Abstract
During pregnancy, reactive oxygen species (ROS) serve as crucial signaling molecules for fetoplacental circulatory physiology. Oxidative stress is thought to sustain the pathogenesis and progression of hypoxic-ischemic encephalopathy (HIE). A retrospective study was performed on the brains and placentas of fetuses and newborns between 36–42 weeks of gestation (Group_1: Fetal intrauterine deaths, Group_2: Intrapartum deaths, Group_3: Post-partum deaths, Control group sudden neonatal death); all groups were further divided into two subgroups (Subgroup_B [brain] and Subgroup_P [placenta]), and the study was conducted through the immunohistochemical investigations of markers of oxidative stress (NOX2, 8-OHdG, NT, iNOS), IL-6, and only on the brain samples, AQP4. The results for the brain samples suggest that NOX2, 8-OHdG, NT, iNOS, and IL-6 were statistically significantly expressed above the controls. iNOS was more expressed in the fetal intrauterine death (Group_1) and less expressed in post-partum death (Group_3), while in intrapartum death (Group_2), the immunoreactivity was very low. IL-6 showed the highest expression in the brain cortex of the fetal intrauterine death (Group_1), while intrapartum death (Group_2) and post-partum death (Group_3) showed weak immunoreactivity. Post-partum death (Group_3) placentas showed the highest immunoreactivity to NOX2, which was almost double that of the fetal intrauterine death (Group_1) and intrapartum death (Group_2) placentas. Placental tissues of fetal intrauterine death (Group_1) and intrapartum death (Group_2) showed higher expression of iNOS than post-partum death (Group_3), while the IL-6 expression was higher in the fetal intrauterine death (Group_1) than the post-partum death (Group_3). The AQP4 was discarded as a possible marker because the immunohistochemical reaction in the three groups of cases and the control group was negative. The goal of this study, from the point of view of forensic pathology, is to provide scientific evidence in cases of medical liability in the Obstetric field to support the clinical data of the timing of HIE.
1. Introduction
Reactive oxygen species (ROS) are physiologically produced from cell metabolism and play a dual role as both beneficial and toxic compounds [1,2]. At physiological concentrations, ROS play a role in the defense against pathogens, cell signaling, and mitogenic responses [1,3]. At higher concentrations (e.g., in case of either overload of ROS or depletion of natural antioxidants), ROS cause oxidative stress (OS), potentially leading to impaired cell function and decreased cell viability due to damage to proteins, DNA, and cellular lipids [4,5,6,7].
ROS serve as crucial signaling molecules during pregnancy for fetoplacental circulatory physiology [8,9]. In the first trimester, OS drives the expression of vascular endothelial growth factor (VEGF) through hypoxia-inducible factor-1, thus stimulating placental vascular development [9,10]. Furthermore, during the second and third trimesters, OS may stimulate the overexpression of apoptotic regulators at the placental level, potentially leading to massive apoptosis and placental insufficiency [11,12]. For these reasons, OS was supposed to play an active role in the development of different severe placenta-related obstetrical complications, including pre-eclampsia and intrauterine growth restriction [9,10,11,12]. Additionally, on the fetal side, OS may sustain the pathogenesis and progression of hypoxic-ischemic encephalopathy (HIE), as developing neurons are very susceptible to ROS due to high oxygen consumption, lipid-rich content, and relatively low antioxidant defense [13,14].
HIE represents one of the major causes of neonatal death and neurological disability, with an incidence of 1.5 cases per 1000 live births in developed countries and of 10–20 per 1000 live births in low-middle-income countries [15,16,17]. The causes of HIE are many, including umbilical cord knotting, umbilical cord prolapse, shoulder dystocia, placental abruption, and chronic maternal hypoxia [18,19]. The mechanism of damage from hypoxic-ischemic insult involves a series of events, with a dramatic increase in ROS occurring mainly during the reperfusion and reoxygenation phase (during the first 12–48 h after the hypoxic-ischemic insult) [20,21].
In clinical practice, identifying the timing of hypoxic-ischemic insult underlying HIE is challenging for forensic pathologists [22]. It is often difficult to resolve doubts about potential clinical liability in cases of HIE. In this respect, we hypothesized that the measurement of OS-indicative markers in the human brain and placenta could be helpful in the identification of the timing of the hypoxic-ischemic insult. In order to test our hypothesis, we retrospectively investigated oxidative stress processes by evaluating immunohistochemistry samples from forensic autopsies of fetuses/newborns in which the timing of hypoxic-ischemic insult was unknown (i.e., intrauterine deaths, intrapartum deaths, and post-partum deaths), as compared with a control group in which the timing of insult was established (sudden death due to acute events).
The reason for our study is to provide scientific evidence in cases of medical liability in the obstetric field to support the clinical data on the timing of HIE.
For the identification of the timing of the hypoxic-ischemic insult, we investigated the potential usefulness of assessing the expression of specific OS markers NADPH oxidase 2 (NOX2), 8-hydroxy-2′-deoxyguanosine (8-OHdG), nitrotyrosine (NT), nitric oxide synthase (iNOS) and Interleukin 6 (IL-6), both in cortex brain and placenta samples, only in the brain we studied the immunohistochemical expression of Aquaporin 4 (AQP4).
2. Results
A preliminary semiquantitative analysis was performed, both for brain and placenta samples; the markers investigated were NADPH oxidase 2 (NOX2), 8-hydroxy-2′-deoxyguanosine (8-OHdG), nitrotyrosine (NT), nitric oxide synthase (iNOS), and Interleukin 6 (IL-6). Based on the results of the semi-quantitative analysis, the quantitative analysis was performed using ImageJ software (see Section 4. Materials and Methods and Section 2.1 and Section 2.2 of Results).
The immunohistochemical investigation of Aquaporin 4 (AQP4) was not significant because the marker reaction in the Group_1 B, Group_2 B, Group_3 B, and Controls B was negative, so after a preliminary semiquantitative analysis, the AQP4 was discarded as a possible marker.
2.1. Brain Tissue
The quantitative expression of oxidative stress markers, NOX2, 8-OHdG, NT, iNOS, and IL-6, within each group (Group_1 B: Fetal intrauterine deaths, Group_2 B: Intrapartum deaths, Group_3 B: Post-partum deaths, Controls B) is reported in Table 1.

Table 1.
Quantitative expression of NADPH oxidase 2 (NOX2), 8-hydroxy-2′-deoxyguanosine (8-OHdG), nitrotyrosine (NT), nitric oxide synthase (iNOS), and IL-6 in the brain cortex among groups. Data reported as median with interquartile range (IQR) of the percentage of the number of positive colored cells/microscopic area analyzed. p values are calculated by the Kruskal–Wallis test. Comparisons between two independent groups were made by the Mann–Whitney U test.
The expression of NOX2, 8-OHdG, NT, iNOS, and IL-6 was significantly different between study groups (p-values 0.009, 0.003, 0.005, <0.001, and 0.001, respectively). Notably, all those markers were more highly expressed in cases (Group_1 B, Group_2 B, and Group_3 B) compared with Controls B (except for iNOS in Group_2 B vs. Controls B; p-value > 0.05).
Individual comparisons between Group_1 B, Group_2 B, and Group_3 B found no significant differences in terms of NOX2, 8-OHdG, and NT expression. Differently, regarding iNOS and IL-6, the highest expression was found in Group_1 B (p < 0.05 compared to Group_2 B and Group_3 B) (Figure 1 and Figure 2).
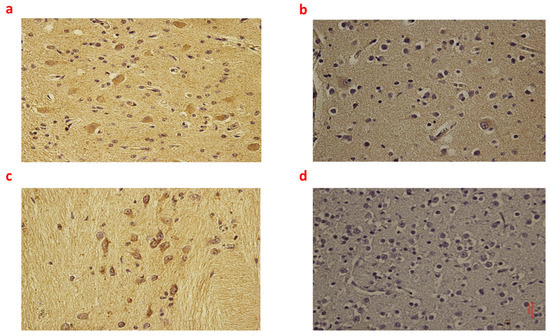
Figure 1.
(a–d): Immunohistochemical expression of inducible Nitric Oxide Synthase (iNOS) in the brain cortex, frontal area: Fetal intrauterine death neuronal and glial expression of iNOS Group_1 B 40× (a); Intrapartum death neuronal and glial expression of iNOS Group_2 B 40× (b); Post-partum death neuronal expression of iNOS Group_3 B 40× (c); Sudden neonatal death negative reaction of iNoS Controls B 40× (d).
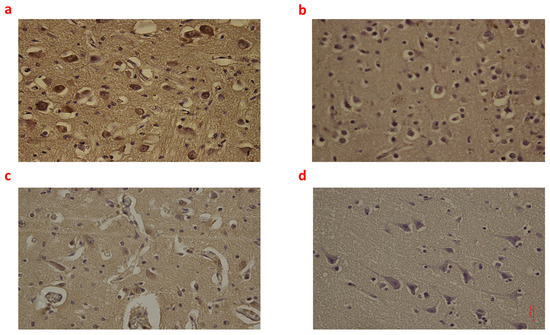
Figure 2.
(a–d): Immunohistochemical expression of Interleukin-6 (IL-6) in the brain cortex, frontal area: Fetal intrauterine death high neuronal and glial expression of IL-6 Group_1 B 40× (a); Intrapartum death low neuronal and glial expression of IL-6 Group_2 B 40× (b); Post-partum death low neuronal and glial expression of IL-6 Group_3 B 40× (c); Sudden neonatal death negative neuronal and glial expression of IL-6 Controls B 40× (d).
Moreover, Group_3 B showed higher immunoreactivity to iNOS than Group_2 B (p < 0.05). Box plots of the immunohistochemical expression of NOX2, 8OHdG, NT, iNOS, and IL-6 in the brain cortex of each group are shown in Figures S1a–S5a.
2.2. Placental Tissue
The quantitative expression of oxidative stress markers in placental tissue within groups (Group_1 P: Fetal intrauterine deaths, Group_2 P: Intrapartum deaths, Group_3 P: Post-partum deaths, Controls P) is reported in Table 2.

Table 2.
Quantitative expression of NADPH oxidase 2 (NOX2), 8-hydroxy-2′-deoxyguanosine (8-OHdG), nitrotyrosine (NT), nitric oxide synthase (iNOS), and IL-6 in the placental tissue among groups. Data reported as median with interquartile range (IQR) of the percentage of the number of positive colored cells/microscopic area analyzed. p values are calculated by the Kruskal–Wallis test. Comparisons between two independent groups were made by the Mann–Whitney U test.
The expression of NOX2, NT, iNOS, and IL-6 differed significantly between groups (p-values 0.01, 0.019, <0.001, and 0.026, respectively), while 8-OHdG positivity was similar (p = ns).
Regarding NOX2 immunohistochemistry, the highest expression was found in Group_3 P (i.e., significantly higher compared to Group_1 P, Group_2 P, and Controls P; all p < 0.05) (Figure 3).
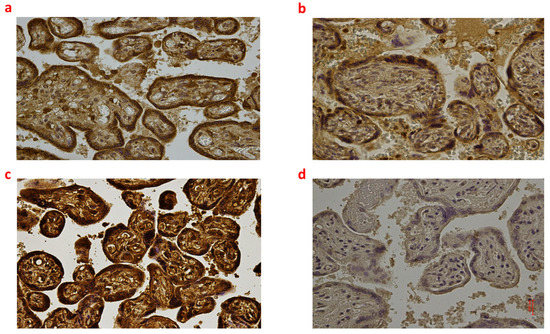
Figure 3.
(a–d): Immunohistochemical expression of NADPH oxidase 2 (NOX2) in the placental tissue, central area: Fetal intrauterine death Group_1 P 40× (a); Intrapartum death Group_2 P 40× (b); Post-partum death Group_3 P 40× (c); Sudden neonatal death Controls P 40× (d).
Moreover, Goup_2 P and Group_3 P showed significantly higher positivity to NT compared to controls (p < 0.05), while no difference in NT immunoreactivity between Group_1 P, Group_2 P, and Group_3 P was found (p = ns).
The highest expression of iNOS was observed in Group_1 P and Group_2 P (i.e., significantly higher compared to Group_3 P and Controls P; all p < 0.05). Nevertheless, the comparison between Group_1 P and Group_2 P did not find significant differences in iNOS positivity (Figure 4).
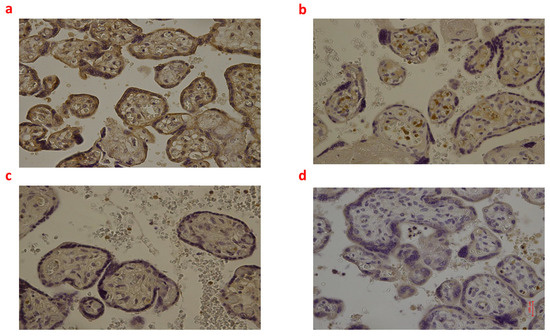
Figure 4.
(a–d): Immunohistochemical expression of inducible Nitric Oxide Synthase (iNOS) in the placental tissue, central area: Fetal intrauterine death Group_1 P 40× (a); Intrapartum death Group_2 P 40× (b); Post-partum death Group_3 P 40× (c); Sudden neonatal death Controls P 40× (d).
Finally, the expression of IL-6 was higher in Group_1 P than in Group_3 P. No other statistical differences were found. Box plots of the immunohistochemical expression of NOX2, 8-OHdG, NT, iNOS, and IL-6 in the brain cortex of each group are shown in Figures S1b–S5b.
3. Discussion
In forensic medicine, the identification of the timing of hypoxic-ischemic injury underlying the HIE remains a challenge. The autopsy alone is often insufficient to answer all doubts during forensic examinations, leading to the potential misinterpretation of unrecognized chronic fetal problems as the consequence of acute perinatal injuries. Therefore, it is often difficult to resolve doubts about potential clinical liability in cases of HIE [22,23].
After a hypoxic-ischemic attack (i.e., impaired cerebral blood flow and oxygen delivery to the brain), the pathologic events of HIE involve the generation of OS, with the release of oxygen and nitrogen species, calcium overloading, ROS generation, ionic imbalance, inflammation, apoptosis, autophagy, and necrosis [24,25].
HIE occurs in two phases (i.e., primary energy failure and secondary energy failure), where the second phase is characterized by massive ROS release 6–48 h after the initial injury, resulting in damage to neuronal cell membranes, necrosis, and apoptosis [26,27,28,29]. In this pilot study, we investigated the potential usefulness of assessing the expression of specific OS markers by immunohistochemistry (i.e., NOX2, 8-OHdG, iNOS, NT, and IL-6) in fetal/neonatal brain cortex and placenta for the identification of the timing of the hypoxic-ischemic insult. Previous in vitro studies about the edema that occurs during the early stage of ischemic stroke and brain trauma show the role of AQP4. The HIE is characterized by brain edema, so we tested AQP4 in our three groups and controls of newborn and fetus brain samples. The immunohistochemical analysis of AQP4 was not significant, so the AQP4 was discarded as a possible marker in HIE. This result suggests that the not complete maturation of brain tissue in fetuses and newborns could be the cause.
On the other hand, NOX2, 8-OHdG, NT, iNOS, and IL-6 were generally more highly expressed in the brain cortex of three groups of cases (fetal intrauterine death, intrapartum death, and post-partum death) compared with controls. Interestingly, the widest differences between the three groups, fetal intrauterine death, intrapartum death, and post-partum death, were found in terms of iNOS and IL-6 expression. Regarding iNOS, the highest expression in brain tissue was observed in fetal intrauterine death (Group_1 B), followed by post-partum death (Group_3 B), while in intrapartum death (Group_2 B), the iNOS immunoreactivity was very low. NOS is an enzyme that catalyzes the production of nitric oxide from l-arginine [30,31]. After asphyxia, nitric oxide generates toxic peroxynitrite (by reacting with superoxide), setting a pre-apoptotic process and resulting in neuronal loss [32,33]. NOS exists in three principal isoforms: endothelial (eNOS), neuronal (nNOS), and inducible NOS (iNOS). nNOS and iNOS are mainly associated with deleterious effects on the brain [31,33,34]. Specifically, murine experiments showed that nNOS is activated immediately after reperfusion, while iNOS is upregulated several hours after the asphyctic injury [34]. This notion is consistent with our finding about the highest expression of iNOS in the cortex of those babies undergoing intrauterine death. Regarding IL-6, fetal intrauterine death (Group_1 B) showed the highest expression in the brain cortex, while intrapartum death (Group_2 B) and post-partum death (Group_3 B) showed weak and similar immunoreactivity. IL-6 is a cytokine with pleiotropic activity produced by different brain cells, mainly by astrocytes [35,36,37]. Rapid increases in the levels of IL-6 can exacerbate an ischemic injury by stimulating the apoptosis of neuronal cells [38,39]. Interestingly, Yoon et al. previously found that IL-6 expression was upregulated in the brain of those babies who died due to chorioamnionitis [40]. In our study, the majority of intrauterine death was of unexplained etiology, likewise in most cases reported in the literature. As IL-6 was maximally up-regulated in Group_1 B (intrauterine death), a potential etiological correlation between intrauterine death and infection may not be excluded.
Therefore, those differences in the immunoreactivity of brain tissue to iNOS and IL-6 between fetal intrauterine death (Group_1 B) (i.e., strong to iNOS and IL-6), intrapartum death (Group_2 B) (low to iNOS and IL-6) and post-partum death (Group_3 B) (low to iNOS and moderate to IL-6) may represent a first step in identifying the timing of lethal injuries for forensic purposes.
In addition to the abovementioned findings on the brain cortex, we found a different expression of NOX2, NT, iNOS, and IL-6 between groups at the placental level, while 8-OHdG positivity was similar. Post-partum death (Group_3 P) placentas showed the highest immunoreactivity to NOX2, which was almost double that of fetal intrauterine death (Group_1 P) and intrapartum death (Group_2 P) placentas. NOX2 is a membrane-bound enzyme catalyzing the production of a superoxide free radical by transferring one electron to oxygen from NADPH [41,42]. Its activity is predominant in the apical microvillous membrane of the syncytiotrophoblast in the term placenta [43,44]. However, the physiological role of this enzyme at the maternal–fetal interface in term pregnancies is still unclear. In a previous study, Manes [45] suggested that NOX2 might respond as an oxygen-sensing mechanism and a signaling molecule at the trophoblast surface. In line with this latter speculation, Matsubara and Sato [46] found a dramatic increase in NOX2 expression at term in placentas with signs of vasculopathy. Concerning our findings, we may hypothesize that the impressive NOX2 upregulation found in post-partum death (Group_3 P) may be due to altered placental blood flow during labor. This result was consistent with the higher (but not significant) NT expression in intrapartum death (Group_2 P) and post-partum death (Group_3 P) placentas compared to fetal intrauterine death (Group_1 P). Notably, NT can be considered as an index of oxidative stress arising from peroxynitrite formation and action, which is found in the placenta after vascular damage [47,48]. Thus, both NOX2 and NT may virtually indicate a pathological response of placental tissue against demands for altered blood flow during labor, such as commonly observed in pregnancies complicated by pre-eclampsia or diabetes [49,50].
Finally, placental tissues of fetal intrauterine death (Group_1 P) and in intrapartum death (Group_2 P) showed higher expression of iNOS than post-partum death (Group_3 P), while the IL-6 expression was higher in fetal intrauterine death (Group_1 P) than post-partum death (Group_3 P). These differences in the placental expression of iNOS and IL-6 between groups almost confirmed our findings observed in the brain cortex.
The results of this study demonstrated that the immunohistochemical expression of specific OS markers in the brain cortex and placentas is different between fetuses/newborns undergoing intrauterine, intrapartum, and post-partum death, providing useful information for identifying the timing of the development of HIE.
The first point for the forensic pathologist is to identify the stage of labor or delivery in which the fetus or newborn died. The tools utilized until now are focused on the study of the lungs to determine whether the fetus had breathed or not before death.
The timing of the injury is the key point to demonstrate that the injury occurred at a different time than the strict intrapartum period. The clinical presentation and neuroimaging of hypoxic-ischemic encephalopathy cannot provide an accurate temporal relationship between the event and the pathologic manifestation. The autopsy cannot provide all the necessary information [51].
In cases of fetal or newborn death during labor or in the last part of pregnancy during medical activity could result in a penal and/or civil suit against the doctors or the hospital, according to the Country Law. The expert witness (e.g., in Italy, a forensic pathologist and an obstetrician) must appoint very qualified personnel to perform the autopsy on the fetus or newborn and study the clinical documentation in depth for the evaluation of the conduct of the doctors who carried out the birth and followed the pregnancy. The evaluation must be scrupulous and supported by the literature, so it is crucial to provide further scientific evidence for the evaluation of the cases using immunohistochemical markers.
Forensic pathology aims to implement immunohistochemical assays to corroborate qualitative evidence with semi-quantitative parameters [52]. The importance of this study is the individuation of markers useful to support the clinical data in cases of medical malpractice in the field of obstetrics.
4. Materials and Methods
4.1. Study Design
This was a retrospective case-control study on fetal/neonatal brains and placental samples collected at two centers (Department of Legal Medicine of Policlinic Umberto I—Sapienza; Department of Legal Medicine of the University of Foggia) from January 2012 to December 2022. The study was designed to investigate whether the use of immunohistochemistry may help establish the timing of hypoxic-ischemic insult in post-mortem fetuses/newborns.
4.2. Subjects and Data Collection
Among cases (n = 25), we analyzed the brains and placentas of babies (fetuses/newborns) born between 36–42 weeks of gestation. One sample of brain and placenta was collected for each case. The study involved three case groups (Group_1: Fetal intrauterine deaths [n = 9], Group_2: Intrapartum deaths [n = 9], Group_3: Post-partum deaths [n = 7]). As a control group (Controls), we evaluated the brains and placentas of babies who had undergone sudden neonatal death (n = 6 cases). In order to simplify the report of each comparison between placentas and brains pertaining to the study groups, all groups were further divided into two subgroups (Subgroup_B [brain] and Subgroup_P [placenta]).
For each baby, we collected data about gestational age at delivery, type of delivery, birth weight, survival time after birth, APGAR score, the potential cause of death (as diagnosed by a forensic pathologist), and the timing of death.
All the characteristics of the study subjects are summarized in Table 3.

Table 3.
General characteristics of included subjects. Cause of death column lists the disease/injury considered as responsible for death at forensic investigation. The Group of each case is indicated in the table.
4.3. Methods
All samples of the brain cortex and placentas were evaluated through immunohistochemical investigations for NADPH Oxidase 2 (NOX2), 8-Hydroxy-2′-deoxyGuanosine (8-OHdG), NitroTyrosine (NT), inducible Nitric Oxide Synthase (iNOS) and Interleukin-6 (IL-6). For brain sections, we also performed immunohistochemical analysis for Aquaporin-4 (AQP4) in order to estimate the development of cerebral edema. For each case, sections of about 4 μm thickness were cut; after the hydration, the slices were pretreated for antigen retrieval and then incubated with primary antibody according to the dilution indicated in Table 4. The utilized detection system (CTS005 HRP-DAB system R&D kit, R&D systems, Inc., Minneapolis, MN, USA) was a refined avidin–biotin system in which a biotinylated secondary antibody reacts with several peroxidize-conjugated streptavidin molecules. The positive reaction was visualized by 3,3′-diaminobenzidine (DAB) peroxidation, according to standard methods.

Table 4.
Antibody dilution.
We evaluated the presence of non-specific markings due to the avidin-biotin system. Hence, we carried out tests using a polymer system (BioCare Goat-on-rodent HRP-Polymer), obtaining the markings of the same areas.
Sections were counterstained with hematoxylin, dehydrated, cover-slipped, and observed in a Nikon Eclipse E600 microscope (Nikon, Tokyo, Japan). Quantification of NOX2, 8-OHdG, NT, iNOS, and IL-6 positive-stained cells was performed by the ImageJ software (imagej.nih.gov/ij/), as previously described [53,54], using the “Manual Cell Counting and Marking” protocol of this software for RGB color, single, not stack images, https://imagej.nih.gov/ij/docs/guide/user-guide.pdf, (accessed on 1 April 2023). One image for each organ (brain and placenta) and the case of the different experimental groups were processed, and a total of 341 slides were analyzed. Quantifications were expressed as the number of positive-stained cells/analyzed area. Histological analyses were performed by researchers who were blind with respect to the information about the cases. The blinding of the data was maintained until the analysis was terminated.
4.4. Statistical Analysis
Statistical analysis was performed by using SPSS v.22.0 (IBM Corp., Armonk, NY, USA). The quantitative results of immunohistochemical analyses of brain and placental sections were expressed as the median and interquartile range (IQR). The Mann–Whitney U test was used to compare medians between two independent groups, while the Kruskal–Wallis test was used for comparisons of median values among three or more groups.
A value of p < 0.05 was considered statistically significant.
5. Conclusions
In this study, we found that the immunohistochemical expression of specific OS markers in the brain cortex and placentas was different between fetuses/newborns undergoing intrauterine, intrapartum, and post-partum death.
iNOS showed the highest expression in brain tissue in fetal intrauterine death, followed by post-partum death, while in intrapartum death, the iNOS immunoreactivity was very low.
Regarding IL-6, fetal intrauterine death showed the highest expression in the brain cortex, while intrapartum death and post-partum death showed weak and similar immunoreactivity.
Therefore, those differences in the immunoreactivity of brain tissue to iNOS and IL-6 between fetal intrauterine death (strong reaction to iNOS and IL-6), intrapartum death (low reaction to iNOS and IL-6) and post-partum death (low reaction to iNOS and moderate reaction to IL-6) may represent a first step in identifying the timing of HIE.
For placenta samples, post-partum death placentas showed the highest immunoreactivity to NOX2, which was almost double that compared to fetal intrauterine death and intrapartum death placentas.
Placental tissues of fetal intrauterine death and intrapartum death showed higher expression of iNOS than post-partum death, while the IL-6 expression was higher in fetal intrauterine death than post-partum death. These differences in the placental expression of iNOS and IL-6 between groups almost confirmed the findings observed in the brain cortex. Therefore, such a diagnostic approach may provide useful information for identifying the timing of the lethal injury above HIE. Immunohistochemistry is becoming increasingly important for forensic purposes, especially in identifying the timing of lesions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241512221/s1.
Author Contributions
Conceptualization, G.S. and M.N.; methodology B.B. and S.D.S.; investigation, P.F. and L.A.; resources, P.G., S.D. and A.V.; writing—original draft preparation, B.B., M.N. and S.D.S.; writing—review and editing, E.T., V.F., M.N. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable. This study was performed by using human post-mortem brain and placenta samples collected during autopsies ordered by the prosecutor and used at the end of the investigations. According to Italian law, no authorizations from the ethics committee regarding the use of these post-mortem samples were required.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to Dott.ssa Santina Cantatore for the laboratory support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of Oxidative Stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, R.; Spinelli, F.; Contino, M.; Colabufo, N.A. The Pivotal Role of Copper in Neurodegeneration: A New Strategy for the Therapy of Neurodegenerative Disorders. Mol. Pharm. 2018, 15, 808–820. [Google Scholar] [CrossRef]
- Sohal, R.S. Role of Oxidative Stress and Protein Oxidation in the Aging Process. Free Radic. Biol. Med. 2002, 33, 37–44. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K.-I. DNA Damage Responses to Oxidative Stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Mylonas, C.; Kouretas, D. Lipid Peroxidation and Tissue Damage. Vivo 1999, 13, 295–309. [Google Scholar]
- Myatt, L.; Cui, X. Oxidative Stress in the Placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Wu, F.; Tian, F.-J.; Lin, Y.; Xu, W.-M. Oxidative Stress: Placenta Function and Dysfunction. Am. J. Reprod. Immunol. 2016, 76, 258–271. [Google Scholar] [CrossRef]
- Bosco, C.; González, J.; Gutiérrez, R.; Parra-Cordero, M.; Barja, P.; Rodrigo, R. Oxidative Damage to Pre-Eclamptic Placenta: Immunohistochemical Expression of VEGF, Nitrotyrosine Residues and von Willebrand Factor. J. Matern.-Fetal Neonatal Med. 2012, 25, 2339–2345. [Google Scholar] [CrossRef]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.-L. Oxidative Stress in Placental Pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Poston, L.; Burton, G.J. Placental-Related Diseases of Pregnancy: Involvement of Oxidative Stress and Implications in Human Evolution. Hum. Reprod. Update 2006, 12, 747–755. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, P.; Fujino, M.; Zhuang, J.; Guo, H.; Sheikh, I.; Zhao, L.; Li, X.-K. Oxidative Stress in Hypoxic-Ischemic Encephalopathy: Molecular Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2016, 17, 2078. [Google Scholar] [CrossRef]
- Qin, X.; Cheng, J.; Zhong, Y.; Mahgoub, O.K.; Akter, F.; Fan, Y.; Aldughaim, M.; Xie, Q.; Qin, L.; Gu, L.; et al. Mechanism and Treatment Related to Oxidative Stress in Neonatal Hypoxic-Ischemic Encephalopathy. Front. Mol. Neurosci. 2019, 12, 88. [Google Scholar] [CrossRef]
- Greco, P.; Nencini, G.; Piva, I.; Scioscia, M.; Volta, C.A.; Spadaro, S.; Neri, M.; Bonaccorsi, G.; Greco, F.; Cocco, I.; et al. Pathophysiology of Hypoxic-Ischemic Encephalopathy: A Review of the Past and a View on the Future. Acta Neurol. Belg. 2020, 120, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Escobar, M.; Weiss, M.D. Hypoxic-Ischemic Encephalopathy: A Review for the Clinician. JAMA Pediatr. 2015, 169, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, R.C. Hypoxic-Ischemic Encephalopathy. Am. J. Perinatol. 2000, 17, 113–120. [Google Scholar] [CrossRef]
- Kumar, S.; Paterson-Brown, S. Obstetric Aspects of Hypoxic Ischemic Encephalopathy. Early Hum. Dev. 2010, 86, 339–344. [Google Scholar] [CrossRef]
- Nasiell, J.; Papadogiannakis, N.; Löf, E.; Elofsson, F.; Hallberg, B. Hypoxic Ischemic Encephalopathy in Newborns Linked to Placental and Umbilical Cord Abnormalities. J. Matern.-Fetal Neonatal Med. 2016, 29, 721–726. [Google Scholar] [CrossRef]
- Fellman, V.; Raivio, K.O. Reperfusion Injury as the Mechanism of Brain Damage after Perinatal Asphyxia. Pediatr. Res. 1997, 41, 599–606. [Google Scholar] [CrossRef]
- Inder, T.E.; Volpe, J.J. Mechanisms of Perinatal Brain Injury. Semin. Neonatol. 2000, 5, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, V.; Viola, R.V.; La Russa, R.; Santurro, A.; Frati, P. A Controversial Medicolegal Issue: Timing the Onset of Perinatal Hypoxic-Ischemic Brain Injury. Mediat. Inflamm. 2017, 2017, 6024959. [Google Scholar] [CrossRef]
- Donn, S.M.; Chiswick, M.L.; Fanaroff, J.M. Medico-Legal Implications of Hypoxic-Ischemic Birth Injury. Semin. Fetal Neonatal Med. 2014, 19, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.A.; Brandon, D.H. Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs. Rev. 2011, 11, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Cotten, C.M.; Shankaran, S. Hypothermia for Hypoxic-Ischemic Encephalopathy. Expert Rev. Obstet. Gynecol. 2010, 5, 227–239. [Google Scholar] [CrossRef]
- Alvarez-Díaz, A.; Hilario, E.; de Cerio, F.G.; Valls-i-Soler, A.; Alvarez-Díaz, F.J. Hypoxic-Ischemic Injury in the Immature Brain--Key Vascular and Cellular Players. Neonatology 2007, 92, 227–235. [Google Scholar] [CrossRef]
- Buonocore, G.; Perrone, S.; Bracci, R. Free Radicals and Brain Damage in the Newborn. Biol. Neonate 2001, 79, 180–186. [Google Scholar] [CrossRef]
- Ferriero, D.M. Neonatal Brain Injury. N. Engl. J. Med. 2004, 351, 1985–1995. [Google Scholar] [CrossRef]
- Lorek, A.; Takei, Y.; Cady, E.B.; Wyatt, J.S.; Penrice, J.; Edwards, A.D.; Peebles, D.; Wylezinska, M.; Owen-Reece, H.; Kirkbride, V. Delayed (“secondary”) Cerebral Energy Failure after Acute Hypoxia-Ischemia in the Newborn Piglet: Continuous 48-Hour Studies by Phosphorus Magnetic Resonance Spectroscopy. Pediatr. Res. 1994, 36, 699–706. [Google Scholar] [CrossRef]
- Favié, L.M.A.; Cox, A.R.; van den Hoogen, A.; Nijboer, C.H.A.; Peeters-Scholte, C.M.P.C.D.; van Bel, F.; Egberts, T.C.G.; Rademaker, C.M.A.; Groenendaal, F. Nitric Oxide Synthase Inhibition as a Neuroprotective Strategy Following Hypoxic–Ischemic Encephalopathy: Evidence From Animal Studies. Front. Neurol. 2018, 9, 258. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Zhao, F.; Wang, H.; Qu, Y.; Mu, D. Nitric Oxide Synthase in Hypoxic or Ischemic Brain Injury. Rev. Neurosci. 2015, 26, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Dawson, V.L.; Kizushi, V.M.; Huang, P.L.; Snyder, S.H.; Dawson, T.M. Resistance to Neurotoxicity in Cortical Cultures from Neuronal Nitric Oxide Synthase-Deficient Mice. J. Neurosci. 1996, 16, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Cipolloni, L.; De Simone, S. Nitrous oxide intoxication: Systematic literature review and proposal of new diagnostic possibilities. Egypt. J. Forensic Sci. 2022, 12, 59. [Google Scholar] [CrossRef]
- Misra, U.K.; Singh, S.K.; Kalita, J.; Kumar, A. Astrocyte Activation Following Nitrous Oxide Exposure Is Related to Oxidative Stress and Glutamate Excitotoxicity. Brain Res. 2020, 1730, 146645. [Google Scholar] [CrossRef] [PubMed]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a Major Cytokine in the Central Nervous System. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.G.; Tang, L.P.; Sparacio, S.M.; Benveniste, E.N. Signal Transduction Pathways Mediating Astrocyte IL-6 Induction by IL-1 Beta and Tumor Necrosis Factor-Alpha. J. Immunol. 1994, 152, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The Role of Interleukin-6 Signaling in Nervous Tissue. Biochim. Biophys. Acta 2016, 1863, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Spooren, A.; Kolmus, K.; Laureys, G.; Clinckers, R.; De Keyser, J.; Haegeman, G.; Gerlo, S. Interleukin-6, a Mental Cytokine. Brain Res. Rev. 2011, 67, 157–183. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Park, C.-W.; Chaiworapongsa, T. Intrauterine Infection and the Development of Cerebral Palsy. BJOG 2003, 110 (Suppl. S20), 124–127. [Google Scholar] [CrossRef] [PubMed]
- Lassègue, B.; San Martín, A.; Griendling, K.K. Biochemistry, Physiology and Pathophysiology of NADPH Oxidases in the Cardiovascular System. Circ. Res. 2012, 110, 1364–1390. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH Oxidases: An Overview from Structure to Innate Immunity-Associated Pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.-L.; Brockman, D.; Campos, B.; Myatt, L. Expression of NADPH Oxidase Isoform 1 (Nox1) in Human Placenta: Involvement in Preeclampsia. Placenta 2006, 27, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, E.; Gomes, S.Z.; Lorenzon, A.R.; Hoshida, M.S.; Amarante-Paffaro, A.M. NADPH Oxidase as an Important Source of Reactive Oxygen Species at the Mouse Maternal-Fetal Interface: Putative Biological Roles. Reprod. Biomed. Online 2012, 25, 31–43. [Google Scholar] [CrossRef]
- Manes, C. Human Placental NAD(P)H Oxidase: Solubilization and Properties. Placenta 2001, 22, 58–63. [Google Scholar] [CrossRef]
- Matsubara, S.; Sato, I. Enzyme Histochemically Detectable NAD(P)H Oxidase in Human Placental Trophoblasts: Normal, Preeclamptic, and Fetal Growth Restriction-Complicated Pregnancy. Histochem. Cell Biol. 2001, 116, 1–7. [Google Scholar] [CrossRef]
- Stanek, J.; Eis, A.L.; Myatt, L. Nitrotyrosine Immunostaining Correlates with Increased Extracellular Matrix: Evidence of Postplacental Hypoxia. Placenta 2001, 22 (Suppl. A), S56–S62. [Google Scholar] [CrossRef] [PubMed]
- Stanek, J.; Al-Ahmadie, H.A. Laminar Necrosis of Placental Membranes: A Histologic Sign of Uteroplacental Hypoxia. Pediatr. Dev. Pathol. 2005, 8, 34–42. [Google Scholar] [CrossRef]
- Myatt, L.; Rosenfield, R.B.; Eis, A.L.; Brockman, D.E.; Greer, I.; Lyall, F. Nitrotyrosine Residues in Placenta. Evidence of Peroxynitrite Formation and Action. Hypertension 1996, 28, 488–493. [Google Scholar] [CrossRef]
- Lyall, F.; Gibson, J.L.; Greer, I.A.; Brockman, D.E.; Eis, A.L.; Myatt, L. Increased Nitrotyrosine in the Diabetic Placenta: Evidence for Oxidative Stress. Diabetes Care 1998, 21, 1753–1758. [Google Scholar] [CrossRef]
- De Simone, S.; Maglietta, F.; Ferrara, M.; Spagnolo, L.; Ricci, P.; De Carlo, D.; Salerno, M.; Sessa, F.; Bertozzi, G. Homicide or Car Accident: The Case of the ‘Guilty’ Fibre. Med. Leg. J. 2019, 87, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, P.; Diani, L.; De Simone, S.; Bosco, M.A.; Cipolloni, L.; Neri, M. Forensic Diagnosis of Freshwater or Saltwater Drowning Using the Marker Aquaporin 5: An Immunohistochemical Study. Medicina 2022, 58, 1458. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, S.; Neri, M.; Trabace, L.; Turillazzi, E. The NADPH oxidase NOX2 mediates loss of parvalbumin interneurons in traumatic brain injury: Human autoptic immunohistochemical evidence. Sci. Rep. 2017, 7, 8752. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, S.; Neri, M.; Maffione, A.B.; Frisoni, P.; Morgese, M.G.; Trabace, L.; Turillazzi, E. Increased iNOS and Nitrosative Stress in Dopaminergic Neurons of MDMA-Exposed Rats. Int. J. Mol. Sci. 2019, 20, 1242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).