SLUG and SNAIL as Potential Immunohistochemical Biomarkers for Renal Cancer Staging and Survival
Abstract
:1. Introduction
2. Results
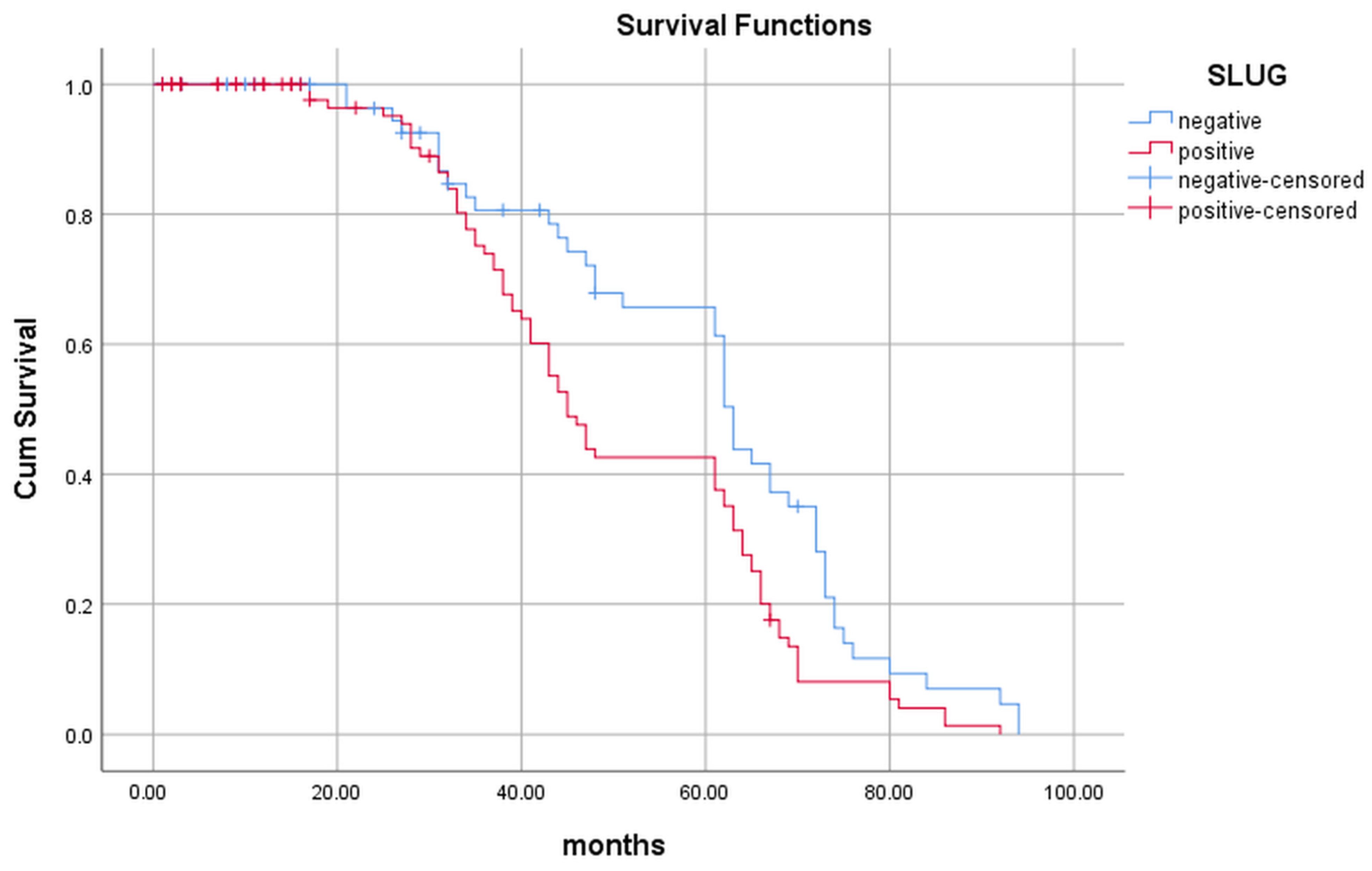
2.1. Evaluation of the Slug Expression and Its Influence on the Patients’ Survival
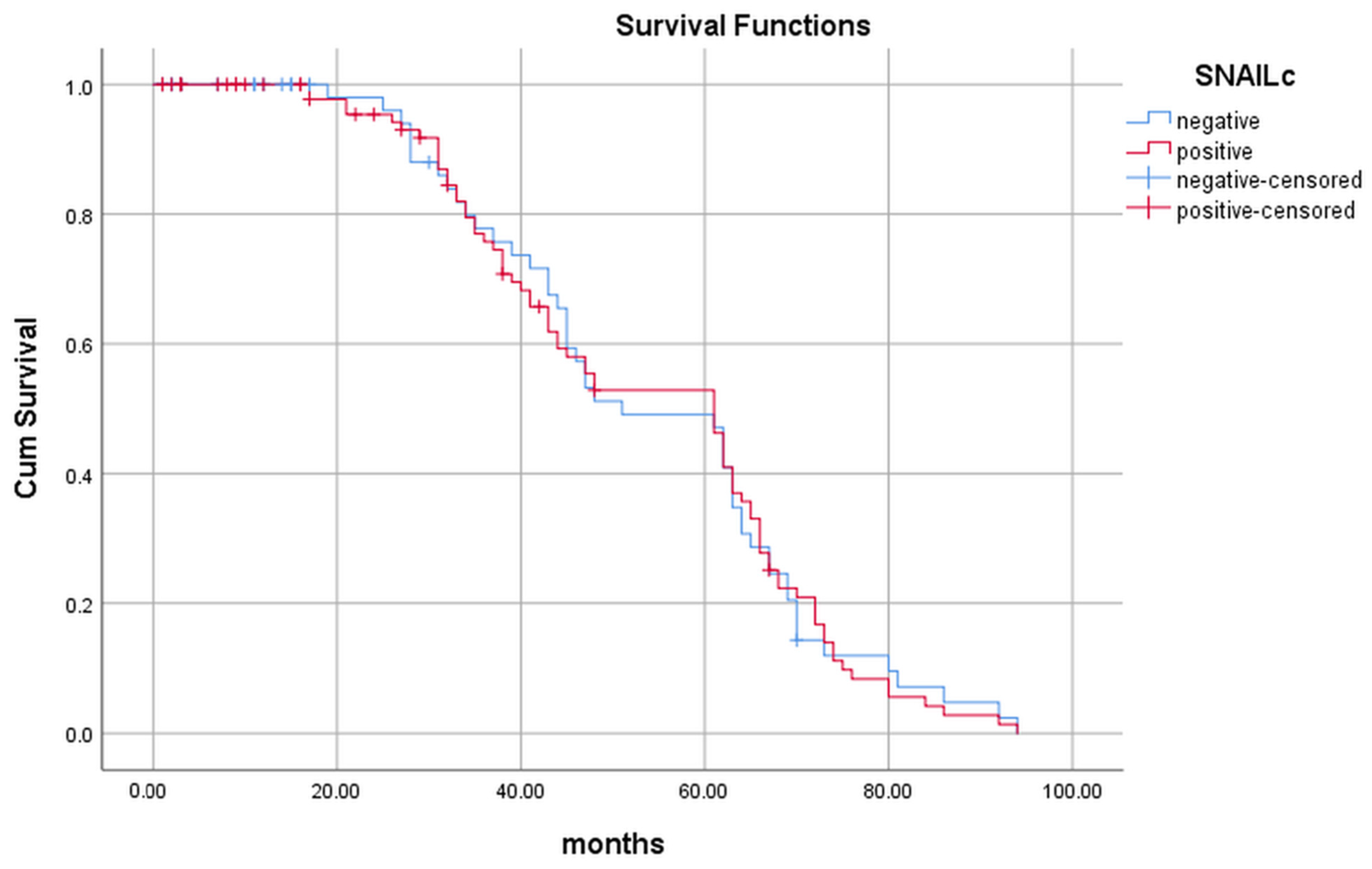
2.2. Evaluation of the Snail Expression and Its Influence on the Patients’ Survival
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Pathological Evaluation and Ethics
4.3. Tissue Microarray
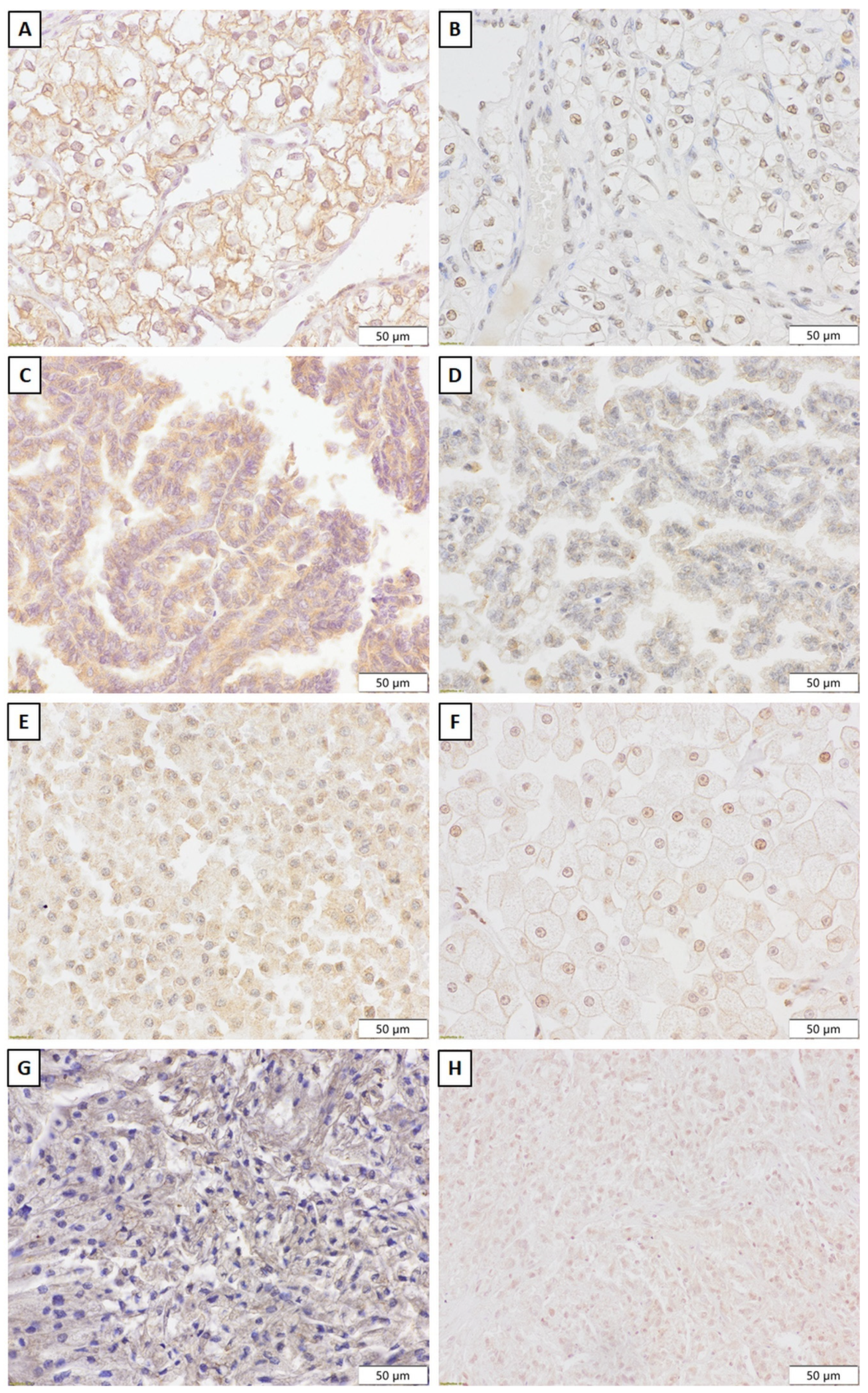
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Decastro, G.J.; McKiernan, J.M. Epidemiology, clinical staging, and presentation of renal cell carcinoma. Urol. Clin. N. Am. 2008, 35, 581–592. [Google Scholar] [CrossRef]
- Li, H.L.; Han, L.; Chen, H.R.; Meng, F.; Liu, Q.H.; Pan, Z.Q.; Bai, J.; Zheng, J.N. PinX1 serves as a potential prognostic indicator for clear cell renal cell carcinoma and inhibits its invasion and metastasis by suppressing MMP-2 via NF-κB-dependent transcription. Oncotarget 2015, 6, 21406–21420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, A.Y.; Harrison, D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: Standards and controversies. World J. Urol. 2018, 36, 1913–1926. [Google Scholar] [CrossRef] [Green Version]
- Nolazco, J.I.; Soerensen, S.J.C.; Chung, B.I. Biomarkers for detection and surveillance of renal cancer. Urol. Clin. N. Am. 2023, 50, 191–204. [Google Scholar] [CrossRef]
- Kang, Y.; Massague, J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell 2004, 118, 277–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [Green Version]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Guo, F.; Parker, K.B.C.; Yang, D.; Hu, L.; Shmulevich, I.; Sood, A.K.; Xue, F.; Zhang, W. Post-transcriptional regulatory network of epithelial-to mesenchymal and mesenchymal-to-epithelial transitions. J. Hematol. Oncol. 2014, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging biological principles of metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [Green Version]
- Santamaria, P.G.; Moreno-Bueno, G.; Portillo, F.; Cano, A. EMT: Present and future in clinical oncology. Mol. Oncol. 2017, 11, 718–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voon, D.C.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. The EMT spectrum and therapeutic opportunities. Mol. Oncol. 2017, 11, 878–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaffer, C.L.; San, J.B.P.; Lim, E.; Weinberg, R.A. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016, 35, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Aiello, N.M.; Kang, Y. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 2019, 216, 1016–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Weinberg, R.A. Epithelial-to mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Bolos, V.; Peinado, H.; Perez-Moreno, M.A.; Fraga, M.F.; Esteller, M.; Cano, A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: A comparison with Snail and E47 repressors. J. Cell Sci. 2003, 116, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Sun, X.; Xu, J.; Xuan, Y.; Zhao, Y.; Qui, T.; Hou, F.; Qin, Y.; Wang, Y.; Lu, T.; et al. Relevance and prognostic ability of Twist, Slug and tumor spread through air spaces in lung adenocarcinoma. Cancer Med. 2020, 9, 1986–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, C.C.; Carneiro, F.; Hoefer, H.; Becker, K.F. Role of the epithelial-mesenchymal transition regulator Slug in primary human cancers. Front. Biosci.-Landmark 2009, 14, 3035–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Inomoto, C.; Uchida, T.; Furuya, H.; Komiyama, T.; Kajiwara, H.; Kobayashi, H.; Nakamura, N.; Miyajima, A. Verification of the International Society of Urological Pathology recommendations in Japanese patients with clear cell renal cell carcinoma. Int. J. Oncol. 2018, 52, 1139–1148. [Google Scholar] [CrossRef] [Green Version]
- Leibovich, B.C.; Lohse, C.M.; Cheville, J.C.; Zaid, H.B.; Boorjian, S.A.; Frank, I.; Thompson, R.H.; Parker, W.P. Predicting oncologic outcomes in renal cell carcinoma after surgery. Eur. Urol. 2018, 73, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W. Epithelial–mesenchymal transition in human cancer: Comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol. Ther. 2015, 150, 33–46. [Google Scholar] [PubMed]
- Sugimoto, M.; Kohashi, K.; Itsumi, M.; Shiota, M.; Abe, T.; Yamada, Y.; Kuroiwa, K.; Naito, S.; Oda, Y. Epithelial to mesenchymal transition in clear cell renal cell carcinoma with rhabdoid features. Pathobiology 2016, 83, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Come, C.; Magnino, F.; Bibeau, F.; De Santa Barbara, P.; Becker, K.F.; Theillet, C.; Savagne, P. Snail and Slug Play Distinct Roles during Breast Carcinoma Progression. Clin. Cancer Res. 2006, 12, 5395–5402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Lo, H.W.; Hsu, S.C.; Xia, W.; Cao, X.; Shih, J.Y.; Wei, Y.; Abbruzzese, J.L.; Hortobagyi, G.N.; Hung, M.C. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007, 67, 9066–9076. [Google Scholar] [CrossRef] [Green Version]
- Oft, M.; Peli, J.; Rudaz, C.; Schwarz, H.; Beug, H.; Reichmann, E. TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes. Dev. 1996, 10, 2462–2477. [Google Scholar] [CrossRef] [Green Version]
- Dong, P.; Karaayvaz, M.; Jia, N.; Kaneuchi, M.; Hamada, J.; Watari, H.; Sudo, S.; Ju, J.; Sakuragi, N. Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis. Oncogene 2013, 32, 3286–3295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brannon, A.R.; Reddy, A.; Seiler, M.; Arreola, A.; Moore, D.T.; Pruthi, R.S.; Wallen, E.M.; Nielsen, M.E.; Liu, H.; Nathanson, K.L.; et al. Molecular stratification of clear cell renal cell carcinoma by consensus clustering reveals distinct subtypes and survival patterns. Genes Cancer 2010, 1, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zongming, M.; Tibshirani, R.; Higgins, J.P.; Ljungberg, B.; Brooks, J.D. Alteration of gene expression signatures of cortical differentiation and wound response in lethal clear cell renal cell carcinomas. PLoS ONE 2009, 4, e6039. [Google Scholar] [CrossRef]
- Mikami, S.; Katsube, K.; Oya, M.; Ishida, M.; Kosaka, T.; Mizuno, R.; Mukai, M.; Okada, Y. Expression of Snail and Slug in renal cell carcinoma: E-cadherin repressor Snail is associated with cancer invasion and prognosis. Lab. Investig. 2011, 91, 1443–1458. [Google Scholar] [CrossRef] [Green Version]
- Mytsyk, Y.; Dosenko, V.; Skrzypczyk, M.A.; Borys, Y.; Diychuk, Y.; Kucher, A.; Kowalskyy, V.; Pasichnyk, S.; Mystyk, O.; Manyuk, L. Potential clinical applications of microRNAs as biomarkers for renal cell carcinoma. Cent. European J. Urol. 2018, 71, 295–303. [Google Scholar]
- Conant, J.L.; Peng, Z.; Evans, M.F.; Naud, S.; Cooper, K. Sarcomatoid renal cell carcinoma is an example of epithelial–mesenchymal transition. J. Clin. Pathol 2011, 64, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Y.; Liu, H.; Zhang, W.; An, H.; Xu, J. Snail predicts recurrence and survival of patients with localized clear cell renal cell carcinoma after surgical resection. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 69.e1–69.e10. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Matrisian, L.M. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst. 1997, 89, 1260–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.H.; Yang, W.H.; Chang, S.Y.; Tai, S.K.; Tzeng, C.H.; Kao, J.Y.; Wu, K.J.; Yang, M.H. Regulation of membrane-type 4 matrix metalloproteinase by SLUG contributes to hypoxia-mediated metastasis. Neoplasia 2009, 11, 1371–1382. [Google Scholar] [CrossRef] [Green Version]
- Harada, K.; Miyake, H.; Kusuda, Y.; Fujisawa, M. Expression of epithelial–mesenchymal transition markers in renal cell carcinoma: Impact on prognostic outcomes in patients undergoing radical nephrectomy. BJU Int. 2012, 110, E1131–E1137. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. Snail, More than EMT. Cell Adh. Migr. 2010, 4, 199–203. [Google Scholar] [CrossRef]
- Gnemmi, V.; Bouillez, A.; Gaudelot, K.; Hémon, B.; Ringot, B.; Pottier, N.; Glowacki, F.; Villers, A.; Vindrieux, D.; Cauffiez, C.; et al. MUC1 drives epithelial—Mesenchymal transition in renal carcinoma through Wnt/b –catenin pathway and interaction with SNAIL promoter. Cancer Lett. 2018, 346, 225–236. [Google Scholar] [CrossRef]
- Moch, H. The WHO/ISUP grading system for renal carcinoma. Pathologe 2016, 37, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Andreiana, B.C.; Stepan, A.E.; Taisescu, O.; Al Khatib, A.M.; Florescu, M.M.; Simionescu, C.E.; Crisan, A.E. Immunoexpression of Snail, Twist1 and Slug in clear cell renal cell carcinoma. Rom. J. Morph. Embryiol. 2019, 60, 463–468. [Google Scholar]
- Zaldumbide, L.; Erramuzpe, A.; Guarch, R.; Pulido, R.; Cortés, J.M.; López, J.I. Snail heterogeneity in clear cell renal cell carcinoma. BMC Cancer 2016, 16, 194. [Google Scholar] [CrossRef] [Green Version]
- Lughezzani, G.; Burger, M.; Margulis, V.; Matin, S.F.; Novara, G.; Roupret, M.; Shariat, S.F.; Wood, C.C.; Zigeuner, R. Prognostic Factors in Upper Urinary Tract Urothelial Carcinomas: A Comprehensive Review of the Current Literature. Eur. Urol. 2012, 62, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Elloul, S.; Elstrand, M.B.; Nesland, J.M.; Trope, C.G.; Kvalheim, G.; Goldberg, I.; Reich, R.; Davidson, B. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer 2005, 103, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual, 7th ed.; Edge, S.B., Byrd, D.R., Compton, C.C., Eds.; Springer: New York, NY, USA, 2010; pp. 479–489. [Google Scholar]


| Slug | |||
|---|---|---|---|
| Positive (n = 100) | Negative (n = 66) | p Value | |
| Age (years) | |||
| 59.9 ± 11.0 | 62.7 ± 10.6 | 0.129 | |
| Gender | |||
| Male | 33 (58.9%) | 23 (37.7%) | 0.908 |
| Female | 67 (60.9%) | 43 (39.1%) | |
| Tumor size (mm) | |||
| 65 (10–340) | 54 (20–180) | 0.145 | |
| Invasion in adipose tissue | |||
| Yes | 11 (55%) | 9 (45%) | 0.172 |
| No | 89 (71.8%) | 35 (28.2%) | |
| Penetration to renal sinus | |||
| Yes | 18 (94.7%) | 1 (5.3%) | 0.005 * |
| No | 82 (55.7%) | 65 (44.3%) | |
| Permeation to renal vein | |||
| Yes | 14 (67.1%) | 6 (32.9%) | 0.085 |
| No | 86 (58.9%) | 60 (41.1%) | |
| Tumor type | |||
| ccRCC | 62 (57.4%) | 46 (42.6%) | 0.712 |
| pRCC—low grade | 3 (42.9%) | 4 (57.1%) | |
| pRCC—high grade | 9 (60%) | 6 (40%) | |
| chRCC | 18 (72%) | 7 (28%) | |
| CDC—Bellini | 5 (83.3%) | 1 (16.7%) | |
| MCRNLMP | 3 (60%) | 2 (40%) | |
| Nuclear grade (only ccRCC) | |||
| I and II | 37 (48%) | 40 (52%) | 0.001 * |
| III and IV | 25 (80.6%) | 6 (19.4%) | |
| Sarcomatoid component | |||
| Yes | 13 (92.3%) | 1 (7.7%) | 0.032 * |
| No | 87 (57.2%) | 65 (42.8%) | |
| TNM stage | |||
| I | 28 (56%) | 22 (44%) | 0.208 |
| II | 11 (61.1%) | 7 (38.9%) | |
| III | 40 (75.5%) | 13 (24.5%) | |
| IV | 6 (54.5%) | 5 (45.5%) | |
| Metastasis | |||
| Yes | 6 (54.5%) | 5 (45.5%) | 0.536 |
| No | 94 (60.6%) | 61 (39.4%) | |
| Outcome | |||
| Survived | 76 (60.8%) | 49 (39.2%) | 0.574 |
| Died | 24 (58.5%) | 17 (41.5%) | |
| Kaplan Maier Univariant Analysis | Cox regression Multivariant Analysis | |||
|---|---|---|---|---|
| Prognostic Factor | Average—Months | p Value | Hazard Ratio | p Value |
| (95%CI) | (95% CI) | |||
| Gender | ||||
| Female | 48 (43–66) | 0.251 | ||
| Male | 61 (45–62) | |||
| Invasion to perirenal adipose tissue | ||||
| Yes | 61 (46–63) | 0.384 | ||
| No | 65 (43–70) | |||
| Penetration to renal sinus | ||||
| Yes | 45 (41–64) | 0.411 | ||
| No | 62 (61–64) | |||
| Permeation to renal vein | ||||
| Yes | 63 (35–64) | 0.446 | ||
| No | 61 (47–63) | |||
| Sarcomatoid component | ||||
| Yes | 39 (33–47) | 0.032 * | 2.19 (0.87–5.54) | 0.097 |
| No | 61 (47–63) | |||
| TNM staging | ||||
| I and II | 62 (47–64) | 0.180 | ||
| III and IV | 45 (38–62) | |||
| Slug expression | ||||
| Yes | 45 (41–64) | 0.002 * | 1.73 (1.16–2.57) | 0.046 * |
| No | 63 (62–72) | |||
| Metastasis | ||||
| Yes | 41 (41-NA) | 0.956 | ||
| No | 61 (45–62) | |||
| Snail Nuclear Expression | Snail Cytoplasmatic Expression | |||||
|---|---|---|---|---|---|---|
| Positive (n = 72) | Negative (n = 94) | p Value | Positive (n = 124) | Negative (n = 42) | p Value | |
| Tumor size (mm) | ||||||
| 71.8 ± 47.6 | 66.4 ± 30.6 | 0.416 | 70.5 ± 41.6 | 63.7 ± 29.1 | 0.251 | |
| Penetration to renal sinus | ||||||
| Yes | 10 (52.3%) | 9 (47.4%) | 0.462 | 17 (90.0%) | 2 (10.0%) | 0.090 |
| No | 62 (42.2%) | 85 (57.8%) | 107 (72.8%) | 40 (27.2%) | ||
| Permeation to renal vein | ||||||
| Yes | 16 (80.0%) | 4 (20.0%) | <0.001 | 18 (90.0%) | 2 (10.0%) | 0.090 |
| No | 56 (38.3%) | 90 (61.7%) | 106 (72.6%) | 40 (27.4%) | ||
| Invasion to perirenal adipose tissue | ||||||
| Yes | 13 (65.0%) | 7 (35.0%) | 0.051 | 18 (90.0%) | 2 (10%) | 0.090 |
| No | 59 (40.4%) | 87 (59.6%) | 106 (72.6%) | 40 (27.4%) | ||
| Tumor type | ||||||
| ccRCC | 49 (45.4%) | 59 (54.6%) | 0.467 | 80 (74.1%) | 28 (25.9%) | 0.143 |
| pRCC—low grade | 2 (28.6%) | 5 (71.4%) | 4 (57.1%) | 3 (42.9%) | ||
| pRCC—high grade | 4 (26.7%) | 11 (73.3%) | 14 (93.3%) | 1 (6.7%) | ||
| chRCC | 13 (52.0%) | 12 (48.0%) | 20 (80.0%) | 5 (20.0%) | ||
| CDC (Bellini) | 3 (50.0%) | 3 (50.0%) | 4 (66.7%) | 2 (33.3%) | ||
| MCRNLMP | 1 (20.0%) | 4 (80.0%) | 2 (60.0%) | 3 (40.0%) | ||
| Nuclear grade (only ccRCC) | ||||||
| I | 11 (57.9%) | 8 (42.1%) | 0.041 * | 16 (84.2%) | 3 (15.8%) | 0.343 |
| II | 20 (33.9%) | 39 (66.1%) | 40 (67.8%) | 19 (32.2%) | ||
| III | 11 (68.8%) | 5 (31.2%) | 14 (87.5%) | 2 (12.5%) | ||
| IV | 7 (53.8%) | 6 (46.2%) | 10 (76.9%) | 3 (23.1%) | ||
| Sarcomatoid component | ||||||
| Yes | 9 (64.3%) | 5 (35.7%) | 0.160 | 10 (71.4%) | 4 (28.6%) | 0.756 |
| No | 63 (41.4%) | 89 (58.6%) | 114 (75.0%) | 38 (25.0%) | ||
| TNM stage | ||||||
| I | 15 (27.8%) | 39 (72.2%) | <0.001 | 31 (57.4%) | 23 (42.6%) | 0.003 * |
| II | 6 (33.3%) | 12 (66.7%) | 11 (61.1%) | 7 (38.9%) | ||
| III | 25 (46.3%) | 29 (53.7%) | 44 (81.5%) | 10 (18.5%) | ||
| IV | 11 (100%) | 0 (0%) | 11 (100%) | 0 (0%) | ||
| Survival (months) | ||||||
| 39.3 ± 25.4 | 47.3 ± 23.0 | 0.037 * | 42.2 ±24.4 | 48.4 ± 23.7 | 0.160 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zivotic, M.; Kovacevic, S.; Nikolic, G.; Mioljevic, A.; Filipovic, I.; Djordjevic, M.; Jovicic, V.; Topalovic, N.; Ilic, K.; Radojevic Skodric, S.; et al. SLUG and SNAIL as Potential Immunohistochemical Biomarkers for Renal Cancer Staging and Survival. Int. J. Mol. Sci. 2023, 24, 12245. https://doi.org/10.3390/ijms241512245
Zivotic M, Kovacevic S, Nikolic G, Mioljevic A, Filipovic I, Djordjevic M, Jovicic V, Topalovic N, Ilic K, Radojevic Skodric S, et al. SLUG and SNAIL as Potential Immunohistochemical Biomarkers for Renal Cancer Staging and Survival. International Journal of Molecular Sciences. 2023; 24(15):12245. https://doi.org/10.3390/ijms241512245
Chicago/Turabian StyleZivotic, Maja, Sanjin Kovacevic, Gorana Nikolic, Ana Mioljevic, Isidora Filipovic, Marija Djordjevic, Vladimir Jovicic, Nikola Topalovic, Kristina Ilic, Sanja Radojevic Skodric, and et al. 2023. "SLUG and SNAIL as Potential Immunohistochemical Biomarkers for Renal Cancer Staging and Survival" International Journal of Molecular Sciences 24, no. 15: 12245. https://doi.org/10.3390/ijms241512245
APA StyleZivotic, M., Kovacevic, S., Nikolic, G., Mioljevic, A., Filipovic, I., Djordjevic, M., Jovicic, V., Topalovic, N., Ilic, K., Radojevic Skodric, S., Dundjerovic, D., & Nesovic Ostojic, J. (2023). SLUG and SNAIL as Potential Immunohistochemical Biomarkers for Renal Cancer Staging and Survival. International Journal of Molecular Sciences, 24(15), 12245. https://doi.org/10.3390/ijms241512245







