G Protein-Coupled Receptor 40 Agonist LY2922470 Alleviates Ischemic-Stroke-Induced Acute Brain Injury and Functional Alterations in Mice
Abstract
1. Introduction
2. Results
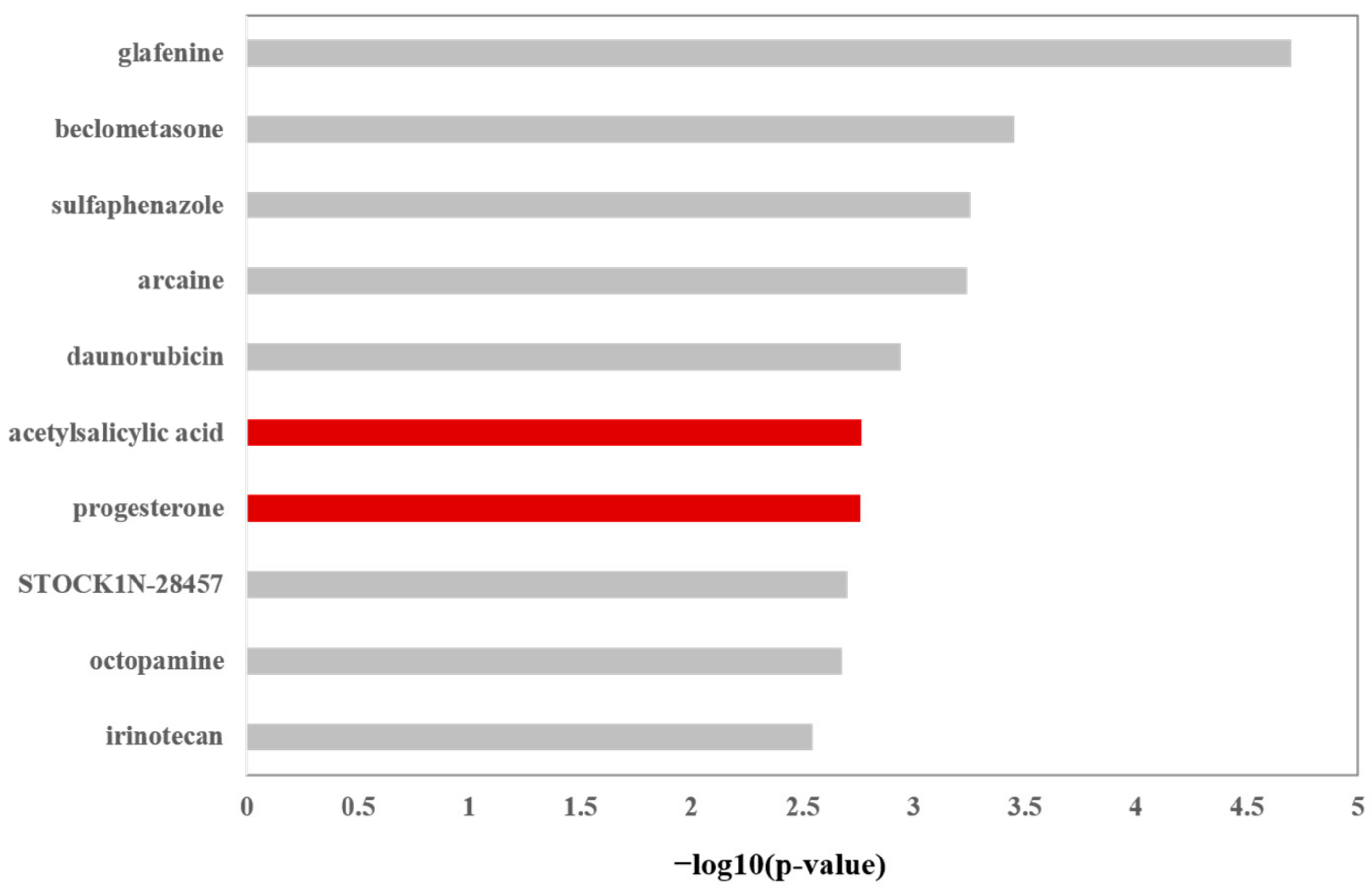
2.1. Top Small Molecules Showing Similar Induced Gene Signature with LY2922470
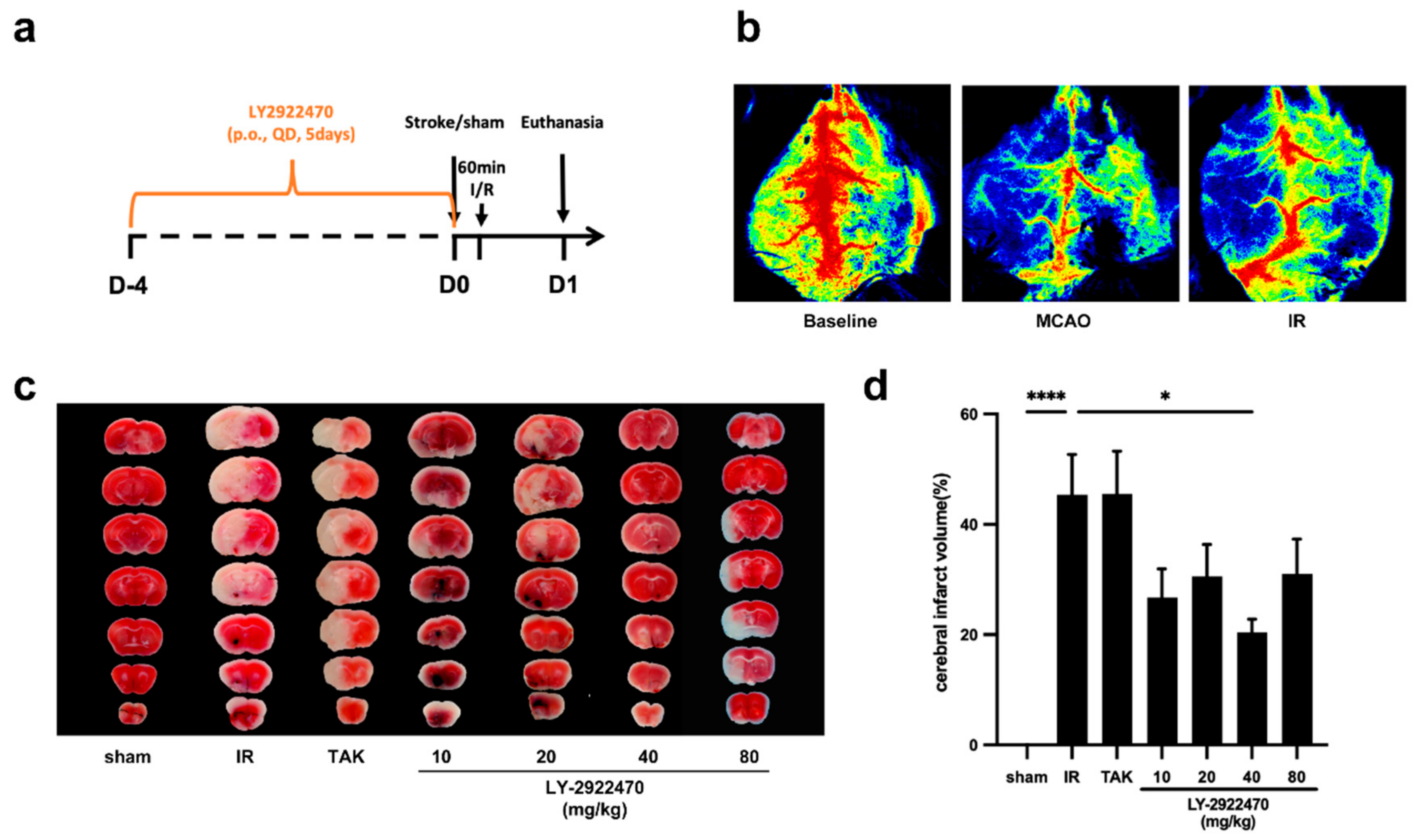
2.2. Pre-Treatment with LY2922470 Could Lessen Cerebral Ischemic Injury
2.3. Acute Protective Effect of LY2922470 on Brain after Stroke
3. Methods and Materials
3.1. Screening Small Molecules Showing Transcriptomic Gene Signatures Similar to LY2922470
3.2. Experimental Animals
3.3. Drug Preparation and Treatments
3.4. Middle Cerebral Artery Occlusion (MCAO)
3.5. Infarct Volume Measurements
3.6. Statistical Analysis
4. Conclusions and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mackman, N. Triggers, targets and treatments for thrombosis. Nature 2008, 451, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Baylis, R.A.; Smith, N.L.; Klarin, D.; Fukaya, E. Epidemiology and Genetics of Venous Thromboembolism and Chronic Venous Disease. Circ. Res. 2021, 128, 1988–2002. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Scwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e99. [Google Scholar] [CrossRef]
- Heit, J.A. Epidemiology of venous thromboembolism. Nat. Rev. Cardiol. 2015, 12, 464–474. [Google Scholar] [CrossRef]
- Chavda, V.; Madhwani, K.; Chaurasia, B. Stroke and immunotherapy: Potential mechanisms and its implications as immune-therapeutics. Eur. J. Neurosci. 2021, 54, 4338–4357. [Google Scholar] [CrossRef]
- Catanese, L.; Tarsia, J.; Fisher, M. Acute Ischemic Stroke Therapy Overview. Circ. Res. 2017, 120, 541–558. [Google Scholar] [CrossRef]
- Patel, P.; Yavagal, D.; Khandelwal, P. Hyperacute Management of Ischemic Strokes: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 1844–1856. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Zhang, Q.; Wang, H.; Xiu, W.; Xu, P.; Deng, Y.; Huang, W.; Wang, D.O. Crocetin antagonizes parthanatos in ischemic stroke via inhibiting NOX2 and preserving mitochondrial hexokinase-I. Cell Death Dis. 2023, 14, 50. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Ames, A. CNS energy metabolism as related to function. Brain Res. Rev. 2000, 34, 42–68. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-J.; Lee, W.-S.; Yang, K.-T. Methyl palmitate protects heart against ischemia/reperfusion-induced injury through G-protein coupled receptor 40-mediated activation of the PI3K/AKT pathway. Eur. J. Pharmacol. 2021, 905, 174183. [Google Scholar] [CrossRef]
- Xiao, J.; Cai, T.; Fang, Y.; Liu, R.; Flores, J.J.; Wang, W.; Gao, L.; Liu, Y.; Lu, Q.; Tang, L.; et al. Activation of GPR40 attenuates neuroinflammation and improves neurological function via PAK4/CREB/KDM6B pathway in an experimental GMH rat model. J. Neuroinflammation 2021, 18, 1–16. [Google Scholar] [CrossRef]
- Nishimura, Y.; Moriyama, M.; Kawabe, K.; Satoh, H.; Takano, K.; Azuma, Y.-T.; Nakamura, Y. Lauric Acid Alleviates Neuroinflammatory Responses by Activated Microglia: Involvement of the GPR40-Dependent Pathway. Neurochem. Res. 2018, 43, 1723–1735. [Google Scholar] [CrossRef]
- Hamdouchi, C.; Kahl, S.; Lewis, A.P.; Cardona, G.R.; Zink, R.W.; Chen, K.; Eessalu, T.E.; Ficorilli, J.V.; Marcelo, M.C.; Otto, K.A.; et al. The Discovery, Preclinical, and Early Clinical Development of Potent and Selective GPR40 Agonists for the Treatment of Type 2 Diabetes Mellitus (LY2881835, LY2922083, and LY2922470). J. Med. Chem. 2016, 59, 10891–10916. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, Q.; Geng, X.; Yang, J.; Huang, W.; Qian, H. Free fatty acid receptor agonists for the treatment of type 2 diabetes: Drugs in preclinical to phase II clinical development. Expert Opin. Investig. Drugs 2016, 25, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Roh, E.; Choi, K.M.; Yoo, H.J.; Hwang, H.-J.; Baik, S.H. GPR40 Agonism Modulates Inflammatory Reactions in Vascular Endothelial Cells. Diabetes Metab. J. 2022, 46, 506–511. [Google Scholar] [CrossRef]
- Negoro, N.; Sasaki, S.; Mikami, S.; Ito, M.; Suzuki, M.; Tsujihata, Y.; Ito, R.; Harada, A.; Takeuchi, K.; Suzuki, N.; et al. Discovery of TAK-875: A Potent, Selective, and Orally Bioavailable GPR40 Agonist. ACS Med. Chem. Lett. 2010, 1, 290–294. [Google Scholar] [CrossRef]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.-P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using Gene-Expression Signatures to Connect Small Molecules, Genes, and Disease. Science 2006, 313, 1929–1935. [Google Scholar] [CrossRef]
- Kiepura, A.; Stachyra, K.; Olszanecki, R. Anti-Atherosclerotic Potential of Free Fatty Acid Receptor 4 (FFAR4). Biomedicines 2021, 9, 467. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Tang, C.; Li, H.; Lei, J.; Zhu, L.; Kou, L.; Li, H.; Luo, S.; Li, C.; Chen, W.; et al. Eicosapentaenoic acid prevents inflammation induced by acute cerebral infarction through inhibition of NLRP3 inflammasome activation. Life Sci. 2019, 242, 117133. [Google Scholar] [CrossRef] [PubMed]
- Hauge, M.; Vestmar, M.A.; Husted, A.S.; Ekberg, J.P.; Wright, M.J.; Di Salvo, J.; Weinglass, A.B.; Engelstoft, M.S.; Madsen, A.N.; Lückmann, M.; et al. GPR40 (FFAR1) – Combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol. Metab. 2015, 4, 3–14. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhou, W.; Cui, Q.; Cui, C. G Protein-Coupled Receptor 40 Agonist LY2922470 Alleviates Ischemic-Stroke-Induced Acute Brain Injury and Functional Alterations in Mice. Int. J. Mol. Sci. 2023, 24, 12244. https://doi.org/10.3390/ijms241512244
Lu Y, Zhou W, Cui Q, Cui C. G Protein-Coupled Receptor 40 Agonist LY2922470 Alleviates Ischemic-Stroke-Induced Acute Brain Injury and Functional Alterations in Mice. International Journal of Molecular Sciences. 2023; 24(15):12244. https://doi.org/10.3390/ijms241512244
Chicago/Turabian StyleLu, Yingyu, Wanlu Zhou, Qinghua Cui, and Chunmei Cui. 2023. "G Protein-Coupled Receptor 40 Agonist LY2922470 Alleviates Ischemic-Stroke-Induced Acute Brain Injury and Functional Alterations in Mice" International Journal of Molecular Sciences 24, no. 15: 12244. https://doi.org/10.3390/ijms241512244
APA StyleLu, Y., Zhou, W., Cui, Q., & Cui, C. (2023). G Protein-Coupled Receptor 40 Agonist LY2922470 Alleviates Ischemic-Stroke-Induced Acute Brain Injury and Functional Alterations in Mice. International Journal of Molecular Sciences, 24(15), 12244. https://doi.org/10.3390/ijms241512244







