Oxidative Stress Markers in Human Brain and Placenta May Reveal the Timing of Hypoxic-Ischemic Injury: Evidence from an Immunohistochemical Study
Abstract
:1. Introduction
2. Results
2.1. Brain Tissue
2.2. Placental Tissue
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Subjects and Data Collection
4.3. Methods
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Biochemistry of Oxidative Stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giampietro, R.; Spinelli, F.; Contino, M.; Colabufo, N.A. The Pivotal Role of Copper in Neurodegeneration: A New Strategy for the Therapy of Neurodegenerative Disorders. Mol. Pharm. 2018, 15, 808–820. [Google Scholar] [CrossRef]
- Sohal, R.S. Role of Oxidative Stress and Protein Oxidation in the Aging Process. Free Radic. Biol. Med. 2002, 33, 37–44. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K.-I. DNA Damage Responses to Oxidative Stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Mylonas, C.; Kouretas, D. Lipid Peroxidation and Tissue Damage. Vivo 1999, 13, 295–309. [Google Scholar]
- Myatt, L.; Cui, X. Oxidative Stress in the Placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Wu, F.; Tian, F.-J.; Lin, Y.; Xu, W.-M. Oxidative Stress: Placenta Function and Dysfunction. Am. J. Reprod. Immunol. 2016, 76, 258–271. [Google Scholar] [CrossRef]
- Bosco, C.; González, J.; Gutiérrez, R.; Parra-Cordero, M.; Barja, P.; Rodrigo, R. Oxidative Damage to Pre-Eclamptic Placenta: Immunohistochemical Expression of VEGF, Nitrotyrosine Residues and von Willebrand Factor. J. Matern.-Fetal Neonatal Med. 2012, 25, 2339–2345. [Google Scholar] [CrossRef]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.-L. Oxidative Stress in Placental Pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Poston, L.; Burton, G.J. Placental-Related Diseases of Pregnancy: Involvement of Oxidative Stress and Implications in Human Evolution. Hum. Reprod. Update 2006, 12, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Zhu, P.; Fujino, M.; Zhuang, J.; Guo, H.; Sheikh, I.; Zhao, L.; Li, X.-K. Oxidative Stress in Hypoxic-Ischemic Encephalopathy: Molecular Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2016, 17, 2078. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Cheng, J.; Zhong, Y.; Mahgoub, O.K.; Akter, F.; Fan, Y.; Aldughaim, M.; Xie, Q.; Qin, L.; Gu, L.; et al. Mechanism and Treatment Related to Oxidative Stress in Neonatal Hypoxic-Ischemic Encephalopathy. Front. Mol. Neurosci. 2019, 12, 88. [Google Scholar] [CrossRef] [Green Version]
- Greco, P.; Nencini, G.; Piva, I.; Scioscia, M.; Volta, C.A.; Spadaro, S.; Neri, M.; Bonaccorsi, G.; Greco, F.; Cocco, I.; et al. Pathophysiology of Hypoxic-Ischemic Encephalopathy: A Review of the Past and a View on the Future. Acta Neurol. Belg. 2020, 120, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Escobar, M.; Weiss, M.D. Hypoxic-Ischemic Encephalopathy: A Review for the Clinician. JAMA Pediatr. 2015, 169, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, R.C. Hypoxic-Ischemic Encephalopathy. Am. J. Perinatol. 2000, 17, 113–120. [Google Scholar] [CrossRef]
- Kumar, S.; Paterson-Brown, S. Obstetric Aspects of Hypoxic Ischemic Encephalopathy. Early Hum. Dev. 2010, 86, 339–344. [Google Scholar] [CrossRef]
- Nasiell, J.; Papadogiannakis, N.; Löf, E.; Elofsson, F.; Hallberg, B. Hypoxic Ischemic Encephalopathy in Newborns Linked to Placental and Umbilical Cord Abnormalities. J. Matern.-Fetal Neonatal Med. 2016, 29, 721–726. [Google Scholar] [CrossRef]
- Fellman, V.; Raivio, K.O. Reperfusion Injury as the Mechanism of Brain Damage after Perinatal Asphyxia. Pediatr. Res. 1997, 41, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Inder, T.E.; Volpe, J.J. Mechanisms of Perinatal Brain Injury. Semin. Neonatol. 2000, 5, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, V.; Viola, R.V.; La Russa, R.; Santurro, A.; Frati, P. A Controversial Medicolegal Issue: Timing the Onset of Perinatal Hypoxic-Ischemic Brain Injury. Mediat. Inflamm. 2017, 2017, 6024959. [Google Scholar] [CrossRef]
- Donn, S.M.; Chiswick, M.L.; Fanaroff, J.M. Medico-Legal Implications of Hypoxic-Ischemic Birth Injury. Semin. Fetal Neonatal Med. 2014, 19, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.A.; Brandon, D.H. Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs. Rev. 2011, 11, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotten, C.M.; Shankaran, S. Hypothermia for Hypoxic-Ischemic Encephalopathy. Expert Rev. Obstet. Gynecol. 2010, 5, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Díaz, A.; Hilario, E.; de Cerio, F.G.; Valls-i-Soler, A.; Alvarez-Díaz, F.J. Hypoxic-Ischemic Injury in the Immature Brain--Key Vascular and Cellular Players. Neonatology 2007, 92, 227–235. [Google Scholar] [CrossRef]
- Buonocore, G.; Perrone, S.; Bracci, R. Free Radicals and Brain Damage in the Newborn. Biol. Neonate 2001, 79, 180–186. [Google Scholar] [CrossRef]
- Ferriero, D.M. Neonatal Brain Injury. N. Engl. J. Med. 2004, 351, 1985–1995. [Google Scholar] [CrossRef]
- Lorek, A.; Takei, Y.; Cady, E.B.; Wyatt, J.S.; Penrice, J.; Edwards, A.D.; Peebles, D.; Wylezinska, M.; Owen-Reece, H.; Kirkbride, V. Delayed (“secondary”) Cerebral Energy Failure after Acute Hypoxia-Ischemia in the Newborn Piglet: Continuous 48-Hour Studies by Phosphorus Magnetic Resonance Spectroscopy. Pediatr. Res. 1994, 36, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Favié, L.M.A.; Cox, A.R.; van den Hoogen, A.; Nijboer, C.H.A.; Peeters-Scholte, C.M.P.C.D.; van Bel, F.; Egberts, T.C.G.; Rademaker, C.M.A.; Groenendaal, F. Nitric Oxide Synthase Inhibition as a Neuroprotective Strategy Following Hypoxic–Ischemic Encephalopathy: Evidence From Animal Studies. Front. Neurol. 2018, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, J.; Zhao, F.; Wang, H.; Qu, Y.; Mu, D. Nitric Oxide Synthase in Hypoxic or Ischemic Brain Injury. Rev. Neurosci. 2015, 26, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Dawson, V.L.; Kizushi, V.M.; Huang, P.L.; Snyder, S.H.; Dawson, T.M. Resistance to Neurotoxicity in Cortical Cultures from Neuronal Nitric Oxide Synthase-Deficient Mice. J. Neurosci. 1996, 16, 2479–2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipolloni, L.; De Simone, S. Nitrous oxide intoxication: Systematic literature review and proposal of new diagnostic possibilities. Egypt. J. Forensic Sci. 2022, 12, 59. [Google Scholar] [CrossRef]
- Misra, U.K.; Singh, S.K.; Kalita, J.; Kumar, A. Astrocyte Activation Following Nitrous Oxide Exposure Is Related to Oxidative Stress and Glutamate Excitotoxicity. Brain Res. 2020, 1730, 146645. [Google Scholar] [CrossRef] [PubMed]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a Major Cytokine in the Central Nervous System. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.G.; Tang, L.P.; Sparacio, S.M.; Benveniste, E.N. Signal Transduction Pathways Mediating Astrocyte IL-6 Induction by IL-1 Beta and Tumor Necrosis Factor-Alpha. J. Immunol. 1994, 152, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The Role of Interleukin-6 Signaling in Nervous Tissue. Biochim. Biophys. Acta 2016, 1863, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Spooren, A.; Kolmus, K.; Laureys, G.; Clinckers, R.; De Keyser, J.; Haegeman, G.; Gerlo, S. Interleukin-6, a Mental Cytokine. Brain Res. Rev. 2011, 67, 157–183. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Park, C.-W.; Chaiworapongsa, T. Intrauterine Infection and the Development of Cerebral Palsy. BJOG 2003, 110 (Suppl. S20), 124–127. [Google Scholar] [CrossRef] [PubMed]
- Lassègue, B.; San Martín, A.; Griendling, K.K. Biochemistry, Physiology and Pathophysiology of NADPH Oxidases in the Cardiovascular System. Circ. Res. 2012, 110, 1364–1390. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH Oxidases: An Overview from Structure to Innate Immunity-Associated Pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.-L.; Brockman, D.; Campos, B.; Myatt, L. Expression of NADPH Oxidase Isoform 1 (Nox1) in Human Placenta: Involvement in Preeclampsia. Placenta 2006, 27, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevilacqua, E.; Gomes, S.Z.; Lorenzon, A.R.; Hoshida, M.S.; Amarante-Paffaro, A.M. NADPH Oxidase as an Important Source of Reactive Oxygen Species at the Mouse Maternal-Fetal Interface: Putative Biological Roles. Reprod. Biomed. Online 2012, 25, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Manes, C. Human Placental NAD(P)H Oxidase: Solubilization and Properties. Placenta 2001, 22, 58–63. [Google Scholar] [CrossRef]
- Matsubara, S.; Sato, I. Enzyme Histochemically Detectable NAD(P)H Oxidase in Human Placental Trophoblasts: Normal, Preeclamptic, and Fetal Growth Restriction-Complicated Pregnancy. Histochem. Cell Biol. 2001, 116, 1–7. [Google Scholar] [CrossRef]
- Stanek, J.; Eis, A.L.; Myatt, L. Nitrotyrosine Immunostaining Correlates with Increased Extracellular Matrix: Evidence of Postplacental Hypoxia. Placenta 2001, 22 (Suppl. A), S56–S62. [Google Scholar] [CrossRef] [PubMed]
- Stanek, J.; Al-Ahmadie, H.A. Laminar Necrosis of Placental Membranes: A Histologic Sign of Uteroplacental Hypoxia. Pediatr. Dev. Pathol. 2005, 8, 34–42. [Google Scholar] [CrossRef]
- Myatt, L.; Rosenfield, R.B.; Eis, A.L.; Brockman, D.E.; Greer, I.; Lyall, F. Nitrotyrosine Residues in Placenta. Evidence of Peroxynitrite Formation and Action. Hypertension 1996, 28, 488–493. [Google Scholar] [CrossRef]
- Lyall, F.; Gibson, J.L.; Greer, I.A.; Brockman, D.E.; Eis, A.L.; Myatt, L. Increased Nitrotyrosine in the Diabetic Placenta: Evidence for Oxidative Stress. Diabetes Care 1998, 21, 1753–1758. [Google Scholar] [CrossRef]
- De Simone, S.; Maglietta, F.; Ferrara, M.; Spagnolo, L.; Ricci, P.; De Carlo, D.; Salerno, M.; Sessa, F.; Bertozzi, G. Homicide or Car Accident: The Case of the ‘Guilty’ Fibre. Med. Leg. J. 2019, 87, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, P.; Diani, L.; De Simone, S.; Bosco, M.A.; Cipolloni, L.; Neri, M. Forensic Diagnosis of Freshwater or Saltwater Drowning Using the Marker Aquaporin 5: An Immunohistochemical Study. Medicina 2022, 58, 1458. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, S.; Neri, M.; Trabace, L.; Turillazzi, E. The NADPH oxidase NOX2 mediates loss of parvalbumin interneurons in traumatic brain injury: Human autoptic immunohistochemical evidence. Sci. Rep. 2017, 7, 8752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavone, S.; Neri, M.; Maffione, A.B.; Frisoni, P.; Morgese, M.G.; Trabace, L.; Turillazzi, E. Increased iNOS and Nitrosative Stress in Dopaminergic Neurons of MDMA-Exposed Rats. Int. J. Mol. Sci. 2019, 20, 1242. [Google Scholar] [CrossRef] [Green Version]
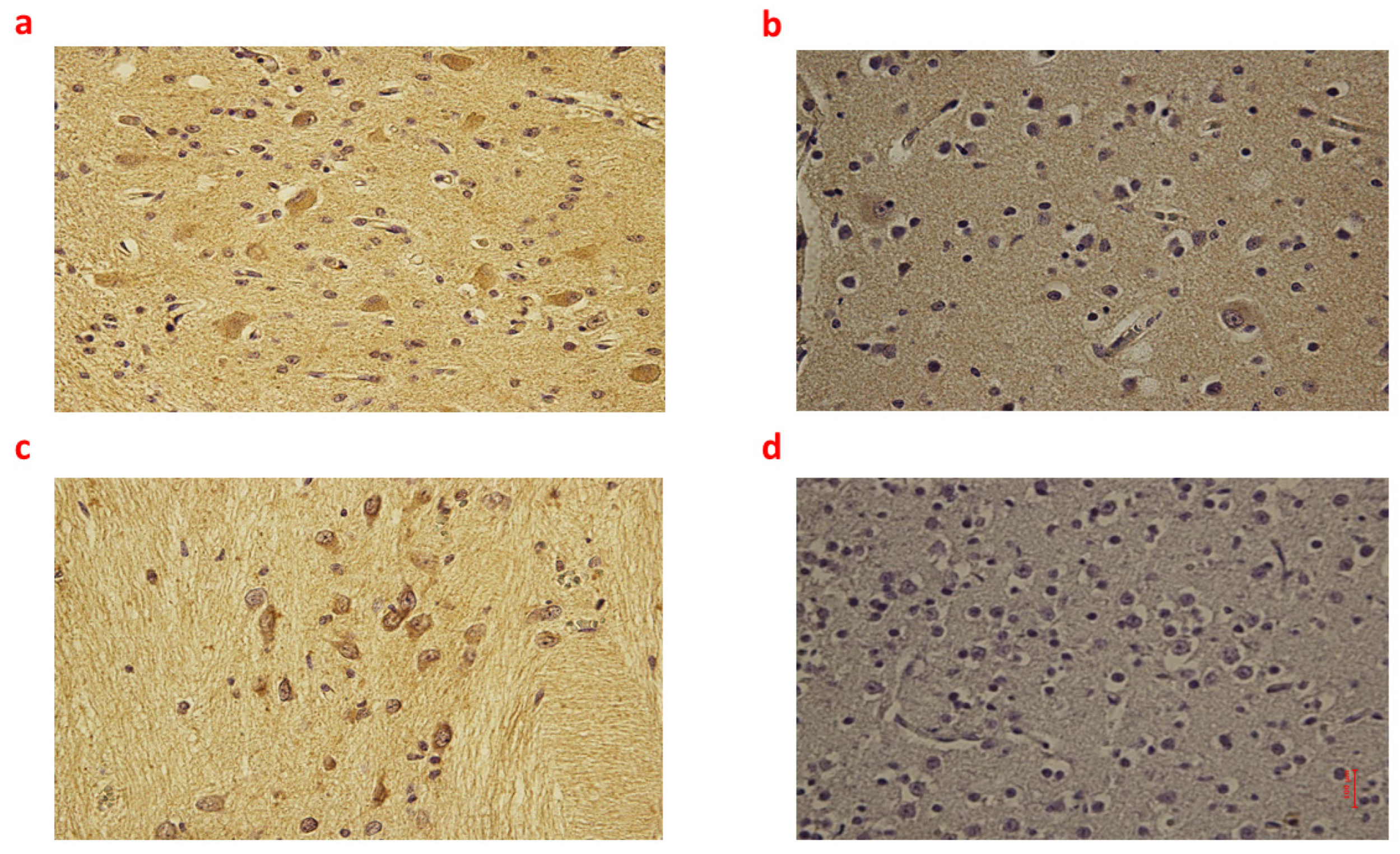
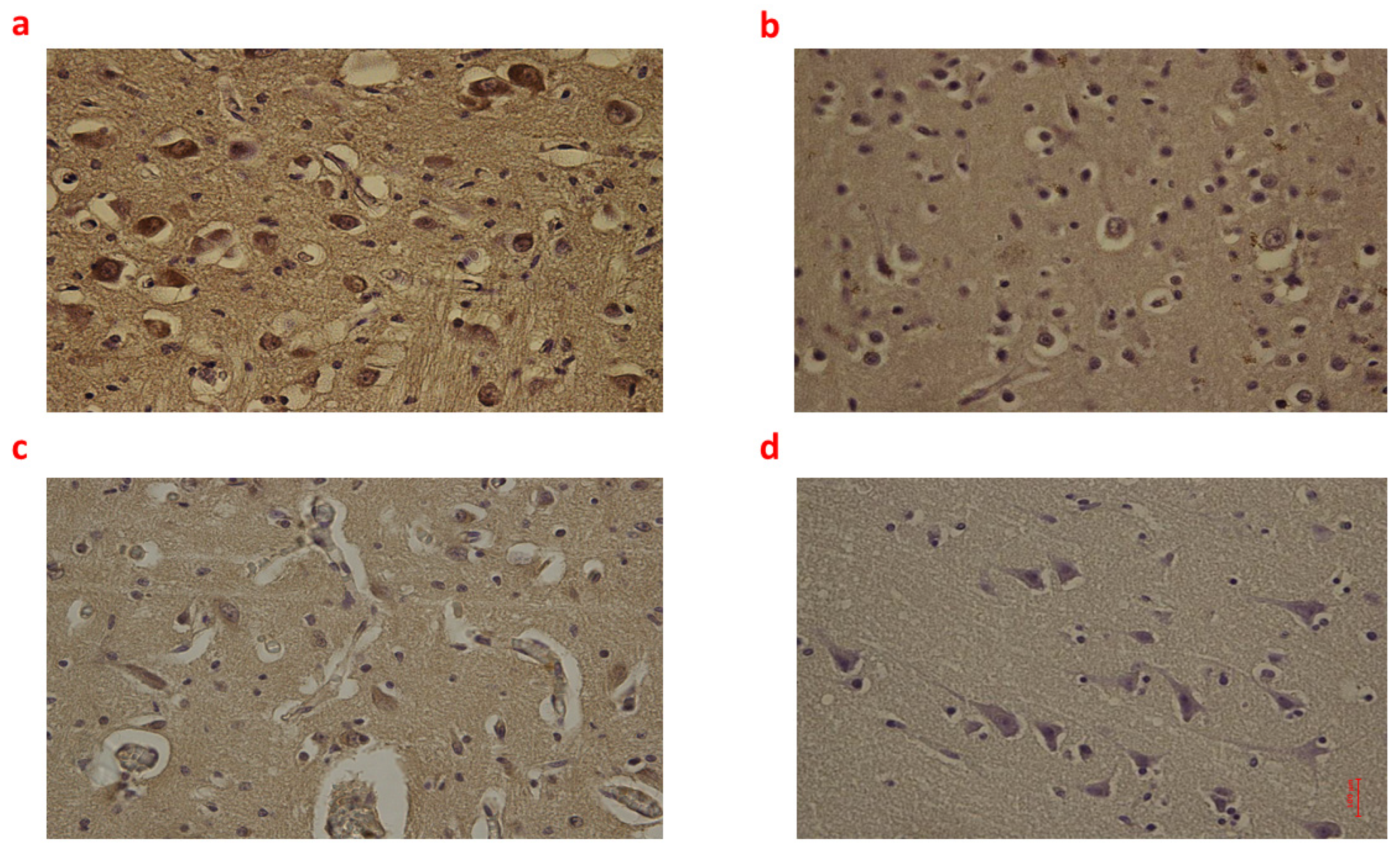
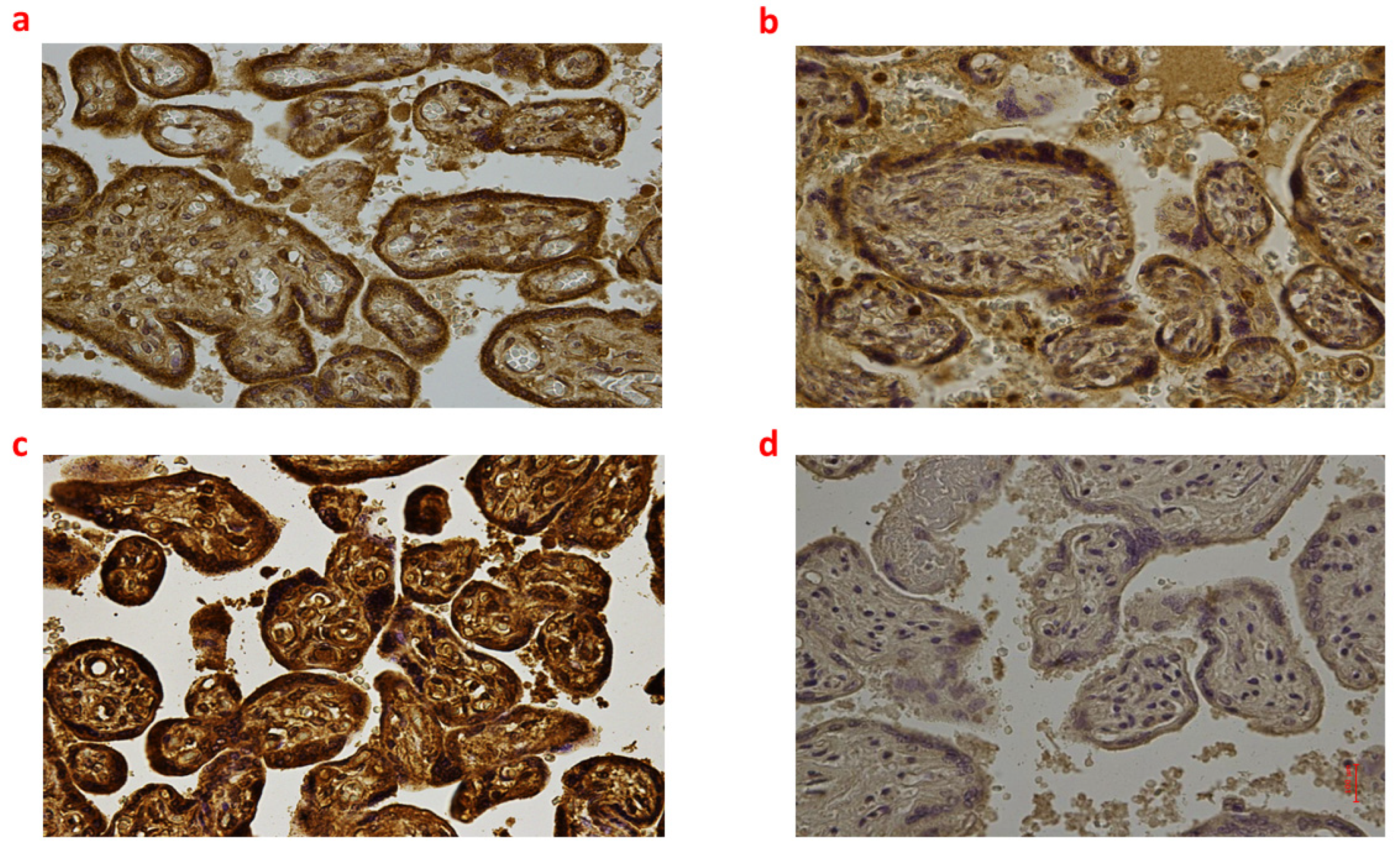
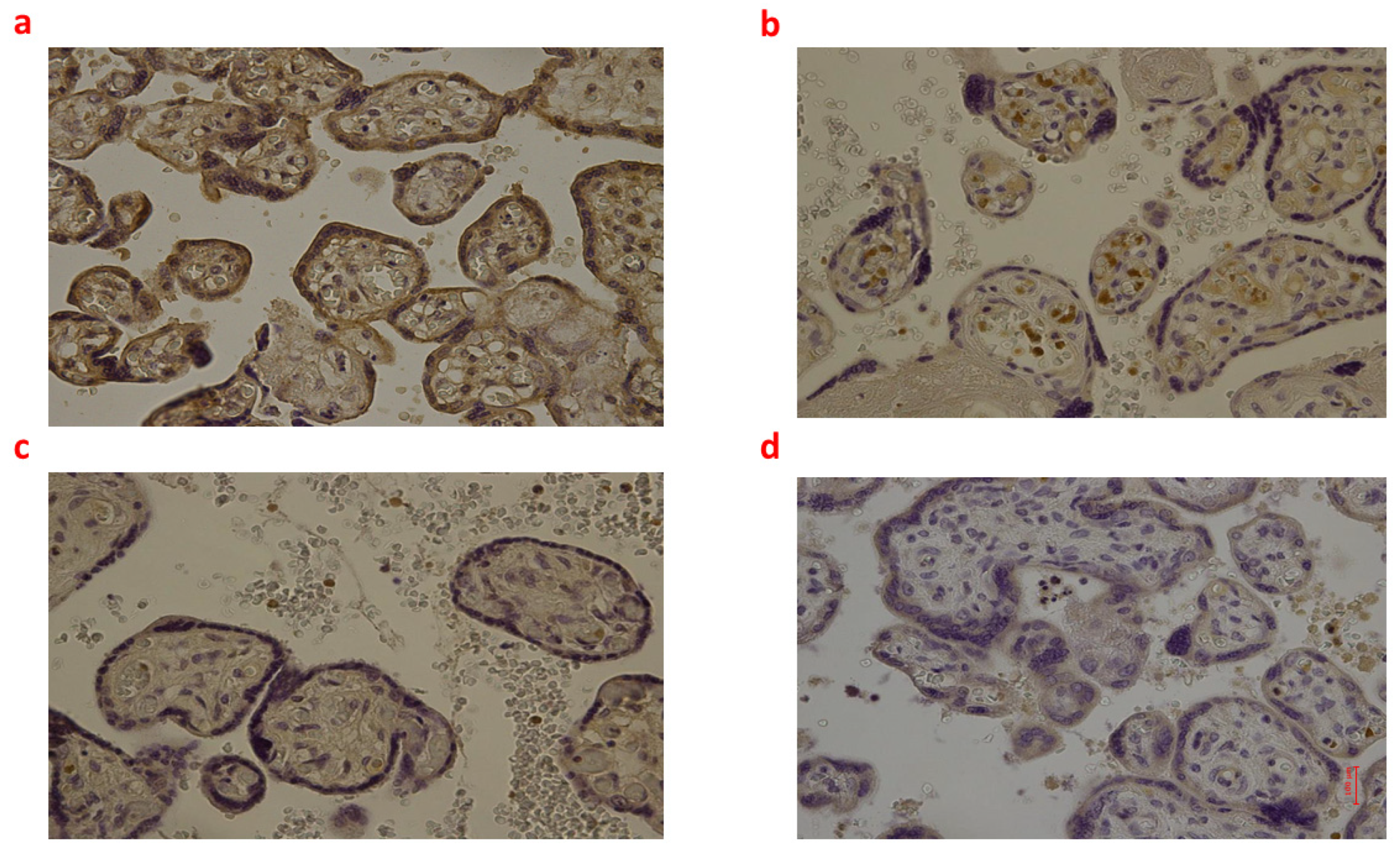
| Group_1 B (n = 9) | Group_2 B (n = 8) | Group_3 B (n = 6) | Controls B (n = 6) | p-Value | |
|---|---|---|---|---|---|
| NOX2 | 4.549 (7.785) 1 | 4.303 (7.132) 1 | 6.841 (2.683) 1 | 0.258 (1.307) | 0.009 |
| 8-OHDG | 11.130 (4.294) 1 | 6.410 (3.976) 1 | 10.800 (7.906) 1 | 1.040 (1.065) | 0.003 |
| NT | 6.926 (5.175) 1 | 4.239 (3.390) 1 | 2.729 (3.292) 1 | 0.140 (0.062) | 0.005 |
| iNOS | 3.851 (2.697) 1,2,3 | 1.037 (1.424) 2,4 | 2.240 (1.146) 1,3,4 | 0.723 (0.643) | <0.001 |
| IL-6 | 4.501 (6.486) 1,2,3 | 0.664 (0.518) 1,2 | 0.535 (0.253) 1,3 | 0.100 (0.046) | 0.001 |
| Group_1 P (n = 9) | Group_2 P (n = 8) | Group_3 P (n = 6) | Controls P (n = 6) | p-Value | |
|---|---|---|---|---|---|
| NOX2 | 10.875 (9.920) 3 | 11.839 (6.369) | 20.519 (9.974) 1,3,4 | 8.588 (3.572) | 0.01 |
| 8-OHDG | 4.107 (11.105) | 6.151 (5.658) | 6.504 (2.657) 1 | 2.861 (3.138) | 0.201 |
| NT | 8.349 (14.111) | 13.943 (12.504) 1 | 15.979 (3.661) 1 | 4.882 (3.560) | 0.019 |
| iNOS | 1.714 (2.199) 1,3 | 0.714 (0.850) 1,4 | 0.154 (0.146) 3,4 | 0.105 (0.087) | <0.001 |
| IL-6 | 0.168 (3.355) 3 | 0.004 (0.876) | 0.020 (0.028) 3 | 0.057 (0.192) | 0.026 |
| Patient Id | Group | Gender | Gestational Age at Birth (Weeks + Days) | Exitus (Type) | Birth (Type) | Weight (Grams) | Survival Time | Apgar Score | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Female | 40 + 2 | Intrauterine | Cesarean | 2749 | - | - | Placental infarction |
| 2 | 1 | Female | 38 | Intrauterine | Vaginal | 3300 | - | - | Fetal asphyxia |
| 3 | 1 | Male | 39 + 2 | Intrauterine | Vaginal | 2680 | - | - | Fetal asphyxia |
| 4 | 1 | Female | 38 | Intrauterine | Cesarean | 3000 | - | - | Placental infarction |
| 5 | 1 | Female | 38 | Intrauterine | Vaginal | 3359 | - | - | Heart failure |
| 6 | 1 | Female | 39 + 4 | Intrauterine | Vaginal | 3200 | - | - | Fetal asphyxia |
| 7 | 1 | Male | 39 + 1 | Intrauterine | Cesarean | 4045 | - | - | Fetal asphyxia |
| 8 | 1 | Female | 40 | Intrauterine | Vaginal | 2996 | - | - | Fetal asphyxia |
| 9 | 1 | Male | 37 + 6 | Intrauterine | Vaginal | 3680 | - | - | Fetal asphyxia |
| 10 | 2 | Male | 41 + 3 | Intrapartum | Cesarean | 3129 | - | - | Prolonged umbilical cord compression |
| 11 | 2 | Female | 41 + 2 | Intrapartum | Cesarean | 3850 | - | - | Unexplained fetal death |
| 12 | 2 | Female | 41 | Intrapartum | Cesarean | 3800 | 40 min | - | Acute hyschemia |
| 13 | 2 | Female | 38 | Intrapartum | Vacuum-assisted vaginal | 4710 | - | 1′: 0 5′: 0 | Acute asphyxia due to umbilical cord knot |
| 14 | 2 | Female | 36 | Intrapartum | Vaginal | 2500 | - | - | Acute asphyxia due to umbilical cord loop |
| 15 | 2 | Female | 41 + 3 | Intrapartum | Cesarean | 3050 | - | - | Acute asphyxia due to umbilical cord loop |
| 16 | 2 | Female | 41 | Intrapartum | Cesarean | 2800 | - | - | Acute asphyxia due to umbilical cord compression |
| 17 | 2 | Male | 41 | Intrapartum | Cesarean | 3450 | - | 1′: 0 | Brain edema |
| 18 | 2 | Female | 41 | Intrapartum | Cesarean | 2800 | - | - | Acute asphyxia due to umbilical cord compression |
| 19 | 3 | Female | 40 + 1 | Post-partum | Vaginal | 3320 | 6 h | 1′: 6 5′: 8 | Acute asphyxia due to umbilical cord compression |
| 20 | 3 | Female | 41 + 3 | Post-partum | Vaginal | 3000 | 6 h | 1′: 2 5′: 1 | Pneumonia |
| 21 | 3 | Male | 38 + 4 | Post-partum | Vaginal | 3390 | 3 days | 1′: 0 5′: 0 10′: 2 20′: 4 | Cerebral hemorrhage |
| 22 | 3 | Male | 36 + 2 | Post-partum | Vacuum-assisted vaginal | 2400 | 1 day | 1′: 9 5′: 10 | Respiratory distress |
| 23 | 3 | Female | 38 + 4 | Post-partum | Cesarean | 2900 | 2 days | 1′: 1 5′: 1 | Cerebral hemorrhage |
| 24 | 3 | Female | 41 + 6 | Post-partum | Cesarean | 3600 | 1 day | 1′: 2 5′: 3 | Meconium aspiration syndrome |
| 25 | 3 | Female | 40 + 1 | Post-partum | Vaginal | 3320 | 6 h | 1′: 6 5′: 8 | Respiratory distress |
| 26 | Controls | Male | 37 | Suicide (maternal hanging) | - | 2750 | - | - | Placental insufficiency |
| 27 | Controls | Female | 38 | Maternal thromboembolism | - | 2900 | - | - | Acute hypoxia |
| 28 | Controls | Male | 38 + 3 | Cardiac malformation | Cesarean | 2835 | - | - | Aortic coarctation |
| 29 | Controls | Female | 36 + 1 | Maternal aortic aneurysm rupture | - | 2615 | - | - | Placental insufficiency |
| 30 | Controls | Female | 41 + 3 | Maternal thromboembolism | - | 3512 | - | - | Acute hypoxia |
| 31 | Controls | Female | 38 + 2 | Cardiac malformation | Cesarean | 2904 | - | - | Aortic coarctation |
| Antibody against | Concentration of Primary Antibody |
|---|---|
| NOX2 (Santa Cruz, CA, USA) | 1:50 |
| 8-OHdG (JaICA, Tokyo, Japan) | 1:10 |
| NT (Santa Cruz, CA, USA) | 1:600 |
| iNOS (Santa Cruz, CA, USA) | 1:100 |
| IL-6 (Santa Cruz, CA, USA) | 1.200 |
| AQP4 (Santa Cruz, CA, USA) | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldari, B.; De Simone, S.; Cipolloni, L.; Frisoni, P.; Alfieri, L.; D’Errico, S.; Fineschi, V.; Turillazzi, E.; Greco, P.; Vitagliano, A.; et al. Oxidative Stress Markers in Human Brain and Placenta May Reveal the Timing of Hypoxic-Ischemic Injury: Evidence from an Immunohistochemical Study. Int. J. Mol. Sci. 2023, 24, 12221. https://doi.org/10.3390/ijms241512221
Baldari B, De Simone S, Cipolloni L, Frisoni P, Alfieri L, D’Errico S, Fineschi V, Turillazzi E, Greco P, Vitagliano A, et al. Oxidative Stress Markers in Human Brain and Placenta May Reveal the Timing of Hypoxic-Ischemic Injury: Evidence from an Immunohistochemical Study. International Journal of Molecular Sciences. 2023; 24(15):12221. https://doi.org/10.3390/ijms241512221
Chicago/Turabian StyleBaldari, Benedetta, Stefania De Simone, Luigi Cipolloni, Paolo Frisoni, Letizia Alfieri, Stefano D’Errico, Vittorio Fineschi, Emanuela Turillazzi, Pantaleo Greco, Amerigo Vitagliano, and et al. 2023. "Oxidative Stress Markers in Human Brain and Placenta May Reveal the Timing of Hypoxic-Ischemic Injury: Evidence from an Immunohistochemical Study" International Journal of Molecular Sciences 24, no. 15: 12221. https://doi.org/10.3390/ijms241512221






