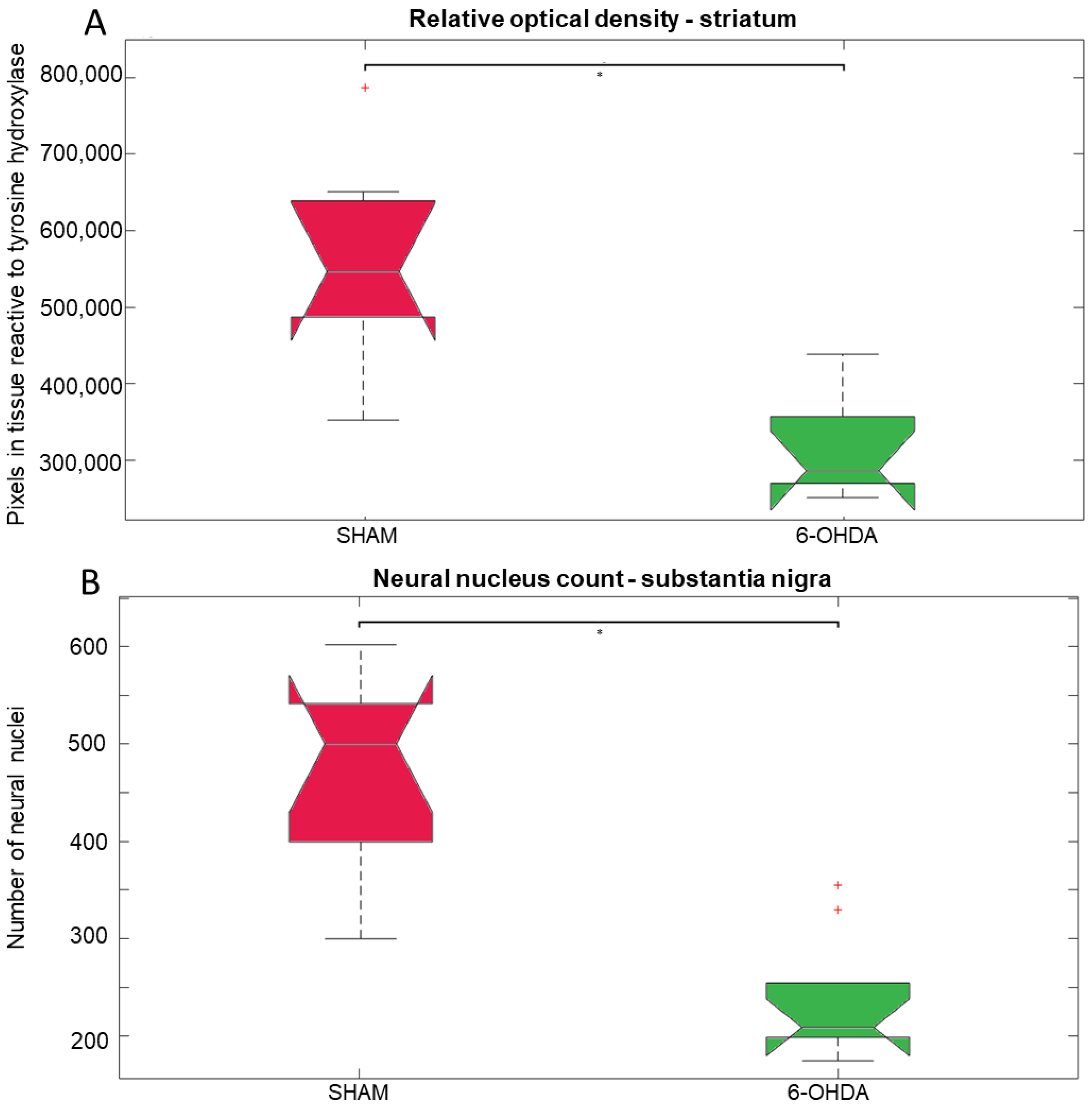
2.1. Tyrosine Hydroxylase Immunohistochemistry
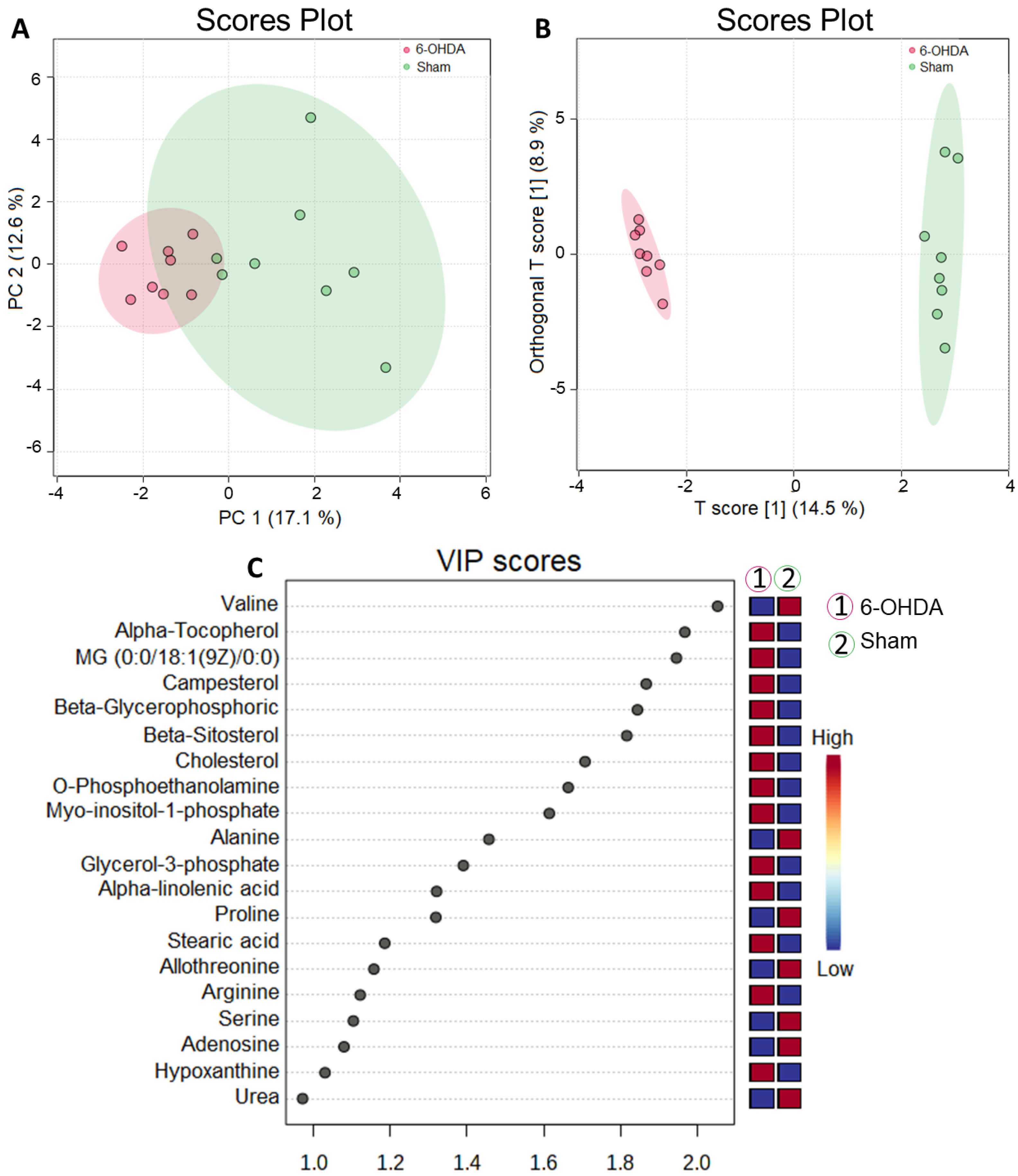
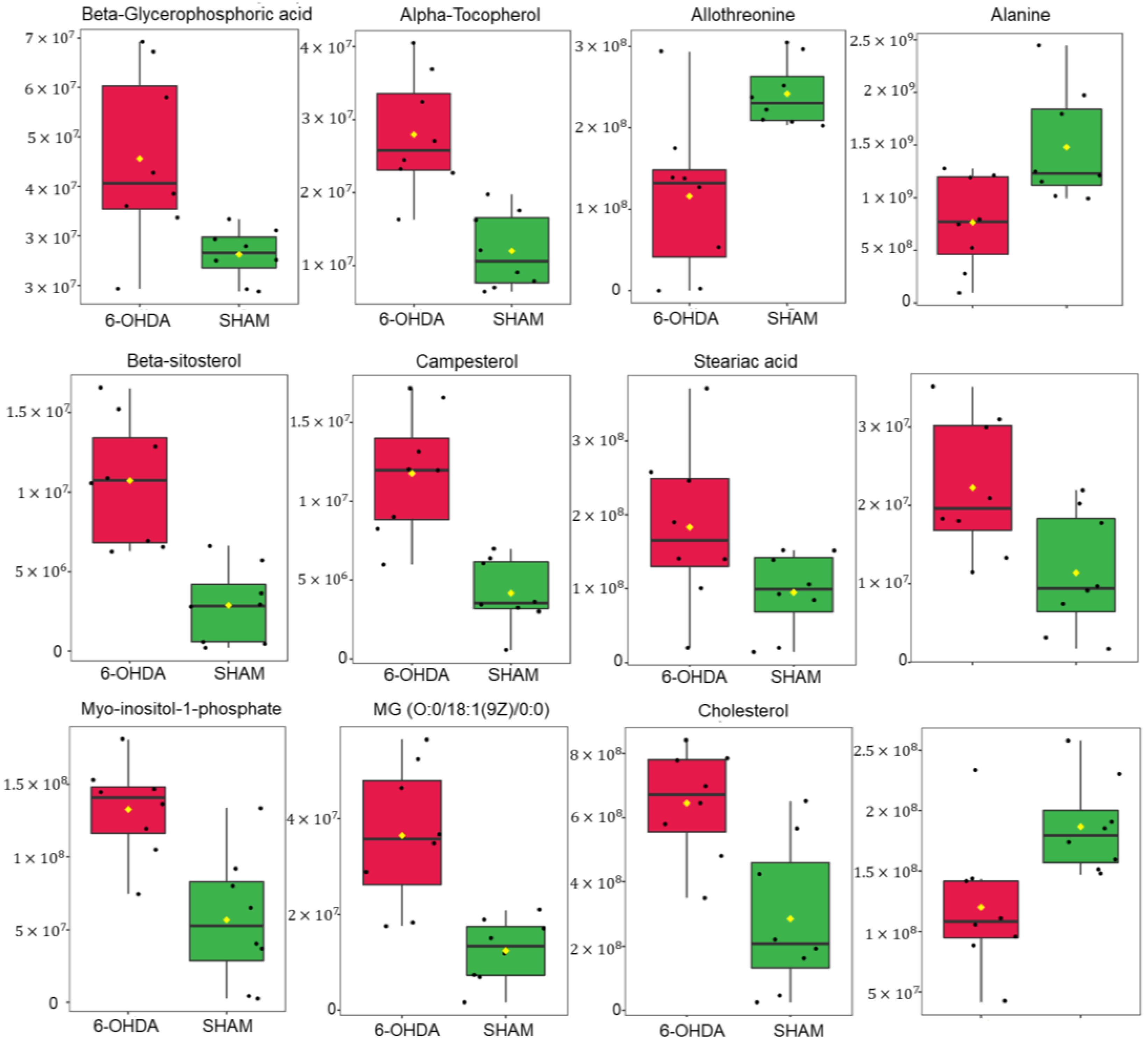
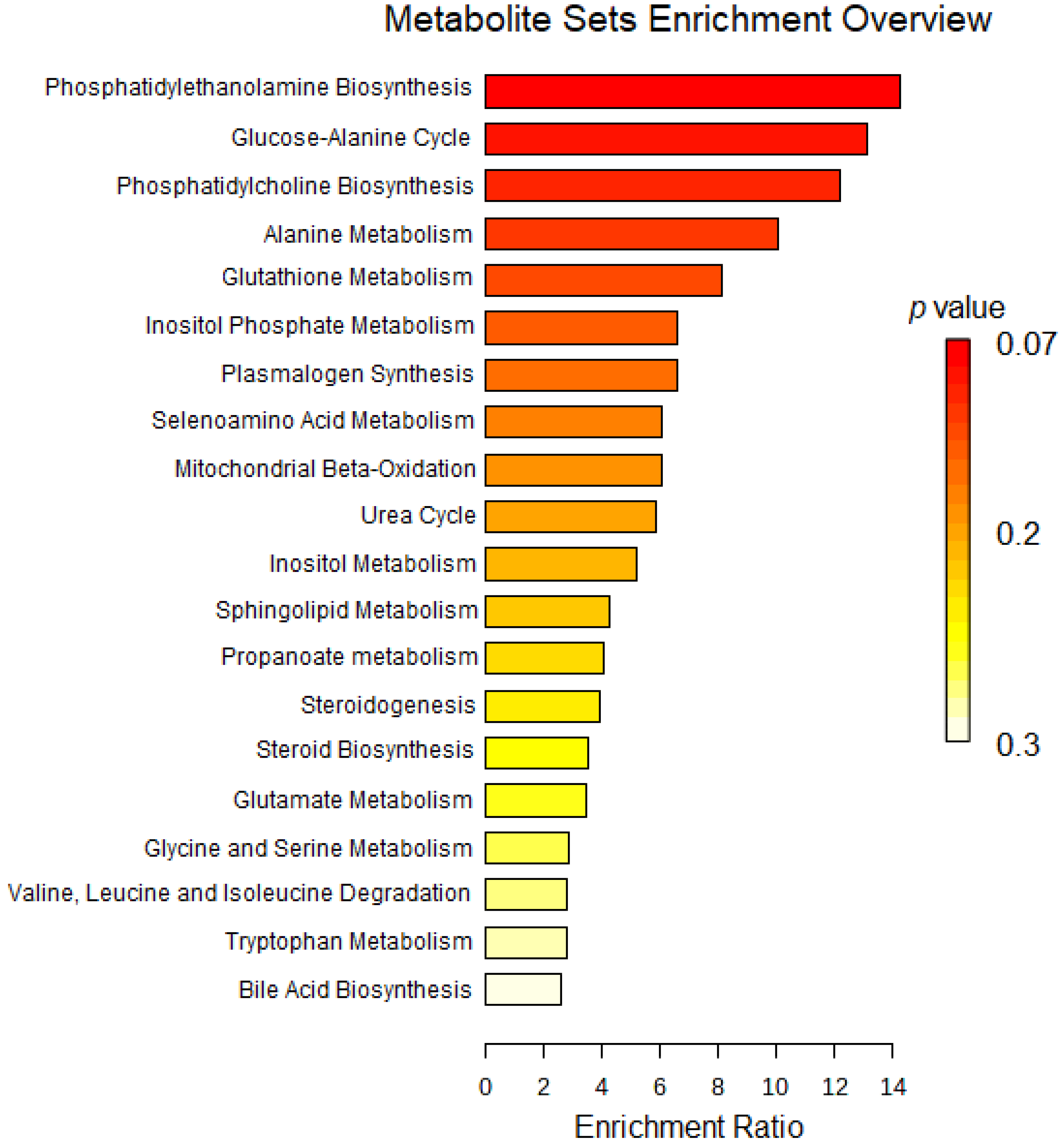
2.2. Untargeted Metabolomics
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Study Design
4.3. 6-OHDA Lesion
4.4. Immunohistochemistry
4.5. Metabolomics
4.6. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteu, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global trends in the incidence, prevalence, and years lived with disability of Parkinson’s disease in 204 countries/territories from 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar]
- Değirmenci, H.; Bakirci, E.M.; Hamur, H. Cardiac Effects of Parkinson’s Disease. Open J. Park. Dis. Treat. 2020, 3, 006–007. [Google Scholar]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [PubMed]
- Mischley, L.K.; Standish, L.J.; Weiss, N.S.; Padowski, J.M.; Kavanagh, T.J.; White, C.C.; Rosenfeld, M.E. Glutathione as a Biomarker in Parkinson’s Disease: Associations with Aging and Disease Severity. Oxidative Med. Cell. Longev. 2016, 2016, 9409363. [Google Scholar] [CrossRef]
- Bjørklund, G.; Peana, M.; Maes, M.; Dadar, M.; Severin, B. The glutathione system in Parkinson’s disease and its progression. Neurosci. Biobehav. 2021, 120, 470–478. [Google Scholar]
- Zhang, X.; Zhang, R.; Awan, M.U.N.; Bai, J. The Mechanism and Function of Glia in Parkinson’s Disease. Frontiers 2022, 16, 2022. [Google Scholar]
- Gopinath, A.; Mackie, P.M.; Phan, L.T.; Tansey, M.G.; Khoshbouei, H. The complex role of inflammation and gliotransmitters in Parkinson’s disease. Neurobiol. Dis. 2023, 176, 105940. [Google Scholar]
- Badanjak, K.; Fixemer, S.; Smajić, S.; Skupin, A.; Grünewald, A. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4676. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; I. Rojo, A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [PubMed]
- Copas, A.N.M.; McComish, S.F.; Fletcher, J.M.; Caldwell, M.A. The Pathogenesis of Parkinson’s Disease: A Complex Interplay Between Astrocytes, Microglia, and T Lymphocytes? Front. Neurol. 2021, 12, 666737. [Google Scholar] [CrossRef]
- Ilaiwy, A.; Liu, M.; Parry, T.L.; Bain, J.R.; Newgard, C.B.; Schisler, J.C.; Muehlbauer, M.J.; Despa, F.; Willis, M.S. Human amylin proteotoxicity impairs protein biosynthesis, and alters major cellular signaling pathways in the heart, brain and liver of humanized diabetic rat model in vivo. Metabolomics Off. J. Metabolomic Soc. 2016, 12, 95. [Google Scholar]
- Tini, G.; Scagliola, R.; Monacelli, F.; La Malfa, G.; Porto, I.; Brunelli, C.; Rosa, G.M. Alzheimer’s Disease and Cardiovascular Disease: A Particular Association. Cardiol. Res. Pract. 2020, 2020, 2617970. [Google Scholar] [CrossRef]
- Buergel, T.; Steinfeldt, J.; Ruyoga, G.; Pietzner, M.; Bizzarri, D.; Vojinovic, D.; Upmeier Zu Belzen, J.; Loock, L.; Kittner, P.; Christmann, L.; et al. Metabolomic profiles predict individual multidisease outcomes. Nat. Med. 2022, 28, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Park, T.S.; Yamashita, H.; Blaner, W.S.; Goldberg, I.J. Lipids in the heart: A source of fuel and a source of toxins. Curr. Opin. Lipidol. 2007, 18, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Le, W. Recent advances and perspectives of metabolomics-based investigations in Parkinson’s disease. Mol. Neurodegener. 2019, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar]
- Yoon, H.; Shaw, J.L.; Haigis, M.C.; Greka, A. Lipid metabolism in sickness and in health: Emerging regulators of lipotoxicity. Mol. Cell 2021, 81, 3708–3730. [Google Scholar]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Poursharifi, P.; Madiraju, S.R.M.; Prentki, M. Monoacylglycerol signalling and ABHD6 in health and disease. Diabetes Obes. Metab. 2017, 19, 76–89. [Google Scholar] [CrossRef]
- Deng, H.; Li, W. Monoacylglycerol lipase inhibitors: Modulators for lipid metabolism in cancer malignancy, neurological and metabolic disorders. Acta Pharm. Sin. B 2020, 10, 582–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, X.; Zhang, L.; Fang, Y.; Zheng, Q.; Liu, X.; Yu, W.; Chen, S.; Ying, J.; Hua, F. Lipid metabolism and storage in neuroglia: Role in brain development and neurodegenerative diseases. Cell Biosci. 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Toczylowska, B.; Zieminska, E.; Michałowska, M.; Chalimoniuk, M.; Fiszer, U. Changes in the metabolic profiles of the serum and putamen in Parkinson’s disease patients–In vitro and in vivo NMR spectroscopy studies. Brain Res. 2020, 1748, 147118. [Google Scholar] [CrossRef]
- Poli, A.; Marangoni, F.; Corsini, A.; Manzato, E.; Marrocco, W.; Martini, D.; Medea, G.; Visioli, F. Phytosterols, Cholesterol Control, and Cardiovascular Disease. Nutrients 2021, 13, 2810. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Liu, G.; Yamawaki, T.M.; Tao, C.; Alexander, S.T.; Ly, K.; Fordstrom, P.; Shkumatov, A.A.; Li, C.M.; Rajamani, S.; et al. Phytosterol accumulation results in ventricular arrhythmia, impaired cardiac function and death in mice. Sci. Rep. 2021, 11, 17449. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Horn, K.; Pott, J.; Gross, A.; Kleber, M.E.; Delgado, G.E.; Mishra, P.P.; Kirsten, H.; Gieger, C.; Müller-Nurasyid, M.; et al. Genome-wide meta-analysis of phytosterols reveals five novel loci and a detrimental effect on coronary atherosclerosis. Nat. Commun. 2022, 13, 143. [Google Scholar] [CrossRef]
- Bodine, S.C.; Brooks, H.L.; Bunnett, N.W.; Coller, H.A.; Frey, M.R.; Joe, B.; Kleyman, T.R.; Lindsey, M.L.; Marette, A.; Morty, R.E.; et al. An American Physiological Society cross-journal Call for Papers on “Inter-Organ Communication in Homeostasis and Disease”. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L42–L49. [Google Scholar] [CrossRef]
- Gödecke, A.; Haendeler, J. Intra-and Interorgan Communication in the Cardiovascular System: A Special View on Redox Regulation. Antioxid. Redox Signal. 2017, 26, 613–615. [Google Scholar] [CrossRef]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The bioavailability and biological activities of phytosterols as modulators of cholesterol metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The role of lipids in Parkinson’s disease. Cells 2019, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Zampelas, A.; Magriplis, E. New insights into cholesterol functions: A friend or an enemy? Nutrients 2019, 11, 1645. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Gomes, F.A.; Flores, R.A.; Bruxel, M.A.; da Silva, F.N.; Moreira, E.L.G.; Zoccal, D.B.; Prediger, R.D.; Rafacho, A. Glucose Homeostasis Is Not Affected in a Murine Model of Parkinson’s Disease Induced by 6-OHDA. Front. Neurosci. 2019, 12, 1020. [Google Scholar] [CrossRef]
- Qiu, J.; Peng, G.; Tang, Y.; Li, S.; Liu, Z.; Zheng, J.; Wang, Y.; Liu, H.; Wei, L.; Su, Y.; et al. Lipid profiles in the cerebrospinal fluid of rats with 6-hydroxydopamine-induced lesions as a model of Parkinson’s disease. Front. Aging Neurosci. 2023, 14, 1077738. [Google Scholar] [CrossRef]
- Shah, A.; Han, P.; Wong, M.Y.; Chang, R.C.; Legido-Quigley, C. Palmitate and Stearate are Increased in the Plasma in a 6-OHDA Model of Parkinson’s Disease. Metabolites 2019, 9, 31. [Google Scholar] [CrossRef]
- Kataoka, H.; Maeda, M.; Makita, M. O-phosphoethanolamine content in mouse tissues during development. Agric. Biol. Chem. 1991, 55, 289–290. [Google Scholar]
- Kennergren, C.; Mantovani, V.; Lönnroth, P.; Nyström, B.; Berglin, E.; Hamberger, A. Extracellular amino acids as markers of myocardial ischemia during cardioplegic heart arrest. Cardiology 1999, 91, 31–40. [Google Scholar] [CrossRef]
- Gull, M.; Pasek, M.A. The role of glycerol and its derivatives in the biochemistry of living organisms, and their prebiotic origin and significance in the evolution of life. Catalysts 2021, 11, 86. [Google Scholar] [CrossRef]
- Bargui, R.; Solgadi, A.; Prost, B.; Chester, M.; Ferreiro, A.; Piquereau, J.; Moulin, M. Phospholipids: Identification and Implication in Muscle Pathophysiology. Int. J. Mol. Sci. 2021, 22, 8176. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.K.; Novak, J.E.; Agranoff, B.W. Inositol and higher inositol phosphates in neural tissues: Homeostasis, metabolism and functional significance. J. Neurochem. 2002, 82, 736–754. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, D.R. Myo-Inositol and Its Derivatives: Their Emerging Role in the Treatment of Human Diseases. Front. Pharmacol. 2019, 10, 1172. [Google Scholar] [CrossRef]
- Gonzalez-Uarquin, F.; Rodehutscord, M.; Huber, K. Myo-inositol: Its metabolism and potential implications for poultry nutrition-a review. Poult. Sci. 2020, 99, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; van de Graaf, S.F. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Zhu, Y.; Chen, Q.; Wang, S.; Chen, L.; Liu, T.; Tang, H.; Yao, H. Comprehensive metabolomic and lipidomic alterations in response to heat stress during seed germination and seedling growth of Arabidopsis. Front. Plant Sci. 2023, 14, 1132881. [Google Scholar] [CrossRef]
- Harper, A.; Miller, R.; Block, K. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef]
- Zhu, G.; Yin, C.; Tian, Z.; Wang, T.; Sun, W.; Xiang, Q.; Guo, G. Metabolomic Analysis of Plasma From Patients With Acute Mountain Sickness Using Chromatography-Mass Spectrometry. Medicine 2015, 94, e1988. [Google Scholar] [CrossRef] [PubMed]
- Rong, W.; Li, J.; Pan, D.; Zhou, Q.; Zhang, Y.; Lu, Q.; Wang, L.; Wang, A.; Zhu, Y.; Zhu, Q. Cardioprotective Mechanism of Leonurine against Myocardial Ischemia through a Liver–Cardiac Crosstalk Metabolomics Study. Biomolecules 2022, 12, 1512. [Google Scholar] [CrossRef] [PubMed]
- Glatz, J.F.; Nabben, M.; Young, M.E.; Schulze, P.C.; Taegtmeyer, H.; Luiken, J.J. Re-balancing cellular energy substrate metabolism to mend the failing heart. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2020, 1866, 165579. [Google Scholar] [CrossRef]
- Ragni, M.; Greco, C.M.; Felicetta, A.; Ren, S.V.; Kunderfranco, P.; Ruocco, C.; Carullo, P.; Larcher, V.; Tedesco, L.; Severi, I. Dietary essential amino acids for the treatment of heart failure with reduced ejection fraction. Cardiovasc. Res. 2023, 119, 982–997. [Google Scholar] [CrossRef]
- Ranea-Robles, P.; Pavlova, N.N.; Bender, A.; Pereyra, A.S.; Ellis, J.M.; Stauffer, B.; Yu, C.; Thompson, C.B.; Argmann, C.; Puchowicz, M.; et al. A mitochondrial long-chain fatty acid oxidation defect leads to transfer RNA uncharging and activation of the integrated stress response in the mouse heart. Cardiovasc. Res. 2022, 118, 3198–3210. [Google Scholar] [CrossRef]
- She, J.; Guo, M.; Li, H.; Liu, J.; Liang, X.; Liu, P.; Zhou, B.; Liu, S.; Deng, Y.; Lou, B.; et al. Targeting amino acids metabolic profile to identify novel metabolic characteristics in atrial fibrillation. Clin. Sci. Lond. Engl. 2018, 132, 2135–2146. [Google Scholar] [CrossRef]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int. Rev. Cell Mol. Biol. 2016, 321, 29–88. [Google Scholar] [PubMed]
- Van der Veen, J.N.; Lingrell, S.; da Silva, R.P.; Jacobs, R.L.; Vance, D.E. The concentration of phosphatidylethanolamine in mitochondria can modulate ATP production and glucose metabolism in mice. Diabetes 2014, 63, 2620–2630. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Witt, S.N. Ethanolamine and phosphatidylethanolamine: Partners in health and disease. Oxidative Med. Cell. Longev. 2017, 2017, 4829180. [Google Scholar] [CrossRef] [PubMed]
- Tavasoli, M.; Lahire, S.; Reid, T.; Brodovsky, M.; McMaster, C.R. Genetic diseases of the Kennedy pathways for membrane synthesis. J. Biol. Chem. 2020, 295, 17877–17886. [Google Scholar] [CrossRef]
- Van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859 9 Pt B, 1558–1572. [Google Scholar] [CrossRef]
- Bozelli, J.C., Jr.; Azher, S.; Epand, R.M. Plasmalogens and Chronic Inflammatory Diseases. Front. Physiol. 2021, 12, 730829. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, N.; Zhao, J.; Xie, Z.; Yang, Z.; Tian, B. Advances in the Biosynthetic Pathways and Application Potential of Plasmalogens in Medicine. Front. Cell Dev. Biol. 2020, 8, 765. [Google Scholar] [CrossRef]
- Sarabhai, T.; Roden, M. Hungry for your alanine: When liver depends on muscle proteolysis. J. Clin. Investig. 2019, 129, 4563–4566. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kumaran, S.S.; Goyal, V.; Bose, S.; Jain, S.; Dwivedi, S.N.; Srivastava, A.K.; Jagannathan, N.R. Metabolomic analysis of serum using proton NMR in 6-OHDA experimental PD model and patients with PD. Neurochem. Int. 2020, 134, 104670. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Yadav, S. The glutathione cycle: Glutathione metabolism beyond the γ-glutamyl cycle. Iubmb Life 2018, 70, 585–592. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Charisis, S.; Ntanasi, E.; Stamelou, M.; Xiromerisiou, G.; Maraki, M.; Veskoukis, A.S.; Yannakoulia, M.; Kosmidis, M.H.; Anastasiou, C.A.; Giagkou, N.; et al. Plasma Glutathione and Prodromal Parkinson’s Disease Probability. Mov. Disord. 2022, 37, 200–205. [Google Scholar] [CrossRef]
- Chatree, S.; Thongmaen, N.; Tantivejkul, K.; Sitticharoon, C.; Vucenik, I. Role of Inositols and Inositol Phosphates in Energy Metabolism. Molecules 2020, 25, 5079. [Google Scholar] [CrossRef]
- Tu-Sekine, B.; Kim, S.F. The Inositol Phosphate System—A Coordinator of Metabolic Adaptability. Int. J. Mol. Sci. 2022, 23, 6747. [Google Scholar] [CrossRef]
- Cestari, I. Phosphoinositide signaling and regulation in Trypanosoma brucei: Specialized functions in a protozoan pathogen. PLOS Pathog. 2020, 16, e1008167. [Google Scholar] [CrossRef]
- Wundenberg, T.; Mayr, G.W. Synthesis and biological actions of diphosphoinositol phosphates (inositol pyrophosphates), regulators of cell homeostasis. Biol. Chem. 2012, 393, 979–998. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007; p. 456. [Google Scholar]
- Shimohama, S.; Sawada, H.; Kitamura, Y.; Taniguchi, T. Disease model: Parkinson’s disease. Trends Mol. Med. 2003, 9, 360–365. [Google Scholar] [CrossRef]
- Perlbarg, V.; Lambert, J.; Butler, B.; Felfli, M.; Valabrègue, R.; Privat, A.L.; Lehéricy, S.; Petiet, A. Alterations of the nigrostriatal pathway in a 6-OHDA rat model of Parkinson’s disease evaluated with multimodal MRI. PLoS ONE 2018, 13, e0202597. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, A.; Ferrarini, A.; Rey-Stolle, F.; García, A.; Barbas, C. From sample treatment to biomarker discovery: A tutorial for untargeted metabolomics based on GC-(EI)-Q-MS. Anal. Chim. Acta 2015, 900, 21–35. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva da Fonsêca, V.; Goncalves, V.d.C.; Augusto Izidoro, M.; Guimarães de Almeida, A.-C.; Luiz Affonso Fonseca, F.; Alexandre Scorza, F.; Finsterer, J.; Scorza, C.A. Parkinson’s Disease and the Heart: Studying Cardiac Metabolism in the 6-Hydroxydopamine Model. Int. J. Mol. Sci. 2023, 24, 12202. https://doi.org/10.3390/ijms241512202
Silva da Fonsêca V, Goncalves VdC, Augusto Izidoro M, Guimarães de Almeida A-C, Luiz Affonso Fonseca F, Alexandre Scorza F, Finsterer J, Scorza CA. Parkinson’s Disease and the Heart: Studying Cardiac Metabolism in the 6-Hydroxydopamine Model. International Journal of Molecular Sciences. 2023; 24(15):12202. https://doi.org/10.3390/ijms241512202
Chicago/Turabian StyleSilva da Fonsêca, Victor, Valeria de Cassia Goncalves, Mario Augusto Izidoro, Antônio-Carlos Guimarães de Almeida, Fernando Luiz Affonso Fonseca, Fulvio Alexandre Scorza, Josef Finsterer, and Carla Alessandra Scorza. 2023. "Parkinson’s Disease and the Heart: Studying Cardiac Metabolism in the 6-Hydroxydopamine Model" International Journal of Molecular Sciences 24, no. 15: 12202. https://doi.org/10.3390/ijms241512202
APA StyleSilva da Fonsêca, V., Goncalves, V. d. C., Augusto Izidoro, M., Guimarães de Almeida, A.-C., Luiz Affonso Fonseca, F., Alexandre Scorza, F., Finsterer, J., & Scorza, C. A. (2023). Parkinson’s Disease and the Heart: Studying Cardiac Metabolism in the 6-Hydroxydopamine Model. International Journal of Molecular Sciences, 24(15), 12202. https://doi.org/10.3390/ijms241512202





